Abstract
Background
The neutrophil to lymphocyte ratio (NLR) was recently shown to be a remarkable prognostic factor in tumors. Moreover, some studies have indicated that the combination of NLR and platelet to lymphocyte ratio (PLR) could be a better prognostic factor. As the combined prognostic value of NLR and PLR in non-small cell lung cancer (NSCLC) is not clear, we conducted this study to explore this further.
Methods
A total of 366 primary NSCLC patients with stage III or IV were finally included. The neutrophil, platelet, and lymphocyte counts were recorded before treatment was initiated. NLR and PLR were calculated and NLR > 2.68 or PLR > 119.50 was defined as elevated. Univariate and multivariate survival analyses were conducted to test their prognostic value.
Results
The median of NLR and PLR were 3.14 and 152.63, respectively, in all patients. It was indicated that PLR is linearly associated with NLR. PLR is associated with survival, but is not an independent prognostic factor. Removing NLR, PLR is an independent prognostic factor (overall survival [OS]: hazard ratio [HR] = 1.918, P = 0.003; progression-free survival [PFS]: HR = 1.822, P = 0.007 in condition of NLR ≤ 2.68). It was also indicated that elevated NLR is an independent prognostic factor (OS: HR = 1.778, P = 0.009; PFS: HR = 1.535, P = 0.022) in all patients.
Conclusions
PLR is a useful complement of NLR, thus, advanced NSCLC patients could be divided into three prognostic groups prior to treatment: poor: NLR > 2.68; moderate: NLR ≤ 2.68 and PLR > 119.50; and good: NLR ≤ 2.68 and PLR ≤ 119.50.
Keywords: Neutrophil to lymphocyte ratio, non-small cell lung cancer, platelet to lymphocyte ratio, prognosis, systemic inflammatory
Introduction
Regardless of the fact that a number of biomarkers have been found to be related to the prognosis of non-small cell lung cancer (NSCLC), they are still not routinely used in clinical practice, partially because of high costs or the lack of sufficient evidence and standardization.1 Thus, it is worthwhile and necessary to make full use of routine clinical analysis to better predict prognosis. Increased systemic inflammatory response (SIR) has been demonstrated to be associated with poor prognosis and some parameters of SIR can easily be obtained in clinical practice.2 A recent high-quality meta-analysis indicated that the neutrophil to lymphocyte ratio (NLR), a SIR indicator, was a prognostic factor in different solid tumors, including NSCLC.3 Despite this, patients with elevated NLR could only account for 25–55% of all NSCLC patients and the number of patients with no elevated NLR could not be further stratified.4,5 Therefore, NLR optimization is necessary.
Platelet to lymphocyte ratio (PLR), another SIR indicator based on routine blood analysis, was also shown to be associated with survival in NSCLC patients.6,7 A number of studies have demonstrated that the platelet is a complement of neutrophil in cancer angiogenesis and metastasis.8,9 In other studies, it was also shown that the combination of NLR and PLR (CNP) and the combination of platelet count and NLR (COP-NLR) were better predictors of survival in patients with esophageal and colorectal cancers.10,11 Until now, no study has been conducted to evaluate the combined value of NLR and PLR in NSCLC patients. The aims of this study were to identify the combined prognostic value of NLR and PLR in NSCLC patients and whether PLR could make NLR a better prognostic indicator.
Materials and methods
Patient selection
This study was conducted at the Department of Respiratory Medicine, Jinling Hospital (Nanjing, China) and was approved by the Jingling Hospital's Institutional Review Committee on Human Research. We conducted this retrospective study comprising 366 patients diagnosed with NSCLC from January 2007 to August 2012. The inclusion criteria were: (i) all included patients received no chemotherapeutics before routine blood and C-reactive protein (CRP) analysis; (ii) patients were diagnosed as primary NSCLC by pathologic examination (histology and/or cytology examination) and staged according to the tumor node metastasis (TNM) criteria of NSCLC (Union for International Cancer Control, 7th edition); and (iii) patients were diagnosed with stage IIIB or IV, including those in stage IIIA who were not suitable for surgery. The TNM stage assessment was based on computed tomography (CT) scans of the thorax and upper abdomen, magnetic resonance imaging (MRI) or CT scans of the brain, and bone emission CT scans. Patients with a history of other tumors and autoimmune diseases, evidence of current infection, or treated with immunomodulation drugs, were excluded from this study. A total of 384 patients were primarily enrolled and 18 patients were excluded: one patient had been diagnosed with renal pelvic carcinoma; two had been diagnosed with connective tissue disease; seven patients had received immunomodulation up to one month before blood analysis; and eight patients had current fungal or bacterial infections. Finally, 366 NSCLC patients were included in this study.
Clinical data collection
Patients’ demographic data were recorded at admission. Data on blood cell counts and CRP were extracted in a retrospective fashion from the electronic medical records database. All data were recorded before diagnosis or one day prior to chemotherapy. Progression-free survival (PFS) was defined as the time from chemotherapy initiation until disease progression or death of any cause before disease progression. Overall survival (OS) was defined as the time from chemotherapy initiation until the time of death by any cause or the time of last follow-up (23 December 2013). The peripheral blood cell counts were measured with a hematology analyzer (Sysmex XE2100, Sys-mex, Kobe, Japan).
Neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and combination NLR and PLR (CNP) evaluation
The NLR was defined as the ratio of absolute neutrophil count divided by the absolute lymphocyte count, and PLR was defined as the ratio of absolute platelet count divided by the absolute lymphocyte count. Receiver operating characteristic (ROC) curves were separately plotted to verify the optimum cut-off point for NLR and PLR. As shown in Figure 1, the optimum cut-off values for NLR and PLR were 2.68 and 119.50, respectively. Patients were divided into four groups based on NLR and PLR: group 1: NLR ≤ 2.68 and PLR ≤ 119.50; group 2: NLR ≤ 2.68 and PLR > 119.50; group 3: NLR > 2.68 and PLR ≤ 119.50; and group 4: NLR >2.68 and PLR >119.50.
Figure 1.
