Abstract
We herein report a case of solitary pulmonary metastasis from malignant melanoma that presented as a pulmonary ground glass nodule. A 57-year-old man who had undergone resection of a malignant melanoma of the right bulbar conjunctiva at the age of 51 was referred to our hospital for management of ground glass opacity in his left lung. Because radiological examination suggested the nodule was an adenocarcinoma in situ, computed tomography (CT) follow-up was planned. CT examination performed nine months later showed that the nodule had grown from 6 mm to 8 mm. Moreover, CT performed one and a half years after first detection revealed that the nodule had grown up to 10 mm. The patient, therefore, underwent partial resection of the lung for diagnosis and treatment. Pathological examination of the resected specimen revealed atypical cells with melanin granules proliferating in a lepidic-like fashion. The cells were positive on S-100 staining, indicating a pulmonary metastasis from malignant melanoma. Thus, metastatic tumors from malignant melanoma can present as ground glass opacities.
Keywords: Bronchioloalveolar, differential diagnosis, melanoma, neoplasm metastasis, solitary pulmonary nodule
Introduction
The increasing use of computed tomography (CT) has resulted in the discovery of small ground glass opacities (GGOs) in the lung. GGOs are defined as having hazy increased attenuation of the lung with preservation of bronchial and vascular margins.1 Various types of pulmonary diseases may have a GGO appearance, including infection, collagen vascular disease, drug toxicity, fibrosis, and pulmonary adenocarcinomas.1 However, metastatic lesions to the lung rarely present as GGOs. Here, we present a case of solitary pulmonary metastasis from a malignant melanoma of the bulbar conjunctiva presenting as a GGO six years after initial treatment.
Case report
A 57-year-old man was referred to our institution for management after a chest CT scan had revealed a GGO measuring 6 mm adjacent to the visceral pleura in the left lower lobe (segment 8; Fig 1a). Six years previously, he had undergone partial resection of a malignant melanoma in the right bulbar conjunctiva, followed by local injection of β-interferon as postoperative adjuvant therapy. A primary lung adenocarcinoma or atypical adenomatous hyperplasia was suspected, and follow-up with CT was planned. According to a CT scan nine months later, the nodule had grown from 6 mm to 8 mm. Further follow-up CT, which was performed one and a half years after first detection uncovered the nodule had grown up to 10 mm (Fig 1b); thus, its doubling time was 230 days. Therefore, we decided to resect the nodule.
Figure 1.
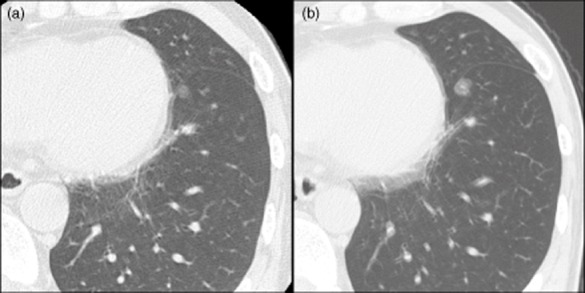
Chest computed tomography scan showing that the ground glass opacity grew from (a) 6 mm to (b) 10 mm during 18 months of observation.
During surgery, a patchy black lesion was identified on the surface of the lung and subjected to wedge resection (Fig 2). Pathological examination revealed proliferation of atypical cells in a lepidic-like fashion. Invasion, characterized by destroyed alveolar walls, was also seen in some areas (Fig 3a). On immunohistochemical staining, the atypical cells were positive for S-100 (Fig 3b). Additionally, Elastic van Gieson staining showed that the elastic membranes of the alveolar septa were intermittently disrupted (Fig 3c). The Ki-67 labeling index was 33.4% (Fig 3d). Based on these findings, a diagnosis of pulmonary metastasis from the previously treated malignant melanoma was made. The patient developed multiple pulmonary metastases showing GGO appearance 15 months after pulmonary resection. Therefore, chemotherapy was commenced.
Figure 2.
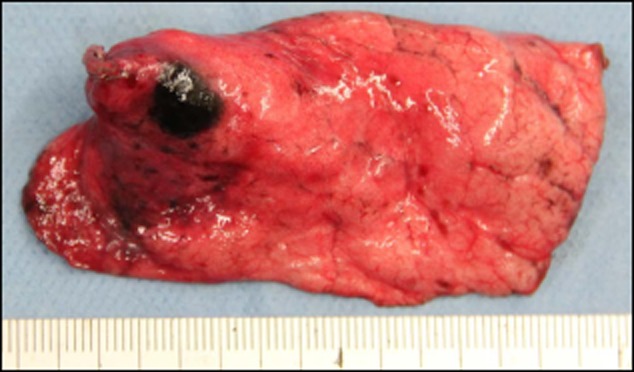
Partially resected lung: a patchy black lesion is apparent on its surface.
Figure 3.
