Abstract
Although surgical resection is the primary means of curing both primary and metastatic lung cancers, about 80% of lung cancers cannot be removed by surgery. As most patients with unresectable lung cancer receive only limited benefits from traditional radiotherapy and chemotherapy, many new local treatment methods have emerged, including local ablation therapy. The Minimally Invasive and Comprehensive Treatment of Lung Cancer Branch, Professional Committee of Minimally Invasive Treatment of Cancer of the Chinese Anti-Cancer Association has organized multidisciplinary experts to develop guidelines for this treatment modality. These guidelines aim at standardizing thermal ablation procedures and criteria for selecting treatment candidates and assessing outcomes; and for preventing and managing post-ablation complications.
Keywords: Guidelines, lung tumor, thermal ablation
Introduction
Lung cancer is the deadliest and most common cancer, with an annual incidence of about 2.5 million and more than 1.6 million deaths each year, worldwide.1 The picture is even worse in China. According to the 2012 China Annual Cancer Report, the annual incidence of lung cancer is 57.63 per 100 000 and annual mortality 48.87 per 100 000, ranking China first in the world in absolute terms.2 For early-stage non-small cell lung cancer (NSCLC), surgical resection remains the therapeutic choice for curative intent,3 but for various reasons about 80% of lung cancers cannot be removed surgically. Most patients with unresectable lung cancer can derive only limited benefits from traditional radiotherapy and chemotherapy. Therefore, many new local treatment methods have emerged, including local ablation therapy. Local thermal ablation is a minimally invasive technique that has been increasingly used to treat early-stage lung cancer.4 Percutaneous thermal ablation has been proved to be effective in treating lung metastases.5 The Minimally Invasive and Comprehensive Treatment of Lung Cancer Branch, Professional Committee of Minimally Invasive Treatment of Cancer of the Chinese Anti-Cancer Association organized multidisciplinary experts to develop these guidelines for thermal ablation of primary and metastatic lung tumors to provide guidance for clinicians.
Local thermal ablation techniques
Tumor thermal ablation is a therapeutic technique that directly causes irreversible injury or coagulation necrosis of tumor cells in one or more tumor lesions in a specific organ by utilizing the biological effects of heat. The techniques include radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, laser ablation, and high- intensity focused ultrasound (HIFU),6 although laser ablation and HIFU are not commonly used to treat lung tumors.
Radiofrequency ablation (RFA)
RFA is currently the most widely used ablation technique for the treatment of solid tumors. With radiofrequency electrodes inserted into the tumor tissue and the application of 200–650 kHz frequency alternating current, mutual friction and collisions of ions within the tumor tissue produce thermal biological effects to raise the local temperature up to 60–120°C. When the tissue is heated to >60°C, cell coagulation necrosis may occur. RFA volume depends on the transfer of heat produced by local RFA and thermal convection between the blood circulation and extracellular fluid.6–9 In December 2007, the US Food and Drug Administration approved RFA for the treatment of lung cancer.9 Since 2009, the NSCLC National Comprehensive Cancer Network (NCCN) Guidelines and China's Primary Lung Cancer Diagnostic and Treatment Practices (2011 edition) have both recommended RFA to treat patients with early-stage lung cancer who cannot tolerate surgical resection.
Microwave ablation (MWA)
MWA generally uses either of two frequencies, 915 MHz or 2450 MHz. In a microwave electromagnetic field, water molecules, protein molecules, and other polar molecules within tumor tissue vibrate at high speeds, resulting in collision and mutual friction between molecules. This can produce temperatures of 60–150°C in a short time, leading to coagulation necrosis of the cells.10–12 As ablation can concentrate microwave energy in a certain range, the desired target area can be effectively radiated. MWA has a higher convection and a lower “heat-sink” effect in the lungs.13–17
Cryoablation
Argon-helium cryoablation is a currently mature cryotherapy, based on the principle that high-pressure argon gas may be cooled to −140°C, and helium can rapidly rise from −140°C to 20–40°C. The temperature gradient change can lead to: (i) protein denaturation of target tissues; (ii) cell lysis as a result of changes of the internal and external osmotic pressures and “freezing;” and (iii) tissue necrosis resulting from microvascular thrombosis.18–20 The “ice ball” observed under computed tomography (CT) or magnetic resonance imaging (MRI) can directly distinguish the ablation region and tumor boundary to determine the margin of freezing injury, which is generally within 4–6 mm of the margin of the ice ball.19,20
These three ablation techniques can all be used for local treatment of lung cancer, but have different advantages. For tumors with diameters ≤ 3 cm, all ablation methods can achieve good therapeutic effects. RFA electrodes can be adjusted to protect adjacent organs. For tumors larger than 3 cm, particularly those larger than 5 cm, MWA is significantly better than the other two techniques because of its consistently higher intratumoral temperatures, larger tumor ablation volumes, shorter ablation times, improved convection profile, and a larger power field that enhances treatment of perivascular tissue and, thus, limits the “heat sink” effect. Furthermore, multiple MWA antennas can be positioned into the target tissue and activated simultaneously, maximizing the ablation zone size. The “ice ball” formed in cryoablation produces a clear, easily monitored margin, and can be used to treat tumors near high-risk adjacent organs. Cryoablation is also less likely to cause local pain. For tumors ≤1 cm from the pleura or complicated with bone destruction as a result of bone metastases, cryoablation is significantly better than RFA and MWA. However, cryoablation consumes platelets during treatment, and thus, should be avoided for patients with poor blood clotting function.