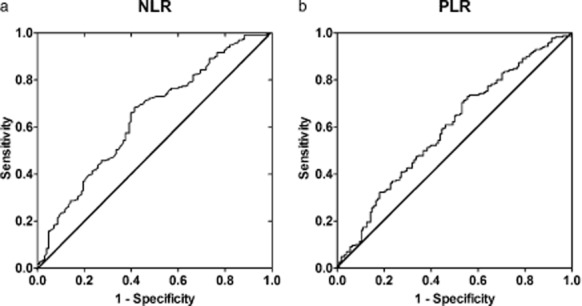
The receiver operating characteristic (ROC) curves for (a) neutrophil to lymphocyte ratio (NLR) (b) and platelet to lymphocyte ratio (PLR) in non-small cell lung cancer patients. (a) The area under the curve (AUC) is 0.643 and the cut-off value is 2.68, with a sensitivity of 0.683 and a specificity of 0.586; (b) The AUC is 0.596 and the cut-off value is 119.50, with a sensitivity of 0.730 and a specificity of 0.445 for PLR.
Statistical analysis
All statistical analyses were performed using Predictive Analytics Software (PASW) Statistics 18.0 (IBM Corporation, Armonk, NY, USA). Data were presented as mean ± standard deviation (s.d) or the absolute number of subjects. ROC curves were constructed to estimate the optimal cut-off value of pretreatment NLR and PLR. Normality and variance homogeneity tests were performed and the Kruskal-Wallis test was applied when analysis of variance was not applicable. Comparison of baseline clinical characteristics between patients in different groups was conducted by chi-squared or Fisher's exact tests. A Cox regression model was utilized for PFS and OS. The survival curve was estimated by Kaplan-Meier analysis and the log-rank test was used to examine the differences in survival between these four groups. Variables with a p value of ≤0.05 were included in subsequent multivariate analysis. A Cox proportional hazards regression model was used to verify independent prognostic factors. In all analyses, a p value of ≤0.05 was considered statistically significant.
Results
Baseline patient characteristics
A total of 366 stage III and IV NSCLC patients were finally included in this study. As shown in Table 1, 246 of the 366 patients were male and the majority of patients (88.25%) were aged between 45 and 80 years old. The median NLR and PLR were 3.14 and 152.63, respectively, in all patients. The median OS and PFS were 359 and 165 days, respectively. Of the 366 patients, 237 had never smoked. Diagnoses included: 237 patients with adenocarcinoma (AC) and 119 with squamous carcinoma (SCC). There were 80 patients with TNM stage III and 286 patients with TNM stage IV.
Table 1.
Relationship between clinical characteristics and the CNP in advanced NSCLC patients
| Variable | Total | NLR ≤ 2.68 | NLR > 2.86 | P | ||
|---|---|---|---|---|---|---|
| PLR ≤ 119.50 | PLR >119.50 | PLR ≤ 119.50 | PLR > 119.50 | |||
| Number of patients | 366 | 87 | 37 | 66 | 176 | |
| Gender | 0.007† | |||||
| Female | 120 | 31 | 7 | 32 | 50 | |
| Male | 246 | 56 | 30 | 34 | 126 | |
| Age (years) | 0.806† | |||||
| <45 | 33 | 7 | 2 | 7 | 17 | |
| 45–65 | 169 | 45 | 19 | 30 | 75 | |
| 65–80 | 154 | 31 | 16 | 27 | 80 | |
| ≥80 | 10 | 4 | 0 | 2 | 4 | |
| Smoking | 0.024† | |||||
| Non-smoker | 157 | 40 | 12 | 38 | 67 | |
| Smoker | 209 | 47 | 25 | 28 | 109 | |
| Histology | 0.016† | |||||
| AC | 237 | 63 | 27 | 50 | 97 | |
| SCC | 119 | 22 | 10 | 16 | 71 | |
| Others | 7 | 1 | 0 | 0 | 6 | |
| Differentiation | 0.062† | |||||
| Well/moderate | 85 | 27 | 9 | 19 | 30 | |
| Poor | 253 | 55 | 28 | 41 | 129 | |
| Tumor stage | 0.018† | |||||
| T1 | 46 | 14 | 3 | 11 | 18 | |
| T2 | 125 | 36 | 10 | 28 | 51 | |
| T3 | 38 | 8 | 2 | 2 | 26 | |
| T4 | 154 | 29 | 22 | 25 | 78 | |
| Node stage | 0.577† | |||||
| N0 | 48 | 16 | 3 | 9 | 20 | |
| N1 | 33 | 6 | 5 | 8 | 14 | |
| N2 | 194 | 49 | 18 | 32 | 95 | |
| N3 | 90 | 16 | 10 | 17 | 47 | |
| Metastasis stage | 0.480† | |||||
| M0 | 80 | 16 | 9 | 13 | 42 | |
| M1a | 107 | 29 | 6 | 23 | 49 | |
| M1b | 179 | 42 | 22 | 30 | 85 | |
| TNM stage | 0.728† | |||||
| III | 80 | 16 | 9 | 13 | 42 | |
| IV | 286 | 71 | 28 | 53 | 134 | |
| Routine blood analysis | ||||||
| Neutrophil (×109 cells/ml) | 4.59 (2.40) | 3.42 (1.74) | 3.24 (1.47) | 5.93 (2.50) | 5.18 (2.52) | <0.001‡ |
| Platelet (×109 cells/ml) | 213.00 (106.25) | 181.00 (79.00) | 259.00 (91.00) | 154.00 (56.00) | 225.00 (104.75) | 0.003‡ |
| Lymphocyte (×109 cells/ml) | 1.42 (0.79) | 2.01 (0.80) | 1.53 (0.54) | 1.70 (0.58) | 1.13 (0.52) | 0.109‡ |
| NLR | 3.14 (2.53) | 1.73 (0.81) | 2.16 (0.63) | 3.20 (1.30) | 4.55 (2.72) | <0.001‡ |
| PLR | 152.63 (104.11) | 95.67 (27.37) | 152.40 (51.75) | 90.87 (17.91) | 203.4438 (100.12) | <0.001‡ |
| CRP (mg/L) | 7.75 (31.13) | 3.70 (5.90) | 6.60 (11.60) | 5.40 (28.20) | 17.95 (54.33) | <0.001‡ |
| PFS (days) | 165.00 (214.75) | 230.0 (234.0) | 173.0 (233.0) | 149.0 (191.0) | 125.0 (172.75) | 0.001‡ |
| OS (days) | 359.00 (339.25) | 459.0 (294.0) | 376.0 (353.0) | 321.0 (245.0) | 311.0 (353.5) | <0.001‡ |
Data were shown as absolute number or median (interquartile range); †Chi-squared or Fisher's exact test; ‡Kruskal-Wallis test. AC, adenocarcinoma; CRP, C-reactive protein; CNP, combination of NLR and PLR; NLR, neutrophil to lymphocyte ratio; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; PLR, platelet to lymphocyte ratio; SCC, squamous carcinoma; TNM, tumor node metastasis.
