Abstract
Background
The optimal chemotherapy route for non-small cell lung cancers involving the phrenic nerve and diaphragm is unclear. The pharmacokinetic properties of paclitaxel following intravenous (IV) or intrapleural (IP) administration were analyzed in the plasma, lung, and diaphragm in a rat model. The purpose of this study was to determine whether IP injection increased paclitaxel concentration in the diaphragm.
Methods
Paclitaxel was administered by IV or IP to male Sprague-Dawley rats. The concentration of drug in the plasma, lung, and diaphragm was determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The pharmacokinetic parameters area under the curve (AUC), mean residence time (MRT), peak plasma concentration (Cmax), and half-life (t1/2) were analyzed.
Results
Paclitaxel concentration in the plasma, lung, and diaphragm decreased quickly following IV administration. However, after IP injection, paclitaxel reached a high concentration in the plasma, lung, and diaphragm that declined gradually. Significant differences in all parameters, except Cmax in the lung, were observed between the two routes of administration (all P < 0.05). Plasma exposure to paclitaxel IP was 41.1% of that observed after IV in the first 24 hours (P < 0.05). IP also significantly increased exposure of paclitaxel in comparison with IV administration to 267.3% and 905.7% of IV administration in the lung and diaphragm, respectively (P < 0.05).
Conclusion
These results suggest that IP administration may reduce systemic distribution of paclitaxel and increase the concentration in the lung and diaphragm. This could increase therapeutic efficacy by increasing the available drug and reduce systemic toxicity.
Keywords: Diaphragm, intrapleural administration, lung, paclitaxel, plasma
Introduction
Effective cancer chemotherapeutic agents are of vital importance in the treatment of thoracic cancers including non-small cell lung cancer (NSCLC). NSCLC is the most common form of lung cancer.1 Most of the patients that present with NSCLC are ineligible for surgery because of local invasive tumors or distant metastases, leaving chemotherapy as the only treatment option.1 Patients that present with advanced NSCLC have a low survival rate over five years.2 Metastases involving the diaphragm reduce the five-year survival rate to only 33%.3 This is well below the survival rate of patients with NSCLC that involves only the pleura (54.8%).3 Standard chemotherapeutics are administered by intravenous (IV) injection. However, IV administration can have an increased risk of toxicity.4 Furthermore, it has been reported that systemically administered IV chemotherapy may be less effective than other routes of delivery for the presence of the pleural-blood barrier.4 Therefore, more effective chemotherapeutic regimens and routes of administration with low toxicity are still needed to effectively manage these patients.
One alternative to IV administration is the intrapleural (IP) route. Patients with malignant pleural effusion are usually treated by IP infusion, either alone or in combination with systemic chemotherapy. Drug administration into the pleural cavity is considered to be safe, effective, and associated with few adverse effects.5 Following IP administration, drug concentrations attain significantly higher levels in the lung tissue than after IV administration.4 This route likely enhances tumor cell death by increasing the local concentration of chemotherapeutic agents and direct contact with tumors. However, increasing the IV dose of paclitaxel can lead to limiting toxicity in many patients.6 The IP route may provide an alternative by increasing the tissue concentration of paclitaxel at lower doses. Finally, chemical pleurisy and pleural adhesions induced by stimulating the pleura are beneficial to control the generation of pleural effusion, without increased adverse effects.7 However, these observations were made during clinical experience and have not been confirmed by animal models.8,9 Recent clinical studies of paclitaxel formulations administering intrapleurally have focused on assessing the drug concentration in the plasma or pleural fluid.10 However, little is known about whether chemotherapeutic agents can achieve their optimal therapeutic levels in the diaphragm during IP chemotherapy.
The anticancer drug paclitaxel (marketed as Taxol) is a taxane diterpene amide that is widely used with good therapeutic effects against various kinds of cancers, such as ovarian, breast, NSCLC, and esophageal cancers.11 The response rate to paclitaxel in lung tumors, however, is significantly lower, averaging only 30 to 40%.12 Paclitaxel is a naturally derived anti-cancer drug thought to inhibit tumor growth by binding to tubulin.1,12 This blocks mitosis by promoting polymerization of microtubules and simultaneously inhibiting de-polymerization.12 Paclitaxel is a high molecular weight drug with very limited aqueous solubility, which prevents easy absorption into the blood vessels.13 In patients with pleural tumors or lung cancers with pleural metastasis, paclitaxel can be sustained in the cavity for a period of 48 hours after IP injection.5 Moreover, the anti-cancer efficacy of paclitaxel is positively correlated with its concentration,14 suggesting that higher local concentrations are desirable.
