Abstract
Using 16S rRNA gene-based approaches, we analyzed the responses of ileal and colonic bacterial communities of weaning piglets to dietary addition of four fermentable carbohydrates (inulin, lactulose, wheat starch, and sugar beet pulp). An enriched diet and a control diet lacking these fermentable carbohydrates were fed to piglets for 4 days (n = 48), and 10 days (n = 48), and the lumen-associated microbiota were compared using denaturing gradient gel electrophoresis (DGGE) analysis of amplified 16S rRNA genes. Bacterial diversities in the ileal and colonic samples were measured by assessing the number of DGGE bands and the Shannon index of diversity. A higher number of DGGE bands in the colon (24.2 ± 5.5) than in the ileum (9.7 ± 4.2) was observed in all samples. In addition, significantly higher diversity, as measured by DGGE fingerprint analysis, was detected in the colonic microbial community of weaning piglets fed the fermentable-carbohydrate-enriched diet for 10 days than in the control. Selected samples from the ileal and colonic lumens were also investigated using fluorescent in situ hybridization (FISH) and cloning and sequencing of the 16S rRNA gene. This revealed a prevalence of Lactobacillus reuteri in the ileum and Lactobacillus amylovorus-like populations in the ileum and the colon in the piglets fed with fermentable carbohydrates. Newly developed oligonucleotide probes targeting these phylotypes allowed their rapid detection and quantification in the ileum and colon by FISH. The results indicate that addition of fermentable carbohydrates supports the growth of specific lactobacilli in the ilea and colons of weaning piglets.
The diets, microbiota, and gastrointestinal (GI) tract interactions of mammals are extremely complex and are the result of millions of years of coevolution between the higher vertebrates and their microbiota. As a consequence, any major changes in lifestyle and diet are likely to place stress on the stability of these interactions and affect the entire GI tract ecophysiology. In contrast to the gradual weaning of human babies, piglets within a production environment are weaned at an early stage with solid feed and transported to production farms. This combination of stress factors can lead to diarrhea, a reduced growth rate, and in some cases even death (54). In order to enhance growth and suppress the activity of the gut microbiota, antimicrobial compounds have been fed to weaning pigs for more than 4 decades (6). Nowadays, the emergence of antibiotic resistance in the human commensal bacteria has raised concerns about the impact of anitimicrobial compounds for agricultural use (53) and has accelerated the search for alternative nutritional strategies, such as the addition of probiotics and prebiotics. These approaches have become an increasingly important consideration in swine nutrition because of accumulating evidence of their potential benefits in animals and humans and the possibility that they could replace antibiotics in feed (5, 21, 61, 64). The development of such dietary strategies requires a combination of in vitro, in vivo, and challenge studies involving both expertise in animal nutrition and an evaluation of the composition and activity of the indigenous microbiota throughout the GI tract.
In the past, the microbial community in the GI tracts of pigs has been studied intensively, but most attention was paid to easily cultivable commensal bacteria and a number of opportunistic pathogens (10, 57). Many of the strictly anaerobic GI tract bacteria are still difficult to cultivate and therefore remain undetectable by conventional techniques (59, 63). Recent phylogenetic analysis based on the in vitro amplification of the 16S rRNA gene and other phylogenetic markers by PCR techniques have revealed dramatically higher diversity than described previously by cultivation (20, 28, 41). While molecular approaches based on PCR can introduce different types of bias (22, 65), a combination of PCR and fingerprinting techniques, such as denaturing gradient gel electrophoresis (DGGE) and terminal restriction fragment length polymorphism, has led to new insights into GI microbial ecology and the effects of different dietary strategies and host factors on bacterial-community composition (69).
It has been recognized that a stable indigenous microbiota in the intestine can prevent colonization by pathogens (46, 62). This so-called colonization resistance may be of utmost importance for animals, especially at stressful times, such as weaning. The promotion of colonization resistance through the addition of prebiotics has been suggested as a comparatively easy way to improve enteric health (4, 7, 66). Prebiotics have been used to induce the colonization of bacteria, such as lactobacilli and bifidobacteria, considered to be beneficial for the host (13, 14, 61). Stimulation of the Lactobacillus population within the GI tracts of piglets is of specific importance, not only due to the potential effect of the bacteria on gut function and health (57, 58), but also because of their possible antagonistic activities toward other bacteria (19, 51). Lactobacilli establish early in the piglet intestine, and although succession occurs throughout the pig's lifetime, they remain a predominant part of the intestinal bacterial community (3, 38, 57, 60). Numerous studies have suggested that some prebiotics may specifically stimulate intestinal lactobacilli. The application of lactobacilli as probiotics or therapeutic supplements has also been studied (44, 45). However, little is known of the response of the bacterial community to such dietary interventions.
This work describes changes in the predominant ileal and colonic bacterial populations in weaning piglets that were fed a diet containing four added fermentable carbohydrates, namely, inulin, lactulose, wheat starch, and sugar beet pulp. The data indicate that the incorporation of these four ingredients in the diet results in outgrowth of lactobacilli in the small intestine and higher diversity in the colon. Two particular phylotypes related to Lactobacillus amylovorus and Lactobacillus reuteri were the most prevalent throughout the guts of piglets fed with the prebiotics, as demonstrated by DGGE of 16S rRNA gene amplicons in combination with sequence analysis. Newly developed DNA oligonucleotide probes targeting these key species allowed their rapid detection and quantification in the ilea and colons of piglets by fluorescent in situ hybridization (FISH).
MATERIALS AND METHODS
Animals, diets, and sampling.
All of the procedures involving animals were conducted in accordance with the Dutch law on experimental animals and were approved by the Animal Experimental Committee of Wageningen University.
Three identical but independent feeding experiments including a total of 108 piglets (crossbred Hypor × Pietrain) were started immediately at the time of weaning (25 to 28 days old). Each experiment used 36 piglets. At the start of the experiment (day 1), four piglets were sacrificed. The remaining 32 piglets were offered one of two diets (16 piglets per diet): the HF diet containing four added fermentable carbohydrates, namely, lactulose, inulin, sugar beet pulp, and wheat starch, and the LF diet with a low concentration of fermentable carbohydrates (Table 1). The diets were composed in such a way that the total energy and protein contents were comparable. On days 4 and 10 of each experiment, eight piglets were sacrificed per treatment.
TABLE 1.
Compositions of the diets
| Diet or ingredient | Amt (g/kg)
|
|
|---|---|---|
| LFa | HFb | |
| Maize starch | 504.8 | 368.1 |
| Sugarbeet pulp | 0 | 50.0 |
| Inulin | 0 | 7.5 |
| Lactulose (∼50% DM)c | 0 | 20.0 |
| Wheat starch | 0 | 50.0 |
| Fish meal | 200.0 | 200.0 |
| Soya isolate | 50.0 | 45.0 |
| Dextrose | 150.0 | 150.0 |
| Soy oil | 15.0 | 30.0 |
| Cellulose (Arbocel) | 50.0 | 50.0 |
| Premix | 10.0 | 10.0 |
| Chalk | 2.5 | 1.5 |
| Monocalcium phosphate | 1.5 | 1.5 |
| KHCO3 | 12.0 | 12.0 |
| l-Lysine HCl | 0.6 | 0.7 |
| dl-Methionine | 2.0 | 2.0 |
| l-Threonine | 1.0 | 1.1 |
| l-Tryptophan | 0.6 | 0.6 |
LF, diet with a low concentration of fermentable carbohydrates.
HF, diet containing lactulose, inulin, sugar beet pulp, and wheat starch.
DM, dry matter.
The samples were divided into aliquots, one of which was used for genomic-DNA extraction, followed by 16S rRNA gene-targeted PCR-DGGE analysis, cloning, and sequencing. In parallel, aliquots from the same samples were fixed for FISH and for determination of the lactic acid concentration.
