Abstract
Background
To establish a prediction model in selecting fit patients with resected pIIIA-N2 non-small cell lung cancer (NSCLC) for postoperative radiotherapy (PORT), and evaluate the model in clinical practice.
Methods
Between January 2003 and December 2005, 221 patients with resected pIIIA-N2 NSCLC were retrospectively analyzed. The effect of PORT on overall survival (OS) of patients with different clinicopathological factors was evaluated and the results were used to establish a prediction model to select patients fit for PORT.
Results
Compared with the control, PORT significantly improved the OS of patients with a smoking index ≤400 (P = 0.033), cN2 (P = 0.003), pT3 (P = 0.014), squamous cell carcinoma (SCC) (P = 0.013), or ≥4 positive nodes (P = 0.025). Patients were divided from zero to all five factors into low, middle, and high PORT index (PORT-I) groups (scored 0–1, 2, and 3–5, respectively). PORT did not improve OS (3-year, P = 0.531), disease free survival (DFS) (P = 0.358), or loco-regional recurrence free survival (LRFS) (P = 0.412) in the low PORT-I group. PORT significantly improved OS (P = 0.033), and tended to improve DFS (P = 0.064), but not LRFS (P = 0.287) in the middle PORT-I group. PORT could significantly improve OS (P = 0.000), DFS (P = 0.000), and LRFS (P = 0.006) in the high PORT-I group.
Conclusion
The prediction model is valuable in selecting patients with resected pIIIA-N2 NSCLC fit for PORT. PORT is strongly recommended for patients with three or more of the five factors of smoking index ≤400, cN2, pT3, SCC, and ≥4 positive nodes.
Keywords: IIIA-N2, non-small-cell lung cancer, postoperative radiotherapy, prediction model, surgery
Introduction
As a heterogeneous group of diseases, completely resected pathological stage IIIA-N2 non-small cell lung cancer (NSCLC) has five-year overall survival (OS) rates ranging from 7% to 34%.1–4 In view of the high loco-regional recurrence failure of up to 40% even after adjuvant chemotherapy,5–7 postoperative radiotherapy (PORT) has been incorporated into multidisciplinary management. However, the definitive role of PORT in resected pIIIA-N2 NSCLC is unknown.8,9 The improvement of modern radiotherapy techniques has seen a resurgence of interest in the field.10,11 However, no study on selecting fit patients with pIIIA-N2 NSCLC for PORT has been reported. Our previous study showed that PORT can improve the OS and tumor control in patients with pIIIA-N2 NSCLC.12 Based on these findings, the effect of PORT in patients with different clinicopathological features was further analyzed, and a new prediction model was established to select proper candidates with resected pIIIA-N2 NSCLC for PORT. The value of the model was also assessed.
Materials and methods
Patient selection
Between January 2003 and December 2005, 221 patients with pIIIA-N2 NSCLC (according to the 1997 American Joint Committee on Cancer Staging System), who survived ≥4 months after complete resection, were included in this study. The medical records and follow-up data of the patients were retrospectively analyzed.
Treatment
The treatment details were described in our previous study.12 All patients underwent lobectomy or ipsilateral pneumonectomy, with complete mediastinal lymph node dissection or systematic mediastinal lymph node sampling. Adjuvant chemotherapy was administered with a cisplatin or paclitaxel-based regimen, with a median of four cycles. Some patients did not receive adjuvant chemotherapy because of asthenia, refusal by the patient, or choice of physician. The administration of PORT was mainly based on the radiation oncologist's decision. Radiotherapy techniques included three-dimensional conformal radiotherapy (3DCRT) and conventional two-dimensional radiotherapy (2DRT). The clinical target volume (CTV) included the subcarinal nodes, ipsilateral mediastinum, and ipsilateral hilum. The planning target volume was defined as CTV plus 0.8 cm margins. Thirty doses of 2 Gy were given up to a total dose of 60 Gy, using six to eight MV X-rays.
Data analysis
Patients were followed-up one month after radiotherapy, every three months for the first year, then every three to six months thereafter. OS was defined as from the day of surgery to the time of death from any cause or last follow-up. Disease free survival (DFS) was defined as from the day of surgery to the time of death, tumor recurrence, or last follow-up. Loco-regional recurrence free survival (LRFS) was defined as from the day of surgery to the time of loco-regional tumor recurrence or last follow-up. The Kaplan–Meier method and log-rank test were used for survival analyses. A Pearson chi-square test was used to compare the constituent ratios in different groups. A statistically significant difference was set as P < 0.05.
Results
Patients and tumor characteristics
Clinicopathological details of the 221 patients were presented in our previously published study.12 Of all the patients, 161 (72.9%) received adjuvant chemotherapy, and 96 patients (43.4%) received PORT, including 41 treated with 3DCRT and 55 with 2DRT. The factors were comparable between the two groups, except that there were more male patients, preoperative clinical N2 (cN2), squamous cell carcinoma (SCC), and fewer patients receiving adjuvant chemotherapy in the PORT group.