In the present study, a rat model was used to compare the pharmacokinetics of paclitaxel in the plasma, lung, and diaphragm tissue after IV or IP injection. The objective was to determine whether IP administration was able to increase the local concentration of paclitaxel in the diaphragm and reduce plasma exposure. This could potentially increase contact with tumors, penetration into tumor sites, and the local concentration of paclitaxel. This study provides direct experimental evidence to identify whether the IP route is a more optimal therapeutic route of paclitaxel administration in the treatment of thoracic cancers, compared with the IV route.
Materials and methods
Chemicals and reagents
The paclitaxel reference standard (99% purity) was obtained from Knowshine Pharmachemicals Inc. (Shanghai, China, Lot No. LX-P-902-0904006). Docetaxel used as the internal standard (IS) was purchased from Sigma (St. Louis, USA). High performance liquid chromatography (HPLC) grade methanol and acetonitrile were obtained from Fisher Chemicals (Fair Lawn, NJ, USA). Deionized water was prepared in our lab from a purification system (ELGA, London, UK) and analytical grade formic acid was purchased from J.T. Baker (Phillipsburg NJ, USA, Lot No. A20471).
Animals
Adult male Sprague-Dawley (SD) rats (n = 120; body weight 180–220 g) aged 10–12 weeks raised specific pathogen free (SPF) were purchased from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). The rats were raised in an SPF room held at 22–25°C and relative humidity of 65–68% with an alternating 12-hour light/dark cycle. The animals were allowed free access to food and water and given three days for acclimatization before the start of the experiment. All procedures and animal experiments were approved by the Animal Ethical Committee of First Affiliated Hospital, General Hospital of PLA and conducted in accordance with all state regulations.
Treatment protocol and sampling
One hundred and twenty rats were randomly divided into two equal groups (n = 60). Rats received 3 mg/kg paclitaxel15 either intravenously in the tail vein (group I) or in the pleural cavity (group II) at the same dose. In group II, after anesthetization with ether, a small (1–2 mm) incision in the right thoracic wall of the rats was opened and the drug was injected into the pleural cavity using a smoothly polished blunt syringe, at a depth of 1 cm. The rats regained consciousness within approximately five minutes. Approximately 0.5 mL of blood was collected from the retinal venous plexus into heparinized tubes at 0, 15, 30, 60, 120, 240, 360, 480, 720, and 1440 minutes after administration under anesthetization with ether. Six rats from each group were sacrificed for each time point. Plasma was isolated by centrifugation at 4°C for 10 minutes at 3000 rpm from whole blood within four hours of collection and stored at −20°C. In addition, approximately 100 mg of diaphragm and lung tissue was removed and washed with normal saline to remove the residual plasma and connective tissues. Finally, the tissues samples were dried on filter paper, weighed, and stored at −20°C until analysis.
Sample preparation
The tissue samples were homogenized using a high-speed homogenizer (Tissuelyser II, Germany) in deionized water at a ratio of 1:5 (w/v); 100 μL of methanol and 200 μL of methanol containing 500 ng/mL of IS were added to a 100 μL aliquot of rat tissue homogenate. The samples were mixed for one minute and centrifuged at 3500 rpm for 10 minutes; 100 μL of the supernatant was removed and an aliquot of 5 μL was injected into the liquid chromatography-tandem mass spectrometry (LC-MS/MS system).
Liquid chromatography
The samples were analyzed on an Agilent 6420 triple quadrupole mass spectrometer (Agilent Technologies) using an Agilent C18 column (50 × 2.1 mm, particle size 3.5 μm). The mobile phase consisted of water: acetonitrile: 0.1% formic acid (35:65:0.1, v/v/v) delivered at a flow rate of 0.3 mL/minute. The column temperature was maintained at 23°C. The data were collected and analyzed using the Agilent MassHunter Quantitative Analysis software.
Mass spectrometry
The mass spectrometer was run in positive electrospray ionization (ESI), with the electrospray voltage set to 4000 V and gas pressure and temperature set at 20 psi and 350°C, respectively. Mass spectrum was obtained in selective reaction monitoring (SRM) mode by quantifying the [M + Na]+ adduct ion with ion transition of m/z 876.3→593.3, 308.1 for paclitaxel and m/z 830.5→549.3, 304.4 for IS, respectively. The collision energy was 26 V for both the paclitaxel and IS. A selected ion monitoring (SIR) mode was employed for the quantification: m/z 876.3 for paclitaxel and 549.3 and 304.4 for IS.