DNA isolation.
DNA isolation from lumen samples (0.2 g) was done by using the Fast DNA Spin kit (Qbiogene, Inc., Carlsbad, Calif.). Agarose gel (1.2% [wt/vol]) electrophoresis in the presence of ethidium bromide was used to check visually for DNA quality and yield.
PCR amplification.
All primers used in this study are listed in Table 2. Primers S-D-Bact-0968-a-S-GC and S-D-Bact-1401-a-A-17 were used to amplify the V6 to V8 regions of the 16S rRNA gene. PCR was performed using the Taq DNA polymerase kit from Life Technologies (Gaithersburg, Md.). PCR mixtures (50 μl) contained 0.5 μl of Taq polymerase (1.25 U), 20 mM Tris-HCl (pH 8.5), 50 mM KCl, 3.0 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 5 pmol of the primers, 1 μl of DNA diluted to ∼1 ng/μl, and UV-sterilized water. The samples were amplified in a T1 thermocycler (Whatman Biometra, Göttingen, Germany), and the cycling consisted of 94°C for 5 min and 35 cycles of 94°C for 30 s, 56°C for 20 s, 68°C for 40 s, and 68°C for 7 min (final extension). Aliquots (5 μl) were analyzed by electrophoresis on 1.2% (wt/vol) agarose gels containing ethidium bromide to check for product size and quantity.
TABLE 2.
Oligonucleotide primers and probes used in this study
| Primer or probe | Sequence (5′-3′) | Reference |
|---|---|---|
| Primers | ||
| S-D-Bact-0011-a-A-17a | AGA GTT TGA T(C/T)(A/C) TGG CTC AG | 28 |
| S-G-Lab-0159-a-S-2a | GGA AAC AG(A/G) TGC TAA TAC CG | 18 |
| S-*-Univ-0515-a-A-24-GCa | CGC CGG GGG CGC GCC CCG GGC GGG GCG GGG GCA CGG GGG G ATC GTA TTA CCG CGG CTG CTG GCA | 18 |
| S-G-Lab-0677-a-A-17a | CAC CGC TAC ACA TGG AG | 18 |
| S-D-Bact-0968-a-S-GCa | CGC CCG GGG CGC GCC CCG GGC GGG GCG GGG GCA CGG GGG GAA CGC GAA GAA CCT TAC | 27 |
| S-D-Bact-1401-a-A-17a | CGG TGT GTA CAA GAC CC | 27 |
| S-Univ-1100-a-A-15a | GGG TTG CGC TCG TTG | 27 |
| S-D-Bact-1492-a-A-19a | GGT TAC CTT GTT ACG ACT T | 28 |
| T7 | TAA TAC GAC TCA CTA TAG G | Promega |
| Sp6 | GAT TTA GGT GAC ACT ATA G | Promega |
| Probes | ||
| S-D-Bact-0338-a-A-17a | GCT GCC TCC CGT AGG AGT | 16 |
| S-G-Lab-0158-a-A-20a | GGT ATT AGC A(C/T)C TGT TTC CA | 16 |
| L-*-OTU171-0088-a-A-18a | CGC TTT CCC AAC GTC ATT | This study |
| L-*-OTU173-0085-a-A-18a | CCA TCG TCA ATC AGG TGC | This study |
Nomenclature according to Alm et al. (1).
To investigate the Lactobacillus-specific GI tract bacterial community by DGGE, a specific nested-PCR approach was chosen. For the initial amplification, S-D-Bact-0011-a-A-17 and S-G-Lab-0677-a-A-17 primers were employed (18), using the following cycling conditions: predenaturation at 94°C for 5 min; 35 cycles of 94°C for 30 s, 66°C for 20 s, and 68°C for 40 s; and a final extension at 68°C for 7 min. The PCR products were then used as templates in nested PCRs with S-G-Lab-0159-a-S-20 and S-*-Univ-0515-a-A-24-GC. The cycling program was identical to the one used for the amplification of the V6 to V8 regions of the 16S rRNA gene.
DGGE analysis.
The amplicons obtained from the lumen-extracted DNA were separated by DGGE according to the specifications of Muyzer et al. (36) using a Dcode TM system (Bio-Rad Laboratories, Hercules, Calif.). Electrophoresis was performed in an 8% polyacrylamide gel (37.5:1 acrylamide-bisacrylamide; dimensions, 200 by 200 by 1 mm) using a 38 to 48% denaturing gradient (35) for separation of PCR products obtained with primers S-D-Bact-0968-a-S-GC and S-D-Bact-1401-a-A-17, while gradients of 30 to 60% were employed for the separation of the S-G-Lab-0159-a-S-20- and S-*-Univ-0515-a-A-24-GC-generated amplicons. The gels were electrophoresed for 16 h at 85 V in 0.5× TAE buffer (48) at a constant temperature of 60°C and subsequently stained with AgNO3 (49).
Analysis of DGGE gels.
Analysis of all DGGE samples was done as described previously (27). Briefly, all gels were scanned at 400 dots/in and analyzed using Molecular Analyst/PC software (version 1.12; Bio-Rad). First, a number of bands were assessed per lane using the band-searching algorithm in the program. A manual check was done, and the DGGE fragments constituting <1% of the total area of all bands were omitted from further analysis. Second, as a parameter for the structural diversity of the microbial community, the Shannon index of general diversity, H′ (9, 27, 32, 50), was calculated using the following function: H′ = −Σ Pi log Pi, where Pi is the importance probability of the bands in a lane. H′ was calculated on the basis of the bands on the gel tracks that were applied for the generation of the dendrograms by using the intensities of the bands as judged by peak height in the densitometric curves. Pi, was calculated as follows: Pi = ni/n", where ni is the height of a peak and n" is the sum of all peak heights in the densitometric curve. The similarity between the DGGE profiles was determined by calculating a band similarity (Dice) coefficient, SD [SD = 2nAB/(nA + nB), where nA is the number of DGGE bands in lane 1, nB represents the number of DGGE bands in lane 2, and nAB is the number of common DGGE bands] (27, 51, 52).
Statistical analysis.
For statistical analysis, the number of DGGE bands, the Shannon index of general diversity, and the band similarity coefficient (SD) were calculated. Differences between these parameters for the two diets were tested for significance using Tukey's Studentized range test of multiple comparisons (56) according to the following equation: Y = μ + Di + ɛij, where Y is the result, μ is the mean, D is the effect of the diet, and ɛij is the error term. All statistical analyses were performed using the SAS GLM procedure (55).
Generation and screening of 16S rRNA gene clone libraries.
PCR was performed with a Taq DNA polymerase kit from Life Technologies using primers S-D-Bact-0011-a-S-17 and S-D-Bact-1492-a-A-19. Amplification was carried out as described previously (27). The PCR product was purified with the QIAquick PCR purification kit (Westburg, Leusden, The Netherlands) according to the manufacturer's instructions. The purified PCR product was cloned into pGEM-T (Promega, Madison, Wis.) using competent Escherichia coli JM109 as a host. The colonies of ampicillin-resistant transformants were transferred with a sterile toothpick to 15 μl of Tris-EDTA buffer and boiled for 15 min at 95°C. PCR was immediately performed with the vector-specific primers T7 and SP6 to check the sizes of the inserts using the cell lysate as a template. Plasmids containing an insert of ∼1.6 kb were used to amplify the V6 to V8 regions of the 16S rRNA gene. The amplicons were compared with the bands of DGGE profiles that comprised >1% of the total area of all bands. Clones representing an insert corresponding to a dominant band were grown in Luria Broth liquid medium (5 ml) with ampicillin (100 μg ml−1). Plasmid DNA was isolated using the Wizard Plus purification system (Promega) and used for sequence analysis of the cloned 16S rRNA gene by using a Sequenase (T7) sequencing kit (Amersham Life Sciences, Slough, United Kingdom) according to the manufacturer's specifications and using either the T7 and SP6 primers or S-Univ-1100-a-A-15 labeled with IRD-800. Sequences were automatically analyzed on a LiCor (Lincoln, Neb.) DNA Sequencer 4000L and corrected manually. The sequences were also compared to those available in public databases by using BLAST analysis (2). The partial and complete 16S rRNA gene sequences were checked for chimeric constructs by the Ribosomal Database Project CHECK_CHIMERA program (31). None of the sequences were found to be PCR-generated chimeras.
