Abstract
Most tumor cells show different metabolic pathways than normal cells. Even under the conditions of sufficient oxygen, they produce energy by a high rate of glycolysis followed by lactic acid fermentation in the cytosol, which is known as aerobic glycolysis or the Warburg effect. Lung cancer is a malignant tumor with one of the highest incidence and mortality rates in the world at present. However, the exact mechanisms underlying lung cancer development remain unclear. The three key enzymes of glycolysis are hexokinase, phosphofructokinase, and pyruvate kinase. Lactate dehydrogenase catalyzes the transfer of pyruvate to lactate. All four enzymes have been reported to be overexpressed in tumors, including lung cancer, and can be regulated by many oncoproteins to promote tumor proliferation, migration, and metastasis with dependence or independence of glycolysis. The discovery of aerobic glycolysis in the 1920s has provided new means and potential therapeutic targets for lung cancer.
Keywords: Aerobic glycolysis, key enzymes, lung cancer, therapeutic target
Introduction
Lung cancer is one of the most common cancers in the world and many lung cancer patients have a poor prognosis.1–4 The high mortality and poor prognosis mainly result from the difficulty of early diagnosis. Tumor cells exhibit distinct metabolic phenotypes that are essential for them to sustain higher proliferative rates and resist cell death signals. These metabolic phenotypes include increased glucose utilization in energy metabolism and alteration in the flux along key metabolic pathways, such as glycolysis and glutaminolysis. Recent advances in cellular metabolism resulting from metabolic dysregulation and adaptation of cancer cells are providing increasing support for the development of treatments that target tumor metabolic transformation. However, the molecular details of the metabolic transformation in tumor invasion and metastasis remain largely unknown. Characterization of the time-dependent metabolic shift during tumor invasion, development, and metastasis will provide new insights into tumor phenotypes and potentially allow us to design therapies that inhibit tumor cell growth and migration.
Hyperactivity of glycolysis independent of oxygen availability is a hallmark of lung cancer metabolism (the Warburg effect). Glycolytic energy metabolism of tumor cells, including lung cancer cells, is advantageous for perpetual proliferation and meeting the high demand for non-essential amino acids, fatty acids, and nucleotides, although not for efficient production of ATP. Besides glucose, glutamine is significantly consumed by most tumor cells and metabolized to alanine, lactate, and ammonium ions, which are secreted out of the cells, in a process called glutaminolysis. Corroborating these features of cancer metabolism, moreover, inter-organ metabolomic differences were more significant than normal-versus-tumor differences within the same organ, which revealed the complexity in generalizing a tumor-specific, organ-independent metabolic profile. This suggested that cells alter their metabolism along with tumorigenesis while retaining much of the metabolism that is unique to their organs of origin. In the present review, we will discuss the aerobic glycolysis and its key enzymes in lung cancer so as to provide new targets for lung cancer therapy.
Glycolysis process of living cells
Glucose metabolism in vivo
Glucose is the most important energy-producing molecule of organisms. There are three glucose metabolism processes: glycolysis under anaerobic conditions, complete oxidation under aerobic conditions, and the pentose phosphate pathway. Tumor cells prefer glycolysis under aerobic conditions, which is called aerobic glycolysis. Following is a brief overview of the process of glycolysis.
Glycolysis pathway
Glycolysis is the metabolic pathway that converts glucose into pyruvate in cytoplasm, which produces adenosine triphosphate (ATP). The whole pathway of glycolysis, containing 10 steps of chemical reactions with each catalyzed by a specific enzyme, was elucidated by the 1940s.5 Figure 1 shows the 10 steps and 10 specific enzymes, which are hexokinase (HK), phosphoglucose isomerase (PGI), phosphofructokinase (PFK), aldolase, triosephosphate isomerase (TPI), glyceraldehyde 3 phosphate dehydrogenase (GAPDH), phosphoglycerate kinase (PGK), phosphoglycerate mutase (PGM), enolase, and pyruvate kinase (PK).
Figure 1.