Procedure platform
Imaging guidance techniques for percutaneous thermal ablation include CT, MRI, and ultrasound. CT is the most commonly used image-guided technique in lung tumor ablation, followed by MRI. For tumors near or adherent to the chest wall in cases where the whole picture could be observed with ultrasound, ultrasound guidance can be used.
Thoracotomy or video-assisted thoracoscopic assisted techniques are generally used when: (i) the tumor is adjacent to critical structures, such as large blood vessels, the hilum, or heart; or (ii) the lesion was identified as unresectable after an earlier thoracotomy.21
Indications and contraindications
Because percutaneous thermal ablation of primary and metastatic lung tumors is still not an established treatment in general, the indications should be selected by an interdisciplinary board (Fig 1).
Figure 1.
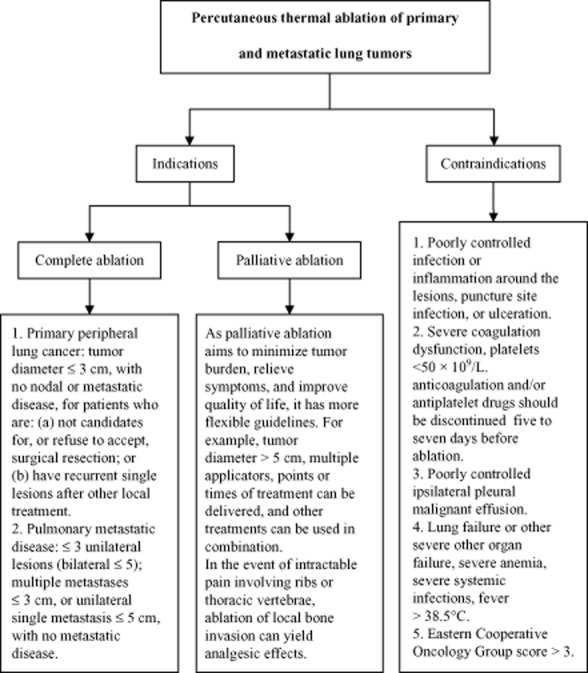
Decision chart for use of thermal ablation.
Complete ablation
Complete ablation refers to complete necrotic lesions of the local tumor tissue and a possible cure through thermal ablation.
Primary peripheral lung cancer
Among patients with primary peripheral lung cancer,22–27 complete ablation may be suitable for: (i) patients who are not candidates for curative surgical resection because of cardiorespiratory comorbidity, insufficient vital lung function, or older age; (ii) patients who refuse to accept surgical resection; or (iii) patients with recurrent single lesions after other local treatment (including three-dimensional conformal radiation or stereotactic body radiotherapy). The maximum tumor diameter for thermal ablation is ≤3 cm, with no evidence of nodal or distant metastases.
Pulmonary metastatic disease
Intrapulmonary metastases,24,25,28–33 such as kidney, colorectal and breast cancers, sarcoma, melanoma, and hepatocellular carcinoma, may have good outcomes, depending on their biological characteristics. If the primary disease can be effectively treated, thermal ablation can be used on pulmonary metastases. However, use of thermal ablation should be limited to ≤3 unilateral lung lesions (bilateral ≤ 5) for multiple metastases, with maximum diameters of ≤3 cm, or ≤5 cm for a unilateral single metastasis with no metastasis at any other site. For patients with bilateral pulmonary metastatic disease, bilateral simultaneous ablation is not recommended.
Palliative ablation
The purpose of palliative thermal ablation is to minimize tumor burden, relieve symptoms caused by the tumor, and improve quality of life. For patients in whom complete ablation cannot be achieved, the indications are more flexible. For example, for target tumors >5 cm, multiple applicators can be delivered and other treatments can be used in combination. In the event of intractable pain involving ribs or thoracic vertebrae, ablation of local bone invasion can yield analgesic effects.25,34–37
Contraindications
Because of excellent tolerance of percutaneous thermal therapy, identifying an absolute contraindication for lung thermal ablation is difficult, with the exception of patients with untreatable coagulopathies.5,7,10,11,24,38,39 However, caution should be exercised for patients with: (i) poorly controlled infection or inflammation around the lesions, puncture site infection, or ulceration; (ii) severe bleeding tendency, platelets <50 × 109/L, or severely disordered coagulation, in whom anticoagulation and/or antiplatelet drugs should be discontinued at least five to seven days before percutaneous ablation; (iii) ipsilateral pleural malignant effusions that are not well controlled; (iv) severe liver, kidney, heart, lung or brain function insufficiency, severe anemia, severe dehydration and nutritional and metabolic disorders that cannot be corrected or improved in the short term, severe systemic infections, high fever (>38.5°C); or (v) Eastern Cooperative Oncology Group (ECOG) score > 3.
Procedure preparation
Patient assessment and imaging
Complete history, physical examination, and recent image data should be carefully reviewed to assess each patient's thermal ablation indications. Selection of procedure should be based on multidisciplinary decisions, with the procedure discussion recorded. Contrast-enhanced chest CT (within 2 weeks) is a key imaging assessment before thermal ablation, in which the tumor's size, location, and relationship with neighboring vital organs, blood vessels, trachea or bronchi can be seen. As relevant staging and examinations are completed, positron emission tomography (PET)-CT scans are recommended if applicable to exclude or detect distant metastases.40
Laboratory tests
Laboratory tests should include routine blood, urine, and stool tests, coagulation, liver and kidney function, blood glucose, tumor markers, blood type and other tests, electrocardiogram (ECG), cardiac ultrasound (optional for elderly patients), and pulmonary function test.
