Abstract
Background
Radiation pneumonitis (RP) is a common side reaction in radiotherapy for esophageal cancer. There are few reports about RP in esophageal cancer patients receiving postoperative intensity modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT). This study aims to analyze clinical or dosimetric factors associated with RP, and provides data for radiotherapy planning.
Methods
We reviewed 68 postoperative esophageal cancer patients who were treated with radiotherapy at the West China Hospital from October 2010 to November 2012 to identify any correlation between the clinical or dosimetric parameters and acute radiation pneumonitis (ARP) or severe acute radiation pneumonitis (SARP) by t-test, chi-square test, and logistic regression analysis.
Results
Of the 68 patients, 33 patients (48.5%) developed ARP, 13 of which (19.1%) developed SARP. Of these 33 patients, 8 (11.8%), 12 (17.6%), 11 (16.2%), and 2 (2.9%) patients were grade 1, 2, 3, and 4 ARP, respectively. Univariate analysis showed that lung infection during radiotherapy, use of VMAT, mean lung dose (MLD), and dosimetric parameters (e.g. V20, V30) are significantly correlated with RP. Multivariate analysis found that lung infection during radiotherapy, MLD ≥ 12 Gy, and V30 ≥ 13% are significantly correlated with an increased risk of RP.
Conclusion
Lung infection during radiotherapy and low radiation dose volume distribution were predictive factors associated with RP and should be accounted for during radiation planning.
Keywords: Esophageal cancer, predictive factor, radiation pneumonitis
Background
Esophageal cancer is the eighth most common malignancy and the sixth leading cause of cancer-related death worldwide.1–3 The incidence of esophageal carcinoma varies widely by region, for example, it occurs 20 to 30 times more often in China than in the US.4,5 The overall five-year survival rate is relatively low, ranging from 15 to 25%, probably related to diagnosis at advanced stages and the propensity for metastases even in superficial tumors.6 Thus, radiotherapy (RT) remains the main treatment for esophageal cancer in order to decrease locoregional recurrence. Although the role of postoperative adjuvant RT for stage II–III esophageal squamous cell carcinoma patients with R0 resection is disputable, it has been observed to improve survival and locoregional control and is, thus, recommended in local practice.6
Radiation pneumonitis (RP) becomes one of the main limiting factors when escalating radiation dose for esophageal cancer, especially in the postoperative setting. RP is one of the most common dose limiting toxicities of thoracic RT, with an incidence around 17 to 47%.7,8 Pulmonary tissue is sensitive to radiation and can develop various forms of injury when radiation dose exceeds its tolerance. RP can be classified into two distinct clinical phases: (i) acute radiation pneumonitis (ARP), an early phase of RP, which often occurs within one to three months after radiation; and (ii) chronic radiation pneumonitis, a late phase of RP, which usually evolves within two years after RT with a manifestation of radiation pulmonary fibrosis (RPF), which usually develops from ARP. The current treatment option for RP is quite limited, particularly for RPF. Once RPF occurs, it usually cannot be reversed, which results in a decline in quality of life, and can even be life threatening.9,10
The onset and severity of RP are related, among other factors, to the volume of irradiated lung, radiation dose and dose rate, fractionation schedule, preexisting pulmonary comorbidity, and concurrent chemotherapy. Dose volume histogram (DVH) parameters V20 and V30 (the percentage of lung volume receiving ≥20 Gy and ≥30 Gy) and mean lung dose (MLD) have long been considered as predictive factors closely correlated with both the risk and severity of RP.11,12
However, most of the available data on RP primarily comes from studies in RT of lung cancer. The incidence of RP in esophageal cancer patients treated with intensity modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT) is rarely discussed, especially in a postoperative setting. When compared with lung cancer, RT of esophageal cancer harbors a unique feature with elongated tumor target volume located in the central thorax. Under this circumstance, RT planning requires different beam arrangement from both sides of the thorax and, inevitably, leads to irradiation of both lungs. Therefore, the aim of this study is to explore the predictive factors associated with RP for resected esophageal cancer patients receiving postoperative RT and seek proper dosimetric parameters to better constrain the irradiated lung volume for optimal RT planning.
Materials and methods
Patients
We retrospectively reviewed patients who had histologically confirmed esophageal cancer and were treated with adjuvant IMRT following radical esophagectomy at the West China Hospital between October 2010 and November 2012. Patients eligible for this study included those with: (i) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2; (ii) normal liver, kidney, and bone marrow functions; (iii) pathologic stage II to IIIc (according to the American Joint Committee on Cancer [AJCC] staging system, 7th edition); and (iv) no chemotherapy or radiotherapy before enrolment. The exclusion criteria included: (i) lost to follow up; and (ii) patients whose DVH was unavailable. All patients granted informed consent.
