Abstract
Background
Studies have revealed mesenchymal cells tend to directionally migrate toward tumor cells and inhibit tumor growth. However, there have been rare reports about adipose-derived mesenchymal stem cells (AMSCs), which achieved stable expression of interleukin (IL)-12 to inhibit lung adenocarcinoma cell migration and invasion. We aimed to achieve stable expression of IL-12 in AMSCs through transgenic technology and utilize the paracrine effect of IL-12 to inhibit lung adenocarcinoma cell migration and invasion.
Methods
Adipose-derived AMSCs were transduced with lentivirus encoding IL-12. IL-12/AMSCs and lung adenocarcinoma A549 cells were co-cultured using a cylinder column to assess cellular attraction, and expression of Ki67 was detected. Dual-chamber transwell experiments were used to assess migration and invasiveness of A549 cells exposed to conditioned media from IL-12/AMSCs.
Results
When A549 cells were co-cultured with lentivirus vectors (LV)-IL-12-green fluorescent protein (GFP)/AMSCs, the intercellular distance was great (346.44 ± 41.07 μm vs. 201.58 ± 27.96 μm vs. 191.45 ± 24.07 μm) (F = 25.414, P < 0.05); the Ki67-positive rate was low (59.13 ± 17.21% vs. 92.31 ± 6.11% vs. 94.25 ± 5.27%) (F = 21.426, P < 0.05). When the lower Transwell chamber contained culture medium from LV-IL-12-GFP/AMSCs, the percentage of the invasive A549 cells was low (31.55 ± 6.21% vs. 70.65 ± 10.46% vs. 68.65 ± 9.50%) (F = 27.494, P < 0.05). The percentages of colonized A549 cells that invaded the culture media of LV-IL-12-GFP/AMSCs were low (4.46 ± 1.21 vs. 10.11 ± 2.07 vs. 9.48 ± 1.4) (F = 23.219, P < 0.05).
Conclusions
AMSCs could target lung carcinoma and mediate stable expression of IL-12, to play a role in tumor treatment.
Keywords: Gene therapy, interleukin-12, lung adenocarcinoma, mesenchymal stem cell
Introduction
Pulmonary adenocarcinoma is a common histological form of lung cancer that contains certain distinct malignant tissue architectural, cytological, or molecular features, including gland and/or duct formation and/or production of significant amounts of mucus.1,2 The incidence of pulmonary adenocarcinoma has been increasing in China and many developed and developing nations in the past few decades.3,4 Its high death rate is a result of the metastasis of late cancer cells.5 Distant metastasis of lung adenocarcinoma frequently occurs in post-surgery lung cancer patients.6 Therefore, high efficiency targeting of scattered metastatic tumor cells at distant sites is critical for improving the outcome of lung cancer therapy.
Recent studies have revealed that mesenchymal cells tend to directionally migrate toward tumor cells and can stably express genes targeted to inhibit tumor growth.7–9 Huang et al. found that bone marrow-derived stem cells (BMSCs) have the ability to migrate into these tumors and even track infiltrating tumor cells, making them promising cellular vehicles for delivering therapeutic agents to glioma cells. Huang et al. evaluated the anti-glioma effect of suicide gene therapy using BMSCs expressing the herpes simplex virus thymidine kinase (HSV–TK) combined with overexpression of connexin 43 (Cx43). They concluded that Cx43 combined with HSV–TK/GCV gene therapy using BMSCs as vehicles was highly effective in a rat glioma model and, therefore, hold great potential as a novel approach for the gene therapy of human malignant gliomas.10
Adipose-derived mesenchymal stem cells (AMSCs) are multipotent and can differentiate into various cell types, including osteocytes, adipocytes, neural cells, and vascular endothelial cells,11–13 as well as BMSCs. In order to assess whether AMSCs could target lung carcinoma and mediate stable expression of IL-12 to play a role in tumor treatment, the present study utilizes AMSCs as an expression vector of interleukin (IL)-12 for the first time, with IL-12 acting in a paracrine fashion to inhibit tumor growth and migration. Furthermore, these therapeutic effects also occurred over distance. This study offers an important theoretical basis and experimental evidence for the future targeted therapy of metastatic lung cancer cells, which could hold promise for patients with advanced lung cancer.