Cloning and sequencing of DGGE bands after Lactobacillus-specific PCR amplification.
Representative bands were excised from DGGE gels using a QIAEXII Gel extraction kit (Westburg) according to the instructions in the manual. After reamplification using the original S-G-Lab-0159-a-S-20 and S-*-Univ-0515-a-A-24-GC primer set, cloning and sequencing analysis were carried out as previously described.
Design and validation of oligonucleotide probes for FISH analysis.
Nearly complete 16S rRNA sequences of L. amylovorus-like and L. reuteri-like isolates (this study) and closely related L. amylovorus-like (OTU171) and L. reuteri-like (OTU173) isolates from pig intestine (28) were aligned, and probes targeting these sequences were designed using the ARB software package (http://www.arb-home.de). Probes were designed taking into consideration the types and positions of nucleotide mismatches with sequences of related species with a G+C content of >50% and a length of ∼18 nucleotides. Sequence comparisons using the ARB, Check Probe, and BLAST programs confirmed that the targeted regions were conserved among the 16S rRNA sequences of OTU171 (L. amylovorus-like) isolated from pig intestine and Lactobacillus kitasatoi isolated from chickens (34) (Fig. 1, band B) and to L. reuteri-like OTU173 (Fig. 1, band A). Probe OTU171-0088-a-A-18 was found to also match the partial sequence of Lactobacillus galinarum (National Center for Biotechnology Information accession no. X97898). However, a comparison among the L. galinarum, OTU171, and L. kitasatoi sequences showed 99 to 100% homology among them based on 600 bp (E. coli positions 20 to 620).
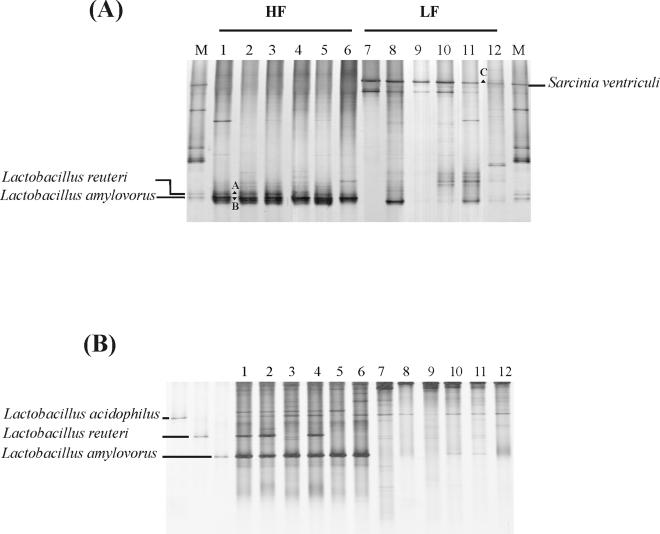
FIG. 1.
Effects of the fermentable-carbohydrate-containing diet on the ileum bacterial community by day 10 of the experiment. (A) DGGE of PCR products of V6 to V8 regions of 16S rRNA gene sequences retrieved from lumen samples on day 10 after weaning. Lanes: 1 to 6, piglets on HF diet; 7 to 12, piglets on LF diet; M, marker. Fragments A, B, and C were identified from 16S rRNA gene clone libraries. (B) Monitoring of the Lactobacillus-like community of piglets. DGGE analysis of amplicons generated by nested PCR with primers S-G-Lab-0159-a-S-20 and S-*-Univ-0515-a-A-24-GC, originating from piglets on the HF diet (lanes 1 to 6) and piglets on the LF diet (lanes 7 to 12). The dominant fragments in Lactobacillus-like patterns were identified by the clones corresponding to L. acidophilus, L. reuteri, and L. amylovorus.
The reference strains L. amylovorus DSMZ 20531 and L. reuteri DSMZ 20015 were used as positive controls (Table 3). Ten reference Lactobacillus strains and Enterococcus faecalis DSMZ 20478, frequently found in the GI tract, were used as negative controls to evaluate the specificities of the newly designed probes. The temperature of hybridization was 50°C, and if needed, formaldehyde was added to increase the specificity (17).
TABLE 3.
Bacterial strains, sources, media for cultivation, and FISH results
| Strain | Sourcea | Mediumb | Probe resultsc
|
|
|---|---|---|---|---|
| I | II | |||
| L. amylovorus | DSMZ 20531 | MRS | + | − |
| L. crispatus | DSMZ 20584 | MRS | − | − |
| L. reuteri | DSMZ 20015 | MRS | − | + |
| L. reuteri | VTT E92142 | MRS | − | + |
| L. acidophilus | VTT E96276 | MRS | − | − |
| L. acidophilus | ATCC 4356 | MRS | − | − |
| Lactobacillus brevis | VTT E91458 | MRS | − | − |
| Lactobacillus buchneri | VTT E93445 | MRS | − | − |
| Lactobacillus plantarum | VTT E79098 | MRS | − | − |
| Lactobacillus rhamnosus LGG | ATCC 53103 | MRS | − | − |
| E. faecalis | DSMZ 20478 | WW | − | − |
DSMZ, German Collection for Microorganisms and Cell Culture, Braunschweig, Germany; VTT, VTT culture collection, FIN-02044, Finland; ATCC, American Type Culture Collection, Manassas, Va.
MRS, lactobacillus MRS broth (Difco, Sparks, Md.); WW, Wilkins West broth.
(I), L-S-OTU171-a-A-0088; (II), L-S-OTU173-a-A-0085; +, hybridization signal; −, no hybridization signal.
The reference strains used in this study were obtained from the sources indicated in Table 3. The strains were cultivated as recommended by the culture collections in the respective catalogues. Exponentially grown cells were harvested at 5,000 × g for 10 min, washed with 0.2-μm-pore-size-filtered phosphate-buffered saline (PBS; per liter, 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4, and 0.24 g of KH2PO4, pH 7.2), and diluted 1:3 with 4% paraformaldehyde in PBS. After fixation at 4°C for 16 h, the cells were stored in 50% ethanol-PBS at −80°C for subsequent FISH analysis.
Collection and preparation of ileal- and colonic-lumen samples for FISH.
Ileal- and colonic-lumen samples from 108 experimental piglets were processed as described previously (12). In short, 0.5 g of lumen samples was resuspended in 4.5 ml of PBS and vortexed with five or six glass beads (diameter, 3 mm) for at least 3 min to homogenize the sample. After centrifugation at 700 × g for 1 min, 1 ml of the supernatant was added to 3 ml of 4% paraformaldehyde in PBS and stored for 16 h at 4°C. After being washed twice with PBS, the fixed cells were stored in 50% ethanol-PBS at −80°C until further use.
Enumeration of bacteria by FISH.
For microscopic analysis, fixed cells were spotted on gelatin-coated glass slides and dried for 20 min at 50°C. The optimal cell concentration for counting using the different probes was determined using dilution series of the lumen samples. After drying of the slides, the cells were dehydrated for 3 min in 50, 70, and finally 96% ethanol-H2O. Ten microliters of hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl [pH 7.5], 0.1% [wt/vol] sodium dodecyl sulfate) containing 10 ng of Cy3-labeled lactobacillus probes/μl or 5 ng of fluorescein-isothiocyanate-labeled S-D-Bact-0338-a-A-17/μl (Table 2) was added to each well, followed by incubation at 50°C for 16 h. After hybridization, the slides were washed in 50 ml of hybridization buffer for 10 min. For total cell counts, DAPI (4′,6-diamino-2-phenylindole) at a final concentration of 100 ng/ml was added to the washing buffer. After the slides were rinsed in double-distilled water, they were immediately air dried and mounted in Vectashield (Vector Laboratories, Burlingame, Calif.). Digital images of the slides were analyzed, and fluorescence-positive cells were counted using Qwin image analysis software (Leica Microsystems, Rijswijk, The Netherlands). For each analysis, 25 microscopic fields were counted.