NLR and PLR analysis
According to the area under the curve (AUC) in Figure 1, the cut-off value is 2.68 with a sensitivity of 0.683 and a specificity of 0.586 for NLR, and 119.50 with a sensitivity of 0.730 and a specificity of 0.445 for PLR. PLR is moderately linearly associated with NLR (Fig. 2, R = 0.644, P < 0.001). NLR gets better specificity (0.586 vs. 0.445) and PLR gets better sensitivity (0.730 vs. 0.683). We divided patients into four groups: group 1: NLR ≤ 2.68 and PLR ≤ 119.50, n = 87; group 2: NLR ≤ 2.68 and PLR > 119.50, n = 37; group 3: NLR > 2.68 and PLR ≤ 119.50, n = 66; and group 4: NLR > 2.68 and PLR > 119.50, n = 176. Among the patients, 213 (58.20%) had an NLR > 2.68 and 242 (66.12%) had a PLR > 119.50. The distribution of clinical characteristics for different groups based on NLR and PLR is presented in Table 1. There are no clinically significant differences among the four groups, except for gender (P = 0.007), smoking status (P = 0.024), histology (P = 0.016), and tumor stage (P = 0.018). Elevated NLR or PLR is associated with neutrophil (P < 0.001) and platelet counts (P = 0.003), but not with lymphocyte count (P = 0.109). Elevated NLR and PLR are associated with increased CRP (P < 0.001) and decreased OS (P < 0.001) and PFS (P < 0.001).
Figure 2.
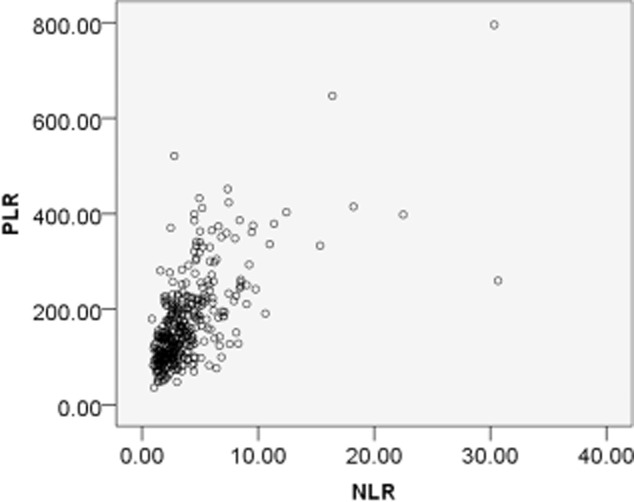
Correlation between neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) in non-small cell lung cancer patients. There was a positive correlation between NLR and PLR: r = 0.644, P < 0.001.
Survival analysis
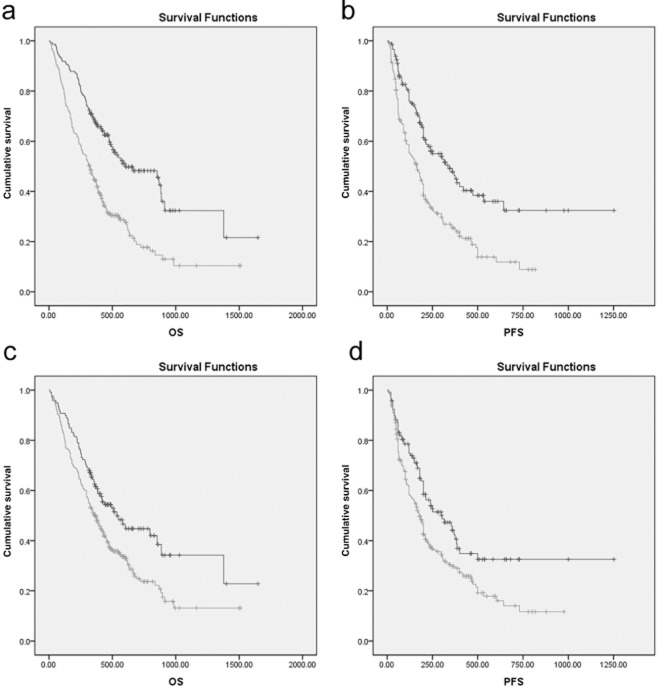
To verify possible prognostic factors, we conducted univariate survival analyses of OS and PFS. Gender (P = 0.023), age (P = 0.006), smoking status (P = 0.005), tumor stage (P = 0.001), node stage (P = 0.017), metastasis stage (P < 0.001), TNM stage (P = 0.001), neutrophil count (P = 0.001), NLR (P < 0.001), PLR (P = 0.003) and CRP (P < 0.001) are possible prognostic factors (Table 2). The Kaplan-Meier survival curves in Figure 3 also indicate that elevated NLR and PLR are associated with decreased OS and PFS.
Table 2.