Pathological examination
For primary lung cancer, percutaneous biopsy or bronchoscopy should be performed before ablation to confirm the diagnosis. When metastatic lesions are not typical, biopsy is recommended before ablation.5,24,41
Drugs and monitoring equipment
Drugs for anesthesia and analgesia, antitussives, hemostatics, and vasodilators and antihypertensive drugs, as well as rescue medicines and equipment, should be prepared preoperatively.
Patient preparation
The patient and/or his/her family or representative must sign an informed consent form. The patient should fast four hours before local anesthesia, or abstain from solid food 12 hours and liquids four hours before general anesthesia. The patient should also undergo surgical skin preparation, if necessary, establishment of intravenous access, and oral antitussive before the procedure.
Anesthesia, procedural and post-procedural care
Anesthesia and disinfection
According to the patient's condition, general anesthesia or local anesthesia can be used for the procedure, while ensuring compliance with aseptic techniques.42,43
Procedural and post-procedural care
After the proper ablation technique is determined, CT is one of the most common and accurate image-guided approaches. During the procedure, the thermal ablation applicator (electrode or antenna or probe) is directly inserted into the target tissue through the skin under CT guidance. After confirming that the ablation applicator is correctly positioned using multi-planar CT imaging, ablation is performed. Selection of lung tissue ablation parameters can be adjusted to the equipment manufacturer's recommended parameters.
A number of ablation patterns can be used based on the tumor size and location: (i) single session at a single point (e.g. lesions ≤ 3 cm); (ii) single session at multiple points (e.g. 3–5 cm); or (iii) single or multiple sessions at multiple points using multi- applicators (e.g. >5 cm, or palliative ablation). During the thermal ablation process, damage to lung tissue surrounding the tumor may lead to an opaque high-density area, called ground-glass opacity (GGO), appearing in the CT image. When the peripheral margin of the GGO in the target tissue expands 5 mm or more beyond the pre-procedure tumor borders, the ablation applicator can be pulled out. Due care should be taken to ablate the withdrawal path of the ablation applicator. A repeat large-range (preferably whole-lung) CT scan should be carried out at the end of the ablation procedure to identify any complications and assess technical success.
Monitoring the patient's heart rate, blood pressure, and oxygen saturation is necessary during the procedure; the patient should be closely observed for breathing, pain, cough, hemoptysis, etc. Symptomatic treatment should be delivered when necessary. Post-procedural attention to vital signs is recommended. Chest X-ray or CT scan can be performed after 24 to 48 hours to check for complications (such as asymptomatic pneumothorax or pleural effusion).
Follow-up and response assessment
Follow-up
Monthly contrast-enhanced chest CT scans are performed for the first three months after the procedure. After that, contrast-enhanced chest CT or PET/CT scans and tumor markers are examined every three months, to detect whether local lesions have been completely ablated or if any new pulmonary lesions or extrapulmonary metastases have emerged. Contrast-enhanced chest CT is the standard method of evaluating ablation efficacy at present. If available, use of PET-CT in combination with contrast-enhanced CT can provide a more accurate assessment of the efficacy after ablation.24,40,44
Post-procedure imaging characteristics and response assessment
Computed tomography-based efficacy assessment
After ablation, variations on contrast-enhanced CT scans will follow a pattern: the lesion will grow for one to three months after ablation, and remain stable or gradually involute and decrease in size after three months.44,45
In the first week, the lesion may show low-density honeycomb-like or cavernous areas, and the area around the ablated tumor will be surrounded by concentric circles of different degrees of decay, known as the “cockade” sign (which is more obvious 24–48 hours after ablation). CT values (Hounsfield units) are reduced, and the lesion is enlarged compared with what it was before ablation, with a GGO-like reaction band around it. A GGO beyond the peripheral border of the tumor is thought to indicate complete tumor ablation.24,46,47 In the intermediate phase (1 week to 3 months), as the ablation zone increases constantly, a sharp enhanced ring may appear around the perimeter (benign periablational enhancement), known as the “egg shell” sign (a thin rim peripheral to the zone of ablation, a relatively symmetric and uniform process with smooth inner margin, that can measure 0.5–3 mm). In the late phase (after 3 months), the ablation zone remains stable compared with the baseline (CT findings at about 1 month after the ablation). Subsequent follow-up CT results of the lesion area may present different modes, such as fibrosis, cavities, nodules, atelectasis, and disappearance.47
Assessment of local efficacy
The lesion at one month after ablation is used as the baseline to determine local efficacy.
Complete ablation is indicated by lesion disappearance, complete cavernous formation, fibrotic progression or scar, solid nodule involution or no change, without contrast-enhanced signs on the CT scan, and/or atelectasis.
Incomplete ablation is indicated by incomplete cavernous formation, with some solid or liquid components remaining and irregular peripheral or internal enhancement signs on CT scans; partial fibrosis, with solid residues in the fibrotic lesion, which presents as irregular peripheral or internal enhancement signs on CT scans; and/or solid nodules with unchanged or increased size, which also present as irregular peripheral or internal enhancement signs on CT scans.