After screening, 74 patients with pathologically confirmed esophageal carcinoma received surgery plus postoperative IMRT or VMAT. Five patients were excluded from this analysis as they were lost to follow up, and the DVH was unavailable for one patient. The characteristics of the 68 patients are shown in Table 1. There were 61 men and 7 women, with ECOG scales ranging from 0 to 1, and an average age of 56.8 years. The tumor sites included 2 cervical, 10 superior thoracic, 24 middle thoracic, and 32 inferior thoracic. There were 27 stage II patients and 41 stage III patients. Among all of the patients, 15 patients had respiratory system disease (chronic obstructive pulmonary disease, chronic bronchitis, emphysema), 52 patients had a smoking history, and 11 patients had pulmonary infection during radiotherapy (all proved microbiologically).
Table 1.
Clinical characteristics of the 68 patients
| Characteristic | Number of patients (%) |
|---|---|
| Gender | |
| Male | 61 (89.7) |
| Female | 7 (10.3) |
| Age (year) | |
| ≤65 | 59 (86.76) |
| >65 | 9 (13.24) |
| Mean | 56.8 (42–74) |
| ECOG scale | |
| 0 | 36 (52.9) |
| 1 | 32 (47.1) |
| Tumor site | |
| Cervical | 2 (2.9) |
| Upper thoracic | 10 (14.7) |
| Mid-thoracic | 24 (35.3) |
| Lower thoracic | 32 (47.1) |
| T stage† | |
| 1 | 7 (10.3) |
| 2 | 11 (16.2) |
| 3 | 28 (41.2) |
| 4 | 22 (32.4) |
| N stage† | |
| 0 | 22 (32.4) |
| 1 | 29 (42.6) |
| 2 | 11 (16.2) |
| 3 | 6 (8.8) |
| G stage† | |
| 2 | 31 (45.6) |
| 3 | 37 (54.4) |
| stage† | |
| II | 27 (39.7) |
| III | 41 (60.3) |
| Chemotherapy | |
| Yes | 40 (58.8) |
| TP regimen | 25(36.8) |
| FP regimen | 15(22.0) |
| No | 28 (41.2) |
| Number of chemotherapy | 2 (0–6.0) |
| RT technique | |
| VMAT | 22 (32.4) |
| IMRT | 46 (67.6) |
| Total dose | |
| ≤45 Gy | 7 (10.29) |
| >45 Gy, ≤50.4 Gy | 61 (89.71) |
| Smoking | |
| Yes | 52 (76.5) |
| No | 16 (23.5) |
| History in respiratory system | |
| Yes | 15 (22.1) |
| No | 53 (77.9) |
| Pulmonary infection in RT | |
| Yes | 11 (16.2) |
| No | 57 (83.8) |
According to the seventh edition of the American Joint Committee on Cancer staging system. ECOG, Eastern Cooperative Oncology Group; FP regimen, cisplatin + 5-Fu; IMRT, intensity modulated radiation therapy; RT, radiation therapy; TP regimen, paclitaxel + cisplatin; VMAT, volumetric modulated arc therapy.
Radiotherapy
Postoperative adjuvant radiotherapy started after about four weeks following esophagectomy. Patients were immobilized with a thermoplastic body frame in the supine position with their arms raised above while undergoing computed tomography (CT) simulation. A free breathing contrast-enhanced helical CT (Siemens, SomatomPlus 4) scan with 3 mm slice thickness was performed from the ear to the second lumbar level in the treatment position. The imaging data were then transmitted to the Elekta's Monaco treatment planning system (TPS) for three-dimensional reconstruction, target and risk organ contouring, and RT planning. Experienced radiation oncologists contoured all of the tumor targets in order to reduce potential bias. In each slice, the clinical target volume (CTV), planning target volume (PTV), and normal organs were contoured. The CTV was defined as the tumor bed and regional lymph node, which was revealed by preoperative barium-meal X-ray, CT scan, and pathological findings. For upper thoracic esophageal cancer, the CTV was contoured from the cricothyroid membrane to 2–3 cm inferior of the carina, covering tumor bed, anastomotic stoma, and lymph nodes in paraesophageal, paratracheal, inferior cervical, supraclavicular, and stations 2 to 7 regions. For middle thoracic esophageal cancer, the CTV was contoured from the first thoracic vertebra to 2–3 cm inferior of the tumor bed, including corresponding mediastinal lymph node drainage areas, such as paraesophageal, paratracheal, inferior cervical, supraclavicular, and stations 2 to 7 lymph nodes. For lower thoracic esophageal cancer, the superior level of CTV was 5 cm superior to the tumor bed and included paraesophageal stations 4 to 7, left gastric, and paracardial lymph node drainage areas. The PTV was generated by adding a margin of 0.5 cm to CTV in all directions based on the quality assurance standards of our center.
The static IMRT plans were generated using four to seven co-planar beams, and the VMAT plans using one or two rotation arcs. Those plans were delivered by the Elekta Synergy linear accelerator with 6-MV photons. The IMRT and VMAT dose distribution was calculated using the VMC (Voxel Monte Carlo) algorithm. The median irradiation dose for the PTV was 50 Gy, with a range of 40.8–50.4 Gy at 1.8–2.0 Gy per fraction and five fractions per week. The prescription dose covered at least 95% of the volume of the PTV and the hot point was limited within 107% of the prescription dose. The dose constraint for the spinal cord was a maximum dose <45 Gy, and for the heart with V40 (volume receiving 40 Gy) ≤40%. The dose constraint for the two lungs was V20 ≤ 30 Gy, mean lung dose (MLD) ≤13 Gy.