Effect of postoperative radiotherapy (PORT) on overall survival of patients with different clinicopathological factors: subgroup analysis
Our previous study has shown that PORT can improve the OS of pIIIA-N2 NSCLC patients. In order to find which subgroup may benefit from PORT, the effect of PORT on the OS of patients with different clinicopathological factors was analyzed, and the result is shown in Table 1. PORT only significantly improved the OS in patients with a smoking index (SI: number of cigarettes smoked per day × number of cigarette-years) ≤400 (P = 0.032), cN2 (P = 0.003), pathological stage T3 (pT3) (P = 0.014), SCC (P = 0.013), or metastatic lymph nodes (LNM) ≥4 (P = 0.025).
Table 1.
Effect of PORT on OS of pIIIA-N2 patients with different clinicopathological factors
| Patient number | MST (month) | P-value | ||||
|---|---|---|---|---|---|---|
| Factors | PORT | Non-PORT | PORT | Non-PORT | ||
| Gender | Male | 77 | 83 | 43.2 | 28.9 | 0.112 |
| Female | 19 | 42 | 56.2 | 32.0 | 0.139 | |
| Age | ≤60 y | 51 | 65 | 43.4 | 26.2 | 0.223 |
| >60 y | 45 | 60 | 46.9 | 34.2 | 0.108 | |
| Smoking Index | ≤400 | 54 | 83 | 49.5 | 32.0 | 0.032 |
| >400 | 42 | 42 | 32.6 | 28.8 | 0.424 | |
| Laterality | Left lung | 46 | 51 | 46.2 | 28.8 | 0.100 |
| Right lung | 50 | 74 | 43.4 | 34.2 | 0.206 | |
| Lobe location | Upper+middle | 60 | 75 | 43.4 | 31.9 | 0.243 |
| Lower | 36 | 50 | 46.9 | 31.5 | 0.092 | |
| Clinical N stage | N0-1 | 31 | 58 | 60.4 | 53.3 | 0.452 |
| N2 | 65 | 67 | 39.2 | 19.7 | 0.003 | |
| Type of surgery | Lobectomy | 84 | 113 | 46.2 | 34.2 | 0.076 |
| Pneumonectomy | 12 | 12 | 24.0 | 16.9 | 0.192 | |
| Pathological T stage | T1-2 | 76 | 107 | 48.0 | 35.6 | 0.124 |
| T3 | 20 | 18 | 24.0 | 13.8 | 0.014 | |
| Histology subtype | SCC | 54 | 35 | 46.2 | 26.2 | 0.013 |
| Non- SCC | 42 | 90 | 34.0 | 34.6 | 0.538 | |
| Lymph node resected | 1–20 | 51 | 54 | 43.2 | 35.6 | 0.385 |
| >20 | 45 | 71 | 46.2 | 28.8 | 0.069 | |
| Positive lymph nodes | 1–3 | 30 | 43 | 68.0 | 52.0 | 0.419 |
| ≥4 | 66 | 82 | 39.3 | 20.6 | 0.025 | |
| Stations of positive N2 | 1 | 62 | 77 | 50.4 | 38.6 | 0.148 |
| 2–4 | 34 | 48 | 34.0 | 22.7 | 0.138 | |
| Chemotherapy | With | 61 | 100 | 48.0 | 33.1 | 0.057 |
| Without | 35 | 25 | 38.3 | 21.6 | 0.219 | |
MST, median survival time; OS, overall survival; PORT, postoperative radiotherapy; SCC, squamous cell carcinoma.
Establishment of a predictive model to evaluate the necessity for PORT for resected pIIIA-N2 non-small cell lung cancer (NSCLC)
Cox regression analysis showed that the hazard ratios (HRs) for all-cause mortality for PORT were: 0.615 (95% confidence interval [CI]: 0.329-0.966) in patients with CI ≤ 400; 0.541 (95% CI: 0.360-0.813) with cN2; 0.414 (95%CI: 0.201-0.853) with pT3; 0.518 (95% CI: 0.306-0.879) with SCC; and 0.642 (95% CI: 0.435-0.948) with LNM ≥ 4. Because the HRs for PORT were similar among the five factors, each factor was equally given one score. Patients with zero to five of all the factors were scored 0 to 5, and the corresponding patient numbers were 11, 42, 82, 62, 22, and two, respectively. As a result of the imbalanced number of patients with a different score, the patients were then merged into low PORT index (PORT-I) (score 0–1), middle PORT-I (score 2), and high PORT-I groups (score 3–5). The numbers of patients in the three groups were 53 (24.0%), 82 (37.1%), and 86 (38.9%), respectively.