PET-CT may be the most accurate means of determining the local response after ablation, but it has a high false-positive rate three months after ablation. For various reasons, PET-CT has not been widely applied in the field of tumor ablation, but if applicable, it is recommended for follow-up assessment of ablation response.48–50
Clinical outcomes assessment
Regular follow-up is based on the local efficacy assessment, to monitor improvement in quality of life (such as pain relief, as evaluated by a numeric rating scale). Overall survival is recorded during follow-up, as survival is the most important indicator of clinical outcomes. Patient survival at one, two, three, and five years should be followed.
Complications and treatment
Percutaneous lung tumor ablation is a relatively safe topical treatment. The incidence of complications is reported according to the classifications of the Imaging-guided Tumor Ablation International Working Group of the Society of Interventional Radiology.51
Major complications can cause death or disability, hospitalization or clinical treatment, increased level of care or prolonged hospitalization, including blood transfusions or thoracic drainage. In the event of death, the relationship with the ablation should be noted. Minor complications include cases of pneumothorax that do not require drainage.
Side effects include adverse outcomes associated with treatment, which may happen often, but rarely cause substantial damage, including pain, pleural reaction, small amounts of pulmonary bleeding, bloody sputum, small amounts of pleural effusion, and post-ablation syndrome. According to the time of occurrence, they are classified as immediate complications (<24 hours after ablation), perioperative complications (24 hours–30 days after ablation) and delayed complications (>30 days after ablation).
Pain
After a procedure under local anesthesia, patients may experience varying degrees of pain. Pain control treatment is often particularly necessary for the ablation of lesions near the pleura. If the pain is severe, opioid medications can be increased, and the proper amount of sedatives can also be used. Post-procedural pain is usually mild and will last for several days, or up to two weeks in certain patients, but moderate or severe pain is rare. Non-steroidal analgesic drugs can be used for pain control.
Post-ablation syndrome
Post-ablation syndrome occurs in approximately two thirds of patients, as a result of the absorption of necrotic substances and release of inflammatory cytokines. This syndrome is transient and self-limiting; its symptoms include low-grade fever, general malaise, fatigue, vomiting, and so on, which generally last three to five days, or (less often) up to about two weeks. Symptomatic treatment is often sufficient, in addition to non-steroidal drugs when necessary. A small dose of short-term glucocorticoids in conjunction with enhanced supportive treatment can be helpful.
Pneumothorax
Pneumothorax is the most common post-ablation complication, with an incidence rate of 10–60%,52,53 and is more common with emphysema, male patients, age > 60 years, tumor <1.5 cm, tumor in the lower lobe, >3 puncture for a single tumor in lung tissue, multiple tumors and multiple puncture and ablations, and a long part of the ablation path through lung tissue or through a large fissure.54–56 Most cases of pneumothorax can be easily treated or are self-limiting. Fewer than 10% of cases need thoracic drainage. If there is still a gas leak after thoracic drainage, continuous suction, pleurodesis, endoscopic injection sclerotherapy, tracheal valve implantation and other means can be used.57 The patient should also be observed for delayed pneumothorax (i.e. ≥72 hours after ablation).58
Pleural effusion
A small amount of pleural effusion is usually observed after 1–60% of ablation procedures,52 and is thought to be a sympathetic response to thermal damage; 1–7% of patients require puncture/catheter drainage for the pleural effusion. Risk factors for pleural effusions include large lesions, ablation of multiple lesions at one time, lesions near the pleura (<10 mm), and prolonged ablation.54
Bleeding
The incidence of bleeding is 3–8% during ablation,51,52 but hemoptysis is extremely rare.52,59,60 In the event of moderate or severe hemoptysis, ablation should be carried out immediately with intravenous hemostatic infusion. As ablation itself can cause blood coagulation, bleeding will gradually stop as the ablation progresses. As a result, the incidence of major bleeding is not high during the procedure. Obviously, large blood vessels or atelectatic lung tissue should be avoided as much as possible. Postoperative hemoptysis is often self-limiting, and can last three to five days. In cases where conservative treatment fails, embolization or exploratory thoracotomy can be performed.
Pleural reaction
Ablation stimulates the vagus nerve that dominates the parietal pleura, and vagus nerve excitement can slow the heart rate, and even cause cardiac arrest. When this happens, the ablation should be suspended to allow complete local anesthesia. Atropine, sedatives and other drugs should be properly applied.
Infection
The incidence of lung infection caused by ablation is 1–6%.52,59–61 Prophylactic antibiotics can be used 30 to 60 minutes before the procedure, and once again in 24 hours, and can be extended to 48 to72 hours for patients older than 70 years, or for those with chronic obstructive pulmonary disease, poorly controlled diabetes, tumors > 4 cm, or low immunity. If the temperature is still > 38.5°C at five days after the procedure, lung infections should be first suspected. Antibiotics should be adjusted according to sputum, blood or pus culture results. Pulmonary or chest abscesses can be drained using tube placement. Also, note that as interstitial pneumonia often occurs after radiotherapy, ablation increases the risk of secondary infection.61
Less common complications
Individual reports included cases of bronchial fistula, acute respiratory distress syndrome, thermal burns or frostbite in non-target areas, cold shock, thrombocytopenia, tumor seeding via needle, nerve damage (brachial plexus, intercostal, phrenic, recurrent laryngeal nerves, etc.), pulmonary or air embolism, and myocardial infarction. Individualized treatment should be given in such cases.