Lactic acid analysis.
The lactic acid concentration in ileal lumen was analyzed by high-performance liquid chromatography (Jasco Instruments) using a column (Supelcogel; C-610H; 30 cm by 7.8-mm internal diameter) and a precolumn (Supelcoguard; C-610H; 5 cm by 4.6-mm internal diameter) with 1% H2SO4 as the mobile phase. The concentrations were determined by UV detection at 210 nm.
Nucleotide sequence accession numbers.
The sequences reported in this study were deposited in GenBank under the following accession numbers: AY493201 to AY493245.
RESULTS
Animal observations.
The animals remained healthy throughout the experimental period. The average weaning weight of the piglets was 7.5 kg. At the end of the experiment, no significant difference in body weight gain between the dietary treatments was observed.
Effects of fermentable carbohydrates on bacterial diversity in the ileum and colon.
A comparative 16S rRNA gene-targeted DGGE fingerprinting analysis of bacterial communities was performed for ileal- and colonic-lumen samples of piglets that were fed two different diets. Samples from piglets that were sacrificed on the day of weaning (n = 12) and 4 (n = 48) and 10 (n = 48) days after weaning were analyzed. The number of DGGE bands and the Shannon index of general diversity were assessed for each sample and subjected to statistical analysis according to GI tract location across all time periods. For all samples, a comparison between the two locations along the GI tract revealed a statistically higher number of DGGE bands (24. 2 ± 5. 5; P < 0.05) in the colonic lumen compared to the ileal lumen (9.7 ± 4. 2). The Shannon index of diversity in the colon (1.43 ± 0.3; P < 0.05) was also higher than in the ileum (0. 86 ± 0. 26). No significant differences in the number of DGGE bands or diversity were detected on day 4 in the ilea or in the colons of piglets fed the different diets. However, by day 10 after weaning, the diversity was significantly higher in the colonic samples from HF piglets, as evidenced by the number of bands (27. 5 ± 5.6; P < 0.05), than in those of the LF group (20.5 ± 6.3). There was no statistical difference between the number of DGGE bands and the diversity index in the ileal samples by day 10.
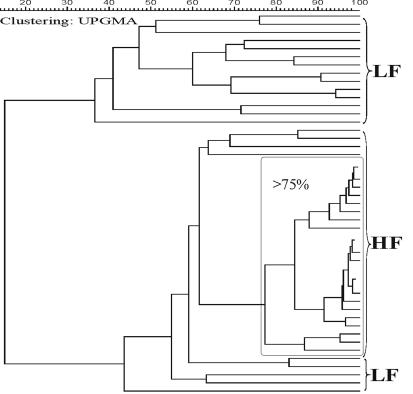
The influence of the diet on the bacterial-community structure in the ileal and colonic lumens of HF and LF group piglets on days 4 and 10 was further elucidated. By day 4, there were no DGGE bands detected in one dietary group that were completely absent in the other (data not shown). In contrast, a simple visual comparison of the DGGE banding patterns by day 10 of the experiment revealed a marked difference between the ileal samples of the two dietary groups. A representative DGGE analysis of the PCR fragments generated with primers S-D-Bact-0968-a-S-GC and S-D-Bact-1401-a-A-17 is shown in Fig. 1A. Two particularly strong bands (A and B) were present in the ileal samples from piglets fed with the HF diet on day 10. Band B was also detected in 18 out of the 24 colonic-lumen samples from pigs fed the HF diet on day 10 and was absent in pigs fed with the LF diet (data not shown). The ileal samples of the LF group, on the other hand, were dominated by another band located in the uppermost part of the DGGE gels (Fig. 1A, band C). Band C was not present in the HF diet samples. To obtain an objective interpretation of the electrophoretic patterns of the HF and LF ilea, the samples were subjected to a numerical analysis based on the Dice similarity coefficient, followed by cluster analysis. The similarity was visualized using the unweighted pair group method with averaging algorithm (Fig. 2). Cluster analysis revealed that all 24 samples from HF diet-fed piglets formed a coherent cluster with similarity indices above 60%. Within this cluster, 20 out of 24 samples grouped together, with similarity indices higher than 75%. The low similarities between HF and LF samples confirmed the visual differences in their DGGE fingerprints (Fig. 1). The average similarity index of the HF colon samples was 45% (data not shown). Taken together, the results obtained after DGGE analysis demonstrate that the bacterial compositions in the ilea and colons of piglets were modulated by the HF diet by day 10.
FIG. 2.
Similarity index of DGGE profiles obtained from ileal-lumen microbiota of 48 piglets fed either HF or LF diet for 10 days. The normalization of the DGGE gels was done with respect to the reference standards included in three gels containing the ileal-lumen samples of HF and LF from the three replicate experiments. The Dice coefficient of similarity between banding patterns of different gels was calculated. This allowed the generation of a dendrogram, and the samples were grouped according to the similarity of their community profiles. UPGMA, unweighted pair group method with averaging.
Identification of cloned 16S rRNA gene sequences in DGGE patterns.
In order to identify changes in the bacterial diversity detected by DGGE analysis, the 16S rRNA genes from the ileal-lumen samples of four HF-fed piglets (10 days) and four LF-fed piglets were amplified and cloned into E. coli, and 15 clones per sample were partially sequenced (Table 4). To identify the dominant bands, A and B, that appeared in 90% of samples 10 days after starting the HF diet, together with a third distinct band (C) in the LH diet (Fig. 1A), the V6 to V8 regions of the 16S rRNA gene were amplified from the cell lysates of a total of 120 transformants. The mobilities of these amplicons during DGGE were compared to those obtained from rRNA gene sequences retrieved from samples from the piglets fed for 10 days with the HF and LF diets. Sixty-one percent of the clones were assigned to one of the dominant bands in the DGGE profiles, while 39% did not match any of the detectable bands. The clones from the eight different clone libraries corresponding to bands A, B, and C were completely sequenced. The 16S rRNA gene sequences of the clones representing band A were identified as L. reuteri-like, or OTU173, while band B showed similarity to OTU171, or L. amylovorus-like (28). Clones matching the position of band C were 98% similar to Sarcinia ventriculi (Fig. 1A).
TABLE 4.