For each patient, lung DVH was calculated directly from the physical dose distribution. The total normal lung volume was defined as the total lung volume minus the volumes of the trachea and main bronchi. The following dosimetric parameters were generated from the DVH: total lung volume (TLV), MLD, and the percentage of lung volume that received more than 5 Gy, 10 Gy, 13 Gy, 15 Gy, 20 Gy, 25 Gy, 30 Gy, and 35 Gy were analyzed.
Chemotherapy
During the observation period, 40 patients (58.8%) received alternating chemotherapy at about four weeks after completion of RT. Of these 40 patients, 15 patients received an FP regimen (cisplatin 20–30 mg/m2 D1-3 + 5-Fu 450–500 mg/m2 D1-5, q3-4w) and 25 patients received a TP regimen (paclitaxel 130 mg/m2 + cisplatin 25–30 mg/m2 D1-3, q3-4w). The median chemotherapy cycles were two (range, 1∼6 cycles).
Radiation pneumonitis (RP) criterion
The acute RP was graded according to the 1995 Radiation Therapy Oncology Group (RTOG) criterion,13 including Grade 0: no change; Grade 1: mild symptoms of dry cough or dyspnea on exertion; Grade 2: persistent cough requiring narcotic, antitussive agents/dyspnea with minimal effort but not at rest; Grade 3: severe cough unresponsive to narcotic antitussive agent or dyspnea at rest/clinical or radiological evidence of acute pneumonitis/intermittent oxygen or steroids; and Grade 4: severe respiratory insufficiency/continuous oxygen or assisted ventilation.
Follow up
Follow-up data were updated in May 2013 with the longest duration being 29 months. Treatment toxicity was assessed weekly during RT, at least twice a month during chemotherapy, and at least every three months for the first year. ARP was defined as grade 1 to 4 RP, and was graded based on symptoms described in the medical records and changes in CT images by a consensus of two radiation oncologists according to RTOG criterion, which occurred within 90 days after the start of RT, while severe acute radiation pneumonitis (SARP) was defined as ARP graded 3 to 4.
Statistical analysis
The relationships between clinical parameters and the incidence of ARP and SARP were analyzed using Mann-Whitney's U test for continuous variables, and a chi-square test for categorical variables. Logistic regression was used for multivariable analysis of impact on the occurrence of ARP and SARP. DVH parameters were listed as mean ± standard deviation (SD) values for patients who developed RP or not. A Student's t-test was used to compare those DVH parameters. The receiver operating characteristics (ROC) curve was also performed to select the most relevant threshold to predict ARP and SARP. The optimal threshold was defined as the point yielding the minimal value for (1-sensitivity)2 + (1-specificity),2 which was the point on the ROC curve closest to the upper left-hand corner.12 Data were considered statistically significant at values of P < 0.05. All statistical analyses were generated using SPSS for Windows, Version 16.0.
Results
The impact of clinical parameters on RP
By the time of follow up, 33 patients (48.5%) had developed ARP. The incidences of grade 1, 2, 3, and 4 ARP were: 8 (11.8%), 19 (27.9%), 4 (5.9%), and 2 patients (2.9%), respectively. Univariate analysis was used to detect the influence of gender, age, tumor site, tumor stage, chemotherapy or lack of, smoking history, respiratory disease history, and pulmonary infection during RT on ARP, and only pulmonary infection during RT showed statistical significance (Table 2). The medium time to occurrence of ARP was 52 days (15–85 days) and 55 days (32–79 days) for SARP after commencing RT.
Table 2.
Univariate analysis of clinical risk factors for ARP (grade 1–4) and SARP (grade 3–4)
| Factor group | Number of patients | ARP number of patients (%) | χ2-value | P-value | SARP number of patients (%) | χ2-value | P-value |
|---|---|---|---|---|---|---|---|
| Gender | 0.513 | 0.474 | 0.507 | ||||
| Male | 61 | 31 (50.82) | 6 (9.84) | ||||
| Female | 7 | 2 (28.57) | 0 (0) | ||||
| Age (year) | 3.224 | 0.073 | 11.11 | 1.000 | |||
| ≤65 | 59 | 27 (45.76) | 5 (8.47) | ||||
| >65 | 9 | 6 (66.67) | 1 | ||||
| Tumor site | 0.006 | 0.938 | 0.540 | 0.463 | |||
| Cervical | 2 | 0 (0) | 0 | ||||
| Upper thoracic | 10 | 7 (70) | 1 (10) | ||||
| Mid-thoracic | 24 | 10 (41.67) | 1 (4.17) | ||||
| Lower thoracic | 32 | 16 (50) | 4 (12.5) | ||||
| Stage | 2.368 | 0.124 | 0.011 | 0.918 | |||
| II | 27 | 10 (37.04) | 3 (11.11) | ||||
| III | 41 | 23 (56.10) | 3 (7.32) | ||||
| Chemotherapy | 0.84 | 0.772 | 0.711 | 0.399 | |||
| Yes | 40 | 20 (50) | 2.667 | 0.102 | 10 (25.00) | 0.327 | 0.567 |
| TP | 25 | 15 (60) | 7 (28) | ||||
| FP | 15 | 5 (33.33) | 3 (20) | ||||
| No | 28 | 13 (46.43) | 0.772 | 1 (3.57) | |||
| Smoking | 0.191 | 0.662 | 0.000 | 1.000 | |||
| Yes | 52 | 26 (50) | 5 (9.62) | ||||
| No | 16 | 7 (43.75) | 1 (6.25) | ||||
| History of respiratory system | 3.552 | 0.059 | 1.472 | 0.225 | |||
| Yes | 15 | 11 (73.33) | 3 (20.00) | ||||
| No | 53 | 22 (41.51) | 3 (5.66) | ||||
| Pulmonary infection in RT | 7.520 | 0.006 | 3.153 | 0.076 | |||
| Yes | 11 | 10 (90.91) | 3 (27.27) | ||||
| No | 57 | 23 (40.35) | 3 (5.26) |
ARP, acute radiation pneumonitis; FP regimen, cisplatin + 5-Fu; RT, radiation therapy; SARP, severe acute radiation pneumonitis; TP regimen, paclitaxel + cisplatin.