Univariate survival analyses in relation to PFS and OS in NSCLC patients
| Variables | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI lower | 95% CI upper | P | HR | 95% CI lower | 95% CI upper | P | |
| Gender | ||||||||
| Female | 1 | 1 | ||||||
| Male | 1.294 | 0.972 | 1.724 | 0.077 | 1.397 | 1.048 | 1.863 | 0.023 |
| Age (years) | ||||||||
| <45 | 1 | 1 | ||||||
| 45–65 | 1.261 | 0.732 | 2.172 | 0.403 | 1.551 | 0.900 | 2.647 | 0.114 |
| 65–80 | 1.570 | 0.912 | 2.702 | 0.104 | 2.142 | 1.244 | 3.689 | 0.006 |
| ≥80 | 2.319 | 1.013 | 5.311 | 0.047 | 3.116 | 1.315 | 7.384 | 0.010 |
| Smoking | ||||||||
| Non-smoker | 1 | 1 | ||||||
| Smoker | 1.269 | 0.971 | 1.659 | 0.081 | 1.477 | 1.128 | 1.933 | 0.005 |
| Differentiation | ||||||||
| Well/ Moderate | 1 | 1 | ||||||
| Poor | 1.208 | 0.877 | 1.665 | 0.247 | 1.190 | 0.863 | 1.640 | 0.289 |
| Histology | ||||||||
| AC | 1 | 1 | ||||||
| SCC | 1.043 | 0.793 | 1.373 | 0.761 | 1.200 | 0.911 | 1.579 | 0.194 |
| Others | 0.725 | 0.268 | 1.959 | 0.526 | 0.716 | 0.265 | 1.940 | 0.512 |
| Tumor stage | ||||||||
| I | 1 | 1 | ||||||
| II | 0.937 | 0.598 | 1.470 | 0.778 | 1.039 | 0.662 | 1.631 | 0.868 |
| III | 1.991 | 1.185 | 3.345 | 0.009 | 2.417 | 1.432 | 4.080 | 0.001 |
| IV | 1.236 | 0.802 | 1.907 | 0.337 | 1.265 | 0.820 | 1.952 | 0.287 |
| Node stage | ||||||||
| N0 | 1 | 1 | ||||||
| N1 | 1.855 | 1.052 | 3.268 | 0.033 | 1.996 | 1.132 | 3.521 | 0.017 |
| N2 | 1.472 | 0.942 | 2.298 | 0.089 | 1.496 | 0.958 | 2.337 | 0.077 |
| N3 | 1.882 | 1.159 | 3.055 | 0.011 | 1.682 | 1.036 | 2.730 | 0.035 |
| Metastasis stage | ||||||||
| M0 | 1 | 1 | ||||||
| M1a | 1.558 | 1.045 | 2.321 | 0.029 | 1.470 | 0.987 | 2.190 | 0.058 |
| M1b | 2.166 | 1.511 | 3.106 | <0.001 | 1.937 | 1.351 | 2.755 | <0.001 |
| TNM stage | ||||||||
| III | 1 | |||||||
| IV | 1.913 | 1.354 | 2.702 | <0.001 | 1.749 | 1.239 | 2.470 | 0.001 |
| Neutrophil (cells/ml) | ||||||||
| ≤3.41 × 109 | 1 | 1 | ||||||
| >3.41 × 109 | 1.783 | 1.279 | 2.486 | 0.001 | 1.756 | 1.260 | 2.447 | 0.001 |
| Platelet (cells/ml) | ||||||||
| ≤177.5 × 109 | 1 | 1 | ||||||
| <177.5 × 109 | 1.248 | 0.923 | 1.687 | 0.150 | 1.221 | 0.903 | 1.651 | 0.195 |
| Lymphocyte (cells/ml) | ||||||||
| ≤2.70 × 109 | 1 | 1 | ||||||
| >2.70 × 109 | 1.002 | 0.472 | 2.128 | 0.996 | 0.883 | 0.415 | 1.878 | 0.747 |
| NLR | ||||||||
| ≤2.68 | 1 | 1 | ||||||
| >2.68 | 1.974 | 1.494 | 2.608 | <0.001 | 2.148 | 1.624 | 2.841 | <0.001 |
| PLR | ||||||||
| ≤119.50 | 1 | 1 | ||||||
| >119.50 | 1.573 | 1.175 | 2.105 | 0.002 | 1.568 | 1.171 | 2.099 | 0.003 |
| CRP (mg/L) | ||||||||
| ≤10.4 | 1 | 1 | ||||||
| >10.4 | 2.230 | 1.683 | 2.955 | <0.001 | 2.281 | 1.724 | 3.019 | <0.001 |
AC, adenocarcinoma; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; NLR, neutrophil to lymphocyte ratio; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; PLR, platelet to lymphocyte ratio; SCC, squamous carcinoma; TNM, tumor node metastasis.
Figure 3.
Kaplan-Meier survival curves stratified by neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) in non-small cell lung cancer patients. (a) Overall survival (OS) stratified by NLR; (b) progression-free survival (PFS) stratified by NLR; (c) OS stratified by PLR; and (d) PFS stratified by PLR.  , NLR <= 2.68;
, NLR <= 2.68;  , NLR > 2.68;
, NLR > 2.68;  , NLR <= 2.68-censored;
, NLR <= 2.68-censored;  , NLR > 2.68-censored.
, NLR > 2.68-censored.
We then conducted multivariate survival analyses of OS and PFS using the same factors to verify independent prognostic factors. As TNM stage is the combination of T, N, and M stages, it was not included in multivariate survival analyses. As shown in Table 3, NLR is an independent prognostic factor (OS: P = 0.009; PFS: P = 0.022) while PLR is not (OS: P = 0.705; PFS: P = 0.309). Previous studies have conflicting results on PLR in multivariate survival analyses. Some have reported that PLR was an independent prognostic factor in NSCLC,6,12 while others have not.13 In our present study, more patients were involved than any previous study by our group and NLR is included in the multivariate survival analyses. As NLR is moderately linearly associated with PLR, we speculated that NLR might partially neutralize the effect of PLR and, thus, conducted further analyses.
Table 3.
Multivariate survival analyses in relation to PFS and OS in NSCLC patients
| Variables | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI lower | 95% CI upper | P | HR | 95% CI lower | 95% CI upper | P | |
| Gender | ||||||||
| Female | 1 | 1 | ||||||
| Male | 1.148 | 0.757 | 1.740 | 0.517 | 1.029 | 0.663 | 1.597 | 0.900 |
| Age (years) | ||||||||
| <45 | 1 | 1 | ||||||
| 45–65 | 0.955 | 0.549 | 1.659 | 0.870 | 0.708 | 0.399 | 1.258 | 0.239 |
| 65–80 | 1.222 | 0.701 | 2.127 | 0.479 | 0.872 | 0.484 | 1.569 | 0.647 |
| ≥80 | 2.237 | 0.949 | 5.275 | 0.066 | 1.552 | 0.601 | 4.009 | 0.364 |
| Smoking | ||||||||
| Non-smoker | 1 | |||||||
| Smoker | 1.111 | 0.757 | 1.630 | 0.592 | 1.298 | .845 | 1.996 | 0.234 |
| Tumor stage | ||||||||
| I | 1 | 1 | ||||||
| II | 1.002 | 0.631 | 1.592 | 0.992 | 1.004 | 0.621 | 1.623 | 0.986 |
| III | 1.497 | 0.869 | 2.581 | 0.146 | 1.540 | 0.843 | 2.815 | 0.160 |
| IV | 1.140 | 0.724 | 1.795 | 0.571 | 1.028 | 0.640 | 1.650 | 0.910 |
| Node stage | ||||||||
| N0 | 1 | 1 | ||||||
| N1 | 1.519 | 0.831 | 2.776 | 0.174 | 1.549 | 0.804 | 2.986 | 0.191 |
| N2 | 1.280 | 0.797 | 2.056 | 0.308 | 1.108 | 0.667 | 1.842 | 0.691 |
| N3 | 1.410 | 0.836 | 2.378 | 0.198 | 0.898 | 0.510 | 1.582 | 0.711 |
| Metastasis stage | ||||||||
| M0 | 1 | 1 | ||||||
| M1a | 1.882 | 1.232 | 2.874 | 0.003 | 1.841 | 1.148 | 2.953 | 0.011 |
| M1b | 2.321 | 1.602 | 3.362 | <0.001 | 2.264 | 1.477 | 3.473 | <0.001 |
| Neutrophil (cells/ml) | ||||||||
| ≤3.41 × 109 | 1 | 1 | ||||||
| >3.41 × 109 | 1.160 | 0.753 | 1.788 | 0.501 | 1.020 | 0.655 | 1.586 | 0.931 |
| NLR | ||||||||
| ≤2.68 | 1 | 1 | ||||||
| >2.68 | 1.535 | 1.064 | 2.212 | 0.022 | 1.778 | 1.157 | 2.732 | 0.009 |
| PLR | ||||||||
| ≤119.50 | 1 | 1 | ||||||
| >119.50 | 1.189 | 0.852 | 1.658 | 0.309 | 1.079 | 0.729 | 1.596 | 0.705 |
| CRP (mg/L) | ||||||||
| ≤10.4 | 1 | 1 | ||||||
| >10.4 | 1.766 | 1.264 | 2.467 | 0.001 | 1.774 | 1.270 | 2.477 | 0.001 |
AC, adenocarcinoma; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; NLR, neutrophil to lymphocyte ratio; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; PLR, platelet to lymphocyte ratio; SCC, squamous carcinoma; TNM, tumor node metastasis.