Ability of the model in predicting the necessity of PORT for resected pIIIA-N2 NSCLC
In order to check the ability of this model in predicting the necessity of PORT, the effect of PORT in the low, middle, and high PORT-I groups was then analyzed. OS is shown in Table 2 and Figure 1. The beneficial effect of PORT on OS increased in a PORT-I dependent manner. Compared with non-PORT patients, PORT deteriorated the OS of patients in the low PORT-I group, although the difference was not statistically significant (P = 0.531). PORT significantly improved the OS of patients in the middle and high PORT-I groups (P = 0.033 and P = 0.000, respectively). The HR for all-cause mortality for PORT in the three groups gradually decreased from 1.340 (95% CI: 0.535-3.351) to 0.542 (95% CI: 0.306-0.960), and 0.413 (95% CI: 0.251-0.680), respectively. When the median survival time (MST) of PORT patients was compared with that of the non-PORT patients, the ratio increased from <1 in the low PORT-I group to 1.87 and 2.26 in middle and high PORT-I groups. For patients without PORT, OS was significantly reduced with the increase of the PORT-I (P < 0.001). The MST of patients was >60 months (not reached), 26.9 months, and 17.0 months in the low, middle and high PORT-I groups, respectively (Fig 2). But for patients receiving PORT, OS in the three groups was not significantly different (P = 0.134), and the MST was 46.2, 50.4, and 35.3 months, respectively (Fig 2). This may be a result of the negative effect of PORT on OS in the low PORT-I group, but positive effect in the middle and high PORT-I groups in an index dependent manner.
Table 2.
Effect of PORT on survival of resected pIIIA-N2 NSCLC according to the PORT index
| Overall survival | DFS | LRFS | |||||
|---|---|---|---|---|---|---|---|
| PORT index | 3-year rate | P-value | 3-year rate | P-value | 3-year rate | P-value | |
| Low (0–1) | Non-PORT | 76.8% | 0.531 | 46.2% | 0.358 | 68.0% | 0.412 |
| PORT | 57.1% | 53.8% | 74.0% | ||||
| Middle (2) | Non-PORT | 44.2% | 0.033 | 27.4% | 0.064 | 64.9% | 0.287 |
| PORT | 69.0% | 42.1% | 74.1% | ||||
| High (3–5) | Non-PORT | 17.5% | <0.001 | 7.3% | <0.001 | 42.7% | 0.006 |
| PORT | 50.8% | 33.2% | 61.6% | ||||
DFS, disease free survival; LRFS, loco-regional recurrence free survival; NSCLC, non-small cell lung cancer; PORT, postoperative radiotherapy.
Figure 1.
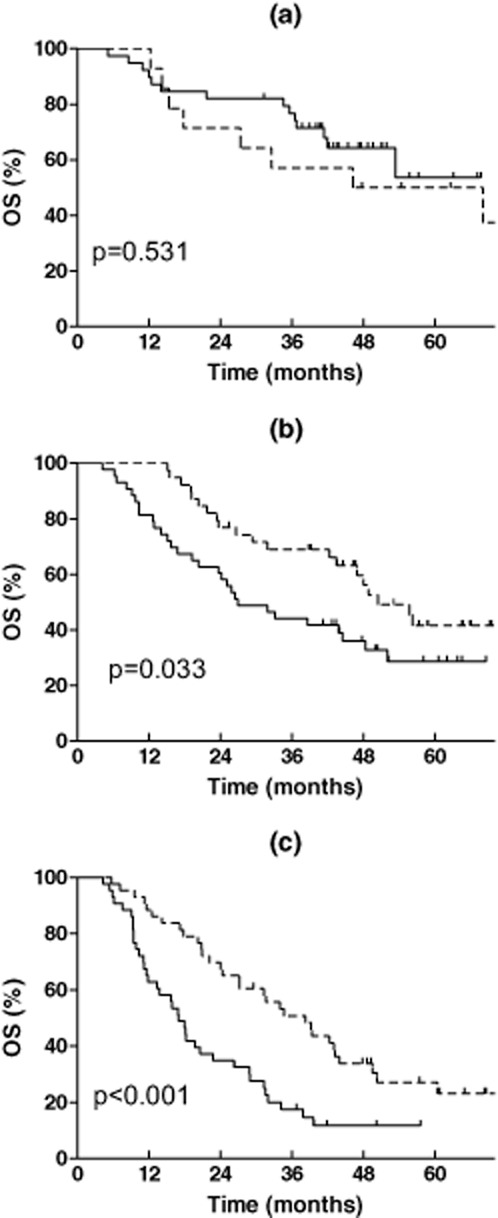
Effect of postoperative radiotherapy (PORT) on overall survival of resected pIIIA-N2 non-small cell lung cancer according to the PORT index. (a) Low PORT index (0–1); (b): middle PORT index (2); (c): high PORT index (3–5).  , Non-PORT;
, Non-PORT;  , PORT.
, PORT.
Figure 2.