Ablation in combination with other therapy
Ablation in combination with other therapy methods is an important part of current cancer research, including combinations of ablation with surgery, chemotherapy, radiation and/or molecular-targeted drugs and the like. The combination of ablation and radiotherapy can improve local tumor control and prolong survival, while not evidently increasing adverse reactions.62–64 Although few studies have been published on ablation with chemotherapy in NSCLC, the combination can control localized tumors and prolong patient survival.65–68
Conclusion
Minimally invasive therapy appears to be the direction of future lung cancer treatment development. In particular, imaging-guided percutaneous thermal ablation techniques have the following advantages in treating lung tumors: minimal invasiveness, good therapeutic effects, safety, fast recovery, relatively simple procedure, and wide applicability. The best reported one, three, and five-year overall survival rates after RFA for NSCLC are 97.7%, 72.9%, and 55.7%, respectively,69 with a procedural mortality rate of <1%.70 Such clinical evidence indicates that this technique will be more widely used in treating lung tumors in the future, and it could become a new treatment modality following surgery, radiotherapy, and chemotherapy. Clinically, however, thermal ablation is used much less often to treat primary and metastatic lung cancer patients than surgery, radiotherapy, and chemotherapy.71–75 Further work is needed to change the knowledge of thermal ablation techniques among traditional oncologists, making the treatments universal and standardized.
However, thermal ablation as the treatment of primary and metastatic lung tumors has some current problems: (i) although thermal ablation technology has become an important facet of multidisciplinary lung cancer treatment, especially for the early lung cancer patients who cannot be tolerate surgical resection (for whom it is likely to become the treatment of choice), large-scale, multi-center, randomized, prospective clinical trials are still lacking; (ii) no prospective, multi-center comparative clinical trials with other conventional treatments (such as stereotactic radiation therapy) have been performed; (iii) few clinical trials use thermal ablation in combination with other treatments (such as radiotherapy, chemotherapy and molecularly targeted therapy);76–79 (iv) as a relatively new modality, thermal ablation has not been subjected to the volume of clinically based refinements to improve local complete ablation rates and reduce local recurrence (for example) as have more traditional modalities; (v) because of differences in equipment manufacturers and performance, and because the technique is just emerging, professional skill varies greatly among clinicians, which complicates the formation of standard guidelines; (vi) compliance with current international criteria in terms of outcome determination after thermal ablation can be difficult, as existing imaging methods do not always reflect outcomes after thermal ablation with a desirable level of precision, so development of recognized outcome criteria in line with thermal ablation technology itself has a long way to go; (vii) palliative ablation in the comprehensive treatment of lung cancer has yet to be fully explored; and (viii) basic research in topics, such as complex thermal field distribution and effects on the immune system, is still lacking.
Disclosure
No authors report any conflict of interest.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics CA Cancer J Clin61. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. . (Published erratum appears in 2011; 134) [DOI] [PubMed] [Google Scholar]
- He J, Zhao P, Chen WQ, editors. Chinese Cancer Registry Annual Report 2012. Beijing: Military Medical Science Press; 2012. pp. 13–16. (In Chinese.) [Google Scholar]
- Scott WJ, Howington J, Feigenberg S, et al. Treatment of non-small cell lung cancer stage I and stage II. Chest. 2007;132(3 Suppl):234S–242. doi: 10.1378/chest.07-1378. [DOI] [PubMed] [Google Scholar]
- Vogl TJ, Naguib NN, Lehnert T, Nour-Eldin NE. Radiofrequency, microwave and laser ablation of pulmonary neoplasms: clinical studies and technical considerations-Review article. Eur J Radiol. 2011;77:346–357. doi: 10.1016/j.ejrad.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Crocetti L, Lencioni R. Radiofrequency ablation of pulmonary tumors. Eur J Radiol. 2010;75:23–27. doi: 10.1016/j.ejrad.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16:192–200. doi: 10.1053/j.tvir.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zhi X, Liu L, et al. Evaluation of three-dimensional reconstruction CT in percutaneous radiofrequency ablation (RFA) of the unresectable lung tumor with a clustered electrode. Zhongguo Fei Ai Za Zhi. 2009;12:775–779. doi: 10.3779/j.issn.1009-3419.2009.07.006. (In Chinese.) [DOI] [PubMed] [Google Scholar]
- Zhi XY, Liu BD, Feng WJ. CT guided percutaneous radiofrequency ablation in treatment of patients with inoperable lung cancer. Zhong Liu Yan Jiu Yu Lin Chuang. 2010;22:19–22. (In Chinese.) [Google Scholar]
