Abstract
We investigated the treatment and prognosis of pleural effusion (PE) in multiple myeloma (MM) patients. From June 2005 to December 2013, 296 MM patients participated in this study. There were 23 PE patients, including 11 men and 12 women, with a median age of 56.8 years (range 37–68 years). A diagnosis of PE was based on physical examination, chest X-ray or computed tomography, and pleural fluid analysis. All patients demonstrated myeloma cells in the pleural fluid, and six patients were positive for PE M protein. PE patients received bortezomib combined with other drugs. Only one patient demonstrated a complete response; 10 patients showed partial responses, and 12 patients developed progressive disease and died. MM linked with myelomatous PE is associated with a poor prognosis. Myelomatous PE is likely a late manifestation of the natural history of MM or an expression of the aggressive behavior of the disease.
Keywords: Bortezomib, multiple myeloma, myelomatous pleural effusion, prognosis
Introduction
Multiple myeloma (MM) results from the malignant proliferation of monoclonal plasma cells that produce monoclonal immunoglobulins. Malignant pleural effusion (PE) in MM patients is rare, observed in <6% of cases.1 Recognizing plasma cells (myeloma cells) in bodily fluids is important for diagnosis, and the presence of these cells is associated with a poor prognosis.2 Our study evaluated a large number of PE cases in myeloma patients. The purpose of our study was to report the frequency, clinical features, laboratory findings, and treatment response in patients with PE and MM.
Materials and methods
Clinical material
Among 296 patients, 23 patients, including 11 men and 12 women, were diagnosed with PE. A diagnosis of PE was based on physical examination, chest X-ray or computed tomography (CT), chest biopsy, or hydrothorax exfoliative cytologic examination. The diagnosis of malignant PE was mainly based on PE exfoliated cytology or M protein detection. The International Staging System (ISS) was also used to stage cases of malignant PE.
Patients were assessed two weeks after completing each chemotherapy cycle. In addition to the International Uniform Response Criteria for MM,3 assessment included chest CT to assess the presence of PE. If the PE cases showed complete absorption, they were classified as complete response. Using chest CT results, partial response was defined as a 51–75% reduction in PE and minimal response was defined as a 25–50% reduction. We also performed blood tests to measure serum creatinine, serum calcium, serum C-reactive protein, serum lactate dehydrogenase, serum beta-2 macroglobulin, and serum albumin levels. Urinary protein electrophoresis was used to measure Bence-Jones proteins, immunofixation was used to detect and type monoclonal antibodies and immunoglobulins, and bone marrow biopsies were also performed. We also assessed the patients for adverse events, which were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
We used the Kaplan–Meier method to estimate overall survival.4 P values of 0.05 or less were considered to be statistically significant. We used the SPSS statistical software package version 13.0 (SPSS Inc., Chicago, IL, USA)to perform all statistical analyses.
Ethics statement
The ethics committee of the Beijing Chao-Yang Hospital, Capital Medical University, approved this study and also waived the requirement to obtain informed consent because the identity of the patients would remain anonymous. All aspects of the study complied with the Declaration of Helsinki.
Results
Of the 296 consecutive MM patients reviewed, 23 (7.8%) showed evidence of PE. Of these 23 patients, three of eight patients had chromosome 13 abnormalities. According to the ISS, 16 patients had stage III MM, five had stage II MM, and two patients had stage I MM. The median patient age was 56.8 years (range 37–68 years) (Table1).
Table 1.
Clinicopathological characteristics of 23 multiple myeloma patients with PE
| Overall | |
|---|---|
| N = 23 | |
| Age, years, median (range) | 56.8 (37–68) |
| Gender, n (%) | |
| Male | 11 (47.8) |
| Female | 12 (52.2) |
| Heavy chain, n (%) | |
| IgG | 3 (13) |
| IgA | 9 (39.1) |
| Light chain, n (%) | |
| κ light chain | 2 (8.7%) |
| λ light chain | 6 (26) |
| Non-secrete | 3 (13) |
| β2MG≥5.5 mg/l, n (%) | 16 (69.5) |
| Bilateral pleural effusion | 20 (86.9) |
IgA, immunoglobin A; IgG, immunoglobin G; PE, pleural effusion.
The median follow-up time was 20 months (range 3–32 months). All patients were characterized by a sense of suppression in the chest and hemorrhagic PE [Fig 1]. X-ray analysis of all patients showed pleural thickening adhesions, and in seven patients, blood gas analysis showed severe hypoxemia and respiratory failure. Three MM patients had PE at the time of diagnosis, whereas the remaining 20 patients developed PE during disease progression. Chest CT revealed the presence of PE in all 23 patients, and hydrothorax exfoliative cytologic examination revealed myeloma cells in all patients [Fig 2]. Six patients tested positive for pleural fluid by immunofixation electrophoresis, and each of these patients demonstrated M protein in the serum.
Figure 1.