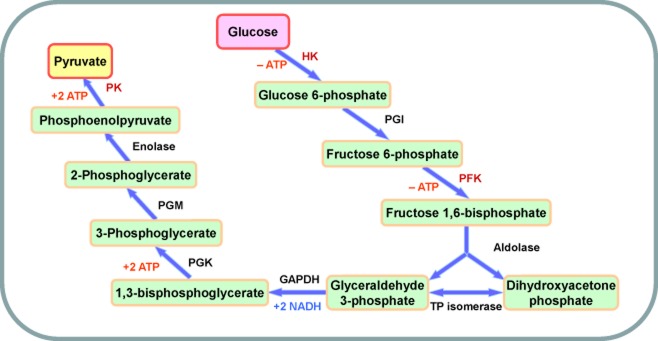
Schematic diagram of glycolysis.
Under hypoxia conditions, pyruvate in both normal and tumor cells is reduced into lactate, also called glycolysis. Tumor cells prefer converting pyruvate into lactate instead of entering the TriCarboxylic Acid (TCA) cycle, even under sufficient oxygen conditions.6
Glycolysis provides intermediates for other metabolic pathways
Many other metabolic pathways are strongly reliant on glycolysis as a source of metabolites: glucose is a breakdown product of glycogen or starch, and is involved in gluconeogenesis; fatty acids can be made using acetyl-CoA from the pyruvate, which is produced in glycolysis; the amino acid, alanine, is synthesized through the glycolytic intermediate, pyruvate; and glucose and glucose-6-phosphate are intermediates in the conversion of other sugars, which, in turn, are involved in nucleotide synthesis.
Most importantly, in the TCA cycle, for instance, pyruvate produced by glycolysis is converted into acetyl-CoA and CO2 within the mitochondria. The acetyl-CoA then enters the TCA cycle (also known as the citric acid or Krebs cycle) where it is fully oxidized to CO2 and H2O, and produces more energy than glycolysis.
Tumor cells prefer the aerobic glycolysis pathway
Discovery of the Warburg effect
In the 1920s, while researching the mode of function of respiratory enzymes, a German scientist, Otto Heinrich Warburg,7 found that tumor cells need more energy than normal cells. In his opinion, the different origins of energy result in the rapid speed of tumor growth. He named this difference between tumor and normal cells “aerobic glycolysis,” known also as the Warburg effect.8 He claimed that tumor cells predominantly produce energy by a high rate of glycolysis followed by lactic acid fermentation in the cytosol, rather than by a comparatively low rate of glycolysis followed by oxidation of pyruvate in mitochondria as in most normal cells, even under conditions of sufficient oxygen.9
More and more research has focused on the Warburg effect since the 1920s, but the exact mechanism of aerobic glycolysis remains unclear. It is obvious that anaerobic metabolism brings more benefits to tumor cells. Therefore, can we slow down the tumor growth when cutting the energy supply of tumor cells by leading them back to aerobic metabolism?10 To answer this question, research on the aerobic glycolysis pathway and functions of key enzymes becomes an important direction of cancer research.11 Drug design on this abnormal metabolic pathway of tumor cells will become a new means for cancer therapy.
Aerobic glycolysis process in tumor cells
The abnormal metabolic pathway between tumor cells and normal cells is shown in the Figure 2. In normal cells, glucose is converted to pyruvate by the glycolysis pathway, with two ATPs consuming and four ATPs producing.12 Pyruvate goes into the TCA cycle in mitochondria with 36 ATPs producing. So one glucose molecule produces 38 ATPs during complete oxidation. In tumor cells, the oxidation of pyruvate is replaced by producing lactate, catalyzed by lactate dehydrogenase (LDH), with no ATP production. One glucose molecule produces only two ATPs in tumor cells. Therefore, we usually use medium with high glucose to culture tumor cells, because they need more energy.
Figure 2.