Pharmacokinetic analysis
The data were analyzed using the Drug and Statistics (DAS) 2.0 pharmacokinetic program (Center for Drug Clinical Research, Shanghai University of Traditional Chinese Medicine, China). Parameters including the peak plasma concentration (Cmax), area under the curve (AUC), terminal elimination half-life (t1/2), and mean residence time (MRT) were obtained.
Statistical analysis
All statistical analyses were performed using the statistical software SAS version 8.1 (SAS Institute Inc., Cary, NC). All data were expressed as mean ± standard deviation (SD). An independent-samples t-test was used to compare the means between groups. P < 0.05 was considered to be statistically significant.
Results
Concentration-time curve following intravenous (IV) or intrapleural (IP) injection
The concentration of paclitaxel varied with the route of administration in each of the plasma and tissues examined. As shown in Figures 3, after IV administration, the concentration of paclitaxel in the plasma, lung, and diaphragm decreased quickly and the pharmacokinetic profiles fit a two- compartment model. However, after IP injection, paclitaxel reached a peak concentration in the plasma, lung, and diaphragm that declined gradually. This kinetics profile was better fit to a three-compartment model. Compared with IV administration, IP injection of paclitaxel resulted in a lower clearance (CL) and significantly prolonged MRT (P < 0.05) (Table 1). In addition, following IP injection, paclitaxel attained a lower plasma concentration with a sustained release effect (Fig 1). The peak plasma concentration was only 3.5% of the corresponding concentration of IV injected paclitaxel. In comparison, the exposure of paclitaxel within 24 hours after IP plasma concentration was 41.1% of that observed in rats receiving paclitaxel IV administration (P < 0.05) (Table 1). Together, these data demonstrate that IP administration reduces both the systemic exposure of paclitaxel and plasma concentration, compared to IV administration. These results suggest that IP injection might decrease the systemic toxicity of paclitaxel.
Figure 3.
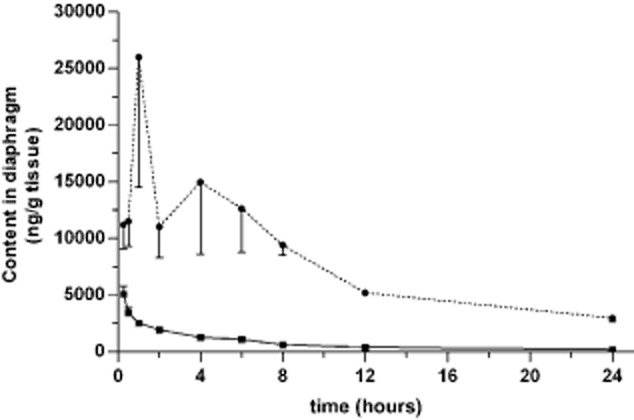
Concentration of paclitaxel in the diaphragm over time. Paclitaxel (3 mg/kg) was administered by either intravenous IV (solid line) or intrapleural (IP) (dotted line) injection. Approximately 100 mg of tissue from the diaphragm was harvested from six rats in each group at the time points indicated. The concentration of paclitaxel was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The mean ± standard deviation (SD) is shown for each time point.  , IV;
, IV;  , IP.
, IP.
Table 1.
Pharmakokinetic parameters of paclitaxel
| AUC0-t (μg·h/L or g·h/g) | AUC0-∞ (μg·h/L or g·h/g) | MRT0-t (h) | Cmax (ng/g or μg/L) | t1/2 (h) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IV | IP | IV | IP | IV | IP | IV | IP | IV | IP | |
| Plasma | 1211.4 ± 189.8 | 498.4 ± 27.4* | 1275.2 ± 192.0 | 637.6 ± 66.2* | 3.70 ± 0.58 | 9.63 ± 0.50* | 975.7 ± 462.7 | 34.5 ± 4.5* | 7.12 ± 2.05 | 10.20 ± 2.25 * |
| Lung | 34856.8 ± 741.5 | 93164.2 ± 2154.5* | 36458.3 ± 780.7 | 135467.9 ± 437.0* | 5.59 ± 0.09 | 9.58 ± 0.29* | 8956.5 ± 277.0 | 7017.9 ± 2289.0 | 5.75 ± 0.36 | 14.08 ± 1.67 * |
| Diaphragm | 18920.5 ± 1244.2 | 171358.4. ± 16789.2* | 19760.6 ± 1313.5 | 175129.9 ± 17202.1* | 5.48 ± 0.10 | 6.59 ± 0.23* | 5108.4 ± 696.3 | 27814.4 ± 9720.1* | 5.68 ± 0.53 | 4.97 ± 1.7 |
The data are shown as mean ± standard deviation (SD), n = 6.