- Casal RF, Tam AL, Eapen GA, et al. Radiofrequency ablation of lung tumors. Clin Chest Med. 2010;31:151–163. doi: 10.1016/j.ccm.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Ward RC, Healey TT, Dupuy DE. Microwave ablation devices for interventional oncology. Expert Rev Med Devices. 2013;10:225–238. doi: 10.1586/erd.12.77. [DOI] [PubMed] [Google Scholar]
- Abbas G. Microwave ablation. Semin Thorac Cardiovasc Surg. 2011;23:81–83. doi: 10.1053/j.semtcvs.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Fan WJ, Ye X, editors. Microwave Ablation Oncology. Beijing: People's Medical Publishing House; 2012. pp. 28–31. (In Chinese.) [Google Scholar]
- Abbas G, Pennathur A, Landreneau RJ, Luketich JD. Radiofrequency and microwave ablation of lung tumors. J Surg Oncol. 2009;100:645–650. doi: 10.1002/jso.21334. [DOI] [PubMed] [Google Scholar]
- Fan W, Li X, Zhang L, Jiang H, Zhang J. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. Am J Roentgenol. 2012;198:46–50. doi: 10.2214/AJR.11.6707. [DOI] [PubMed] [Google Scholar]
- Healey TT, Dupuy DE. Microwave ablation for lung cancer. Med Health R I. 2012;95:52–53. [PubMed] [Google Scholar]
- Andreano A, Huang Y, Meloni MF, Lee FT, Jr, Brace C. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37:2967–2973. doi: 10.1118/1.3432569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocetti L, Bozzi E, Faviana P, et al. Thermal ablation of lung tissue: in vivo experimental comparison of microwave and radiofrequency. Cardiovasc Intervent Radiol. 2010;33:818–827. doi: 10.1007/s00270-010-9869-z. [DOI] [PubMed] [Google Scholar]
- Xiao YY, Tian JL, editors. Argon-Helium Knife Cryoablation Techniques of Tumor. Beijing: People's Military Medical Press, Beijing; 2010. pp. 21–25. (In Chinese.) [Google Scholar]
- Wang H, Littrup PJ, Duan Y, Zhang Y, Feng H, Nie Z. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology. 2005;235:289–298. doi: 10.1148/radiol.2351030747. [DOI] [PubMed] [Google Scholar]
- Feng HS, Duan YJ, Nie ZS, et al. Clinical study on targeted argon-heliun cryotherapy in treatment for 725 cases with pulmonary neoplasm. Zhongguo Zhong Liu. 2007;16:906–909. (In Chinese.) [Google Scholar]
- Schneider T, Warth A, Herpel E, et al. Intraoperative radiofrequency ablation of lung metastases and histologic evaluation. Ann Thorac Surg. 2009;87:379–384. doi: 10.1016/j.athoracsur.2008.10.088. [DOI] [PubMed] [Google Scholar]
- Sonntag PD, Hinshaw JL, Lubner MG, Brace CL, Lee FT., Jr Thermal ablation of lung tumors. Surg Oncol Clin N Am. 2011;20:369–387. doi: 10.1016/j.soc.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology. 2011;260:633–655. doi: 10.1148/radiol.11091126. [DOI] [PubMed] [Google Scholar]
- Pereira PL, Salvatore M. Standards of practice: guidelines for thermal ablation of primary and secondary lung tumors. Cardiovasc Intervent Radiol Cardiovasc Intervent Radiol. 2012;35:247–254. doi: 10.1007/s00270-012-0340-1. . (Published erratum appears in 2012; 444) [DOI] [PubMed] [Google Scholar]
- Yang X, Ye X. Image-guided pulmonary malignant tumor ablation treatment. Zhonghua Lin Chuang Yi Shi Za Zhi. 2012;6:7686–7690. (In Chinese.) [Google Scholar]
- Liu B, Liu L, Li Y, et al. Survival after radiofrequency ablation for 100 cases of lung neoplasms. Zhongguo Fei Ai Za Zhi. 2011;14:335–339. doi: 10.3779/j.issn.1009-3419.2011.04.06. (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung VA, DiPetrillo TA, Dupuy DE. Image-guided tumor ablation for the treatment of recurrent non-small cell lung cancer within the radiation field. Eur J Radiol. 2011;80:e491–499. doi: 10.1016/j.ejrad.2010.09.042. [DOI] [PubMed] [Google Scholar]
- Lee JM, Jin GY, Goldberg SN, et al. Percutaneous radiofrequency ablation for inoperable NSCLC and metastases: preliminary report. Radiology. 2004;230:125–134. doi: 10.1148/radiol.2301020934. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumors: a prospective intention to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9:621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- Chua TC, Glenn D, Morris DL. Extending the survival of patients with melanoma lung metastases through radiofrequency ablation. Acta Oncol. 2010;49:517–519. doi: 10.3109/02841860903473305. [DOI] [PubMed] [Google Scholar]
- Hiraki T, Yamakado K, Ikeda O, et al. Percutaneous radiofrequency ablation for pulmonary metastases from hepatocellular carcinoma: results of a multicenter study in Japan. J Vasc Interv Radiol. 2011;22:741–748. doi: 10.1016/j.jvir.2011.02.030. [DOI] [PubMed] [Google Scholar]
- Palussière J, Italiano A, Descat E, et al. Sarcoma lung metastases treated with percutaneous radiofrequency ablation: results from 29 patients. Ann Surg Oncol. 2011;18:3771–3777. doi: 10.1245/s10434-011-1806-0. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Matsumine A, Yamakado K, et al. Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcomas. Cancer Cancer. 2009;115:3774–3781. doi: 10.1002/cncr.24420. . (Published erratum appears in 2009; 4041) [DOI] [PubMed] [Google Scholar]