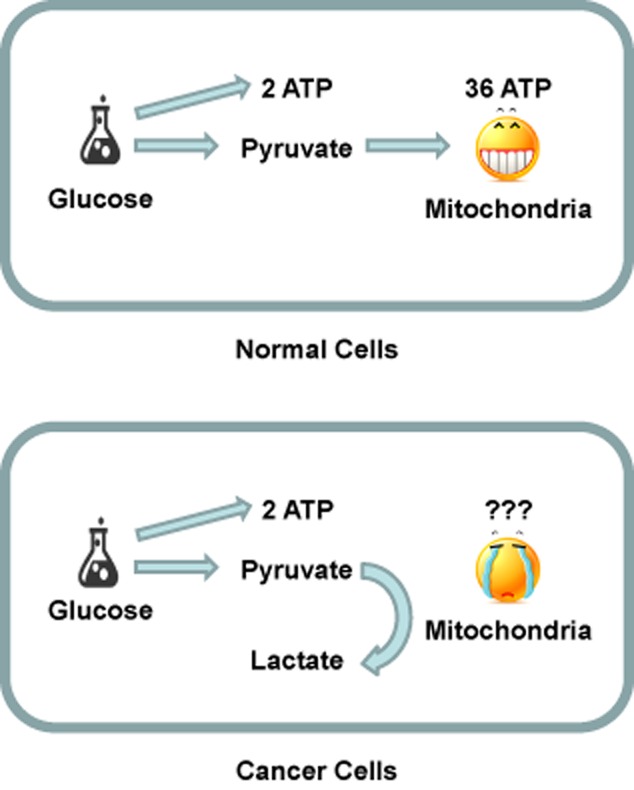
Schematic diagram of abnormal metabolic pathway in tumor cells.
Regulators of aerobic glycolysis
Many signaling pathways are involved in this aerobic glycolysis process. Both the adenosine monophosphate-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) pathways are either directly or indirectly involved in the glycolysis of tumor cells. Famous oncoproteins c-Myc and hypoxia inducible factor 1 (HIF-1) are involved in glycolysis by regulating key enzymes.13 The oncogenic transcription factor c-Myc is known to regulate glycolysis under normoxia conditions through the direct activation of LDH and other glycolytic genes. It can also activate genes associated with mitochondrial biogenesis. HIF-1 is a transcription factor that regulates gene expression in the event of decreased oxygen or hypoxia. It positively regulates GLUT1 and activates PDKs, which block the flow of pyruvate into the TCA cycle. The tumor suppressor p53 also shows an inhibitory effect on glycolysis. Because it directly stimulates oxidative phosphorylation, loss of p53 shifts metabolism from oxidative phosphorylation to glycolysis. Increasingly, research has focused on these regulators, which provide potential therapeutic targets for cancer treatment.
Targeting aerobic glycolysis for lung cancer diagnoses and treatments
Lung cancer is one of the most prevalent and deadly cancers in the world. Like many other kinds of solid tumors, lung cancer prefers aerobic glycolysis in the presence of oxygen for bioenergetic processes (the Warburg effect). This is very important in clinical diagnosis, as a high aerobic glycolysis rate in lung cancer can be used clinically to monitor responses by the uptake of 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG), a radioactive modified HK substrate, with positron emission tomography (PET).
With the exception of clinical diagnosis, the results from basic research have indicated that interrupting aerobic glycolysis with small molecules could inhibit many types of tumor cell survival. But whether the inhibition of glycolysis will be effective in the treatment of lung cancer is unclear. To answer this question, Sinthupibulyakit et al. attempted to kill non-small cell lung cancer (NSCLC) cells with a glycolytic inhibitor, 2-deoxy-D-glucose (2DG). They successfully found that 2DG exhibited a cytotoxic effect on NSCLC in a p53-dependent manner.14 Farah et al. found that oxalic acid, zinc acetate, honey, and D-glucose, which are all inhibitors of glycolysis, showed statistically significant differential negative effects on cell survival between A549 and normal lung fibroblast MRC-5 cell lines, presenting promising indicators for lung cancer therapeutic potential.15,16 Transketolases are involved in the metabolic switch between glycolysis and complete oxidation. In 2011, Kayser et al. showed that TKTL1, which encoded a transketolase-like enzyme, was overexpressed in NSCLC. This regulator of glycolysis represented a novel pharmacodiagnostic marker.17 Mohamed et al.'s 2014 review provided an overview of the current understanding of the role of altered metabolism in cancer, proposing future approaches to lung cancer treatment.18 Besides proliferation and survival, tumor metastasis is an important cause of the poor prognosis of lung cancer. Until now, no reports have suggested the regulation of lung cancer metastasis by altering aerobic glycolysis. This abnormal metabolic pathway should be targeted for lung cancer treatment.