Clones with similarity to known sequences in GenBanka
| No. | Closest relative in GenBank | GenBank accession no. | No. of clones
|
Avg similarity (%) | |
|---|---|---|---|---|---|
| HF | LF | ||||
| 1 | L. amylovorus-like | AF371469 | 22 | 0 | 98 |
| 2 | L. reuteri (DSM 20016 T) | X76328 | 11 | 2 | 98 |
| 3 | L. acidophilus subsp. johnsonii | M99704 | 1 | 19 | 99 |
| 4 | Lactobacillus sp. oral clone CX036 | AY005048 | 7 | 0 | 98 |
| 5 | Lactobacillus sp. strain CLE-4 | AY017059 | 4 | 0 | 99 |
| 6 | Lactobacillus mucosae | AY445125 | 2 | 1 | 97 |
| 7 | L. gallinarum | AJ242968 | 3 | 1 | 97 |
| 8 | S. ventriculi (DSM 286) | X76649 | 0 | 12 | 98 |
| 9 | Salmonella sp. | AF130955 | 1 | 5 | 95 |
| 10 | Pasteurella aerogenes | M75048 | 1 | 4 | 93 |
| 11 | Clostridium lituseburense | M59107 | 1 | 3 | 97 |
| 12 | Haemophilus sp. | M75077 | 1 | 3 | 96 |
| 13 | Moraxella lacunata | AF005161 | 1 | 2 | 97 |
| 14 | Veillonella atypica | X84007 | 1 | 2 | 98 |
| 15 | Leptotrichia sp. (oral clone) | AY349385 | 1 | 2 | 96 |
| 16 | Ruminococcus albus | AF104843 | 1 | 1 | 91 |
| 17 | Ruminococus callidus | X85100 | 0 | 1 | 95 |
| 18 | Holdemania filiformis | Y11466 | 0 | 1 | 91 |
| 19 | Calonectris diomedea | Y16359 | 1 | 1 | 96 |
| 20 | Veillonella parvula | AF439640 | 1 | 0 | 90 |
| Total | 60 | 60 | |||
Clones were retrieved from ileal-lumen samples of four HF-fed piglets and LF-fed piglets at 5 weeks of age. The clones are listed according to their abundances in the eight different 16S rRNA gene clone libraries.
Although the amplification of the V6 to V8 regions using general bacterial primers allowed the visualization of the major differences between the HF and LF samples and the screening of the 16S rRNA gene clone libraries, it yielded poor resolution of Lactobacillus populations. Therefore, specific amplification of the Lactobacillus GI tract bacterial community was used to screen the clones matching one of the dominant bands after V6 to V8 16S region DGGE analysis. Lactobacillus-specific amplification, in combination with DGGE analysis, confirmed the predominance of one particular phylotype related to L. amylovorus, while a band related to L. reuteri was not consistently found in the samples from the piglets fed with the HF diet (Fig. 1B). L. acidophilus was also present in HF and LF samples irrespective of diet.
To confirm the visual match between the 16S rRNA gene clones and the DGGE bands, the HF diet-specific bands were excised from DGGE gels after Lactobacillus-specific PCR, reamplified, and sequenced. Sequencing analysis of the bands (A and B) confirmed their identities as L. reuteri-like and L. amylovorus-like (28). Interestingly, of the four HF diet samples, an average of four other phylotypes related to Lactobacillus mucosae (97%), L. galinarum (97%), and two different Lactobacillus species clones, one oral (98%; accession no. AY005048) and one isolated from swine production facilities (99%; accession no. AY017059), were found (Table 4). However, their sequences did not match any visible DGGE bands. In comparison, the Lactobacillus diversity in LF was predominated by Lactobacillus acidophilus-like related sequences. The results of the clone libraries showed increased lactobacillus diversity in the HF samples, while DGGE analysis suggested a specific outgrowth of L. amylovorus-like phylotypes in the terminal ilea of weaning piglets.
Development and evaluation of FISH probes specific for L. amylovorus and L. reuteri-like isolates.
Potential probes were identified based on the alignment of the complete 16S rRNA sequences of the clones matching DGGE bands A and B (Fig. 1A) and related Lactobacillus spp. (Table 3).
The probes were experimentally validated by performing FISH analysis on a range of Lactobacillus species and other bacteria that are commonly found in large numbers in the pig GI tract (28, 57). A constant temperature of 50°C for 16 h and 0% (vol/vol) formamide in the hybridization buffer were used, resulting in specific hybridization only with the respective target strains (Table 3). Subsequently, the validated probes were used to enumerate target bacteria in individual ileal and colonic samples from piglets fed the different diets for 10 days. To evaluate whether the microbiota was affected by the diet, the total cell counts and the total bacterial and lactobacillus-enterococcus counts for the HF and LF diets were compared (Table 5). Within the HF, the lactobacillus-enterococcus counts were significantly higher (P < 0.05) than in the LF. Hybridization with the L-*-OTU171-0088-a-A-18 probe detected the OTU171 phylotype in 83% of the ileal samples and 75% of the piglet colonic samples from the HF diet, while no hybridization-positive cells were obtained for the LF diet (Table 5). In comparison, an L. reuteri-like related population was detected in 75% of the ileal-lumen samples and 41% of the colonic-lumen samples from the HF diet using the L-*-OTU173-0085-a-A-18 probe.
TABLE 5.
FISH results
| Parameter | Valueb
|
|||
|---|---|---|---|---|
| HF
|
LF
|
|||
| Ileum (n = 24) | Colon (n = 24) | Ileum (n = 24) | Colon (n = 24) | |
| Total cell counta | 2.1 ± 1.1 × 108 | 3.1 ± 2.4 × 1010 | 1.2 ± 1.2 × 108 | 2.3 ± 1.4 × 1010 |
| Total bacterial counta | 1.89 ± 1.4 × 108 | 2.7 ± 1.5 × 1010 | 1.17 ± 1.1 × 108 | 1.94 ± 0.9 × 1010 |
| Lactobacillus-enterococcusa | 1.5 ± 0.6 × 108d | 3.8 ± 2.1 × 108 | 0.4 ± 0.3 × 107 | 6.3 ± 3.1 × 108 |
| L. amylovorus-like (Probe, L-S-OTU171-a-A-0088)c | ||||
| No. of piglets colonized (%) | 20 (83) | 18 (75) | ND | ND |
| Median count | 1.3 × 108 | 3.7 × 109 | ND | ND |
| Range | 0.64-2.4 × 108 | 1.3-5.6 × 109 | ND | ND |
| L. reuteri- like (Probe, L-S-OTU173-a-A-0085)c | ||||
| No. of piglets colonized (%) | 18 (75) | 10 (41.2) | ND | 9 (37.5) |
| Median count | 7.7 × 107 | 5.8 × 107 | ND | 5.5 × 107 |
| Range | 4.4-12 × 107 | 5.3-6.2 × 107 | ND | 5.1-6.3 × 107 |
Results for total cell counts (DAPI staining), total bacterial counts (S-D-Bact-0338-a-A-17), and lactobacillus-enterococcus counts (S-G-Lab-0158-a-A-20).
Mean ± standard deviation (cells per gram). ND, no bacteria detected.
Specific counts for the probes L-S-OTU171-a-A-0088 (L. amylovorus-like) and L-S-OTU173-a-A-0085 (L. reuteri-like) for ileal- and colonic-lumen samples from piglets fed for 10 days on HF or LF diet.
Significant differences (P < 0.05) from the values compared (bold face).
Lactic acid concentration in ileal lumen.
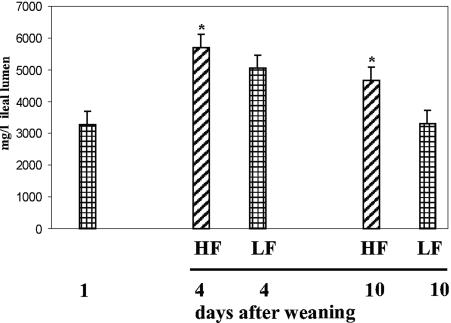
Lactic acid was measured in the lumen samples from the terminal ilea of all piglets from days 1, 4, and 10 after the introduction of the diet (Fig. 3). By days 4 and 10, a significantly higher lactic acid concentration was recovered in the samples from the HF diet than in those from the LF diet. As lactic acid is a common end product of fermentation of lactobacilli, these results were in agreement with the outgrowth of lactobacilli in the terminal ileum, as demonstrated by 16S rRNA gene-based DGGE and FISH analyses, and they suggest that the lactobacilli were not only present but also metabolically active.
FIG. 3.
Luminal lactic acid concentrations in the terminal ilea of piglets. The data are expressed as mean values plus standard errors of the mean for all samples. Asterisks, significantly different concentrations of lactic acid (P < 0.05).