The impact of physical/dosimetric parameters on RP
MLD in all of the patients was 12.10 Gy. Univariate analysis for dosimetric parameters was performed. The results revealed an ARP rate of 75.76% when the MLD ≥ 12 Gy, which was significantly higher than when the MLD < 12 Gy (22.86%) (P = 0.000). The ARP rate was also remarkably increased when V5 ≥ 65% or V25 ≥ 15% or V30 ≥ 13% (P < 0.05). The SARP rate was significantly increased when V30 ≥ 13% (83.33% vs. 36%) (P < 0.05). Compared with IMRT, VMAT could increase the risk of ARP (72.73% vs. 36.96%) (P < 0.05); however, no statistical difference was observed on SARP. The radiation dose above or below 45 Gy didn't show statistical difference on RP (P > 0.05) (Table 3).
Table 3.
Univariate analysis of dosimetric risk factors for ARP (grade 1–4) and SARP (grade 3–4)
| Variable group | Number of patients | ARP number of patients (%) | χ2-value | P-value | SARP number of patients (%) | χ2-value | P-value |
|---|---|---|---|---|---|---|---|
| MLD (Gy) | 19.029 | 0.000 | 1.846 | 0.174 | |||
| <12 | 35 | 8 (22.86) | 1 (2.86) | ||||
| ≥12 | 33 | 25 (75.76) | 5 (15.15) | ||||
| V5 (%) | 7.379 | 0.007 | 0.976 | 0.323 | |||
| <65 | 30 | 9 (30.00) | 1 (3.33) | ||||
| ≥65 | 38 | 24 (63.16) | 5 (13.16) | ||||
| V10 (%) | 7.591 | 0.006 | 0.711 | 0.399 | |||
| <45 | 28 | 2 (11.76) | 1 (3.57) | ||||
| ≥45 | 40 | 8 (28.57) | 5 (12.50) | ||||
| V15 (%) | 7.867 | 0.005 | 0.488 | 0.485 | |||
| <30 | 26 | 7 (26.92) | 1 (3.85) | ||||
| ≥30 | 42 | 26 (61.90) | 5 (11.90) | ||||
| V20 (%) | 7.008 | 0.008 | 0.229 | 0.632 | |||
| <20 | 23 | 6 (26.09) | 1 (4.35) | ||||
| ≥20 | 45 | 27 (60.00) | 5 (11.11) | ||||
| V25 (%) | 7.072 | 0.008 | 2.062 | 0.151 | |||
| <15 | 36 | 12 (33.33) | 1 (2.78) | ||||
| ≥15 | 32 | 21 (65.63) | 5 (15.63) | ||||
| V30 (%) | 10.052 | 0.002 | 7.963 | 0.005 | |||
| <13 | 50 | 18 (36.00) | 1 (2.00) | ||||
| ≥13 | 18 | 15 (83.33) | 5 (27.78) | ||||
| RT technique | 7.624 | 0.006 | 0.261 | 0.610 | |||
| VMAT | 22 | 16 (72.73) | 3 (13.64) | ||||
| IMRT | 46 | 17 (36.96) | 3 (6.52) | ||||
| Total dose (Gy) | 0.099 | 0.753 | 0.027 | 0.869 | |||
| ≤45 | 7 | 3 (42.86) | 0 (0) | ||||
| 45–50.4 | 61 | 30 (49.18) | 6 (9.84) |
ARP, acute radiation pneumonitis; MLD, mean lung dose; IMRT, intensity modulated radiation therapy; RT, radiation therapy; SARP, severe acute radiation pneumonitis; V5, V10, V15, V20, V25, V30, percentage of lung receiving ≥ 5 Gy (through 30 Gy); VMAT, volumetric modulated arc therapy.
Multivariate analysis on RP
On multivariate analysis, pulmonary infection during RT and MLD ≥12 Gy showed statistical significance on ARP (P < 0.05), while V30 ≥13% showed statistical significance on SARP (Table 4).