Materials and methods
Reagents and cells
Human AMSCs were kindly provided by the Research Center at Tianjin 5th Central Hospital and the lung adenocarcinoma cell line A549 was purchased from Shanghai Bogu Biotech Co., Ltd. Lentivirus vectors (LV-IL-12 and LV-green fluorescent protein [GFP]) were constructed by Shanghai Jikai Gene Chemistry Technology Co., Ltd. Other reagents: fetal bovine serum (FBS; Hangzhou Sijiqing), Dulbecco's Modified Eagle's Medium (DMEM) and trypsin (Invitrogen, USA), Transwell plates (Corning, USA); IL-12 enzyme linked immunosorbent assay (ELISA) kit (R&D, USA), anti-human Ki67 antibody (Chemicon, USA), and Texas Red conjugated secondary antibody.
Cell culture
Lung adenocarcinoma cell A549 and AMSCs were both cultured in 10 cm plastic culture dishes using DMEM containing 10% FBS, 100 U/ml penicillin, and 0.1 mg/mL streptomycin. The culture conditions were 37°C, 5% CO2 and saturated humidity. Medium for A549 was changed daily and the cells were passaged every three days. Medium for AMSCs was changed daily and the cells were passaged every four to five days.
Lentiviral transduction of adipose-derived mesenchymal stem cells (AMSCs)
Fourth-passage AMSCs were seeded at 105 cells/mL in culture dishes. Cells were infected on the following day with a virus suspension at a multiplicity of infection of 30. The treatment group was treated with LV-IL-12-GFP virus solution, while the control was treated with an equal amount of LV-GFP virus. GFP expression was observed at 48, 72, and 96 hours following transduction to evaluate transduction efficiency.
Examination of interleukin (IL)-12 protein expression by enzyme linked immunosorbent assay (ELISA)
Supernatants from the first, third, and fifth passages of AMSCs post-transduction were obtained and their IL-12 concentrations were determined by double antibody sandwich ELISA according to the manufacturer's instructions.
Measurements of intercellular distance
A cylindrical column was placed at the bottom of the cell culture plate and AMSCs (or IL-12/AMSCs) were seeded on the outside of the column, while A549 cells were seeded on the inside. The column was removed at 24 hours and the intercellular distances were measured at 48 hours using an inverted microscope. A total of 10 random high-magnitude fields were observed for each condition.
Examination of cells with immunofluorescence
A549 cells were cultured on coverslips within a chamber before being transferred to the cylindrical columns as above. Cells were fixed in formalin at room temperature for 15 minutes, blocked with 5% goat serum solution, and incubated with a 1:1000 dilution of anti-Ki67 antibody at 4°C overnight. Texas Red conjugated secondary antibody was used at a 1:200 dilution and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Positive expression rate (PER) was calculated as: PER = (number of Ki67 positive cells/total number of cells) × 100.
The invasion capacity of the tumor cells determined by Transwell system
Gel matrix (60 μL) was added to the polycarbonate membrane in the upper Transwell chamber, and allowed to solidify at 37°C for 30 minutes. A549 cells were then seeded in the upper Transwell chamber and regular culture medium or that from AMSCs, LV-GFP/AMSCs or LV-IL-12-GFP/AMSCs was added to the lower Transwell chamber. The upper Transwell chambers were removed at 48 hours and the cells that did not penetrate the membrane were wiped off using a wet cotton swab. Coverslips were stained with hematoxylin and then sealed. The number of cells that penetrated the membrane was counted using an inverted microscope.
Determination of the colonizing capability of the tumor cells using a Transwell system
The bottom well of the upper Transwell chamber was pre-coated with 0.35% low-melting temperature agarose. An A549 cell suspension was added to the surface of the solidified agarose. Regular culture medium or that from AMSCs, LV-GFP/AMSCs or LV-IL-12-GFP/AMSCs was then added to the lower Transwell chamber. Three weeks later, the number of colonized cell clusters was calculated according to the average of number of cells within 10 microscopic fields.
Statistical analysis
SPSS 13.0 software (SPSS Inc.) was used for the statistical analysis. The quantitative data were expressed as mean ±standard deviation. Analysis of variance (ANOVA) was performed to compare between-group differences. An χ2 test was performed to compare the count data. A p value <.05 was considered statistically significant.
Results
ELISA results
Cellular expression of GFP was apparent 24 hours following viral transduction, with over 70% of cells showing fluorescence at 72 hours. ELISA results demonstrated that the culture medium of LV-GFP transduced cells contained low levels of IL-12, while sustainably high levels of IL-12 were present in the first, third, and fifth generations of AMSCs infected with LV-IL-12-GFP (P < 0.01; Fig. 1).
Figure 1.