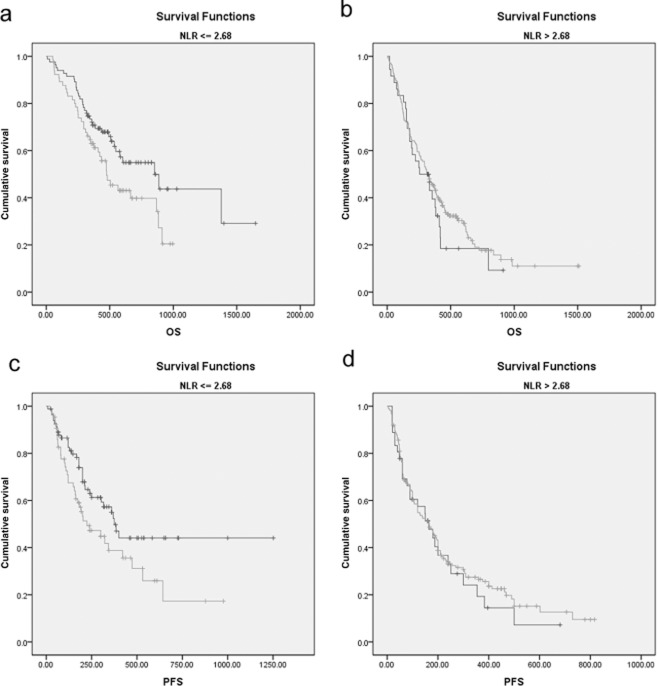
To further explore the association of NLR and PLR, we conducted Kaplan-Meier survival curves of PLR in NLR elevated patients (NLR > 2.68) and NLR non-elevated patients (NLR ≤ 2.68). Elevated PLR is associated with decreased OS and PFS only when NLR is ≤2.68 (Fig. 4). These results indicated that PLR might be a complement of NLR, thus, we conducted further multivariate survival analyses of OS and PFS in patients with an NLR ≤ 2.68. As shown in Table 4, PLR is an independent prognostic factor (OS: P = 0.020; PFS: P = 0.035) when NLR is ≤ 2.68. As shown in Table 1, there is a significant decrease in OS and PFS in PLR-elevated patients compared with PLR-non-elevated patients when NLR is ≤2.68. All of these results show that PLR is a complementary prognostic factor of NLR when NLR is ≤2.68. Patients were divided into three groups prior to treatment based on the combination of pretreatment NLR and PLR (Fig. 5): (i) poor survival: NLR > 2.68; (ii) moderate survival: NLR ≤ 2.68 and PLR > 119.50; and (iii) good survival: NLR ≤ 2.68 and PLR ≤ 119.50.
Figure 4.
Kaplan–Meier survival curves stratified by a combination of neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) in non-small cell lung cancer patients. (a) Overall survival (OS) stratified by PLR when NLR is ≤2.68; (b) OS stratified by PLR when NLR is > 2.68; (c) progression-free survival (PFS) stratified by PLR when NLR is ≤2.68; and (d) PFS stratified by PLR when NLR is > 2.68.  , PLR <= 119.50;
, PLR <= 119.50;  , PLR > 119.50;
, PLR > 119.50;  , PLR <= 119.50-censored;
, PLR <= 119.50-censored;  , PLR > 119.50-censored.
, PLR > 119.50-censored.
Table 4.
Multivariate survival analyses in relation to PFS and OS when NLR ≤ 2.68
| Variables | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI lower | 95% CI upper | P | HR | 95% CI lower | 95% CI upper | P | |
| Gender | ||||||||
| Female | 1 | 1 | ||||||
| Male | 1.689 | 0.689 | 4.143 | 0.252 | 1.396 | 0.600 | 3.252 | 0.439 |
| Age (years) | ||||||||
| <45 | 1 | 1 | ||||||
| 45–65 | 0.157 | 0.050 | 0.492 | 0.001 | 0.204 | 0.067 | 0.620 | 0.005 |
| 65–80 | 0.179 | 0.057 | 0.561 | 0.003 | 0.270 | 0.086 | 0.843 | 0.024 |
| ≥80 | 0.219 | 0.045 | 1.052 | 0.058 | 0.496 | 0.104 | 2.373 | 0.380 |
| Smoking | ||||||||
| Non-smoker | 1 | |||||||
| Smoker | 1.006 | 0.409 | 2.476 | 0.989 | 1.544 | 0.672 | 3.545 | 0.306 |
| Tumor stage | ||||||||
| I | 1 | 1 | ||||||
| II | 0.774 | 0.357 | 1.679 | 0.517 | 0.842 | 0.385 | 1.842 | 0.668 |
| III | 1.841 | 0.437 | 7.756 | 0.406 | 0.900 | 0.193 | 4.191 | 0.893 |
| IV | 0.847 | 0.366 | 1.961 | 0.699 | 0.865 | 0.381 | 1.962 | 0.728 |
| Node stage | ||||||||
| N0 | 1 | 1 | ||||||
| N1 | 1.334 | 0.423 | 4.200 | 0.623 | 1.167 | 0.371 | 3.668 | 0.792 |
| N2 | 0.991 | 0.406 | 2.417 | 0.984 | 0.596 | 0.242 | 1.472 | 0.262 |
| N3 | 0.900 | 0.318 | 2.546 | 0.843 | 0.339 | 0.107 | 1.078 | 0.067 |
| Metastasis stage | ||||||||
| M0 | 1 | 1 | ||||||
| M1a | 2.408 | 0.832 | 6.966 | 0.105 | 1.816 | 0.632 | 5.218 | 0.268 |
| M1b | 4.164 | 1.499 | 11.569 | 0.006 | 3.724 | 1.339 | 10.358 | 0.012 |
| Neutrophil (cells/ml) | ||||||||
| ≤3.41 × 109 | 1 | 1 | ||||||
| >3.41 × 109 | 1.211 | 0.692 | 2.120 | 0.503 | 1.087 | 0.607 | 1.947 | 0.779 |
| PLR | ||||||||
| ≤119.50 | 1 | 1 | ||||||
| >119.50 | 1.879 | 1.047 | 3.374 | 0.035 | 2.046 | 1.122 | 3.732 | 0.020 |
| CRP (mg/L) | ||||||||
| ≤10.4 | 1 | 1 | ||||||
| >10.4 | 2.786 | 1.374 | 5.648 | 0.004 | 3.014 | 1.490 | 6.099 | 0.002 |
CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; NLR, neutrophil to lymphocyte ratio; OS, overall survival; PFS, progression-free survival; PLR, platelet to lymphocyte ratio.