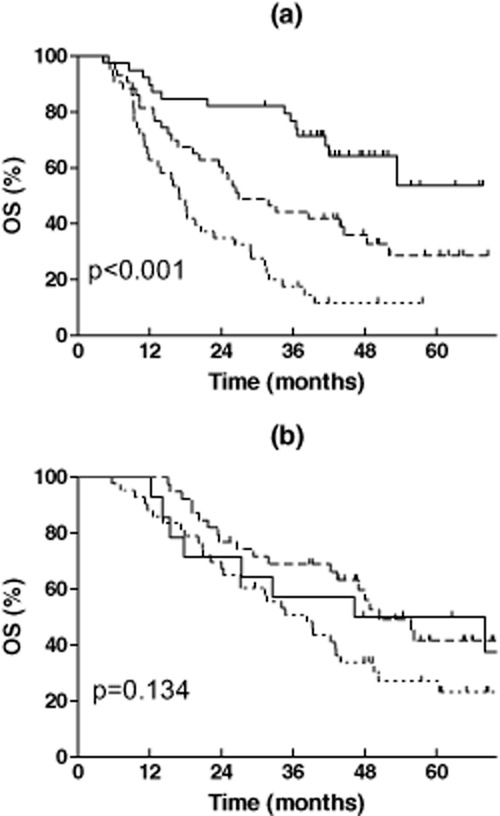
Overall survival of the low, middle, and high postoperative radiotherapy (PORT) index patients in the: (a) non-PORT group, and (b) PORT group.  , Index 0–1;
, Index 0–1;  , Index 2;
, Index 2;  , Index 3–5.
, Index 3–5.
The effect of PORT on DFS and LRFS was also varied in a PORT-I dependent manner, shown in Table 2 and Figures 3 and 4. PORT did not improve the DFS of patients in the low PORT-I group (P = 0.358), tended to improve the DFS in the middle PORT-I group (P = 0.064), and significantly improved the DFS in the high PORT-I group (P = 0.000). The HR for PORT in the three groups gradually decreased from 0.658 (95% CI: 0.268-1.616) to 0.615 (95% CI: 0.366-1.034) and 0.385 (95% CI: 0.233-0.636), respectively. PORT did not improve the LRFS in the low or middle PORT-I groups (P = 0.412 and P = 0.287), but significantly improved the LRFS in the high PORT-I group (P = 0.006). The HR for PORT in the three groups was 0.595 (95% CI: 0.170-2.087), 0.652 (95% CI: 0.295-1.442), and 0.394 (95% CI: 0.198-0.785), respectively.
Figure 3.
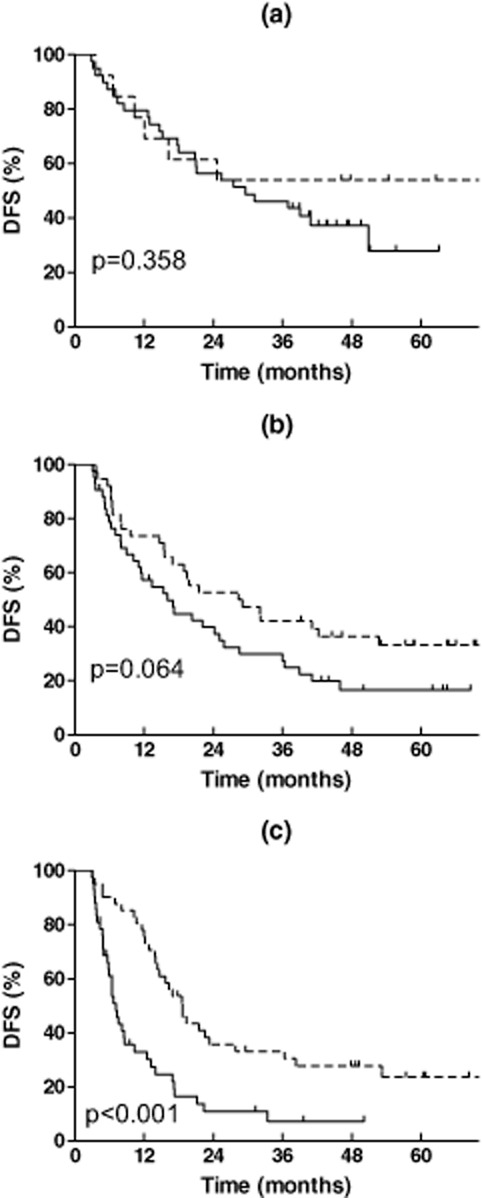
Effect of postoperative radiotherapy (PORT) on disease free survival of resected pIIIA-N2 non-small cell lung cancer according to the PORT index. (a) Low PORT index (0–1); (b) middle PORT index (2); (c) high PORT index (3–5).  , Non-PORT;
, Non-PORT;  , PORT.
, PORT.
Figure 4.