Table 4.
Multivariate analysis of ARP (grade 1–4) and SARP (grade 3–4)
| Variable | B | S.E. | Wald | P-value | Exp (B) | 95%CI lower | Upper | |
|---|---|---|---|---|---|---|---|---|
| ARP | Pulmonary infection in RT | 2.265 | 1.154 | 3.850 | 0.017 | 9.632 | 1.002 | 92.549 |
| MLD (≥12 Gy) | 2.160 | 0.596 | 13.145 | 0.000 | 8.672 | 2.698 | 27.877 | |
| SARP | V30 (≥13%) | 2.936 | 1.139 | 6.646 | 0.010 | 18.846 | 2.022 | 175.689 |
ARP, acute radiation pneumonitis; MLD, mean lung dose; SARP, severe acute radiation pneumonitis; V30, percentage of lung receiving ≥ 30 Gy.
Figure 1a shows the ROC curve for MLD to predict ARP. The area under the curve of ROC was 0.754 (P = 0.000). The optimal threshold for MLD to predict ARP was 12 Gy, with a sensitivity of 0.758 and specificity of 0.771. Figure 1b shows the ROC curve for V30 in prediction of SARP. The area under the curve of ROC was 0.837 (P = 0.000). The optimal threshold for V30 to predict SARP was 13%, with a sensitivity of 0.462 and specificity of 0.909.
Figure 1.
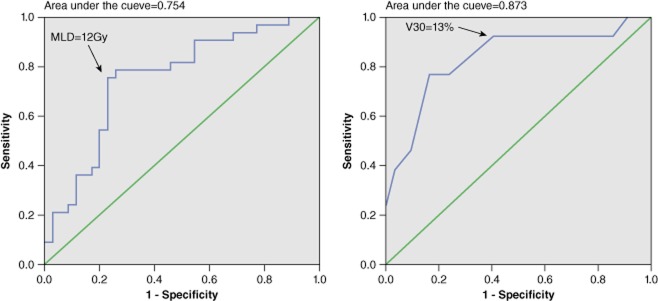
(a) Receiver operating characteristics (ROC) curve for mean lung dose (MLD) to predict acute radiation pneumonitis (ARP); black arrow shows the predicted optimal MLD threshold for ARP. (b) ROC curve for V30 to predict severe acute radiation pneumonitis (SARP); black arrow shows the predicted optimal V30 threshold for SARP.
Discussion
In this study, postoperative adjuvant RT was applied to patients with pathologic T3, T4 or N + esophageal cancer. Postoperative adjuvant RT may decrease local recurrence and benefit patients with esophageal cancer in terms of overall survival.14–16 However, the application of adjuvant RT after radical esophagectomy may risk greater dose-limiting toxicities in normal tissues following gastric pull-up and oppressed lungs,17 and is more challenging for RT planning and delivery. With the advantages of providing highly conformed dose distribution to tumor targets while allowing rapid dose fall-off in surrounding normal tissues, IMRT is becoming the mainstream RT technique for thoracic cancers, particularly for tumors with an irregular or concave shape and close adjacency to critical organs. Application of IMRT may increase the therapeutic ratio of esophageal cancer RT by escalating the dose to PTV and reducing the dose to the heart and lungs.17 However, with IMRT planning, more surrounding lung tissue may be affected by low dose radiation, making it vulnerable to radiation induced toxicity.18,19
In our study, the incidence of ARP in all grades is 48.5% in patients receiving postoperative RT of esophageal cancer, including six (8.8%) patients with SARP. The incidence of RP varies widely among reports because of differences in radiation techniques, evaluation of symptoms, and method of reporting. About 50–90% of patients undergoing irradiation to the lung have been reported to develop radiographic and pulmonary function abnormalities,7 and five to 40% of patients develop symptomatic RP.8,20,21
The development of RP after RT of esophageal cancer can be affected by various patient related factors. Cwikiel et al.22 observed that 11 (15%) out of 73 patients developed ARP after postoperative RT of esophageal cancer. Zhu et al.23 reported that 10 (17.9%) out of 56 patients with esophageal cancer developed ARP, of which seven patients had grade 2 and three patients had grade 3. It has been proposed that male gender, age >60 years, a history of chronic pulmonary disease, and concurrent chemotherapy all have a significant influence on the development of RP.24,25 Parashar et al.24 reported the incidence of RP was as high as 63% in patients receiving alternating chemotherapy, while only 16% in patients without chemotherapy; the incidence was 77% in patients aged 61–70 years. In our study, the average age of patients was 56.8 years (range 46–74 years) and there was no statistical difference in the incidence of RP between subgroups stratified at 65 years. Also in our study, 40 (58.8%) patients received postoperative sequential chemotherapy after completion of RT and no statistical difference of RP risk was detected between patients with and without chemotherapy (60% vs. 33.33%, P = 0.772). Although sequential chemotherapy did not affect our study, chemotherapy regimens were heterogeneous. The impact of chemotherapy upon RP requires further prospective trials for clarification.