Figure 5.
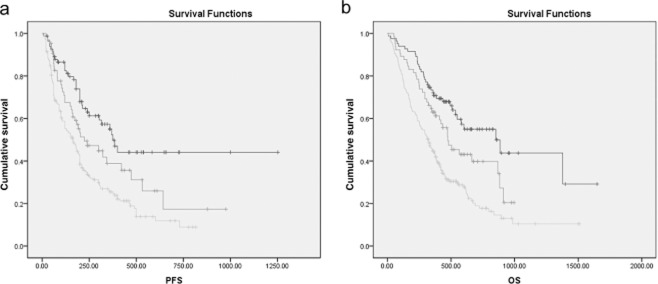
Kaplan-Meier survival curves stratified by a combination of platelet to lymphocyte ratio (PLR) and neutrophil to lymphocyte ratio (NLR) in non-small cell lung cancer patients. (a) Progression-free survival (PFS); (b) overall survival (OS).  , NLR ≤ 2.68 and PLR ≤ 119.50;
, NLR ≤ 2.68 and PLR ≤ 119.50;  , NLR ≤ 2.68 and PLR > 119.50;
, NLR ≤ 2.68 and PLR > 119.50;  , NLR > 2.68 and PLR > 119.50;
, NLR > 2.68 and PLR > 119.50;  , NLR ≤ 2.68 and PLR ≤ 119.50-censored;
, NLR ≤ 2.68 and PLR ≤ 119.50-censored;  , NLR ≤ 2.68 and PLR > 119.50-censored;
, NLR ≤ 2.68 and PLR > 119.50-censored;  , NLR > 2.68 and PLR > 119.50-censored.
, NLR > 2.68 and PLR > 119.50-censored.
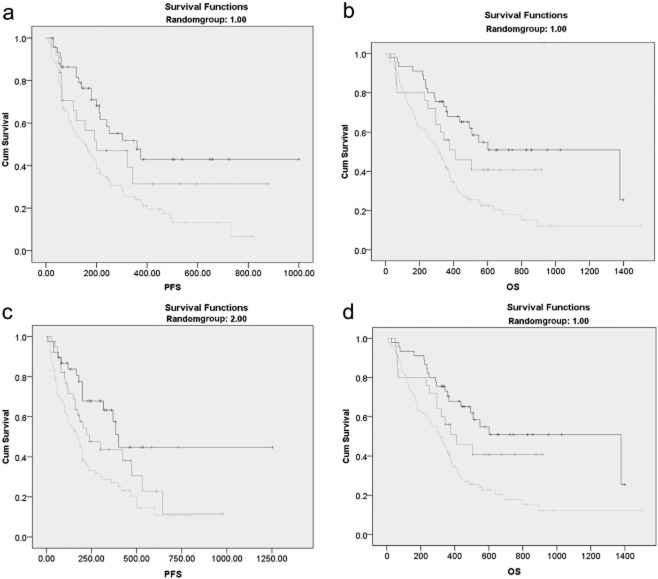
To verify the reliability of the combination of NLR and PLR in survival indication, involved patients were divided into 2 randomized groups and Kaplan–Meier survival curves were plotted in both groups. It was shown that the log rank P value was less than 0.005 in either group (Fig. 6) and this model is reliable.
Figure 6.
Verification of the combination of platelet to lymphocyte ratio (PLR) and neutrophil to lymphocyte ratio (NLR) in non-small cell lung cancer. Patients were divided into two groups at random and Kaplan-Meier survival curves of progression-free survival (PFS) and overall survival (OS) were plotted by the combination of PLR and NLR. (a) PFS in randomized group 1, P = 0.002; (b) OS in randomized group 1, P < 0.001; (c) PFS in randomized group 2, P = 0.001; and (d) OS in randomized group 2, P = 0.001.  , NLR ≤ 2.68 and PLR ≤ 119.50;
, NLR ≤ 2.68 and PLR ≤ 119.50;  , NLR ≤ 2.68 and PLR > 119.50;
, NLR ≤ 2.68 and PLR > 119.50;  , NLR > 2.68;
, NLR > 2.68;  , NLR ≤ 2.68 and PLR ≤ 119.50-censored;
, NLR ≤ 2.68 and PLR ≤ 119.50-censored;  , NLR ≤ 2.68 and PLR > 119.50-censored;
, NLR ≤ 2.68 and PLR > 119.50-censored;  , NLR > 2.68-censored.
, NLR > 2.68-censored.
Discussion
In this study, we demonstrate that PLR, which is moderately linearly associated with NLR, is a complementary prognostic factor of NLR when NLR is not elevated for the first time. Patients with PLR > 119.50 had poorer survival than patients with PLR ≤ 119.50 when NLR is ≤2.68. We also confirmed that elevated NLR (NLR > 2.68) is independently associated with poor survival in stage III and IV NSCLC patients. Thus, advanced NSCLC patients were divided into three prognostic groups prior to treatment with combinations of NLR and PLR.