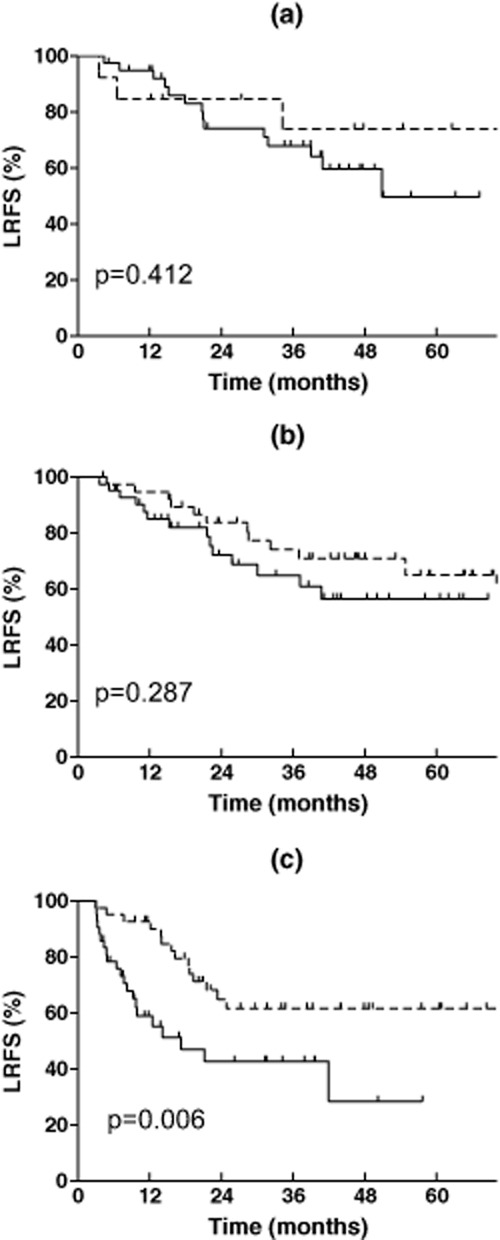
Effect of postoperative radiotherapy (PORT) on loco-regional recurrence free survival of resected IIIA-N2 non-small cell lung cancer according to the PORT index. (a) Low PORT index (0–1); (b) middle PORT index (2); (c) high PORT index (3–5).  Non-PORT;
Non-PORT;  , PORT.
, PORT.
Value of the prediction model in patients with adjuvant chemotherapy
Of the 221 patients, 161 received adjuvant chemotherapy. The number of patients in the low, middle, and high PORT-I groups was 53 (32.9%), 83 (51.6%), and 25 (15.5%), respectively. PORT did not improve the OS of patients in the low PORT-I group (HR = 0.988, 95% CI: 0.314-3.107; P = 0.984). PORT tended to improve the OS in the middle PORT-I group (HR = 0.614, 95% CI: 0.299-1.206; P = 0.179). In the high PORT-I group, PORT could significantly improve the OS (HR = 0.371, 95% CI: 0.204-0.677; P = 0.001). The ratio of MST in PORT patients compared with non-PORT patients also gradually increased from 1.28 in the low PORT-I group to 1.75 and 2.17 in the middle and high PORT-I groups, respectively.
Discussion
The aim of this study was to assess the differential effect of PORT in predefined subgroups of resected pIIIA-N2 NSCLC patients with different clinicopathological features, and then to establish a prediction model to select proper candidates for PORT. PORT has long been administered to improve loco-regional tumor control. However, a clear benefit to survival has not yet been reported. A meta-analysis indicated that PORT was not associated with improved survival in resected pathological stage N2 (pN2) patients.8 This may be a result of the high morbidity and mortality of suboptimal radiotherapy techniques.13 In the modern era, 3DCRT and intensity-modulated radiotherapy have been widely implemented, which has proven effective in reducing the radiation toxicity of normal tissues. Under these conditions, the role of PORT for resected pIIIA-N2 NSCLC required reevaluation.