Myelomatous pleural effusion on chest computed tomography.
Figure 2.
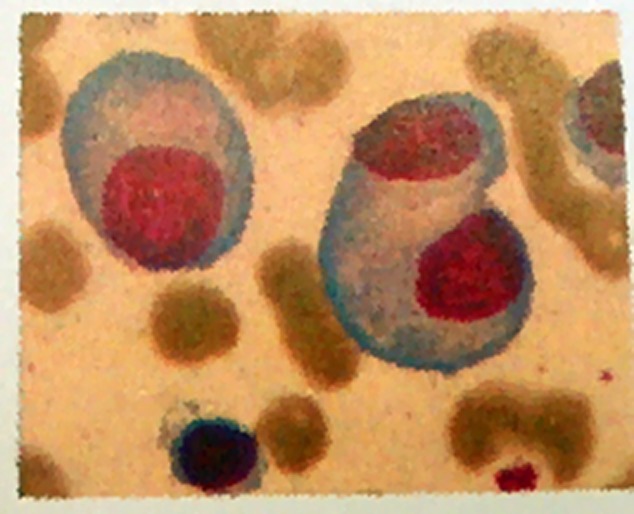
Pleural fluid cytology (Giemsa Stain ×1000).
The treatments and outcomes of MM patients with PE in this study differed from those of the MM patients without PE, as reported in previous studies. Twenty-three patients received chemotherapy, and all patients received bortezomib-containing regimens, which included: bortezomib plus vindesine, epirubicin, and dexamethasone (PVAD); bortezomib plus dexamethasone (BD); and bortezomib plus cisplatin, etoposide, cyclophosphamide, and prednisone (DECP). Two patients received chemotherapy and partial medication treatment with interleukin (IL)-2 and dexamethasone. Among the 23 patients with PE, one patient showed a complete response, 10 patients demonstrated a partial response, and 12 patients developed progressive disease and died. Twelve patients died, including three from respiratory failure, three from cardiac tamponade, and two from cerebral hemorrhage. In addition, three patients were lost to follow-up (Table 2).
Table 2.
Treatment and response of MM patients with PE to therapy
| Method of therapy | Number of patients | ||||
|---|---|---|---|---|---|
| Total | CR | PR | PD | DIED | |
| BDECP | 4 | 0 | 2 | 2 | 2 |
| BD | 1 | 0 | 0 | 1 | 1 |
| PAD | 7 | 0 | 2 | 5 | 5 |
| PVAD | 3 | 0 | 1 | 2 | 2 |
| PCTD | 6 | 0 | 4 | 2 | 2 |
| Chemo + local therapy | 2 | 1 | 1 | 0 | 0 |
BD, bortezomib plus dexamethasone; BDECP, bortezomib plus cisplatin, etoposide, ifosfamide, and prednisone; CR, complete response; MM, multiple myeloma; PAD, bortezomib, epirubicin, plus dexamethasone; PCTD, bortezomib, ifosfamide, thalidomide, plus dexamethasone; PD, progressive disease; PE, pleural effusion; PR, partial response; PVAD, bortezomib plus vindesine, epirubicin, and dexamethasone.
In general, the patients in this study tolerated bortezomib combination therapy well. The most common severe toxicities associated with bortezomib were thrombocytopenia and peripheral neuropathy. Infection was the most common adverse effect among the patients who received DECP and VAD regimens.
Discussion
MM is mainly characterized by malignant plasma cells in the bone marrow, blood, and/or urine containing monoclonal immunoglobulins and osteoporosis or osseous lesions. Malignant plasma cells are mainly found in the bone marrow, but may also affect other bodily organs and tissues, including the breast, bone, lung, and pleura; in particular, malignant plasma in the pleura can lead to PE. The incidence of PE in myeloma is rare; fewer than 100 cases of PE in myeloma patients have been reported.5,6 Our analysis of 23 patients with MM and PE represents the largest case study to date. Our incidence of myeloma patients with PE was 7.8%, which is slightly higher than reported in previous studies.7 In previous studies, 80% of PE cases occurred with the immunoglobin (Ig)A subtype, whereas most of the remaining cases were classified as the IgG subtype.8 More cases of PE in the left side of the chest have been reported.9,10 In our analysis, most of the patients in the present study presented IgA-k MM (9/23) or lambda light chain MM (6/23), IgG-k MM (3/23), and non-secreted type (3/23). In addition, 20 (86.9%) patients had bilateral PE.