Since 2007, NLR has been investigated in a number of tumors by over 100 separate studies. Over half of these were published in the last two years.3 PLR, another systemic inflammatory indicator, has also been revealed to be a survival indicator in different types of tumors.14–16 Recent meta-analyses of NLR3 and PLR17 have also verified that elevated NLR or PLR is associated with poor survival. In NSCLC, 14 studies on NLR and five studies on PLR have been conducted to evaluate their prognostic value (Table 5).4–7,12,13,18–26 These studies used different criteria of elevated NLR and elevated PLR and the number of included patients varied from 23 to 388. Most of these studies used a value of 5.0 for NLR as the threshold. Using this value may obtain ideal specialty but poor sensitivity, and a considerable number of patients with increased risk of death might be excluded from the NLR elevated group. Thus, we conducted ROC curves and obtained a cut-off value of 2.68 for NLR, which is very close to our previous study.5 Among the five previous studies of PLR in NSCLC demonstrating that elevated PLR is associated with decreased survival, only two studies reported that PLR is a potential independent survival indicator by multivariate survival analyses. It seems that other factors might have an influence on PLR. Our study indicated that PLR is not an independent prognostic factor in all NSCLC patients. This may be because of the different staging of included patients (III or IV vs. II or III) and the addition of NLR in multivariate survival analyses. As PLR is linearly associated with NLR (Fig. 2), we further analyzed the influence of NLR on PLR. We found that elevated PLR was independently associated with poor survival in patients with NLR ≤ 2.68. As shown in Figure 4 (a & c), PLR enables us to distinguish patients that should be defined as “low risk,” because their NLR was not elevated. Thus, PLR is a useful complement to NLR in NSCLC in this sense, and makes discrimination of patients with “moderate survival” possible.
Table 5.
Summary of studies on NLR and/or PLR in NSCLC
| Study | Country | No. of patients | Cut-off value | Details of patients | |
|---|---|---|---|---|---|
| NLR | PLR | ||||
| Sarraf, 2009 | UK | 177 | † | ‡ | Ia-IV, 33% stage Ib¶; 59% male; 49% AC§; all underwent surgical resection |
| Teramukai, 2009 | Japan | 388 | 2.093, 2.914 and 4.744 | ‡ | IIIb-IV, 82% stage IV¶; 71% male; 70% AC§; all received chemotherapy |
| Sakai, 2011 | Japan | 23 | † | ‡ | Ia-IIIb, 60.9% stage Ib¶; 60.9% male; 100% AC§; all underwent surgical resection |
| Tomita, 2012£ | Japan | 284 | 2.5 | ‡ | I-III, 63.5% stage I; 63.5% male; 72.8% AC§; all underwent surgical resection |
| Forget, 2013 | Belgium | 255 | 5.0 | ‡ | I-II, 76% stage I¶; 82.4% male; 53.3% AC§; all underwent surgical resection |
| Unal, 2013 | Turkey | 94 | 3.44 | 194 | II-IIIb, 47.9% stage IIIb¶; 93.6% male; 70.2% SCC§; all received chemotherapy, partial received radiotherapy |
| Kacan | Turkey | 270 | 5.0 | ‡ | I-IV, 66.7% stage III¶, 90% male; 41% SCC§; therapy strategy was not stated |
| Cedres, 2012 | Spain | 171 | 5.0 | ‡ | 100% stage IV¶; 83% male; 40.5% AC§; all patients received first-line chemotherapy |
| Lee, 2012 | Korea | 199 | 3.25 | ‡ | III b-IV, 92% stage IV¶; 8.5% male; 100% AC§; all patients received first-line chemotherapy; never smokers |
| Jafri, 2013 | USA | 173 | 5.0 | ‡ | 100% stage IV¶; 67% male; 52% AC§; 66% received chemotherapy |
| Kaya, 2013 | Turkey | 156 | 5 | 150 | IIIa-IV, 40.4% stage IIIa¶; 51.3% male; 54.8% SCC§; 66% received chemotherapy |
| Yao, 2012 | China | 182 | 2.63 | ‡ | III-IV, 68.1 % stage IV¶; 65.4 % male; 70.3 % AC§; all patients received first-line chemotherapy |
| Liu, 2013 | China | 210 | ‡ | 152.6 | III-IV, 70.5 % stage IV¶; 66.2 % male; 66.2 % AC§; all patients received first-line chemotherapy |
| Sanchez-Lara, 2012 | Mexico | 119 | 5.0 | 150 | IIIb-IV, 72.3 % IIIb; 46.2 % male; No histology information; all patients received first-line chemotherapy |
| Pinato, 2014 | UK | 220 | 5.0 | 300 | Ia-IIIa, 34 % Ia; 50 % male; 60 % AC§; all underwent surgical resection |
†It was investigated but not offered; ‡It was not investigated in this study; §The largest portion of histological sub-type was presented; ¶The largest portion of non-small cell lung cancer (NSCLC) stage was presented; £This author conducted two studies in this field and the latter with more patients was chosen. AC, adenocarcinoma; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; SCC, squamous carcinoma.
Although a number of studies have been conducted on NLR and/or PLR in NSCLC, few of them have studied the association of NLR and PLR in NSCLC patients. Furthermore, previous studies have revealed that the combination of platelet count and NLR10 and the combination of PLR and NLR6 were better than NLR alone. In our study, we found that the combination of platelet count and NLR was no better than NLR in NSCLC patients (Fig. 5); however, using the combination of PLR and NLR, PLR could further stratify the patients with poor survival with NLR ≤ 2.68. In survival analysis, no significant difference was found between PLR elevated and PLR non-elevated patients when NLR is > 2.68. However, poorer survival rates were found in PLR elevated patients than in PLR non-elevated patients when NLR is ≤2.68. This indicated that an elevated NLR partially offset the effect of PLR on survival prediction. Thus, we speculated that PLR was a complementary prognostic factor of NLR, but not an independent prognostic factor in all advanced NSCLC patients and the combination of PLR and NLR was a better prognostic indicator than NLR alone.
Chronic inflammation has long been proven to contribute to cancer initiation and progression in a number of studies.2,27–29 Neutrophils, lymphocytes, and platelets are the three main subpopulations in peripheral blood. Neutrophils play the role of a double-edged sword in tumor progression. Previous studies have indicated that although CD11b+/Ly6G+ tumor-associated neutrophils (TANs) are cytotoxic to tumor cells, the tumor microenvironment could also induce pro-tumor phenotype TANs by tumor growth factor -beta (TGF-beta)30 and, thus, increase tumor angiogenesis.31 It has also been demonstrated that circulating neutrophils could promote metastasis by the release of neutrophil extracellular traps (NETs), sequestering circulating tumor cells.8 On the other hand, CD8+ T cells are shown to be key modulators of cytotoxic to tumor cells caused by neutrophils.30 Other subpopulations of lymphocytes, including T helper and NK cells, are also key anti-cancer cells affecting growth and/or metastasis in different cancers.32–34 This might partially explain why an elevated NLR is associated with poor survival. Furthermore, previous studies have demonstrated that platelets, another population of pro-inflammatory cells, might interact with neutrophils and contribute to the release of NETs.35 This might also partially explain the mechanism that PLR complements NLR.
