Abstract
Background
Treatment strategies for brain metastasis from lung cancer have been making progress. The aim of this retrospective analysis was to investigate the post-recurrent prognostic factors in patients with brain metastasis after complete resection of non-small cell lung cancer (NSCLC).
Methods
We retrospectively reviewed the medical records of 40 patients found to have postoperative brain metastasis from NSCLC in our institution from 2002 to 2008. All patients had undergone radical pulmonary resection for the lung cancer. The impact of numerous variables on survival were assessed, including gender, age, carcinoembryonic antigen (CEA), tumor size, N status, histological type, number of brain metastases, tumor size of brain metastasis, presence of symptoms from the brain tumor(s), and use of perioperative chemotherapy.
Results
The median follow-up was 20.6 months (range, 3.4–66 months). The five-year survival rate from the diagnosis of brain recurrence was 22.5%. In univariate analysis, the favorable prognostic factors after brain recurrence included a normal range of CEA, no extracranial metastasis, no symptoms from the brain metastasis, brain metastasis (less than 2 cm), and radical treatment (craniotomy or stereotactic radiosurgery [SRS]). The multivariate Cox model identified that a small brain metastasis and radical treatment were independent favorable prognostic factors.
Conclusions
This study found that the implementation of radical therapy for metastatic brain tumor(s) when the tumor is still small contributed to an increase in patients' life expectancy.
Keywords: Brain metastasis, lung cancer, postoperative recurrence, prognostic factors
Introduction
The central nervous system (CNS) is a frequent site of metastasis of non-small cell lung cancer (NSCLC). Brain metastases occur in 30 to 50% of patients with NSCLC, and confer a worse prognosis and quality of life.1–3 About 50% of stage 3A and 3B NSCLC patients will develop brain metastasis during treatment for lung cancer.4 Some investigators have reported long-term survival after the resection of a solitary recurrence as brain metastasis.5–7 The treatment modalities used for metastatic brain tumors, such as stereotactic radiosurgery (SRS), have been improved in recent years.8 Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), such as erlotinib or gefitinib, have been reported to be an effective treatment for NSCLC patients with brain metastasis and activating mutations in the EGFR gene.9 Therefore, the role and indications for radical therapy for metastatic brain lesions have remained under discussion.
In this study, to elucidate the prognostic factors for long-term survival of brain metastasis from lung cancer, we reviewed the clinical records of cases treated at our institution.
Patients and methods
This was a retrospective single-center study. It included patients with postoperative brain metastasis as the initial relapse site from surgically resected lung cancer. We reviewed medical records and follow-up data from the Osaka Medical Center for Cancer and Cardiovascular Diseases. Between January 2002 and December 2008, 1091 patients underwent R0-resection and received complete follow-up. Of these patients, 40 cases (3.6%) with postoperative brain metastasis as initial relapse site were included in this study. The pathological stages of all patients were determined according to the 7th edition of the International Staging System.10 Standard operations, such as lobectomy or pneumonectomy with complete dissection of the hilar and mediastinal lymph nodes, were performed on all patients.
Follow-up data were obtained by outpatient visits and correspondence with the patients' primary physicians. Our follow-up procedures included physical examinations, chest roentgenography and blood tests, including tests for tumor markers, such as carcinoembryonic antigen (CEA). Chest computed tomography (CT) or 18-fluorodeoxyglucose (FDG) positron emission tomographic (PET) scans were generally performed every six months. In addition, brain CT or magnetic resonance imaging (MRI) was performed annually to detect any brain recurrence. Other metastatic workup also included a bone scan at time of diagnosis to evaluate the patients for bone metastasis.
Postoperative brain recurrence was diagnosed mainly by radiological examination. The histological diagnosis of brain metastasis from NSCLC was confirmed only when we performed craniotomy. In our institution, cranial nerve surgeons determined the operability criteria for brain metastasis. Our inclusion criteria for craniotomy were basically that the tumor location was surgically accessible and we could assure negative surgical margins. After 2004, our indications for SRS were that the tumor size was less than 3 cm, and the number of brain metastases was one to three. In this study, SRS and craniotomy are defined as radical treatments for brain metastasis. Whole brain radiation therapy (WBRT) was performed, not only to prevent recurrence after radical treatments, such as craniotomy or SRS, but also for palliative treatment when the patient could not receive radical treatment for the brain metastasis.