Key enzymes of aerobic glycolysis in lung cancer
More and more reports show that the key enzymes in the glycolysis pathway are upregulated in different kinds of tumors, which aggravates the abnormal metabolic pathway in tumor cells. Altenberg and Greulich showed key enzymes of glycolysis and their expression status in 24 cancers.19 Pyruvate kinase was over-expressed in 21 of 24 cancers, which indicated its importance. Eight out of 10 enzymes of glycolysis were found to be upregulated in lung cancer, which indicated that the key enzymes of the aerobic glycolysis pathway play important roles in lung cancer development.
Hexokinase (HK) and lung cancer development
Introduction of HK
This first step of glycolysis is catalyzed by HK.20 In this step, glucose is activated for subsequent reactions by its phosphorylation at C-6 to yield glucose 6-phosphate, with ATP as the phosphoryl donor. The activity of HK is very important for the rate of glycolysis. HK also requires Mg2+ for its activity. This step not only activates glucose, but also restricts glucose in cells.
HK is a rate-limiting enzyme of glucose oxidation reaction. There are four important isoenzymes in mammalian organisms. HK I/A is found in all mammalian tissues, and is considered as a “housekeeping enzyme.” HK II/B constitutes the principal regulated isoform in many cell types and is increased in many cancers. HK III/C is substrate-inhibited by glucose at physiologic concentrations. Little is known about the regulatory characteristics of this isoform. Finally, mammalian HK IV is also referred to as glucokinase.
HK and lung cancer
In their 2006 review, Mathupala et al. reported that HK II was cancer's double-edged sword, for it acted as both facilitator and gatekeeper of malignancy when bound to mitochondria.21 Robey and Hay also discussed the functions of mitochondria-binding HK, and found that the glucose dependence of the anti-apoptotic effects of growth factors and protein kinase B (AKT) showed a strong correlation between AKT-regulated mitochondrial HK association, which changed the survival of tumor cells.22 Summarily, the interactions between HK and the mitochondrial are not only necessary to facilitate and maintain a highly glycolytic rate in malignant tumors, but are also crucial for tumor survival.
There is a great deal of research on dissociative HK in lung cancer. Early in 1999, Katabi et al., by a reporter gene assay, found that the activations of HK II are 61% and 40% in lung cancer cell lines NCI-H661 and NCI-H460, respectively; it was only 0.9% in primary normal human bronchial epithelial cells (NHBECs).23 Their work revealed a novel use of HK type II as a selective promoter in lung cancer gene therapy. During hypoxia, HIF-1α could promote activity of the glycolysis pathway by regulating HK I in A549 cells.24 Yohena et al. also found a significantly positive correlation between HIF-1α mRNA and HK II mRNA levels in NSCLC.25 In recent years, much research has focused on microRNAs. In 2012, Fang et al. reported that down-regulation of miR-143 expression was inversely associated with HK2 protein level in human lung cancer samples. Furthermore, miR-143 could inhibit glycolysis and cell proliferation by targeting HK II directly.26 By using HK2 conditional knockout mice, Patra et al. showed that HK II is required for tumor initiation and maintenance in Kras-driven lung cancer mouse models.27 Overall, HK, especially HK II, is important for lung cancer cell survival, cell proliferation, and tumor initiation. Inhibition of HK might be a potential therapeutic target for NSCLC.
Phosphofructokinase (PFK) and lung cancer development
Introduction of PFK
This second key step of glycolysis is catalyzed by PFK.20 It is the most important rate-limiting enzyme of glycolysis. PFK-1 catalyzes the conversion of fructose 6-phosphate and ATP to fructose 1, 6-bisphosphate, and adenosine diphosphate (ADP). It is an allosteric enzyme made of four subunits and controlled by several activators and inhibitors. There are three PFK genes in humans: PFKL in the liver, PFKM in the muscles, and PFKP in platelet.
PFK-2 and fructose 2, 6-bisphosphatase (FBPase2) are both activities of the bifunctional enzyme. When serine 32 is phosphorylated, the negative charge causes the conformation of the enzyme to favor the FBPase2 activity. When not phosphorylated, PFK-2's activity is favored.