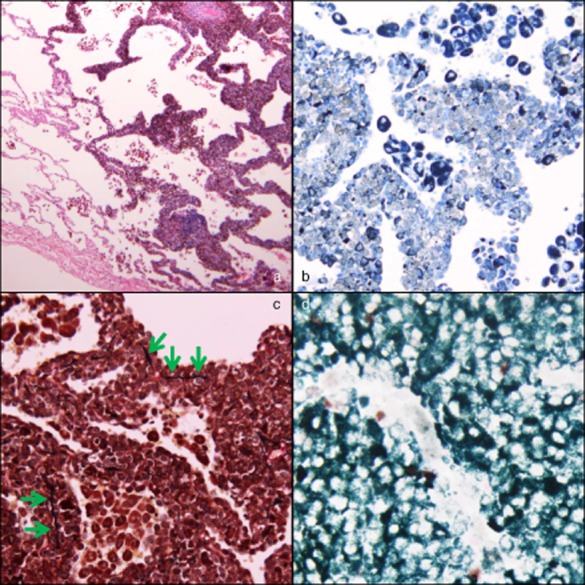
(a) Hematoxylin–eosin staining (×40) showing atypical cells with melanin granules proliferating in a lepidic-like fashion, simulating lepidic predominant adenocarcinoma (in the right half). (b) S-100 staining (×200) shows that the atypical cells are positive (appear gray). (c) Elastic van Gieson staining (×200) shows that the elastic membranes of the alveolar septa are intermittently disrupted (green arrows). (d) Ki-67 staining with May-Giemsa staining (×400) shows that brown and dark green nuclei are positive, but white ones are negative. The Ki-67 labeling index, average proportion of positive cells in three hot spots of the tumor, was 33.4%.
Discussion
The lung is one of the organs in which various types of cancer frequently metastasize. In general, pulmonary metastatic tumors present as solid, well-defined, round multiple lesions on CT scans. Thus, pulmonary metastasis is clinically diagnosed on the basis of such features. Pulmonary metastases from malignant melanoma reportedly also show a solid pattern.2 To our knowledge, this is the third report of pulmonary metastasis from malignant melanoma with a GGO pattern, rather than the typical metastatic tumor appearance.3,4 The characteristics of these three patients are summarized in Table 1. Okita et al. reported rapidly growing multiple GGO lesions in both lungs that were diagnosed as pulmonary metastases from malignant melanoma by biopsy.3 Kang et al. reported a solitary GGO that also grew rapidly.4 This lesion was diagnosed as pulmonary metastatic melanoma by surgical resection of the right upper lobe.
Table 1.
Documented cases of metastatic pulmonary melanoma showing ground glass opacity
| Authors | Age (years) | Gender | Number of lesions | Maximum diameter (cm) | RFI (years) | Estimated doubling time (days) | Treatment |
|---|---|---|---|---|---|---|---|
| Okita et al.3 | 58 | F | multiple | 2.8 | 1.5 | 27 | Chemotherapy |
| Kang et al.4 | 56 | M | solitary | 1.1 | 10 | 47 | Surgery |
| Present case | 57 | M | solitary | 1.0 | 6 | 230 | Surgery |
RFI, recurrence free survival from initial treatment.
These three cases taken together indicate that pulmonary metastasis from malignant melanoma with GGO appearance has a wide range in the rate of tumor growth. In previous reports, the tumor doubling times were reportedly 27 and 47 days, respectively. In contrast, in the present case, growth was relatively slow with a doubling time of 230 days (Table 1). Eskelin et al. reported tumor doubling times of metastatic melanoma that ranged from 34 to 220 days,5 whereas Oda et al. reported that those of atypical adenomatous hyperplasia and bronchioloalveolar carcinoma ranged from 193 to 1288 days.6 Our case indicates that, in patients with a history of malignant melanoma, metastatic lung tumor cannot be excluded, even when lung nodules have a GGO appearance and are growing slowly. Surgical resection is necessary to make a precise diagnosis, and likely to improve patient outcome, even when the nodule is a metastasis from malignant melanoma.7
In conclusion, we herein report a rare case of pulmonary metastasis from malignant melanoma that clinically and radiologically resembled lepidic predominant adenocarcinoma.
Acknowledgments
The authors thank Dr. Shinji Okano for his expert contribution. This work was partially supported by JPJS KAKENHI Grant Number 25830119 (K. Suda).
Disclosure
No authors report any conflict of interest.
References
- Collins J, Stern EJ. Ground-glass opacity at CT: the ABCs. AJR Am J Roentgenol. 1997;169:355–367. doi: 10.2214/ajr.169.2.9242736. [DOI] [PubMed] [Google Scholar]
- Folio LR, Choi Solomon JM, Schaub NP. Automated registration, segmentation, and measurement of metastatic melanoma tumors in serial CT scans. Acad Radiol. 2013;20:604–613. doi: 10.1016/j.acra.2012.12.013. mm, [DOI] [PubMed] [Google Scholar]
- Okita R, Yamashita M, Nakata M, Teramoto N, Bessho A, Mogami H. Multiple ground-glass opacity in metastasis of malignant melanoma diagnosed by lung biopsy. Ann Thorac Surg. 2005;79:e1–2. doi: 10.1016/j.athoracsur.2004.03.096. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Kim MA, Park CM, Lee CH, Goo JM, Lee HJ. Ground-glass nodules found in two patients with malignant melanomas: different growth rate and different histology. Clin Imaging. 2010;34:396–399. doi: 10.1016/j.clinimag.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Eskelin S, Pyrhönen S, Summanen P, Hahka-Kemppinen M, Kivelä T. Tumor doubling times in metastatic malignant melanoma of the uvea: tumor progression before and after treatment. Ophthalmology. 2000;107:1443–1449. doi: 10.1016/s0161-6420(00)00182-2. [DOI] [PubMed] [Google Scholar]
- Oda S, Awai K, Murao K, et al. Volume-doubling time of pulmonary nodules with ground glass opacity at multidetector CT: assessment with computer-aided three-dimensional volumetry. Acad Radiol. 2011;18:63–69. doi: 10.1016/j.acra.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Chua TC, Scolyer RA, Kennedy CW, Yan TD, McCaughan BC, Thompson JF. Surgical management of melanoma lung metastasis: an analysis of survival outcomes in 292 consecutive patients. Ann Surg Oncol. 2012;19:1774–1781. doi: 10.1245/s10434-011-2197-y. [DOI] [PubMed] [Google Scholar]