DISCUSSION
The bacteriological results reported here indicate that the addition of specific fermentable carbohydrates to the diet can lead to a shift in both the composition and activity of the microbial communities of the small and large intestines of weaning piglets. Two particular phylotypes related to L. amylovorus and L. reuteri were the most prevalent populations in the ilea of piglets fed the HF diet for 10 days, as demonstrated by DGGE analysis and a phylotype-specific 16S rRNA-targeted FISH analysis. In addition, bacterial diversity was increased by day 10 in the colons of the HF group, as evidenced by the higher number of DGGE bands and the Shannon index of diversity in the corresponding samples. Given the present global concern about antibiotic replacement in feed, these results show that careful design of the diet can indeed stimulate supposedly beneficial bacteria. They also indicate that other species can be suppressed. These findings, therefore, are not only interesting for piglet microbiology and nutrition at the time of weaning but also provide new insights into the specific effects of prebiotics on the indigenous Lactobacillus communities of piglets.
The availability of fast sequencing techniques offers an unprecedented opportunity to conduct comprehensive surveys of pig microbial communities (20, 28). Results based on comparative sequence analyses of the 16S rRNA and chaperonin-60 genes documented the complexity of the intestinal microbial community and suggested that the majority of the bacterial species colonizing the GI tract in pigs have not yet been characterized. However, cloning and sequencing is time-consuming and may limit the number of samples that can be processed. Thus, the high sample throughput required to determine community responses to experimental treatments, such as introduction of prebiotics or probiotics, needs to be achieved by the analysis of multiple clone libraries. Alternatively, DGGE and a similar technique called temperature gradient gel electrophoresis have been introduced into microbial ecology (35-37) as one attempt to obtain an overview of the structural diversity of microbial communities. As reported previously, DGGE and temperature gradient gel electrophoresis are sensitive enough to detect bacteria that constitute 1% of the total bacterial community (67). The PCR-DGGE detection limit has also been estimated previously by dilution series of pure cultures (68). In addition, the primer pair used in the present study (S-D-Bact-0968-a-S-GC and S-D-Bact-1401-a-A-17) was found to amplify with the same efficiency 16S rRNA genes from the complex soil bacterial communities (11). Individual DGGE bands can be assigned to cultured organisms or retrieved ribosomal sequences (26, 27). This is usually not possible in activated sludge, sediments, soil, and other highly diverse microbial systems because the banding patterns are too complex (9). However, the number, precise positions, and intensities of the bands reflect the number and relative abundance of dominant rRNA sequence types in the sample and thus allow comparison of microbial communities with each other. By applying this approach to piglet GI tract lumen samples, a distinct diversity value for each sample was obtained and changes in community diversity over time in different experiments were observed. In agreement with previous analyses of 16S rRNA gene libraries obtained from pig ileum and colon samples (28), the results reported in this study showed a significantly lower diversity in the ileum than in the colon. Further elucidation of the diet effect by using 16S rRNA gene PCR-DGGE analysis unveiled the impact of the ileum microbiota in the utilization of the fermentable carbohydrates. Marked differences in the bacterial-community compositions between ileal samples from piglets fed with HF or LF diets were demonstrated by day 10 of the experiment (Fig. 1). While this has not been previously demonstrated by culture-independent approaches, there are numerous studies showing that the ilea of pigs harbor diverse and active bacterial populations (reference 24; reviewed in reference 64). Furthermore, the increased diversity in the colons of piglets fed the HF diet, as demonstrated by DGGE, is in agreement with earlier data (27). Such a strong effect of the diet on the porcine colon and fecal bacterial populations has also been demonstrated when the animals were fed different diets (29) containing fermentable carbohydrates and after introduction of an exogenous Lactobacillus strain (51).
The combination of 16S rRNA gene-directed DGGE, cloning, and sequencing in this study identified the phylogenetic changes in the piglets' microbiota and highlighted the outgrowth of L. amylovorus-like populations in the ilea of piglets in the HF-fed group. However, because these approaches are all based on PCR amplification methods, the results cannot be converted into actual bacterial numbers. FISH, in combination with microscopic analysis, has provided a powerful tool for detecting and quantifying various bacterial genera, including Lactobacillus, in human feces (16, 17). Sequences related to L. amylovorus or phylotype OTU171 were recovered from the colonic wall and lumen of a pig (41) and were found to be the most abundant Lactobacillus phylotype in the GI tracts of Danish pigs of different ages and with different feeding regimes (28). The same phylotype was found independently to predominate in the small-intestinal microbiota of weaning piglets based on 16S rRNA gene sequence analyses and DGGE (25). Since the present results suggest a significant stimulation of this bacterium in the presence of the HF diet, a DNA oligonucleotide probe targeting the phylotype was developed and validated for FISH analysis. After validation, the probe was used to quantify the number of hybridized cells in the ileal and colonic lumens of piglets fed for 10 days with the HF or LF diet. The FISH results showed that the sizes of OTU171-related populations varied from 0.64 × 108 to 2.4 × 108/g of ileal lumen in 20 out of 24 piglets (Table 5B). The results were in agreement with the DGGE analysis, where the phylotype was detected as a dominant DGGE band in 80% of the analyzed piglets fed for 10 days with the HF diet.
Various studies of the effects of prebiotic oligosaccharides on the colonic microbiota in humans have reported a stimulation of lactobacilli by inulin and lactulose (reference 47; reviewed by Rastall and Gibson [42]). However, in many of the in vitro and in vivo experiments, further characterization beyond the genus level has not been achieved (42). Populations of lactobacilli related to L. amylovorus and L. reuteri have been identified as common inhabitants of the human and animal intestine. The properties of the type strains are also well established. They are known for the ability to degrade starch. In addition, both strains produce bacteriocins, potentially suppressing other populations within the intestinal microbiota (15). However, the extrapolation of functional properties from well-characterized cultured strains to the related L. amylovorus-like and L. reuteri-like phylotypes may not be justified. In particular, a high level of 16S rRNA gene relatedness (>97.5%) was found between the type strains of L. amylovorus, Lactobacillus crispatus, L. gallinarum, and Lactobacillus kitasatonis (34) and the most abundant L. amylovorus-like phylotype detected in the study. Therefore, a study of the physiological and genomic properties of a large collection of L. amylovorus-like populations isolated from the pig intestine is under way in our laboratory. The results will be described in a separate paper.
In the present study, sugar beet pulp with a significant fermentable-carbohydrate (including cell walls) content was also included. The cell wall component of the diet has previously been found not only to affect microbial fermentation in the GI tracts of pigs (24), but also to play a role in stimulation or inhibition of certain pathogens in the intestine (23). The addition of sugar beet pulp to the diets of pigs was reported to reduce the population of coliforms (43), while others suggested an increased proliferation of pathogenic E. coli if the piglets were fed a fiber-enriched diet (33). The effect of dietary fiber on the development of swine dysentery is also under discussion. As shown by some reports, diets with low fiber and resistant starches protect pigs from infection with Brachyspira hyodysenteriae (8, 39, 40), while others were not able to confirm these findings (29, 30). Our results suggest that the combination of fermentable dietary fiber and oligosaccharides may specifically stimulate the L. amylovorus-like population along the guts of weaning piglets.
Acknowledgments
This research was financially supported by the European Communities project HEALTHYPIGUT (QLK5-LT2000-00522).
We thank Wilma Akkermans-van Vliet, Dick Bongers, Cornelia Malin, and Yanka Georgieva for their technical assistance. We are grateful to Maria Saarela for providing the Lactobacillus VTT strains.