For the analysis of overall survival (OS), each patient's survival time was measured from the date of diagnosis of postoperative brain metastasis until the date of death or the most-recent date of follow-up for surviving patients. We evaluated the following factors: the clinicopathological findings at the time of treatment for the primary lung cancer (type of operation, p-stage, histology, use of adjuvant chemotherapy); and the clinical characteristics at the time of treatment for brain metastasis (gender, age, CEA at the time of brain recurrence, interval to brain metastasis after pulmonary resection, number of brain metastases, tumor size, use of radical therapy, presence of symptoms from the brain tumor, use of an EGFR-TKI, chemotherapy after the treatment for brain metastasis). In this study, we defined the size of the brain metastasis as the size of the biggest brain tumor in the patient if that patient had multiple metastatic brain tumors. On the other hand, if that patient had a single brain metastasis, the size of that tumor was considered to be the size of that metastatic tumor.
Univariate survival analyses were performed using the Kaplan-Meier method, and the differences among the groups were analyzed by the log-rank test. For multivariate analysis, a Cox's proportional hazards regression model was used to evaluate the variables that were significant predictors of survival after the diagnosis of brain recurrence. Univariate and multivariate analyses (SPSS V11.5, Chicago, Illinois) were used to identify the prognostic factors in our population. The Chi-square test was used to compare discrete data. A value of P < 0.05 was considered to be statistically significant.
Results
A summary of the 40 patients is shown in Table 1. The study population consisted of 25 men and 15 women. The median age at the time of diagnosis of the brain recurrence was 65 years. At the initial operation, 33 patients underwent lobectomy and seven patients received bilobectomy or pneumonectomy for the primary tumor. Of these patients, seven were classified to have pathological stage 1A, six were in stage 1B, 10 were 2A, nine were 2B, and eight patients were in stage 3A. The histopathological subtype was adenocarcinoma in 24 cases and others in 16 cases. Twenty of these patients received adjuvant chemotherapy after pulmonary resection.
Table 1.
The clinical and pathological characteristics of the 40 patients with postoperative brain metastasis from non-small cell lung cancer
| Gender (male/female) | 25/15 |
| Age (years) | 65.0 ± 8.9 (49–79) |
| Primary lesion | |
| Operation (Lobe/Bil-lobe or Pneumo) | 33/7 |
| Pathological stage (1a/1b/2a/2b/3a) | 7/6/10/9/8 |
| Histologic classification (Ad/Others ) | 24/16 |
| Adjuvant chemotherapy (Yes/No) | 20/20 |
| Brain lesion | |
| Interval to brain metastasis after surgery (months) | 10.2 ± 8.4 (2.1–32.3) |
| Number of brain meta (Single/Multiple) | 26/14 |
| Tumor size of brain metastasis (mm) | 21.5 ± 15.5 (5–70) |
| Radiotherapy† (Yes/No) | 14/26 |
| Radical treatment‡ (Yes/No) | 29/11 |
| Symptom before Tx (Yes/No) | 26/14 |
| CEA level at the time of recurrence (ng/ml) | 1.4–78.6 |
| Extracranial metastasis (Yes/No) | 17/23 |
| Chemotherapy after treatment for brain (Yes/No) | 12/28 |
| EGFR-TKI (Yes/No) | 7/33 |
SRS and/or WBRT. ‡2 SRS and/or Craniotomy. Ad, adenocarcinoma; CEA, carcinoembryonic antigen; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitors.