PFK and lung cancer
PFK catalyzes the second key step of glycolysis, and is over-expressed in many kinds of cancers.19 PFK-2 has mainly been researched in association with lung cancer. In 2006, Telang et al. found that heterozygotic genomic deletion of the PFK-2 gene in ras transformed mouse lung fibroblasts had the following effects: suppressed of F2, 6BP production; suppressed glycolytic flux to lactate; and suppressed growth of soft agar colonies or tumors in athymic mice.28 This indicated the important function of the PFK-2 gene as a metabolic mediator of oncogenic ras. Minchenko et al. observed the overexpression of PFK mRNA in human lung cancer tissues and A549 cells when compared with corresponding normal tissues. Moreover, hypoxia highly induced PFK isozymes in lung carcinoma cells.29 To answer the question of whether an inhibitor of PFK-2 could suppress lung cancer proliferation, Clem et al. found a competitive inhibitor of PFK-2 called PFK15 (1-(4-pyridinyl)-3-(2-quinolinyl)-2-propen-1-one), which caused a rapid induction of apoptosis in transformed cells and suppressed the glucose uptake and growth of Lewis lung carcinomas in syngeneic mice.30 In 2012, Tang et al. focused their research on PFK-1 and identified miR-320a as a regulator of PFKM expression in lung cancer using microRNA profiling. They also found that manipulation of miR-320a levels alters PFKM and lactate levels in the expected directions in vitro and in vivo.31 Further research will focus on the migration and invasion functions of both PFK-1 and PFK-2, because they currently remain unclear.
PK and lung cancer development
Introduction of PK
This last step of glycolysis is catalyzed by PK (pyruvate kinase).20 PK is a very important key enzyme of glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to ADP, yielding one molecule of pyruvate and one molecule of ATP. This step is irreversible.
Pyruvate kinase is about 57 kDa. There are two isoenzymes: PKL and PKM. PKL exists in the liver, kidneys, and erythrocytes. PKMs can be divided into two types by alternative splicing, PKM1 and PKM2. PKM1 exists in heart muscle, skeletal muscle, and the brain; PKM2 is mainly expressed in the brain and liver. PKM2 is also the main isoform in embryos and tumors. Alternatively, PKR exists mainly in erythrocytes. Figure 3 shows the expression pattern of all pyruvate kinase isoenzymes.
Figure 3.
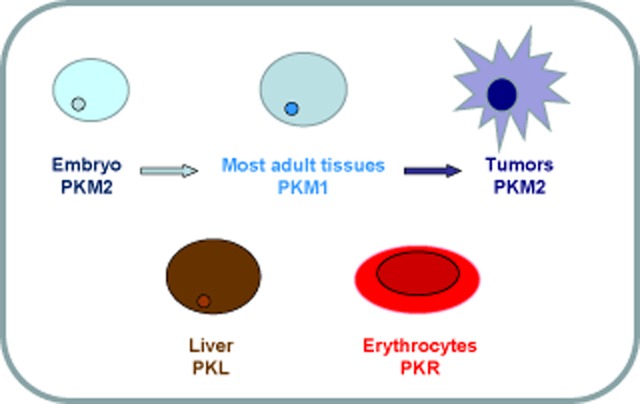
Schematic diagram of pyruvate kinase isoenzymes.
PKM mRNA is regulated by alternative splicing. Selective accessing exon 9 or exon10 decides the two isoenzymes of PKM1 and PKM2. The mechanism of alternative splicing of PKM1 and PKM2 was not discovered until 2010. David et al. found that c-Myc induced PKM2 expression through regulating heterogeneous nuclear ribonucleoprotein (hnRNP). They found that hnRNP1 and hnRNP2 were directly regulated by c-Myc, and both could reduce PKM1 mRNA while inducing PKM2 mRNA. Their outstanding work was published in Nature Letters.32 Figure 4 shows the schematic diagram of alternative splicing of PKM.
Figure 4.
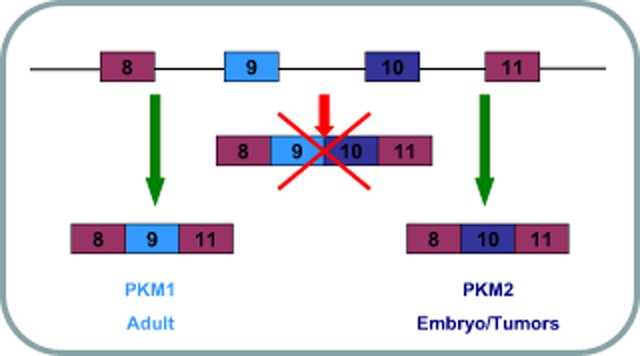
Schematic diagram of alternative splicing of pyruvate kinase (PKM).