REFERENCES
- 1.Alm, E. W., D. B. Oerther, N. Larsen, D. A. Stahl, and L. Raskin. 1996. The oligonucleotide probe database. Appl. Environ. Microbiol. 62:3557-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Bateup, J., S. Dobbinson, K. Munro, M. A. McConnell, and G. W. Tannock. 1998. Molecular analysis of the composition of Lactobacillus populations inhabiting the stomach and caecum of pigs. Microb. Ecol. Health Dis. 10:95-102. [Google Scholar]
- 4.Blaut, M. 2002. Relationship of prebiotics and food to intestinal microflora. Eur. J. Nutr. 41:11-16. [DOI] [PubMed] [Google Scholar]
- 5.Buddington, R. K., K. Kelly-Quagliana, K. K. Buddington, and Y. Kimura. 2002. Non-digestible oligosaccharides and defense functions: lessons learned from animal models. Br. J. Nutr. 87(Suppl. 2):S231-S239. [DOI] [PubMed] [Google Scholar]
- 6.Cromwell, G. L. 2002. Why and how antibiotics are used in swine production. Anim. Biotechnol. 13:7-27. [DOI] [PubMed] [Google Scholar]
- 7.Cummings, J. H., and G. T. Macfarlane. 2002. Gastrointestinal effects of prebiotics. Br. J. Nutr. 87(Suppl. 2):S145-S151. [DOI] [PubMed] [Google Scholar]
- 8.Durmic, Z., D. W. Pethick, J. R. Pluske, and D. J. Hampson. 1998. Changes in bacterial populations in the colon of pigs fed different sources of dietary fibre, and the development of swine dysentery after experimental infection. J. Appl. Microbiol. 85:574-582. [DOI] [PubMed] [Google Scholar]
- 9.Eichner, C. A., R. W. Erb, K. N. Timmis, and I. Wagner-Döbler. 1999. Thermal gradient gel electrophoresis analysis of bioprotection from pollutant shocks in the activated sludge microbial community. Appl. Environ. Microbiol. 65:102-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewing, W. N., and D. J. A. Cole. 1994. The living gut: an introduction to micro-organisms in nutrition. Context Publications, Dungannon, Ireland.
- 11.Felske, A., A. D. L. Akkermans, and W. M. De Vos. 1998. Quantification of 16S rRNAs in complex bacterial communities by multiple competitive reverse transcription-PCR in temperature gradient gel electrophoresis fingerprints. Appl. Environ. Microbiol. 64:4581-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franks, A. H., H. J. M. Harmsen, G. C. Raangs, G. J. Jansen, F. Shut, and G. W. Welling. 1998. Variation of the bacterial population in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson, G. R. 1999. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J. Nutr. 129:1438S-1441S. [DOI] [PubMed] [Google Scholar]
- 14.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 15.Hammes, W. P., N. Weiss, and W. Holzapfel. 1991. The genera Lactobacillus and Carnobacterium, p. 1535-1594. In A. Bolows, H. G. Truper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The procariotes: a handbook on the biology of bacteria: ecophysiology, isolation, identification, applications, vol. 2. Springer-Verlag, New York, N.Y. [Google Scholar]
- 16.Harmsen, H. J. M., P. Elfferich, F. Schut, and G. W. Welling. 1999. A 16S rRNA-targeted probe for detection of lactobacilli and enterococci in faecal samples by fluorescent in situ hybridization. Microb. Ecol. Health Dis. 11:3-12. [Google Scholar]
- 17.Harmsen, H. J. M., G. C. Raangs, T. He, J. E. Degener, and G. W. Welling. 2002. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 68:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heilig, H., E. G. Zoetendal, E. E. Vaughan, P. Marteau, A. D. L. Akkermans, and W. M. de Vos. 2002. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl. Environ. Microbiol. 68:114-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henriksson, A., L. André, and P. Conway. 1995. Distribution of lactobacilli in the porcine gastrointestinal tract. FEMS Microbiol. Ecol. 16:55-60. [Google Scholar]
- 20.Hill, J. E., R. P. Seipp, M. Betts, L. Hawkins, A. G. Van Kessel, W. L. Crosby, and S. M. Hemmingsen. 2002. Extensive profiling of a complex microbial community by high-throughput sequencing. Appl. Environ. Microbiol. 68:3055-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins, M. J., and G. T. Macfarlane. 2003. Nondigestible oligosaccharides enhance bacterial colonization resistance against Clostridium difficile in vitro. Appl. Environ. Microbiol. 69:1920-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii, K., and M. Fukui. 2001. Optimization of annealing temperature to reduce bias caused by a primer mismatch in multitemplate PCR. Appl. Environ. Microbiol. 67:3753-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen, B. B., O. Hojberg, L. L. Mikkelsen, M. S. Hedemann, and N. Canibe. 2003. Enhancing intestinal function to treat and prevent intestinal disease, p. 103-121. Proceedings of the 9th International Symposium on Digestible Physiology in Pigs. University of Alberta, Banff, Canada.
- 24.Jensen, B. B., and H. Jorgensen. 1994. Effect of dietary fiber on microbial activity and microbial gas production in various regions of the gastrointestinal tract of pigs. Appl. Environ. Microbiol. 60:1897-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kluess, J., A. Akkermans, S. Konstantinov, S. Kuhla, M. Kwella, and W. B. Souffrant. 2003. The microbial community and its metabolic activities in the small intestine of weaning piglets, p. 613-618. In W. B. Souffrant and C. C. Metges (ed.), Progress in research on energy and protein metabolism. Wageningen Academic Publishers, Rostock, Germany.
- 26.Konstantinov, S. R., N. Fitzsimons, E. E. Vaughan, and A. D. L. Akkermans. 2002. From composition to functionality of the intestinal microbial communities, p. 59-84. In G. W. Tannock (ed.), Probiotics and prebiotics: where are we going? Caister Academic Press, Wymondham, United Kingdom.
- 27.Konstantinov, S. R., W.-Y. Zhu, B. A. Williams, S. Tamminga, W. M. de Vos, and A. D. L. Akkermans. 2003. Effect of fermentable carbohydrates on faecal bacterial communities as revealed by DGGE analysis of 16S rDNA. FEMS Microbiol. Ecol. 43:225-235. [DOI] [PubMed] [Google Scholar]
- 28.Leser, T. D., J. Z. Amenuvor, T. K. Jensen, R. H. Lindecrona, M. Boye, and K. Moller. 2002. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl. Environ. Microbiol. 68:673-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leser, T. D., R. H. Vindecrona, T. K. Jensen, B. B. Jensen, and K. Moller. 2000. Changes in the colon of pigs fed different experimental diet and after infection with Brachyspira hyodysenteriae. Appl. Environ. Microbiol. 66:3290-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindecrona, R. H., T. J. Jensen, B. B. Jensen, T. Lezer, M. Jiufeng, and K. Moller. 2003. The influence of diet on the development of swine dysentery upon experimental infection. Anim. Sci. 76:81-87. [Google Scholar]
- 31.Maidak, B. L., J. R. Cole, J. C. T. Parker, G. M. Garrity, B. N. Larsen, T. G. L. Li, M. J. McCaughey, G. J. Olsen, R. Overbeek, S. Pramanik, T. M. Schmidt, J. M. Tiedje, and C. R. Woese. 1999. A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res. 27:171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCracken, V. J., J. M. Simpson, R. I. Mackie, and H. R. Gaskins. 2001. Molecular ecological analysis of dietary and antibiotic-induced alterations of the mouse intestinal microbiota. J. Nutr. 131:1862-1870. [DOI] [PubMed] [Google Scholar]