Our present study confirmed the previous study by our group and the recent meta-analysis, which indicated that NLR is a potential biomarker of shorter PFS and OS for advanced NSCLC patients.3,5 There are some differences between our two studies. One main difference is that CRP was an independent prognostic factor in the present study, but not in the previous study. This might be a result of different inclusion criteria and the addition of PLR and other factors in the multivariate survival analyses. Some other recent studies have also demonstrated that elevated CRP is independently associated with poor survival.36
This study was a single-center, retrospective study and the number of included subjects was moderate to small. However, we confirmed our previous findings by involving more patients, and, for the first time, found that PLR could stratify patients with non-elevated NLR. The clinical utility of NLR and PLR still needs to be confirmed by future prospective analysis.
Conclusion
In summary, we have demonstrated that PLR is a useful complement to NLR. Using the combination of NLR and PLR, advanced NSCLC patients were able to be divided into three different prognostic groups prior to treatment: poor (NLR > 2.68), moderate (NLR ≤ 2.68 and PLR > 119.50) and good survival (NLR ≤ 2.68 and PLR ≤ 119.50). Future well-designed, prospective studies are needed to verify this conclusion.
Acknowledgments
This study was supported by National Natural Scientific Foundation of China Grants (no. 81370172 and no. 81170064).
Disclosure
No authors report any conflict of interest.
References
- Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110:435–440. doi: 10.1038/bjc.2013.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- Forget P, Machiels JP, Coulie PG, et al. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol. 2013;20(Suppl 3):S650–660. doi: 10.1245/s10434-013-3136-x. [DOI] [PubMed] [Google Scholar]
- Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62:471–479. doi: 10.1007/s00262-012-1347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wu Y, Wang Z, et al. Pretreatment platelet-to-lymphocyte ratio (PLR) as a predictor of response to first-line platinum-based chemotherapy and prognosis for patients with non-small cell lung cancer. J Thorac Dis. 2013;5:783–789. doi: 10.3978/j.issn.2072-1439.2013.12.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya V, Yildirim M, Demirpence O, Yildiz M, Yalcin AY. Prognostic significance of basic laboratory methods in non-small-cell-lung cancer. Asian Pac J Cancer Prev. 2013;14:5473–5476. doi: 10.7314/apjcp.2013.14.9.5473. [DOI] [PubMed] [Google Scholar]
- Cools-Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Investig. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–287. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer. 2013;109:401–407. doi: 10.1038/bjc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JF, Huang Y, Liu JS. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. OncoTargets Ther. 2013;6:1605–1612. doi: 10.2147/OTT.S52501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal D, Eroglu C, Kurtul N, Oguz A, Tasdemir A. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev. 2013;14:5237–5242. doi: 10.7314/apjcp.2013.14.9.5237. [DOI] [PubMed] [Google Scholar]
- Pinato DJ, Shiner RJ, Seckl MJ, Stebbing J, Sharma R, Mauri FA. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer. 2014;110:1930–1935. doi: 10.1038/bjc.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinato DJ, Mauri FA, Ramakrishnan R, Wahab L, Lloyd T, Sharma R. Inflammation-based prognostic indices in malignant pleural mesothelioma. J Thorac Oncol. 2012;7:587–594. doi: 10.1097/JTO.0b013e31823f45c1. [DOI] [PubMed] [Google Scholar]
- Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–3100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- Kang MH, Go SI, Song HN, et al. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer. 2014;111:452–460. doi: 10.1038/bjc.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS ONE. 2014;9(6):e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- Sakai T, Tsushima T, Kimura D, Hatanaka R, Yamada Y, Fukuda I. A clinical study of the prognostic factors for postoperative early recurrence in patients who underwent complete resection for pulmonary adenocarcinoma. Ann Thorac Cardiovasc Surg. 2011;17:539–543. doi: 10.5761/atcs.oa.11.01660. [DOI] [PubMed] [Google Scholar]
- Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer. 2009;45:1950–1958. doi: 10.1016/j.ejca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011;31:2995–2998. [PubMed] [Google Scholar]
- Kacan T, Babacan NA, Seker M, et al. Could the neutrophil to lymphocyte ratio be a poor prognostic factor for non small cell lung cancers? Asian Pac J Cancer Prev. 2014;15:2089–2094. doi: 10.7314/apjcp.2014.15.5.2089. [DOI] [PubMed] [Google Scholar]
- Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. doi: 10.1186/1471-2407-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedrés S, Torrejon D, Martínez A, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. 2012;14:864–869. doi: 10.1007/s12094-012-0872-5. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim SH, Han JY, Kim HT, Yun T, Lee JS. Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. J Cancer Res Clin Oncol. 2012;138:2009–2016. doi: 10.1007/s00432-012-1281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Lara K, Turcott JG, Juárez E, et al. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: a prospective study. Nutr Cancer. 2012;64:526–534. doi: 10.1080/01635581.2012.668744. [DOI] [PubMed] [Google Scholar]
- Wang H, Taverna D, Stram DO, et al. Genetic variation in the inflammation and innate immunity pathways and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2013;22:2094–2101. doi: 10.1158/1055-9965.EPI-13-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Engels EA, Shi J, et al. Genetic variation in innate immunity and inflammation pathways associated with lung cancer risk. Cancer. 2012;118:5630–5636. doi: 10.1002/cncr.27605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska J, Wu CF, Andzinski L, Leschner S, Weiss S. CXCR2-mediated tumor-associated neutrophil recruitment is regulated by IFN-beta. Int J Cancer. 2014;134:1346–1358. doi: 10.1002/ijc.28551. [DOI] [PubMed] [Google Scholar]
- Logan RW, Zhang C, Murugan S, et al. Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol. 2012;188:2583–2591. doi: 10.4049/jimmunol.1102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- Braumüller H, Wieder T, Brenner E, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Investig. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni XF, Wu P, Wu CP, et al. Elevated serum C-reactive protein, carcinoembryonic antigen and N2 disease are poor prognostic indicators in non-small cell lung cancer. Asia Pac J Clin Oncol. 2014 doi: 10.1111/ajco.12091. . doi: 10.1111/ajco.12091. [DOI] [PubMed] [Google Scholar]