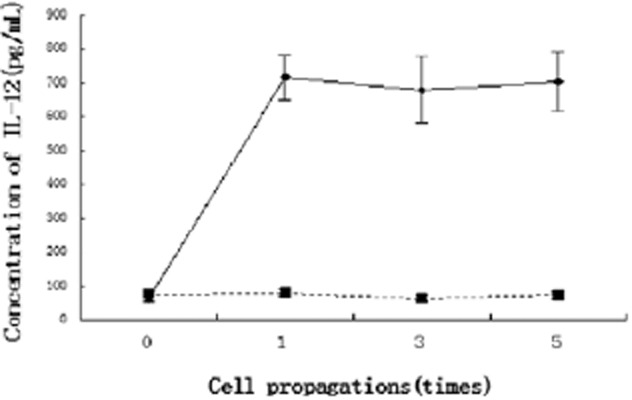
Interleukin (IL)-12 concentration determined by enzyme linked immunosorbent assay (ELISA) (** P < 0.01 compared with cells that were infected with lentivirus vectors-green fluorescent protein [LV-GFP]).  , LV-IL-12-GFP;
, LV-IL-12-GFP;  , LV-GFP.
, LV-GFP.
Comparison of the intercellular distances
When untreated AMSCs were co-cultured with A549 cells, the average distance between the two populations was 191.45 ± 24.07 μm. When AMSCs infected with LV-GFP or LV-IL-12-GFP virus were co-cultured with A549 cells, the average distance between them was (201.58 ± 27.96 μm and 346.44 ± 41.07 μm, respectively)(Fig. 2). This difference in separation distance with LV-IL-12-GFP-infected cells was statistically significant when compared with the previous two conditions (F = 25.414, P < 0.05).
Figure 2.
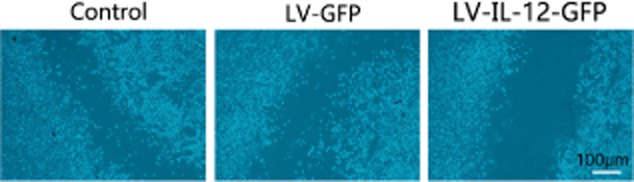
A549 cells exhibit reduced migration capacity in co-culture with with lentivirus vectors-green fluorescent protein (LV-GFP)- interleukin (IL)-12 adipose-derived mesenchymal stem cells (AMSCs).
Determination of cellular proliferative activity
Immunofluorescence revealed that A549 cells co-cultured with AMSCs alone expressed high levels of Ki67 (94.25 ± 5.27% (Fig. 3). For A549 cells co-cultured with AMSCs infected with LV-GFP virus, the Ki67-positive rate was 92.31 ± 6.11% and not statistically different from the control (P > 0.05). However, when A549 cells were co-cultured with AMSCs infected with LV-IL-12-GFP virus, the Ki67-positive rate was 59.13 ± 17.21% and statistically significant (F = 21.426, P < 0.05).
Figure 3.
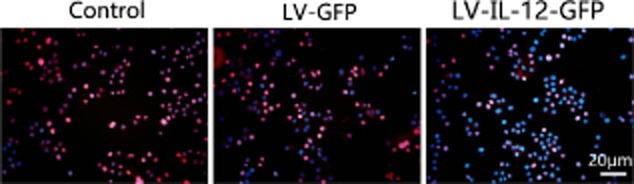
A549 cells co-cultured with lentivirus vectors-green fluorescent protein (LV-GFP)-interleukin (IL)-12 adipose-derived mesenchymal stem cells (AMSCs) exhibit reduced proliferative capacity.
Determination of the invasive capability of the cells
When the lower Transwell chamber contained standard complete medium, the percentage of invasive A549 cells was 11.34 ± 3.72%. When the lower Transwell chamber contained culture medium from AMSCs alone, the percentage of invasive A549 cells was 70.65 ± 10.46%; and culture medium from LV-GFP/AMSCs induced a similar invasion rate of 68.65 ± 9.50%. However, when the lower Transwell chamber contained culture medium from LV-IL-12-GFP/AMSCs, the percentage of the invasive A549 cells was 31.55 ± 6.21%. There were statistically significant differences among all pairwise comparisons (F = 27.494, P < 0.05; Fig. 4), except between the culture medium of AMSCs and LV-GFP/AMSCs.
Figure 4.
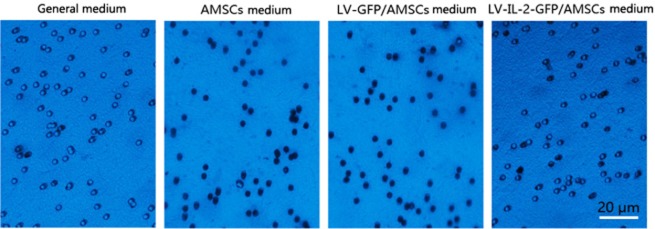
Conditioned media from lentivirus vectors-green fluorescent protein (LV-GFP)- interleukin (IL)-12 adipose-derived mesenchymal stem cells (AMSCs impairs invasiveness of A549 cells.