In our study, chemotherapy did not influence RP. This might be partly explained by the fact that chemotherapy was not administered concurrently with RT; therefore, synergistic toxicity could be avoided. We also found that higher incidence of ARP or SARP was associated with preexisting chronic respiratory disease, pathological stage III, and a history of smoking, although no statistical difference was detected. Our study also showed that pulmonary infection developed during a course of RT significantly increased the risk of ARP and SARP, with incidence rates as high as 90.91% for ARP and 27.27% for SARP. For patients who did not develop pulmonary infection, the incidence was 40.35% for ARP and 5.26% for SARP. This implies that pulmonary infection might be an important risk factor for ARP. Multivariate analysis of this study also showed that pulmonary infection was significantly correlated with the incidence of ARP (P = 0.000). RP often coexists with pulmonary infection. Whether pulmonary infection can induce or exacerbate RP requires further study. Other than clinical influence factors, the dosimetric parameters derived from DVH, such as MLD, V5, V10, V13, V20, and V30 have been found to influence both the occurrence and prediction of ARP. Some studies have reported that an increased risk of RP was associated with MLD ≥ 14 Gy or > 18 Gy.26–29 Nomura et al. performed a study on 125 esophageal cancer patients who received three-dimensional radiotherapy (3D-RT) using conventional anterior-posterior plus off-cord beam arrangement and no prophylactic nodal irradiation.11 An experience based pulmonary dose constraint of V20 < 30% was made, but the actual V20 were about 15.1% (2.0%-25.2%) for all included patients. They found that three cases (2%) developed SARP. As a result of the extensive application of IMRT in RT of thoracic cancer in recent years, low dose radiation to the lungs has significantly increased, compared to 3D-RT. The impact of V5 and V15 on RP has drawn much attention. Some literature has postulated that V5 and V15 may have greater predictive value than V20.14,30 In our study, when MLD ≥ 12 Gy, the risk of ARP and SARP were 75.76% and 33.33%, respectively, while the risk of ARP and SARP were 22.86% and 5.71%, respectively, with MLD < 12 Gy. Based on univariate analysis, MLD and V5, V10, V15, V20, V25, and V30 were correlated with risks of ARP and SARP. Particularly when V5 ≥ 65%, V25 ≥ 15%, and V30 ≥ 13%, the risk of ARP was 63.16%, 65.63%, and 83.33%, respectively, and the risk of SARP was 31.58%, 34.38% and 55.56%, respectively, which were significantly higher than those with DVH parameters less than the cut points (Table 3). These results imply that the risk of RP is greater for patients with a larger volume of low dose radiation than for patients with a smaller volume receiving low dose radiation. Therefore when making RT plans, in addition to MLD and V20, the total lung V5, V10, and V15 should be limited to a certain level. Multivariate analysis in our study has suggested that MLD and V30 are significant predictive factors for RP; MLD is significantly correlated with ARP (P = 0.000), but not significantly correlated with SARP; V30 has greater predictive value for SARP, with significant difference between V30 ≥ 13% and V30 < 13% (P = 0.022) (Fig. 1).
Interestingly, in our study it was noted that VMAT can cause a larger low dose region, which may increase the risk of radiation lung injury (Table 5). In the 22 patients receiving VMAT in our study, the incidences were 72.73% of ARP and 31.82% of SARP, while for the 46 patients receiving IMRT the incidence of ARP and SARP were 36.96% and 13.04%, respectively. Compared to IMRT, using VMAT the lung MLD was higher (P = 0.009) and the low dose region was greater, with statistical difference for V5, V10, V25, and V30 (P < 0.005). VMAT is an extension of IMRT; it can deliver continuous radiation by rotating the gantry of a linear particle accelerator through arcs at varied gantry rotation speed and changing MLC orientation and aperture shape. VMAT can achieve similar homogeneous and conformal dose distribution as compared to IMRT with shorter treatment time, but may increase the region of low dose radiation.31–33 VMAT has been widely used in the treatment of cancers of the head and neck, prostate, rectum, and uterine cervix. VMRT in thoracic RT has also been investigated. Several researchers have reported that the application of VMAT in lung cancer RT could reduce the lung V30 and V20, while increasing V11.34–36 Increasing the low dose region may affect pulmonary function and, subsequently, patients' quality of life and survival.37 Only a few studies on VMAT for esophageal cancer have been published, mostly focused on dosimetric comparison.38,39 No other clinical data has been reported on RP in patients with esophageal cancer treated with VMAT. However, because of the retrospective nature of this study, the predictive value of VMAT on RP, as well as other clinical and dosimetric factors, needs further evaluation from randomized clinical studies.
Table 5.