PK and lung cancer
PK has been reported to be overexpressed in almost every kind of cancer, which shows its important function in glycolysis.19 Though tumor cells prefer expressing PKM2 by alternative splicing, controversy still exists on whether both normal and tumor cells express PKM2. In 2005, Mazurek et al. reported that proliferating cells and tumor cells, in particular, both expressed PKM2, but with different forms. For example, in normal lung proliferating cells, the tetrameric form of PKM2 is a highly active form used to produce pyruvate; however, in lung cancer cells, the dimeric form is always predominant. Though the low active dimeric forms inhibited the glycolysis process, the phosphometabolites above pyruvate kinase, such as PEP, accumulated and were then available as precursors for synthetic processes, such as nucleic acid, amino acid, and phospholipid synthesis, which improved tumor malignancy.33 Because of this theory, Parnell et al. used small-molecule PKM2 activators to enhance the affinity of PKM2 and PEP. They found that these activators influenced the growth of lung cancer cells in vitro and in vivo.34
Conversely, Christofk et al. reported that lung and breast cancers and cervical carcinoma cells were shown to only express PKM2. Using short hairpin RNA to knockdown PKM2 and replacing it with PKM1, they found a reversal of the Warburg effect, judged by reduced lactate production and increased oxygen consumption. They also discovered that it correlated with a reduced ability to form tumors in nude mice xenografts. They demonstrated that PKM2 expression was necessary and important for aerobic glycolysis.35 Guo et al. also used RNA interfering technology to knockdown PKM2, resulting in significantly inhibited tumor growth when combined with cisplatin in A549 lung cancer xenograft models.36 Until now, no reports have shown that PKM functions in lung cancer migration and invasion. However, conceivably, as a very important key enzyme of glycolysis, PKM might play roles in all aspects of tumor development, and could be an efficient target for lung cancer therapy.
Lactate dehydrogenase (LDH) and lung cancer development
Introduction of LDH
LDH catalyzes the interconversion of pyruvate and lactate with concomitant interconversion of nicotinamide adenine dinucleotide (NADH) and NAD+, when oxygen is absent or in short supply.20 At high concentrations of lactate, the enzyme exhibits feedback inhibition and the rate of conversion of pyruvate to lactate is decreased.
LDH is about 140 kDa. It consists of two subunits: H (heart, coded by LDHB) and M (muscle, coded by LDHA). They combine to form five enzyme isoforms: LDH-1 (H4, in the heart); LDH-2 (H3M1, in the reticuloendothelial system); LDH-3 (H2M2, in the lungs); LDH-4 (H1M3, in the kidneys); and LDH-5 (M4, in the liver and striated muscle).
LDH and lung cancer
The LDHA gene is directly regulated by the famous oncoprotein c-Myc. Its overexpression is observed in Rat1a fibroblasts, and in lymphoblastoid, Burkitt lymphoma, and lung cancer cells, which enhance the production of lactate and soft agar clonogenicity.37 HIF directly regulates the transcription of the LDH-5 (LDHA) gene under hypoxia in NSCLC, which is the main reason why LDH-5 (LDHA) is overexpressed in lung cancer. Together with the HIF pathway, LDH-5 (LDHA) has become an important basis for prognosis.38
Koukourakis et al. also found that LDH-5 (LDHA) was expressed preferentially in tumor cells, including lung cancer, while LDH-1 (LDHB) was consistently expressed in all living cells.39 In contrast, Chen et al. found that levels of LDHB were specifically elevated in NSCLC sera compared with non-lung cancer and benign lung disease, as well as in healthy individuals, and progressively increased with clinical stage.40 This finding could offer novel biomarkers related to lung cancer migration and invasion. Kayser et al. also found that LDH5 (LDHA) was over-expressed in NSCLC. Furthermore, they reported that LDH5 (LDHA) correlated positively with the prognostic marker transketolase like 1 protein (TKTL1).41 These results confirmed a close link between the two metabolic enzymes functioning in glucose metabolism and lung cancer prognosis. Using a specific small molecule LDHA inhibitor, Xie et al. reported that LDHA was essential for cancer-initiating cell survival and proliferation, which could be a potential therapeutic target for NSCLC.42
Conclusion
Tumor cells show different metabolism pathways to normal cells. They prefer anaerobic metabolism even under sufficient oxygen conditions, that is, the Warburg Effect.7 This aerobic glycolysis pathway is an important reason for tumor proliferation and migration.6 Each step of glycolysis is catalyzed by specific enzymes, which are upregulated in different kinds of tumors. The three key enzymes are hexokinase, phosphofructokinase and pyruvate kinase. Together with lactate dehydrogenase, these four key enzymes improve tumor proliferation and migration, dependent or independent of glycolysis. Although no evidence has been found on regulation of NSCLC metastasis by these key enzymes, lung cancer cell survival is indeed regulated by aerobic glycolysis and its key enzymes. The anaerobic metabolism of tumor cells provides new means and potential therapeutic targets for tumors, especially lung cancer.