P < 0.05 versus IV. AUC, area under the curve; Cmax, peak concentration; h, hours; IV, intravenous; IP intrapleural; MRT, mean residence time; t1/2, terminal elimination half-life.
Figure 1.
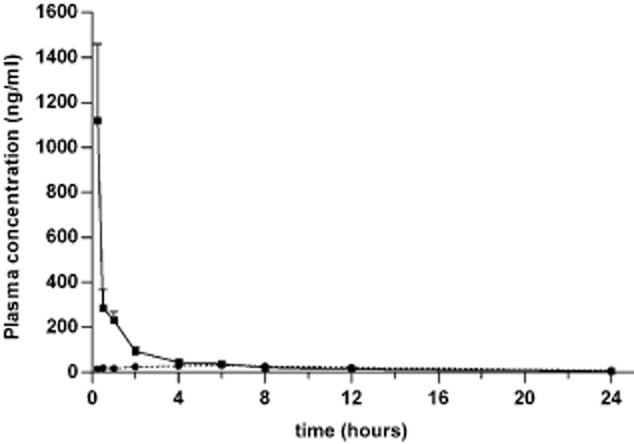
Concentration of paclitaxel in the plasma over time. Paclitaxel (3 mg/kg) was administered by either intravenous (IV) (solid line) or intrapleural (IP) (dotted line) injection. Plasma samples were harvested from six rats in each group at the time points indicated. The concentration of paclitaxel was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The mean ± standard deviation (SD) is shown for each time point.  , IV;
, IV;  , IP.
, IP.
Pharmacokinetics of paclitaxel following IV or IP injection
Similarly, the pharmacokinetics of paclitaxel in the lung (Fig 2) and diaphragm (Fig 3) also showed significant differences between the two routes of administration. IP administration resulted in a lower CL value and prolonged MRT (P < 0.05) in the lung and diaphragm (Table 1). IP also significantly increased exposure of paclitaxel in comparison with IV administration to 267.3% and 905.7% of IV administration in the lung and diaphragm, respectively (P < 0.05) (Table 1). This suggests that IP administration might induce an increased distribution and prolonged efficacy of paclitaxel in the lung and diaphragm compared to IV injection.
Figure 2.
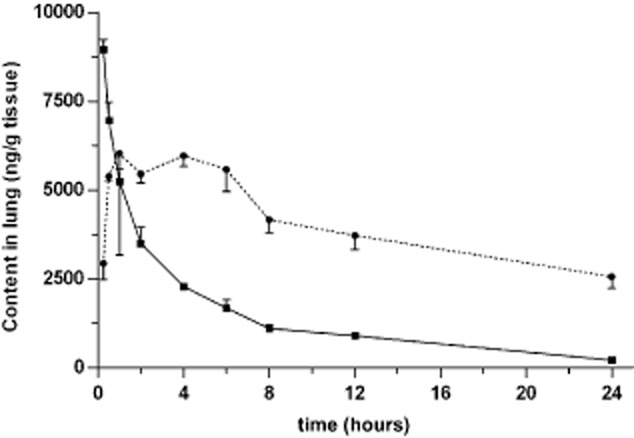
Concentration of paclitaxel in the lung over time. Paclitaxel (3 mg/kg) was administered by either intravenous (IV) (solid line) or intrapleural (IP) (dotted line) injection. Approximately 100 mg of lung tissue was harvested from six rats in each group at the time points indicated. The concentration of paclitaxel was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The mean ± standard deviation (SD) is shown for each time point.  , IV;
, IV;  , IP.
, IP.
Discussion
These results address whether IP administration of paclitaxel can increase the duration of paclitaxel exposure in the diaphragm and reduce overall plasma exposure. This combination might be potentially beneficial to patients that have metastatic NSCLC.
Docetaxel was chosen as the internal standard for quantitation.16,17 It has been validated for sensitivity, accuracy, recovery, and stability and was successfully applied to the pharmacokinetics of paclitaxel. Moreover, the LC-MS/MS method required fewer biological samples and matrix effects, such as apparent ion suppression, were not detected in this method.