Of the 15 patients showing chromosome abnormalities, three showed chromosome 13 cytogenetic abnormalities and five showed chromosome 1 abnormal. In the present study, adverse cytogenetic abnormalities demonstrated no clear effect on the extramedullary plasmacytoma rate or on the chemotherapy response rate, as reported in previous studies.11,12 The presence of PE in myeloma patients may have prompted the myeloma to develop into a more invasive form of disease: if the patients have myelomatous pleural effusion, the disease becomes more invasive. Traditional chemotherapy is less effective in these patients; therefore, the need to develop more effective treatments for this population is urgent. In the present study, MM patients with PE showed some responsiveness to bortezomib combination therapy, but the response rate was poorer than in MM patients without PE.13,14 In particular, patients who received local therapy demonstrated better responses. In addition, the two patients who received IL-2 and bortezomib-containing regimens had longer survival compared to the patients who did not receive IL-2 treatment; the survival time of the former group was nearly 30 months. Nevertheless, most MM patients with PE involvement showed disease relapse within one year and shorter survival times. The progression free and overall survival were 5.7 and 11.7 months, respectively [Fig 3]. Therefore, researchers must develop more effective and durable treatments for MM patients with PE invasion.
Figure 3.
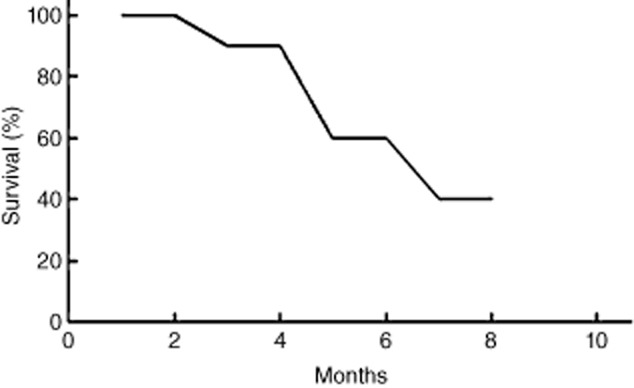
The survival curves of multiple myeloma patients with pleural effusion.
Acknowledgments
We wish to thank Kate S and Christopher M for providing editorial assistance.
Disclosure
No authors report any conflict of interest.
References
- Uskül BT, Türker H, Emre Turan F, et al. Pleural effusion as the first sign of multiple myeloma. Tuberk Toraks. 2008;56:439–442. [PubMed] [Google Scholar]
- Maachi M, Fellahi S, Diop ME, Francois T, Capeau J, Bastard JP. Pleural effusion as a first sign of Ig D lambda multiple myeloma. Ann Med Interne (Paris) 2003;154:70–72. (In French.) [PubMed] [Google Scholar]
- Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- Dinse GE, Lagakos SW. Nonparametric estimation of lifetime and disease onset distributions from incomplete observations. Biometrics. 1982;38:921–932. [PubMed] [Google Scholar]
- Chang H, Chou WC, Lee SY, Huang JY, Hung YH. Myelomatous pleural effusion in patient with plasmblastic myeloma: A case report. Diagn Cytopathol. 2009;37:205–207. doi: 10.1002/dc.21004. [DOI] [PubMed] [Google Scholar]
- Kamble R, Wilson CS, Fassas A, et al. Malignant pleural effusion of multiple myeloma: Prognostic factors and outcome. Leuk Lymph. 2005;46:1137–1142. doi: 10.1080/10428190500102845. [DOI] [PubMed] [Google Scholar]
- Alshati MH, Kumar R, Kannan S. Dyspnea, massive effusion and lytic rib lesion as initial presentation of multiple myeloma in a young man. Can Respir J. 2013;20:253–255. doi: 10.1155/2013/242045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Lee KK, Oh HS, et al. Myelomatous effusion with poor response to chemotherapy. J Korean Med Sci. 2000;15:243–246. doi: 10.3346/jkms.2000.15.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Kim SJ, Min K, et al. Multiple myeloma with myelomatous pleural effusion: A case report and review of the literature. Acta Haematol. 2008;120:108–111. doi: 10.1159/000165694. [DOI] [PubMed] [Google Scholar]
- Iannitto E, Minardi V, Tripodo C. Use of intrapleural bortezomib in myelomatous pleural effusion. Br J Haematol. 2007;139:621–622. doi: 10.1111/j.1365-2141.2007.06735.x. [DOI] [PubMed] [Google Scholar]
- Bladé J, Fernández de Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29:3805–3812. doi: 10.1200/JCO.2011.34.9290. [DOI] [PubMed] [Google Scholar]
- Rosiñol L, Cibeira MT, Martinea J, et al. Thalidomide/dexamethasone (TD) vs. bortezomib (Velcade)/Thalidomide/dexamethasone (VTD) vs. VBMCP/VBAD/Velcade as induction regimens prior autologous stem cell transplantation (ASCT) in multiple myeloma (MM): results of a phase III PETHEMA/GEM trial. Blood. 2009;114:Abstract 130. (22) [Google Scholar]
- Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- Hjorth M, Hjertner Ø, Knudsen LM, et al. Thalidomide and dexamethasone vs. bortezomib and dexamethasone for melphalan refractory myeloma: A randomized study. Eur J Haematol. 2012;88:485–496. doi: 10.1111/j.1600-0609.2012.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