Comparison of dosimetric parameters between VMAT and IMRT
| Variable | Volume (mean ± sd, cm3) VMAT | IMRT | P-value | Ratio of volume and dose (mean ± sd, %) VMAT | IMRT | P-value |
|---|---|---|---|---|---|---|
| TLV | 3171.2 ± 470.64 | 3215.8 ± 729.71 | 0.795 | |||
| V5 | 2344.4 ± 542.91 | 2041.1 ± 738.73 | 0.091 | 0.74 ± 0.10 | 0.63 ± 0.14 | 0.002 |
| V10 | 1591.2 ± 378.25 | 1440.9 ± 547.13 | 0.250 | 0.50 ± 0.11 | 0.44 ± 0.10 | 0.023 |
| V15 | 1013.0 ± 265.56 | 983.48 ± 381.53 | 0.745 | 0.32 ± 0.08 | 0.30 ± 0.07 | 0.319 |
| V20 | 707.49 ± 203.59 | 640.59 ± 233.55 | 0.254 | 0.22 ± 0.06 | 0.19 ± 0.05 | 0.066 |
| V25 | 500.48 ± 169.91 | 428.06 ± 172.64 | 0.109 | 0.16 ± 0.05 | 0.13 ± 0.04 | 0.029 |
| V30 | 363.69 ± 146.39 | 287.86 ± 132.73 | 0.037 | 0.10 ± 0.04 | 0.09 ± 0.03 | 0.011 |
| MLD (Gy) | 13.12 ± 2.36 | 11.59 ± 2.18 | 0.009 |
IMRT, intensity modulated radiation therapy; MLD, mean lung dose; sd, standard deviation; TLV, total lung volume; V5, V10,V15,V20,V25, V30, percentage of lung receiving ≥ 5 Gy (through 30 Gy); VMAT, volumetric modulated arc therapy.
Conclusion
In conclusion, pulmonary infection during RT might be a risk factor of ARP for patients with postoperative adjuvant RT of esophageal cancer, which should be closely controlled in the course of treatment. Dosimetric parameters of dose volume and MLD of the lungs could also be important risk factors. A dose constraint of total lung MLD < 12 Gy and V30 < 13% could be applied for postoperative IMRT of esophageal cancer.
Acknowledgments
This study was approved by Ethical Committee of Sichuan University and was supported by a grant from the National Basic Research Program of China (No. 2011CB935800).
Disclosure
No authors report any conflict of interest.
References
- Nieman DR, Peters JH. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am. 2013;42:187–197. doi: 10.1016/j.gtc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–5606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233–242. doi: 10.2188/jea.JE20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taifu L. Radiotherapy of carcinoma of the esophagus in China – a review. Int J Radiat Oncol Biol Phys. 1991;20:875–879. doi: 10.1016/0360-3016(91)90035-3. [DOI] [PubMed] [Google Scholar]
- Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–1293. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morota M, Gomi K, Kozuka T, et al. Late toxicity after definitive concurrent chemoradiotherapy for thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2009;75:122–128. doi: 10.1016/j.ijrobp.2008.10.075. [DOI] [PubMed] [Google Scholar]
- Graves PR, Siddiqui F, Anscher MS, Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20:201–207. doi: 10.1016/j.semradonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Nomura M, Kodaira T, Furutani K, Tachibana H, Tomita N, Goto Y. Predictive factors for radiation pneumonitis in oesophageal cancer patients treated with chemoradiotherapy without prophylactic nodal irradiation. Br J Radiol. 2012;85:813–818. doi: 10.1259/bjr/13604628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura H, Hashimoto T, Zenda S, et al. Analysis of dose-volume histogram parameters for radiation pneumonitis after definitive concurrent chemoradiotherapy for esophageal cancer. Radiother Oncol. 2010;95:240–244. doi: 10.1016/j.radonc.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- Wang SL, Liao Z, Vaporciyan AA, et al. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys. 2006;64:692–699. doi: 10.1016/j.ijrobp.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control. 2013;20:97–110. doi: 10.1177/107327481302000203. [DOI] [PubMed] [Google Scholar]
- Xiao ZF, Yang ZY, Miao YJ, et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radiat Oncol Biol Phys. 2005;62:82–90. doi: 10.1016/j.ijrobp.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Martin S, Chen JZ, Rashid Dar A, Yartsev S. Dosimetric comparison of helical tomotherapy, RapidArc, and a novel IMRT & Arc technique for esophageal carcinoma. Radiother Oncol. 2011;101:431–437. doi: 10.1016/j.radonc.2011.08.030. [DOI] [PubMed] [Google Scholar]
- Wu VW, Sham JS, Kwong DL. Inverse planning in three-dimensional conformal and intensity-modulated radiotherapy of mid-thoracic oesophageal cancer. Br J Radiol. 2004;77:568–572. doi: 10.1259/bjr/19972578. [DOI] [PubMed] [Google Scholar]
- Shen WB, Zhu SC, Gao HM, et al. [Low dose volume histogram analysis of the lungs in prediction of acute radiation pneumonitis in patients with esophageal cancer treated with three-dimensional conformal radiotherapy] Zhonghua Zhong Liu Za Zhi. 2013;35:45–49. doi: 10.3760/cma.j.issn.0253-3766.2013.01.010. . (In Chinese.) [DOI] [PubMed] [Google Scholar]
- Provatopoulou X, Athanasiou E, Gounaris A. Predictive markers of radiation pneumonitis. Anticancer Res. 2008;28:2421–2432. [PubMed] [Google Scholar]