Results of colonizing experiments
The percentage of colonized A549 cells that invaded the normal complete medium was 7.15 ± 2.71. The percentages of colonized A549 cells that invaded the culture media of AMSCs, LV-GFP/AMSCs, and LV-IL-12-GFP/AMSCs were 10.11 ± 2.07, 9.48 ± 1.49, and 4.46 ± 1.21, respectively. There were statistical differences among all pairwise comparisons (F = 23.219, P < 0.05; Fig. 5), except between the culture medium of AMSCs and of LV-GFP/AMSCs.
Figure 5.
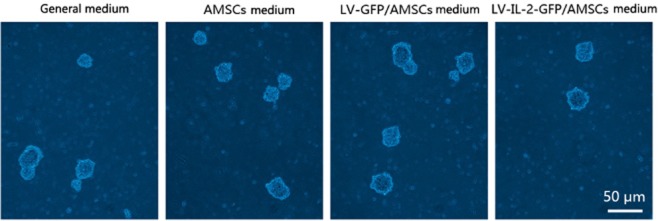
A549 cells demonstrate reduced colonizing capability following exposure to medium from lentivirus vectors-green fluorescent protein (LV-GFP)- interleukin (IL)-12 adipose-derived mesenchymal stem cells (AMSCs).
Discussion
Lung adenocarcinoma is a non-small cell lung cancer with a high mortality rate.14 Recent improvements to treatment protocols involving surgery in combination with radiation therapy or chemotherapy have facilitated increased survival time and quality of life for patients.15 However, effective treatments that inhibit tumor recurrence and lower the mortality rate among late-stage lung cancer patients with distant metastases are still lacking.16 Therefore, whether these distant metastatic lung cancer cells can be effectively targeted and killed is critical for the inhibition of the tumor recurrence.
Lung adenocarcinoma arises from the malignant transformation of lung glandular cells.17 Early molecular events including gene mutation, amplification, and overexpression act to accelerate the tumor cell cycle, inhibit apoptosis, maintain telomerase activity, and promote the tumor angiogenesis and invasion.14 IL-12 is a heterodimeric molecule that is produced by monocytes/macrophages and B cells. It has multiple physiological and pathological functions and can be used in the treatment of infection, autoimmune disease, and tumors.18IL-12 has strong inhibitory effects on tumor growth in the treatment of lung adenocarcinoma; however, its usage in a clinical setting is severely limited because of a short half-life and significant toxic side effects with systemic application.19
Recent studies have shown that mesenchymal stems cells derived from adipose tissue, bone marrow, and the umbilical cord exhibit strong chemotaxis toward tumors and could act as stable vector of a target gene for tumor treatment.8 Here, for the first time, we have used human AMSCs stably expressing IL-12 to target metastatic lung adenocarcinoma cells.
We first obtained uniform AMSCs from adipose tissue and determined the corresponding cellular protein markers. We found that these cells expressed high levels of the mesenchymal stem cell (MSC) markers CD29 and CD105, and extremely low levels of the hematopoietic stem cell markers CD34 and CD45. We confirmed that these cells were AMSCs by multiple marker identification. These cells were then transduced with IL-12 through lentivirus infection. ELISA results indicated that the IL-12 gene could be stably expressed in AMSCs for five consecutive generations without any fall in expression level. We used a cylinder column and dual-chamber Transwell system to separately culture ASMCs and the lung adenocarcinoma cell line A549 to determine the inhibitory effects of IL-12 on A549 cells. The migratory capacity of A549 cells was increased when they were exposed to AMSCs or LV-GFP/AMSCs in a Transwell system. However, this increased migratory capability of tumor cells was impaired when LV-IL-12-GFP/AMSCs were seeded in the lower Transwell chamber. These results showed that AMSCs alone increased the migratory capability of the lung adenocarcinoma cells, while IL-12 inhibited this effect. Conditioned media from LV-IL-12-GFP/AMSC cell culture also inhibited migration of A549 cells and promoted their apoptosis when compared with control group and LV-GFP AMSCs group.
Conclusion
This study confirms that AMSCs and lung adenocarcinoma cells interact with and are attracted to each other. AMSCs could, therefore, be used as a stable expression vector for immunotherapeutic drugs, such as IL-12, to facilitate inhibition of metastatic lung carcinoma. The results provide a firm basis for future in vivo studies.