- de Baère T. Lung tumor radiofrequency ablation: where do we stand? Cardiovasc Intervent Radiol. 2011;34:241–251. doi: 10.1007/s00270-010-9860-8. [DOI] [PubMed] [Google Scholar]
- Baisi A, Raveglia F, De Simone M, Cioffi U. Palliative role of percutaneous radiofrequency ablation for severe hemoptysis in an elderly patient with inoperable lung cancer. J Thorac Cardiovasc Surg. 2010;140:1196–1197. doi: 10.1016/j.jtcvs.2010.01.049. [DOI] [PubMed] [Google Scholar]
- Lane MD, Le HB, Lee S, et al. Combination radiofrequency ablation and cementoplasty for palliative treatment of painful neoplastic bone metastasis: experience with 53 treated lesions in 36 patients. Skeletal Radiol. 2011;40:25–32. doi: 10.1007/s00256-010-1010-5. [DOI] [PubMed] [Google Scholar]
- Guenette JP, Lopez MJ, Kim E. Solitary painful osseous metastases: correlation of imaging features with pain palliation after radiofrequency ablation-a multicenter American College of Radiology Imaging Network study. Radiology. 2013;268:907–915. doi: 10.1148/radiol.13122398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTaggart RA, Dupuy DE. Thermal ablation of lung tumors. Tech Vasc Interv Radiol. 2007;10:102–113. doi: 10.1053/j.tvir.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23:727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Shim SS, Lee KS, Kim BT, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET-CT and CT alone for preoperative staging. Radiology. 2005;236:1011–1019. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]
- Van Cutsem EJ, Oliveira J. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(Suppl 2):ii33–34. doi: 10.1093/annonc/mdn079. [DOI] [PubMed] [Google Scholar]
- Hoffmann RT, Jakobs TF, Lubienski A, et al. Percutaneous radiofrequency ablation of pulmonary tumors—is there a difference between treatment under general anesthesia and under conscious sedation? Eur J Radiol. 2006;59:168–174. doi: 10.1016/j.ejrad.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Pouliquen C, Kabbani Y, Saignac P, et al. Radiofrequency ablation of lung tumours with the patient under thoracic epidural anaesthesia. Cardiovasc Intervent Radiol. 2011;34(Suppl. 2):S178–181. doi: 10.1007/s00270-010-9843-9. [DOI] [PubMed] [Google Scholar]
- Abtin FG, Eradat J, Gutierrez AJ, Lee C, Fishbein MC, Suh RD. Radiofrequency ablation of lung tumors:imaging features of the postablation zone. Radiographics. 2012;32:947–969. doi: 10.1148/rg.324105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke K, King J, Glenn D, Morris DL. Radiologic appearance and complications of percutaneous computed tomography- guided radiofrequency-ablated pulmonary metastases from colorectal carcinoma. J Comput Assist Tomogr. 2003;27:750–757. doi: 10.1097/00004728-200309000-00012. [DOI] [PubMed] [Google Scholar]
- Anderson EM, Lees WR, Gillams AR, et al. Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumors. Cardiovasc Intervent Radiol. 2009;32:478–483. doi: 10.1007/s00270-008-9482-6. [DOI] [PubMed] [Google Scholar]
- Palussière J, Marcel B, Descat E, et al. Lung tumors treated with percutaneous radiofrequency ablation: computed tomography imaging follow-up. Cardiovasc Intervent Radiol. 2011;34:989–997. doi: 10.1007/s00270-010-0048-z. [DOI] [PubMed] [Google Scholar]
- Deandreis D, Leboulleux S, Dromain C, et al. Role of FDG-PET-CT and chest CT in the follow-up of lung lesions treated with radiofrequency ablation. Radiology. 2011;258:270–276. doi: 10.1148/radiol.10092440. [DOI] [PubMed] [Google Scholar]
- Yoo DC, Dupuy DE, Hillman SL, et al. Radiofrequency ablation of medically inoperable stage IA non-small cell lung cancer: are early posttreatment PET findings predictive of treatment outcome? AJR Am J Roentgenol. 2011;197:334–340. doi: 10.2214/AJR.10.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera LJ, Fernando HC, Perry Y, et al. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg. 2003;125:929–937. doi: 10.1067/mtc.2003.18. [DOI] [PubMed] [Google Scholar]
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–739. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol. 2008;15:1765–1774. doi: 10.1245/s10434-008-9848-7. [DOI] [PubMed] [Google Scholar]
- Carrafiello G, Mangini M, Fontana F, et al. Complications of microwave and radiofrequency lung ablation: personal experience and review of the literature. Radiol Med. 2012;117:201–213. doi: 10.1007/s11547-011-0741-2. [DOI] [PubMed] [Google Scholar]
- Hiraki T, Tajiri N, Mimura H, et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: incidence and risk factors. Radiology. 2006;241:275–283. doi: 10.1148/radiol.2411051087. [DOI] [PubMed] [Google Scholar]
- Nour-Eldin NE, Naguib NN, Saeed AS, et al. Risk factors involved in the development of pneumothorax during radiofrequency ablation of lung neoplasms. AJR Am J Roentgenol. 2009;193:W43–48. doi: 10.2214/AJR.08.1457. [DOI] [PubMed] [Google Scholar]
- Gillams AR, Lees WR. Analysis of the factors associated with radiofrequency ablation-induced pneumothorax. Clin Radiol. 2007;62:639–644. doi: 10.1016/j.crad.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Abu-Hijleh M, Blundin M. Emergency use of an endobronchial one-way valve in the management of severe air leak and massive subcutaneous emphysema. Lung. 2010;188:253–257. doi: 10.1007/s00408-009-9204-0. [DOI] [PubMed] [Google Scholar]