A generally accepted method of assessing the PORT effect in pIIIA-N2 NSCLC after complete resection is to conduct randomized clinical trials (RCTs). To our knowledge, there have been three such phase III RCTs using modern irradiation techniques. The earliest one, CALGB 9734, began in 1998 but failed after two years because of slow accrual. One ongoing phase III RCT, known as “LUNG ART,” began in 2007 and aims to enrol 700 patients by its conclusion in 2017.14 The other ongoing phase III multi-center RCT, conducted by our institute, plans to accrue 500 patients.15 By May 2012, we had 128 patients enrolled, fewer than scheduled. Conducting RCTs is rather difficult. The fact that only some pN2 patients may benefit from PORT, which is likely to be outweighed by the deleterious effect in a subgroup of unsuitable pN2 patients receiving PORT, makes RCTs even harder. Even if a positive conclusion on PORT can finally be drawn from RCTs, PORT will continue to be performed without considering the potential individual differences, which is still far from individualized treatment. Moreover, an increasing number of high quality cohort studies have shown that PORT could improve the survival of pIIIA-N2 NSCLC patients under the modern treatment model.11,12,16–20
A more feasible and reasonable method is to select suitable candidates or the high-risk subset of the pIIIA-N2 NSCLC population who may benefit from PORT. In our analysis of pIIIA-N2 NSCLC patients with 26 different clinicopathological factors, PORT significantly only improved the OS in patients with SI ≤400, cN2, pT3, SCC, or LNM ≥4. Smoking is one of the major poor prognostic factors of NSCLC,21–23 including resected NSCLC.24 Although a literature-based meta-analysis showed that ongoing smoking protected against radiation pneumonitis (RP; P = 0.008),25 current smokers still have a poorer prognosis for survival after definitive radiotherapy,26 or poorer locoregional control after PORT for NSCLC.27 Our study revealed that PORT significantly improved the OS of patients with SI ≤400. This may be because of fewer comorbidities, better tolerance, and better tumor response to PORT in non-smokers or light smokers than in heavy smokers. Further studies are needed to identify the biological basis of the smoking effect on radiotherapy in NSCLC patients.
When compared with cN0-1, pT1-2 and LNM < 4 in pIIIA-N2 NSCLC, cN2, pT3, and LNM ≥4 normally represent advanced stage and a higher tumor burden, which were closely related with poorer prognosis and more treatment failures, even after complete resection.28 Theoretically, patients with these high risk factors can benefit more from PORT. A study including >4000 cases also reported that patients with LNM ≥4 had an improved five-year OS in association with PORT (11% vs. 18%; P = 0.001).29 A Chinese study of PORT on resected pN2 NSCLC showed that PORT was related to a higher five-year survival in patients with larger tumors (>3 cm) compared to non-PORT patients (33% vs. 15%; P = 0.002), but not in those with tumor ≤3 cm.18 Our results are in accord with these findings.
A study including 13 010 resected lung cancers showed that SCC was associated with lower OS compared with adenocarcinoma;30 however, the prognostic significance of SCC is still controversial, especially in completely resected pN2 NSCLC.12,31 As SCC has a lower propensity to metastasize (especially to the brain) when compared with adenocarcinoma, we believe that PORT could improve the OS of SCC patients with NSCLC. Varlotto et al. reported that SCC had a higher local failure rate (21% vs. 14%) and lower distant failure rate (7% vs. 11%) than adenocarcinoma in patients with resected NSCLC.32 Thus, after complete resection and effective adjuvant chemotherapy, more rigorous local treatment using PORT to eradicate the micro-residues of the tumor can finally reflect an improvement in OS in patients with SCC. The effect of PORT on eliminating loco-regional tumors can also lower the risk of potential tumor metastasis from these sites, as proven by our previous study.12
Some studies found that PORT could improve the survival of patients with other factors, such as extracapsular extension negative and multi-station N2 nodes;18,33 however, these results were not repeated in our study. This may be a result of a selection bias of patients, as well as limited details on the record of clinicopathological factors in our study.
In order to select patients fit for PORT in a more precise and efficient way, a prediction model was established with the integration of the five factors. As with the international index in non-Hodgkin's lymphoma,34 we used PORT-I to divide pIIIA-N2 NSCLC patients into low (score 0–1), middle (score 2) and high (score 3–5) PORT-I groups. We posed that the higher the PORT-I, the more benefit (necessity) would be gained. Three commonly used endpoints including OS, DFS, and LRFS were adopted to check the ability of the model in assessing the necessity of PORT according to the PORT-I. Compared with the control, PORT did not significantly improve the OS, DFS or LRFS in the low PORT-I group. PORT significantly improved the OS, and tended to improve DFS, but not LRFS in the middle PORT-I group. PORT could significantly improve the OS, DFS, and LRFS in the high PORT-I group. In view of the results, the prediction model using PORT-I to select resected pIIIA-N2 NSCLC patients fit for PORT is fairly helpful in clinical practice. According to the model, patients with a high PORT-I should be recommended for PORT, while the patients with a low PORT-I should avoid this treatment method. These PORT-I groups could be excluded from future phase III RCTs. To our knowledge, this is the first prediction model addressing individualized PORT in IIIA-N2 NSCLC patients after complete resection. This multi-factor model may be more accurate and useful, though more complex, than a recently published method using only the lymph node ratio to predict the benefit of PORT in NSCLC.35
Clinical evidence has confirmed the benefit of adjuvant chemotherapy for completely resected pIIIA-N2 NSCLC, and postoperative chemotherapy has been the standard of care in the treatment of these subgroup populations.5–7 However, the addition of adjuvant chemotherapy was correlated with more lung toxicities induced by PORT. Our previous study revealed that the incidence of RP was significantly higher in patients treated with adjuvant chemotherapy than in those without adjuvant chemotherapy (21.9% vs. 5.3%; P = 0.039).36 A recently published systematic review also confirmed that sequential chemotherapy significantly increased RP (odds ratio = 1.6, P = 0.01).25 Therefore, it is pivotal to check if our prediction model is also feasible in patients who received adjuvant chemotherapy. Our analysis showed that PORT did not improve OS in the low PORT-I group, tended to improve OS in the middle PORT-I group, and significantly improved the OS in the high PORT-I group. We believe that the prediction model is also helpful in approaching individualized PORT under the present triple-modality treatment model.