Acknowledgments
This study was supported by grants from the State Key Development Program of Basic Research of China (No. 2010CB529405, to Qinghua Zhou), the National High Technology Research and Development Program of China (No. 2012AA02A502, to Qinghua zhou), the National Natural Science Foundation of China (No.81302002, to Xuebing Li), the Key Project of Science and Technology of Sichuan Province (No. 06SG005-002-2, to Qinghua Zhou) and the Tianjin Natural Science Foundation (No. 14JCQNJC12300, to Xuebing Li).
Disclosure
No authors report any conflict of interest.
References
- Zhou QH, Fan YG, Wu N, et al. Demonstration program of population-based lung cancer screening in China: rationale and study design. Thorac Cancer. 2014;5:197–203. doi: 10.1111/1759-7714.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WQ, Zheng RS, Zhang SW, Zou XN, Zhao P, He J. Lung cancer incidence and mortality in China. 2009. Thorac Cancer. 2013;4:102–108. doi: 10.1111/1759-7714.12025. [DOI] [PubMed] [Google Scholar]
- Chen WQ, Zhang SW, Zou XN. Evaluation on the incidence, mortality and tendency of lung cancer in China. Thorac Cancer. 2010;1:35–40. doi: 10.1111/j.1759-7714.2010.00011.x. [DOI] [PubMed] [Google Scholar]
- Fan Y, Yao YB, Li L, et al. nm23-H1 gene driven by hTERT promoter induces inhibition of invasive phenotype and metastasis of lung cancer xenograft in mice. Thorac Cancer. 2013;4:41–52. doi: 10.1111/j.1759-7714.2012.00140.x. [DOI] [PubMed] [Google Scholar]
- Zwerschke W, Mazurek S, Stöckl P, Hütter E, Eigenbrodt E, Jansen-Dürr P. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem J. 2003;376:403–411. doi: 10.1042/BJ20030816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto Heinrich Warburg [Cited 12 Aug 2014.] Available from URL: http://en.wikipedia.org/wiki/Otto_Heinrich_Warburg.
- Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Pan JG, Mak TW. Metabolic targeting as an anticancer strategy: dawn of a new era? Sci STKE. 2007;2007:pe14. doi: 10.1126/stke.3812007pe14. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- Nutritional Oncology Cancer Cell Metabolism [Cited 12 Aug 2014.] Available from URL: http://nutritionaloncology.org/cancerCellMetabolism.html.
- Cell Signaling Technology Cellular Metabolism Resources [Cited 12 Aug 2014.] Available from URL: http://www.cellsignal.com/pathways/glucose-metabolism.jsp.