In the present study, paclitaxel was administered at a dose of 3 mg/kg body weight to Sprague Dawley rats. This dose was chosen for the following reasons: (i) the dose was determined to be less than or equal to the clinical paclitaxel dose in patients; (ii) to avoid toxicity in the animals from receiving a single bolus of the drug rather than a slow drip; and (iii) to avoid tissue toxicity and maintain a consistent dose, for both the IV and IP routes of administration. The human equivalent dose to the 3 mg/kg administered here was calculated using the body surface area ratio of mouse/human.18,19 For a patient weighing 70 kg with a body surface area between 1.7 m2 and 1.8 m2, the human equivalent dose of paclitaxel was calculated to be 33.6 mg total. This is equivalent to a dose between 18.7 mg/m2 and 19.8 mg/m2. In a clinical setting, paclitaxel is usually given by IV drip over three hours at a dose between 135 and 175 mg/m2. The administered paclitaxel dose was clearly much lower than the human equivalent therapeutic dose. However, this does not detract from the finding that the drug concentration was significantly higher in the diaphragm than the plasma. Previous pharmacokinetic studies have administered intravenous paclitaxel doses ranging from 5 mg/kg–15 mg/kg to rats.15,20 However, the safest dose of paclitaxel for intravenous use in rats is reported to be less than 5 mg/kg.15 Therefore, to minimize potential toxicity for the animals, the dose of 3 mg/kg was established.
The current study showed that the route of paclitaxel administration, either IV or IP, does impact the pharmacokinetic parameters of the drug. The IP route resulted in a significantly lower plasma concentration of paclitaxel than the IV route and a sustained release. This suggests that the IP route might be less toxic systemically than the IV route. Also, exposure to paclitaxel was significantly increased following the IP route of injection. This suggests that after IP administration, the distribution of paclitaxel and the concentrations in the lung and diaphragm were well maintained for a long time. In fact, one clinical study has shown IP paclitaxel was able to clear tumors from four out of 15 patients evaluated.15 Interestingly, the results obtained by IP injection in our study appear similar to the increased local concentration and retention time obtained by administering liposomal paclitaxel formulations, which are safer and more effective in patients than traditional paclitaxel.1 These results support the use of IP injection clinically to administer paclitaxel.
The concentration of paclitaxel in the lung and diaphragm exhibited a bimodal distribution. The first peak that appears after absorption is likely a result of the strong liposolubility of paclitaxel, which appears colorless or light yellow. Once in the tissue, because of the long MRT pleural cavity, paclitaxel might be dissolved and rapidly distributed into the fat-soluble tissues in the pleural cavity, like fat and pleura. This could result in the reabsorption of paclitaxel and the second peak concentration, thereby prolonging the action time in the pleural cavity. The bimodal pharmacokinetics observed in this study may explain why paclitaxel is able to persist at high levels in lung and diaphragm tissue.
Conclusion
In conclusion, we provide experimental evidence that the IP route of paclitaxel administration provides higher lung and diaphragm concentrations of the drug than the IV route. These results indicated that IP paclitaxel may be a more optimal treatment to thoracic cancers with high efficacy and low general toxicity in clinics. However, further studies are still required to determine the optimal practice for using paclitaxel to treat thoracic tumors in a clinical setting.
Acknowledgments
We thank our laboratory colleagues for their help and Dr. Lingdi Yan for editing the manuscript.
Disclosure
No authors report any conflict of interest.