As a retrospective analysis, our study has some limitations. First, all of the patients came from our single institution; therefore the number of cases is limited. The results should be interpreted cautiously as selection bias may exist. Second, more than half of the patients in the PORT group received conventional 2D techniques. This may underestimate the beneficial effect of PORT because of treatment toxicities. Third, although the prediction model showed exciting results in selecting patients fit for PORT in the training cohort, an additional testing cohort should be adopted for independent validation in a future study. Finally, our prediction model is based purely on clinicopathlogical features. Understanding and exploiting the molecular biomarkers have greatly improved individualized treatment in NSCLC.37 Further study integrating clinical and molecular information of patients may be a potential method for selecting PORT candidates more precisely.
Conclusion
In patients with completely resected pIIIA-N2 NSCLC, those with SI ≤400, cN2, pT3, SCC, or LNM ≥4 may benefit from PORT. The prediction model based on PORT-I is valuable in selecting fit patients for PORT. PORT is strongly recommended for patients with a high PORT-I and should be avoided in patients with a low PORT-I. Integration of the findings of ongoing phase III RCTs and molecular biomarkers will continue to improve the prediction model, with the aim of individualized PORT for completely resected pIIIA-N2 NSCLC.
Acknowledgments
This study was supported by the Capital Health Development Research Grant for Youth Scholars (2011-4002-05) and the Funding for Talents Training Project in Beijing (2012D009008000001).
Disclosure
No authors report any conflict of interest.
References
- Vansteenkiste JF, De Leyn PR, Deneffe GJ, Lerut TE, Demedts MG. Clinical prognostic factors in surgically treated stage IIIA-N2 non-small cell lung cancer: analysis of the literature. Lung Cancer. 1998;19:3–13. doi: 10.1016/s0169-5002(97)00072-x. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Ahn YC, Lim do H. Analyses on prognostic factors following tri-modality therapy for stage IIIa non-small cell lung cancer. Lung Cancer. 2007;55:329–336. doi: 10.1016/j.lungcan.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Casali C, Stefani A, Natali P, Rossi G, Morandi U. Prognostic factors in surgically resected N2 non-small cell lung cancer: the importance of patterns of mediastinal lymph nodes metastases. Eur J Cardiothorac Surg. 2005;28:33–38. doi: 10.1016/j.ejcts.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Andre F, Grunenwald D, Pignon JP, et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol. 2000;18:2981–2989. doi: 10.1200/JCO.2000.18.16.2981. [DOI] [PubMed] [Google Scholar]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- Douillard J, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- PORT Meta-analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet. 1998;352:257–263. [PubMed] [Google Scholar]
- PORT Meta-analysis Trialists Group. Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2005;(2) doi: 10.1002/14651858.CD002142.pub2. CD002142. [DOI] [PubMed] [Google Scholar]
- Uno T, Sumi T, Kihara A, et al. Postoperative radiotherapy for non-small-cell lung cancer: results of the 1999–2001 patterns of care study nationwide process survey in Japan. Lung Cancer. 2007;56:357–362. doi: 10.1016/j.lungcan.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Lally B, Zelterman D, Colasanto JM, Haffty BG, Detterbeck FC, Wilson LD. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. 2006;24:2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- Dai H, Hui Z, Ji W, et al. Postoperative radiotherapy for resected pathological stage IIIA-N2 non-small cell lung cancer: a retrospective study of 221 cases from a single institution. Oncologist. 2011;16:641–650. doi: 10.1634/theoncologist.2010-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro AJ. What now for postoperative radiotherapy for lung cancer? Lancet. 1998;352:250–251. doi: 10.1016/S0140-6736(98)22030-7. [DOI] [PubMed] [Google Scholar]
- Le Péchoux C. Role of postoperative radiotherapy in resected non-small cell lung cancer: a reassessment based on new data. Oncologist. 2011;16:672–681. doi: 10.1634/theoncologist.2010-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone Pain Trial Working Party. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol. 1999;52:111–121. [PubMed] [Google Scholar]
- Douillard J, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72:695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- Zou B, Xu Y, Li T, et al. A multicenter retrospective analysis of survival outcome following postoperative chemoradiotherapy in non-small-cell lung cancer patients with N2 nodal disease. Int J Radiat Oncol Biol Phys. 2010;77:321–328. doi: 10.1016/j.ijrobp.2009.05.044. [DOI] [PubMed] [Google Scholar]