- Clasen S, Kettenbach J, Kosan B, et al. Delayed development of pneumothorax after pulmonary radiofrequency ablation. Cardiovasc Intervent Radiol. 2009;32:484–490. doi: 10.1007/s00270-008-9489-z. [DOI] [PubMed] [Google Scholar]
- Schneider T, Heussel CP, Herth FJ, Dienemann H. Thermal ablation of malignant lung tumors. Dtsch Arztebl. 2013;110:394–400. doi: 10.3238/arztebl.2013.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol. 2011;197:W576–580. doi: 10.2214/AJR.11.6408. [DOI] [PubMed] [Google Scholar]
- Nomura M, Yamakado K, Nomoto Y. Complications after lung radiofrequency ablation: risk factors for lung inflammation. Br J Radiol. 2008;81:244–249. doi: 10.1259/bjr/84269673. [DOI] [PubMed] [Google Scholar]
- Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest. 2006;129:738–745. doi: 10.1378/chest.129.3.738. [DOI] [PubMed] [Google Scholar]
- Grieco CA, Simon CJ, Mayo-Smith WW, DiPetrillo TA, Ready NE, Dupuy DE. Percutaneous image-guided thermal ablation and radiation therapy: outcomes of combined treatment for 41 patients with inoperable stage I/II non-small cell lung cancer. J Vasc Interv Radiol. 2006;17:1117–1124. doi: 10.1097/01.RVI.0000228373.58498.6E. [DOI] [PubMed] [Google Scholar]
- Wang YG, Yang B. The treatment efficacy observation of the advanced non-small cell lung cancer with radiofrequency ablation combined radiotherapy. Chin J Phys Med Rehabil. 2012;34:879–880. [Google Scholar]
- Li X, Zhao M, Wang J, et al. Percutaneous CT-guided radiofrequency ablation as supplemental therapy after systemic chemotherapy for selected advanced non-small cell lung cancers. Am J Roentgenol. 2013;201:1362–1367. doi: 10.2214/AJR.12.10511. [DOI] [PubMed] [Google Scholar]
- Lee H, Jin GY, Han YM. Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc Intervent Radiol. 2012;35:343–350. doi: 10.1007/s00270-011-0194-y. [DOI] [PubMed] [Google Scholar]
- Ye X, Yang X, Zheng AM, et al. The clinical research of microwave ablation combined chemotherapy treated advanced non-small-cell lung cancers. Guo Ji Zhong Liu Xue Za Zhi. 2013;40:396–397. (In Chinese.) [Google Scholar]
- Liu WJ, Zeng XT, Liu XJ, et al. The meta-analysis of radiofrequency ablation combined with chemotherapy treated advanced non-small-cell lung cancer. Lin Chuang Zhong Liu Xue Za Zhi. 2012;17:530–538. (In Chinese.) [Google Scholar]
- de Baere T, Farouil G, Deschamps F. Lung cancer ablation: what is the evidence? Semin Intervent Radiol. 2013;30:151–156. doi: 10.1055/s-0033-1342956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraki T, Gobara H, Fujiwara H. Lung cancer ablation: complications. Semin Intervent Radiol. 2013;30:169–175. doi: 10.1055/s-0033-1342958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Han HJ, Park SJ, et al. Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer. Eur J Radiol. 2012;81:395–399. doi: 10.1016/j.ejrad.2010.12.091. [DOI] [PubMed] [Google Scholar]
- Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg. 2010;211:68–72. doi: 10.1016/j.jamcollsurg.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Renaud S, Falcoz PE, Olland A, Massard G. Is radiofrequency ablation or stereotactic ablative radiotherapy the best treatment for radically treatable primary lung cancer unfit for surgery? Interact Cardiovasc Thorac Surg. 2013;16:68–73. doi: 10.1093/icvts/ivs423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal H, Mahmood S, Rajashanker B, Shah R. Is radiofrequency ablation more effective than stereotactic ablative radiotherapy in patients with early stage medically inoperable non-small cell lung cancer? Interact Cardiovasc Thorac Surg. 2012;15:258–265. doi: 10.1093/icvts/ivs179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy DE. Treatment of medically inoperable non-small-cell lung cancer with stereotactic body radiation therapy versus image-guided tumor ablation: can interventional radiology compete? J Vasc Interv Radiol. 2013;24:1139–1145. doi: 10.1016/j.jvir.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Yang X, Ye X, Han XY, et al. The clinical research of microwave ablation in combination with radiation and chemotherapy treated III-stage peripheral non-small-cell lung cancers. Zhonghua Lin Chuang Yi Shi ZaZhi. 2013;7:9431–9435. (In Chinese.) [Google Scholar]
- Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–351. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadaleta CD, Solbiati L, Mattioli V, et al. Unresectable lung malignancy: combination therapy with segmental pulmonary arterial chemoembolization with drug-eluting microspheres and radiofrequency ablation in 17 patients. Radiology. 2013;267:627–637. doi: 10.1148/radiol.12120198. [DOI] [PubMed] [Google Scholar]
- Gómez FM, Palussière J, Santos E, et al. Radiofrequency thermocoagulation of lung tumors. Where we are, where we are headed. Clin Transl Oncol. 2009;11:28–34. doi: 10.1007/s12094-009-0307-0. [DOI] [PubMed] [Google Scholar]