- Sinthupibulyakit C, Ittarat W, St Clair WH, St Clair DK. p53 Protects lung cancer cells against metabolic stress. Int J Oncol. 2010;37:1575–1581. doi: 10.3892/ijo_00000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah IO, Lewis VL, Ayensu WK, Cameron JA. Therapeutic implications of the warburg effect: role of oxalates and acetates on the differential survival of mrc-5 and a549 cell lines. Biomed Sci Instrum. 2012;48:119–125. [PubMed] [Google Scholar]
- Farah IO, Lewis VL, Ayensu WK, Cameron JA. Therapeutic implications of the warburg effect assessing the survival of MRC5 and a549 cell lines upon exposure to honey and d glucose – biomed 2013. Biomed Sci Instrum. 2013;49:101–108. [PubMed] [Google Scholar]
- Kayser G, Sienel W, Kubitz B, et al. Poor outcome in primary non-small cell lung cancers is predicted by transketolase TKTL1 expression. Pathology. 2011;43:719–724. doi: 10.1097/PAT.0b013e32834c352b. [DOI] [PubMed] [Google Scholar]
- Mohamed A, Deng X, Khuri FR, Owonikoko TK. Altered glutamine metabolism and therapeutic opportunities for lung cancer. Clin Lung Cancer. 2014;15:7–15. doi: 10.1016/j.cllc.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry, Fourth Edition. New York: W. H. Freeman; 2004. [Google Scholar]
- Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- Katabi MM, Chan HL, Karp SE, Batist G. Hexokinase type II: a novel tumor-specific promoter for gene-targeted therapy differentially expressed and regulated in human cancer cells. Hum Gene Ther. 1999;10:155–164. doi: 10.1089/10430349950018959. [DOI] [PubMed] [Google Scholar]
- Luo F, Liu X, Yan N, et al. Hypoxia-inducible transcription factor-1alpha promotes hypoxia-induced A549 apoptosis via a mechanism that involves the glycolysis pathway. BMC Cancer. 2006;6:26. doi: 10.1186/1471-2407-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohena T, Yoshino I, Takenaka T, et al. Upregulation of hypoxia-inducible factor-1alpha mRNA and its clinical significance in non-small cell lung cancer. J Thorac Oncol. 2009;4:284–290. doi: 10.1097/JTO.0b013e31819852d5. [DOI] [PubMed] [Google Scholar]
- Fang R, Xiao T, Fang Z, et al. MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting hexokinase 2 gene. J Biol Chem. 2012;287:23227–23235. doi: 10.1074/jbc.M112.373084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra KC, Wang Q, Bhaskar PT, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telang S, Yalcin A, Clem AL, et al. Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene. 2006;25:7225–7234. doi: 10.1038/sj.onc.1209709. [DOI] [PubMed] [Google Scholar]
- Minchenko OH, Ogura T, Opentanova IL, et al. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene family overexpression in human lung tumor. Ukr Biokhim Zh. 2005;77:46–50. [PubMed] [Google Scholar]
- Clem BF, O'Neal J, Tapolsky G, et al. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther. 2013;12:1461–1470. doi: 10.1158/1535-7163.MCT-13-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Lee M, Sharpe O, et al. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. FASEB J. 2012;26:4710–4721. doi: 10.1096/fj.11-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Parnell KM, Foulks JM, Nix RN, et al. Pharmacologic activation of PKM2 slows lung tumor xenograft growth. Mol Cancer Ther. 2013;12:1453–1460. doi: 10.1158/1535-7163.MCT-13-0026. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Guo W, Zhang Y, Chen T, et al. Efficacy of RNAi targeting of pyruvate kinase M2 combined with cisplatin in a lung cancer model. J Cancer Res Clin Oncol. 2011;137:65–72. doi: 10.1007/s00432-010-0860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H, Dolde C, Lewis BC, et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E Tumour and Angiogenesis Research Group. Lactate dehydrogenase isoenzymes 1 and 5: differential expression by neoplastic and stromal cells in non-small cell lung cancer and other epithelial malignant tumors. Tumour Biol. 2003;24:199–202. doi: 10.1159/000074430. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang H, Xu A, et al. Elevation of serum l-lactate dehydrogenase B correlated with the clinical stage of lung cancer. Lung Cancer. 2006;54:95–102. doi: 10.1016/j.lungcan.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Kayser G, Kassem A, Sienel W, et al. Lactate-dehydrogenase 5 is overexpressed in non-small cell lung cancer and correlates with the expression of the transketolase-like protein 1. Diagn Pathol. 2010;5:22. doi: 10.1186/1746-1596-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Hanai JI, Ren JG, et al. Targeting lactate dehydrogenase-A inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