- Du F, Yuan Z, Wang J, et al. The role of postoperative radiotherapy on stage N2 non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2009;12:1164–1168. doi: 10.3779/j.issn.1009-3419.2009.11.07. (In Chinese.) [DOI] [PubMed] [Google Scholar]
- Patel SH, Ma Y, Wernicke AG, Nori D, Chao KS, Parashar B. Evidence supporting contemporary post-operative radiation therapy (PORT) using linear accelerators in N2 lung cancer. Lung Cancer. 2014;84:156–160. doi: 10.1016/j.lungcan.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Billiet C, Decaluwé H, Peeters S, et al. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: a meta-analysis. Radiother Oncol. 2014;110:3–8. doi: 10.1016/j.radonc.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Ferketich AK, Niland JC, Mamet R, et al. Smoking status and survival in the national comprehensive cancer network non-small cell lung cancer cohort. Cancer. 2013;119:847–853. doi: 10.1002/cncr.27824. [DOI] [PubMed] [Google Scholar]
- Kogure Y, Ando M, Saka H, et al. Histology and smoking status predict survival of patients with advanced non-small-cell lung cancer. Results of West Japan Oncology Group (WJOG) Study 3906L. J Thorac Oncol. 2013;8:753–758. doi: 10.1097/JTO.0b013e31828b51f5. [DOI] [PubMed] [Google Scholar]
- Tsao AS, Liu D, Lee JJ, Spitz M, Hong WK. Smoking affects treatment outcome in patients with advanced nonsmall cell lung cancer. Cancer. 2006;106:2428–2436. doi: 10.1002/cncr.21884. [DOI] [PubMed] [Google Scholar]
- Hanagiri T, Sugio K, Mizukami M, et al. Significance of smoking as a postoperative prognostic factor in patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:1127–1132. doi: 10.1097/JTO.0b013e318186fafb. [DOI] [PubMed] [Google Scholar]
- Vogelius IR, Bentzen SM. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol. 2012;51:975–983. doi: 10.3109/0284186X.2012.718093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JL, Rosenzweig KE, Ostroff JS. The effect of smoking status on survival following radiation therapy for non-small cell lung cancer. Lung Cancer. 2004;44:287–293. doi: 10.1016/j.lungcan.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Nguyen SK, Masson-Côté L, Fortin A, Dagnault A. Influence of smoking status on treatment outcomes after post-operative radiation therapy for non-small-cell lung cancer. Radiother Oncol. 2010;96:89–93. doi: 10.1016/j.radonc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Yoshino I, Yoshida S, Miyaoka E, et al. Surgical outcome of stage IIIA- cN2/pN2 non-small-cell lung cancer patients in Japanese lung cancer registry study in 2004. J Thorac Oncol. 2012;7:850–855. doi: 10.1097/JTO.0b013e31824c945b. [DOI] [PubMed] [Google Scholar]
- Rescigno J. Use of postoperative radiotherapy for node- positive non-small-cell lung cancer. Clin Lung Cancer. 2002;4:35–44. doi: 10.3816/clc.2002.n.014. [DOI] [PubMed] [Google Scholar]
- Asamura H, Goya T, Koshiishi Y, et al. A Japanese Lung Cancer Registry study: prognosis of 13 010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- Goldstraw P, Mannam GC, Kaplan DK, Michail P. Surgical management of non-small-cell lung cancer with ipsilateral mediastinal node metastasis (N2 disease) J Thorac Cardiovasc Surg. 1994;107:19–27. [PubMed] [Google Scholar]
- Varlotto JM, Recht A, Flickinger JC, Medford-Davis LN, Dyer AM, Decamp MM. Factors associated with local and distant recurrence and survival in patients with resected nonsmall cell lung cancer. Cancer. 2009;115:1059–1069. doi: 10.1002/cncr.24133. [DOI] [PubMed] [Google Scholar]
- Moretti L, Yu DS, Chen H, et al. Prognostic factors for resected non-small cell lung cancer with pN2 status: implications for use of postoperative radiotherapy. Oncologist. 2009;14:1106–1115. doi: 10.1634/theoncologist.2009-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- Urban D, Bar J, Solomon B, Ball D. Lymph node ratio may predict the benefit of postoperative radiotherapy in non-small-cell lung cancer. J Thorac Oncol. 2013;8:940–946. doi: 10.1097/JTO.0b013e318292c53e. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ji W, Ou G, et al. Risk factors for radiation-induced lung toxicity in patients with non-small cell lung cancer who received postoperative radiation therapy. Lung Cancer. 2012;77:326–330. doi: 10.1016/j.lungcan.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Nana-Sinkam SP, Powell CA. Molecular biology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e30S–39. doi: 10.1378/chest.12-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]