References
- Hu L, Liang G, Yuliang W, Bingjing Z, Xiangdong Z, Rufu X. Assessing the effectiveness and safety of liposomal paclitaxel in combination with cisplatin as first-line chemotherapy for patients with advanced NSCLC with regional lymph-node metastasis: study protocol for a randomized controlled trial (PLC-GC trial) Trials. 2013;14:45–51. doi: 10.1186/1745-6215-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawabata N, Asamura H, Goya T, et al. Japanese Lung Cancer Registry Study: first prospective enrollment of a large number of surgical and nonsurgical cases in 2002. J Thorac Oncol. 2010;5:1369–1375. doi: 10.1097/JTO.0b013e3181e452b9. [DOI] [PubMed] [Google Scholar]
- Sakakura N, Mori S, Ishiguro F, et al. Subcategorization of resectable non-small cell lung cancer involving neighboring structures. Ann Thorac Surg. 2008;86:1076–1083. doi: 10.1016/j.athoracsur.2008.06.034. [DOI] [PubMed] [Google Scholar]
- Liu J, Meisner D, Kwong E, Wu XY, Johnston MR. Translymphatic chemotherapy by intrapleural placement of gelatin sponge containing biodegradable Paclitaxel colloids controls lymphatic metastasis in lung cancer. Cancer Res. 2009;69:1174–1181. doi: 10.1158/0008-5472.CAN-08-1753. [DOI] [PubMed] [Google Scholar]
- Perng RP, Wu MF, Lin SY, Chen YM, Lin JY, Whang-Peng J. A phase I feasibility and pharmacokinetic study of intrapleural paclitaxel in patients with malignant pleural effusions. Anticancer Drugs. 1997;8:565–573. doi: 10.1097/00001813-199707000-00003. [DOI] [PubMed] [Google Scholar]
- Alberola V, Cortesi E, Juan O. Weekly paclitaxel in the treatment of metastatic and/or recurrent non-small cell lung cancer. Crit Rev Oncol Hematol. 2002;44(Suppl):S31–41. doi: 10.1016/s1040-8428(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki Y, Shibata K, Yoshioka M. Intrapleural perfusion hyperthermo-chemotherapy for malignant pleural dissemination and effusion. Ann Thorac Surg. 1995;59:127–131. doi: 10.1016/0003-4975(94)00614-D. [DOI] [PubMed] [Google Scholar]
- Zhou JX. Practical Medical Oncology. Beijing: People's Medical Publishing House; 2003. p. 75. [Google Scholar]
- Wu YL. The Theory and Practice to Multi-Disciplinary Comprehensive Treatment of Lung Cancer. Beijing: People's Medical Publishing House; 2000. p. 277. [Google Scholar]
- Wang X, Zheng H, Zhu Z, Wei Y, Chen L. Clinical pharmacokinetics of paclitaxel liposome with a new route of administration in human based on the analysis with ultra performance liquid chromatography. J Pharm Sci. 2010;99:4746–4752. doi: 10.1002/jps.22169. [DOI] [PubMed] [Google Scholar]
- Dang C, D'Andrea G, Lake D, et al. Prolonged dose-dense epirubicin and cyclophosphamide followed by paclitaxel in breast cancer is feasible. Clin Breast Cancer. 2008;8:418–424. doi: 10.3816/CBC.2008.n.050. [DOI] [PubMed] [Google Scholar]
- Shimomura M, Yaoi T, Itoh K, et al. Drug resistance to paclitaxel is not only associated with ABCB1 mRNA expression but also with drug accumulation in intracellular compartments in human lung cancer cell lines. Int J Oncol. 2012;40:995–1004. doi: 10.3892/ijo.2011.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer I, Cao H, Persson J, et al. Coadministration of epithelial junction opener JO-1 improves the efficacy and safety of chemotherapeutic drugs. Clin Cancer Res. 2012;18:3340–3351. doi: 10.1158/1078-0432.CCR-11-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bree E, Rosing H, Filis D, et al. Cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy with paclitaxel: a clinical and pharmacokinetic study. Ann Surg Oncol. 2008;15:1183–1192. doi: 10.1245/s10434-007-9792-y. [DOI] [PubMed] [Google Scholar]
- Shord SS, Camp JR. Intravenous administration of paclitaxel in Sprague-Dawley rats: what is a safe dose? Biopharm Drug Dispos. 2006;27:191–196. doi: 10.1002/bdd.503. [DOI] [PubMed] [Google Scholar]
- Suno M, Ono T, Iida S, et al. Improved high-performance liquid chromatographic detection of paclitaxel in patient's plasma using solid-phase extraction, and semi-micro-bore C18 separation and UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860:141–144. doi: 10.1016/j.jchromb.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Andersen A, Warren DJ, Brunsvig PF, Aamdal S, Kristensen GB, Olsen H. High sensitivity assays for docetaxel and paclitaxel in plasma using solid-phase extraction and high- performance liquid chromatography with UV detection. BMC Clin Pharmacol. 2006;6:2–7. doi: 10.1186/1472-6904-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation for animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Pinkel D. The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res. 1958;18:853–856. [PubMed] [Google Scholar]
- Marchettini P, Stuart OA, Mohamed F, Yoo D, Sugarbaker PH. Docetaxel: pharmacokinetics and tissue levels after intraperitoneal and intravenous administration in a rat model. Cancer Chemother Pharmacol. 2002;49:499–503. doi: 10.1007/s00280-002-0439-1. [DOI] [PubMed] [Google Scholar]