Disclosure
No authors report any conflict of interest.
References
- Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- Morales-Oyarvide V, Mino-Kenudson M. High-grade lung adenocarcinomas with micropapillary and/or solid patterns: a review. Curr Opin Pulm Med. 2014;20:317–323. doi: 10.1097/MCP.0000000000000070. [DOI] [PubMed] [Google Scholar]
- Sakashita S, Sakashita M, Sound Tsao M. Genes and pathology of non-small cell lung carcinoma. Semin Oncol. 2014;41:28–39. doi: 10.1053/j.seminoncol.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Yousem SA. Pulmonary intestinal-type adenocarcinoma does not show enteric differentiation by immunohistochemical study. Mod Pathol. 2005;18:816–821. doi: 10.1038/modpathol.3800358. [DOI] [PubMed] [Google Scholar]
- Dacic S, Nikiforova MN. Present and future molecular testing of lung carcinoma. Adv Anat Pathol. 2014;21:94–99. doi: 10.1097/PAP.0000000000000012. [DOI] [PubMed] [Google Scholar]
- Bittner N, Ostoros G, Géczi L. New treatment options for lung adenocarcinoma–in view of molecular background. Pathol Oncol Res. 2014;20:11–25. doi: 10.1007/s12253-013-9719-9. [DOI] [PubMed] [Google Scholar]
- Belmar-Lopez C, Mendoza G, Oberg D, et al. Tissue-derived mesenchymal stromal cells used as vehicles for anti-tumor therapy exert different in vivo effects on migration capacity and tumor growth. BMC Med. 2013;11:139. doi: 10.1186/1741-7015-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorgieva D, Zaidman N, Bosnakovski D. Mesenchymal stem cells for anti-cancer drug delivery. Recent Pat Anticancer Drug Discov. 2013;8:310–318. doi: 10.2174/15748928113089990040. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan A, Torok-Storb B, Pillai MM. Primary marrow-derived stromal cells: isolation and manipulation. Methods Mol Biol. 2013;1035:75–101. doi: 10.1007/978-1-62703-508-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Liu XZ, Kang CS, Wang GX, Zhong Y, Pu PY. The anti-glioma effect of suicide gene therapy using BMSC expressing HSV/TK combined with overexpression of Cx43 in glioma cells. Cancer Gene Ther. 2010;17:192–202. doi: 10.1038/cgt.2009.64. [DOI] [PubMed] [Google Scholar]
- Sun LY, Pang CY, Li DK, et al. Antioxidants cause rapid expansion of human adipose-derived mesenchymal stem cells via CDK and CDK inhibitor regulation. J Biomed Sci. 2013;20:53–64. doi: 10.1186/1423-0127-20-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Guan FX, Li Y, Tang YJ, Yang F, Yang B. New methods for inducing the differentiation of amniotic-derived mesenchymal stem cells into motor neuron precursor cells. Tissue Cell. 2013;45:295–305. doi: 10.1016/j.tice.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Li Q, Qi LJ, Guo ZK, Li H, Zuo HB, Li NN. CD73+ adipose-derived mesenchymal stem cells possess higher potential to differentiate into cardiomyocytes in vitro. J Mol Histol. 2013;44:411–422. doi: 10.1007/s10735-013-9492-9. [DOI] [PubMed] [Google Scholar]
- Van Schil PE, Asamura H, Rusch VW, et al. Surgical implications of the new IASLC/ATS/ERS adenocarcinoma classification. Eur Respir J. 2012;39:478–486. doi: 10.1183/09031936.00027511. [DOI] [PubMed] [Google Scholar]
- Yatabe Y, Shibata N. Molecular diagnosis of lung cancer in association with treatment of choice. Rinsho Byori. 2012;60:786–795. (In Japanese.) [PubMed] [Google Scholar]
- Yang M, Shen H, Qiu C, et al. High expression of miR-21 and miR-155 predicts recurrence and unfavourable survival in non-small cell lung cancer. Eur J Cancer. 2013;49:604–615. doi: 10.1016/j.ejca.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Kerr KM. Classification of lung cancer: proposals for change? Arch Pathol Lab Med. 2012;136:1190–1193. doi: 10.5858/arpa.2012-0240-SA. [DOI] [PubMed] [Google Scholar]
- Kerkar SP, Muranski P, Kaiser A, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70:6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekaii-Saab TS, Roda JM, Guenterberg KD, et al. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol Cancer Ther. 2009;8:2983–2991. doi: 10.1158/1535-7163.MCT-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]