- 33.McDonald, D. E., D. W. Pethick, B. P. Mullan, and D. J. Hampson. 2001. Increasing viscosity of the intestinal contents alters small intestinal structure and intestinal growth, and stimulates proliferation of enterotoxigenic Escherichia coli in newly-weaned pigs. Br. J. Nutr. 86:487-498. [DOI] [PubMed] [Google Scholar]
- 34.Mukai, T., K. Arihara, A. Ikeda, K. Nomura, F. Suzuki, and H. Ohori. 2003. Lactobacillus kitasatonis sp. nov., from chicken intestine. Int. J. Syst. Evol. Microbiol. 53:2055-2059. [DOI] [PubMed] [Google Scholar]
- 35.Muyzer, G., T. Brinkhoff, U. Nübel, C. Santegoeds, H. Schäfer, and C. Wawer. 1998. Denaturing gradient gel electrophoresis (DGGE) in microbial ecology, p. 1-27. In A. D. L. Akkermans, J. D. van Elsas, and F. J. de Bruijn (ed.), Molecular microbial ecology manual, vol. 3.4.4. Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- 36.Muyzer, G., E. C. de Waal, and G. A. Uitterlinden. 1993. Profiling of complex populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muyzer, G., and K. Smalla. 1998. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek 73:127-141. [DOI] [PubMed] [Google Scholar]
- 38.Naito, S., H. Hayashidani, K. Kaneko, M. Ogawa, and Y. Benno. 1995. Development of intestinal lactobacilli in normal piglets. J. Appl. Bacteriol. 79:230-236. [DOI] [PubMed] [Google Scholar]
- 39.Pluske, J. R., D. W. Pethick, D. E. Hopwood, and D. J. Hampson. 2002. Nutritional influence on some major enteric disease in pigs. Nutr. Res. Rev. 15:333-371. [DOI] [PubMed] [Google Scholar]
- 40.Pluske, J. R., P. M. Siba, D. W. Pethick, Z. Durmic, B. P. Mullan, and D. J. Hampson. 1996. The incidence of swine dysentery in pigs can be reduced by feeding diets that limit the amount of fermentable substrate entering the large intestine. J. Nutr. 126:2920-2933. [DOI] [PubMed] [Google Scholar]
- 41.Pryde, S. E., A. J. Richardson, C. S. Stewart, and H. J. Flint. 1999. Molecular analysis of the microbial diversity present in the colonic wall, colonic lumen, and cecal lumen of a pig. Appl. Environ. Microbiol. 65:5372-5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rastall, R. A., and G. R. Gibson. 2002. Prebiotic oligosaccharides: evaluation of biological activities and potential future developments, p. 107-148. In G. W. Tannock (ed.), Probiotics and prebiotics: where are we going?, vol. 1. Caister Academic Press, Wymondham, United Kingdom. [Google Scholar]
- 43.Reid, C. A., and K. Hilman. 1999. The effect of retogradation and amylose/amylopectin ratio on starches and carbohydrates fermentation and microbial populations in the porcine colon. Anim. Sci. 68:503-510. [Google Scholar]
- 44.Reid, G. 1999. The scientific basis for probiotic strains of Lactobacillus. Appl. Environ. Microbiol. 65:3763-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reid, G., J. Howard, and B. S. Gan. 2001. Can bacterial interference prevent infection? Trends Microbiol. 9:424-428. [DOI] [PubMed] [Google Scholar]
- 46.Rolfe, R. 1997. Colonization resistance. In R. I. Mackie, B. A. White, and R. E. Isaacson (ed.), Gastrointestinal microbiology, vol. 2. Chapman and Hall, New York, N.Y.
- 47.Rycroft, C. E., M. R. Jones, G. R. Gibson, and R. A. Rastall. 2001. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J. Appl. Microbiol. 91:878-887. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Sanguinetti, C. J., E. Dias Neto, and A. J. G. Simpson. 1994. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. BioTechniques 17:915-919. [PubMed] [Google Scholar]
- 50.Shannon, C. E., and W. Weaver. 1963. The mathematical theory of communication. University of Illinois Press, Urbana, Ill.
- 51.Simpson, J. M., V. J. McCracken, H. R. Gaskins, and R. I. Mackie. 2000. Denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA amplicons to monitor changes in fecal bacterial populations of weaning pigs after introduction of Lactobacillus reuteri strain MM53. Appl. Environ. Microbiol. 66:4705-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson, J. M., V. J. McCracken, B. A. White, H. R. Gaskins, and R. I. Mackie. 1999. Application of denaturant gradient gel electrophoresis for the analysis of the porcine gastrointestinal microbiota. J. Microbiol. Methods 36:167-179. [DOI] [PubMed] [Google Scholar]
- 53.Smith, D. L., A. D. Harris, J. A. Johnson, E. K. Silbergeld, and J. G. Morris, Jr. 2002. Animal antibiotic use has an early but important impact on the emergence of antibiotic resistance in human commensal bacteria. Proc. Natl. Acad. Sci. USA 99:6434-6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spreeuwenberg, M. A. M., J. M. A. J. Verdonk, H. R. Gaskins, and M. W. A. Verstegen. 2001. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J. Nutr. 131:1520-1527. [DOI] [PubMed] [Google Scholar]
- 55.Statistical Analysis Systems Institute. 1989. SAS/STAT user's guide, version 6, 4th ed., vol. 2. Statistical Analysis Systems Institute, Cary, N.C.
- 56.Steel, R. G. D., and J. H. Torrie. 1980. Principles and procedures of statistics, a biometrical approach, 2nd ed. McGraw-Hill, Tokyo, Japan.
- 57.Stewart, C. S. 1997. Microorganisms in hindgut fermentors, p. 142-186. In R. I. Mackie, B. A. White, and R. E. Isaacson (ed.), Gastrointestinal microbiology, vol. 2. Chapman and Hall, New York, N.Y. [Google Scholar]
- 58.Tannock, G. 1999. Probiotics. A critical review. Horizon Scientific Press, Wymondham, Norfolk, United Kingdom.
- 59.Tannock, G. W. 2001. Molecular assessment of intestinal microflora. Am. J. Clin. Nutr. 73:410S-414S. [DOI] [PubMed] [Google Scholar]
- 60.Tannock, G. W., R. Fuller, and K. Pedersen. 1990. Lactobacillus succession in the piglet digestive tract demonstrated by plasmid profiling. Appl. Environ. Microbiol. 56:1310-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teitelbaum, J. E., and W. A. Walker. 2002. Nutritional impact of pre- and probiotics as protective gastrointestinal organisms. Annu. Rev. Nutr. 22:107-138. [DOI] [PubMed] [Google Scholar]
- 62.Van der Waaij, D. 1989. The ecology of the human intestine and its consequences for the overgrowth of pathogens such as Clostridium difficile. Annu. Rev. Microbiol. 43:69-87. [DOI] [PubMed] [Google Scholar]
- 63.Vaughan, E. E., F. Schut, H. G. H. J. Heilig, E. G. Zoetendal, W. M. de Vos, and A. D. L. Akkermans. 2000. A molecular view of the intestinal ecosystem. Curr. Issues Intest. Microbiol. 1:1-12. [PubMed] [Google Scholar]
- 64.Verstegen, M. W., and B. A. Williams. 2002. Alternatives to the use of antibiotics as growth promotors for monogastric animals. Anim. Biotechnol. 13:113-127. [DOI] [PubMed] [Google Scholar]
- 65.von Wintzingerode, F., U. B. Göbel, and E. Stackebrandt. 1997. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 21:213-229. [DOI] [PubMed] [Google Scholar]
- 66.Williams, B. A., M. W. A. Verstegen, and S. Tamminga. 2001. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr. Res. Rev. 14:207-227. [DOI] [PubMed] [Google Scholar]
- 67.Zoetendal, E. G., A. D. L. Akkermans, and W. M. de Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zoetendal, E. G., K. Ben-Amor, A. D. Akkermans, T. Abee, and W. M. de Vos. 2001. DNA isolation protocols affect the detection limit of PCR approaches of bacteria in samples from the human gastrointestinal tract. Syst. Appl. Microbiol. 24:405-410. [DOI] [PubMed] [Google Scholar]
- 69.Zoetendal, E. G., C. T. Collier, S. Koike, R. I. Mackie, and H. R. Gaskins. 2004. Molecular ecological analysis of the gastrointestinal microbiota: a review. J. Nutr. 134:465-472. [DOI] [PubMed] [Google Scholar]