- Rodrigues G, Lock M, D'Souza D, Yu E, Van Dyk J. Prediction of radiation pneumonitis by dose – volume histogram parameters in lung cancer – a systematic review. Radiother Oncol. 2004;71:127–138. doi: 10.1016/j.radonc.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Cwikiel M, Albertsson M, Hambraeus G. Acute and delayed effects of radiotherapy in patients with oesophageal squamous cell carcinoma treated with chemotherapy, surgery and pre- and postoperative radiotherapy. Acta Oncol. 1994;33:49–53. doi: 10.3109/02841869409098375. [DOI] [PubMed] [Google Scholar]
- Zhu SC, Shen WB, Liu ZK, Li J, Su JW, Wang YX. Dosimetric and clinical predictors of radiation-induced lung toxicity in esophageal carcinoma. Tumori. 2011;97:596–602. doi: 10.1177/030089161109700510. [DOI] [PubMed] [Google Scholar]
- Parashar B, Edwards A, Mehta R, et al. Chemotherapy significantly increases the risk of radiation pneumonitis in radiation therapy of advanced lung cancer. Am J Clin Oncol. 2011;34:160–164. doi: 10.1097/COC.0b013e3181d6b40f. [DOI] [PubMed] [Google Scholar]
- Dang J, Li G, Lu X, Yao L, Zhang S, Yu Z. Analysis of related factors associated with radiation pneumonitis in patients with locally advanced non-small-cell lung cancer treated with three-dimensional conformal radiotherapy. J Cancer Res Clin Oncol. 2010;136:1169–1178. doi: 10.1007/s00432-010-0764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacsson U, Lennernäs B, Grusell E, Jung B, Montelius A, Glimelius B. Comparative treatment planning between proton and x-ray therapy in esophageal cancer. Int J Radiat Oncol Biol Phys. 1998;41:441–450. doi: 10.1016/s0360-3016(98)00047-9. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhao KL, Guerrero TM, et al. Four-dimensional computed tomography-based treatment planning for intensity-modulated radiation therapy and proton therapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2008;72:278–287. doi: 10.1016/j.ijrobp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Tsujii H. Proton beam therapy with high-dose irradiation for superficial and advanced esophageal carcinomas. Clin Cancer Res. 2003;9:3571–3577. [PubMed] [Google Scholar]
- Sugahara S, Tokuuye K, Okumura T, et al. Clinical results of proton beam therapy for cancer of the esophagus. Int J Radiat Oncol Biol Phys. 2005;61:76–84. doi: 10.1016/j.ijrobp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Schallenkamp JM, Miller RC, Brinkmann DH, Foote T, Garces YI. Incidence of radiation pneumonitis after thoracic irradiation: dose-volume correlates. Int J Radiat Oncol Biol Phys. 2007;67:410–416. doi: 10.1016/j.ijrobp.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Hoogeman MS, Nuyttens JJ, Levendag PC, Heijmen BJ. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys. 2008;70:609–618. doi: 10.1016/j.ijrobp.2007.08.066. [DOI] [PubMed] [Google Scholar]
- Zimmerman J, Korreman S, Persson G, et al. DMLC motion tracking of moving targets for intensity modulated arc therapy treatment: a feasibility study. Acta Oncol. 48:245–250. doi: 10.1080/02841860802266722. [DOI] [PubMed] [Google Scholar]
- Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol. 2011;84:967–996. doi: 10.1259/bjr/22373346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CL, Verbakel WF, Cuijpers JP, Slotman BJ, Lagerwaard FJ, Senan S. Stereotactic radiotherapy for peripheral lung tumors: a comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol. 2010;97:437–442. doi: 10.1016/j.radonc.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Brock J, Bedford J, Partridge M, et al. Optimising stereotactic body radiotherapy for non-small cell lung cancer with volumetric intensity-modulated arc therapy – a planning study. Clin Oncol (R Coll Radiol) 2012;24:68–75. doi: 10.1016/j.clon.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Holt A, van Vliet-Vroegindeweij C, Mans A, Belderbos JS, Damen EM. Volumetric-modulated arc therapy for stereotactic body radiotherapy of lung tumors: a comparison with intensity-modulated radiotherapy techniques. Int J Radiat Oncol Biol Phys. 2011;81:1560–1567. doi: 10.1016/j.ijrobp.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Semenenko VA, Molthen RC, Li C, et al. Irradiation of varying volumes of rat lung to same mean lung dose: a little to a lot or a lot to a little? Int J Radiat Oncol Biol Phys. 2008;71:838–847. doi: 10.1016/j.ijrobp.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Lin CY, Huang WY, Jen YM, et al. Dosimetric and efficiency comparison of high-dose radiotherapy for esophageal cancer: volumetric modulated arc therapy versus fixed-field intensity-modulated radiotherapy. Dis Esophagus. 2013;18:12144. doi: 10.1111/dote.12144. [DOI] [PubMed] [Google Scholar]
- Hawkins MA, Bedford JL, Warrington AP, Tait DM. Volumetric modulated arc therapy planning for distal oesophageal malignancies. Br J Radiol. 2012;85:44–52. doi: 10.1259/bjr/25428720. [DOI] [PMC free article] [PubMed] [Google Scholar]