The interval to brain metastasis after surgery ranged from four to 32 months (median: 10 months). Twenty-six patients had solitary brain metastasis and 14 patients had multiple metastatic lesions. The CEA status at the time of recurrence ranged from 1.4 to 78.6 ng/mL. Twenty-six patients suffered from neurological symptoms caused by the metastatic brain tumor(s). Of these patients, 23 had only brain metastasis and 17 had brain metastasis with extracranial recurrence. Twenty-nine patients received radical therapy, such as SRS or craniotomy, and 11 patients received WBRT.
In this study, the follow-up period after the time of brain recurrence ranged from 3.4–66 months (median: 20.6 months). The five-year OS rate after the time of recurrence was 22.5%.
Table 2 summarizes the results of the univariate regression model analysis. In this analysis, the favorable prognostic factors after brain recurrence included: a normal range of CEA; no extracranial metastasis; no symptoms from the brain metastasis; small metastatic brain tumor; and radical treatment (craniotomy or SRS). The median OS of patients with a high CEA level was 13.3 months, while that of patients with a normal CEA level was 36.3 months. For the patients with extracranial metastasis, the median OS was 16.6 months, compared with 26.2 months for the patients without extracranial metastasis. The median survival of patients who received radical treatment was 27.0 months compared with 10.8 months for patients who did not receive radical treatment. For the patients with symptoms from the brain metastasis, the median OS was 17.2 months. Statistically significant differences were seen between the two groups in all of these parameters (P < 0.05).
Table 2.
Results of univariate analysis of the prognostic factors for overall survival from the date of postoperative brain recurrence
| Cases | P-value | Median Survival (months) | |
|---|---|---|---|
| Number of brain metastasis | |||
| Single/Multiple | 26/14 | 0.91 | 20.4/17.1 |
| EGFR-TKI | |||
| Yes/No | 6/34 | 0.90 | 26.1/18.7 |
| Interval to brain metastasis from surgery | |||
| >1 year/<1 year | 18/22 | 0.39 | 21.1/17.4 |
| Adjuvant chemotherapy | |||
| Yes/No | 20/20 | 0.08 | 26.2/14.2 |
| CEA level at the time of rec | |||
| <5 ng/mL/>5 ng/mL | 20/20 | 0.03 | 36.3/13.3 |
| Extracranial metastasis | |||
| Yes/No | 17/23 | 0.03 | 16.6/26.2 |
| Tumor size of brain metastasis | |||
| <2 cm/>2 cm | 20/20 | <0.01 | 43.6/13.3 |
| Radical treatment (Craniotomy or SRS) | |||
| Yes/No | 29/11 | <0.01 | 27.0/10.8 |
| Symptom from brain metastasis | |||
| Yes/No | 26/14 | <0.01 | 17.2/45.4 |
CEA, carcinoembryonic antigen; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitors; SRS, stereotactic radiosurgery.
These five variables showing differences with values of P < 0.05 in the univariate analysis were selected for subsequent multivariate analysis (Table 3). The multivariate analysis using Cox's proportional hazards model revealed that a small size of brain metastasis and radical therapy were independent favorable prognostic factors in this patient group (P = 0.04, P = 0.01). Figure 1 shows the Kaplan-Meier survival plot generated from curves stratified by these factors.
Table 3.
Results of multivariate analysis of the prognostic factors for overall survival from the date of postoperative brain recurrence
| Factor | Odds ratio (95% CI) | P-value |
|---|---|---|
| CEA level at the time of rec | ||
| <5 ng/mL/>5 ng/mL | 0.966 (0.376–2.292) | 0.940 |
| Extracranial metastasis | ||
| Yes/No | 1.605 (0.786–3.590) | 0.181 |
| Symptom from brain metastasis | ||
| Yes/No | 2.061 (0.719–5.909) | 0.178 |
| Tumor size of brain metastasis | ||
| <2 cm/>2 cm | 2.509 (1.023–6.151) | 0.041 |
| Radical treatment (Craniotomy or SRS) | ||
| Yes/No | 3.619 (1.514–8.652) | <0.01 |
CEA, carcinoembryonic antigen; CI, confidence interval; SRS, stereotactic radiosurgery.
Figure 1.
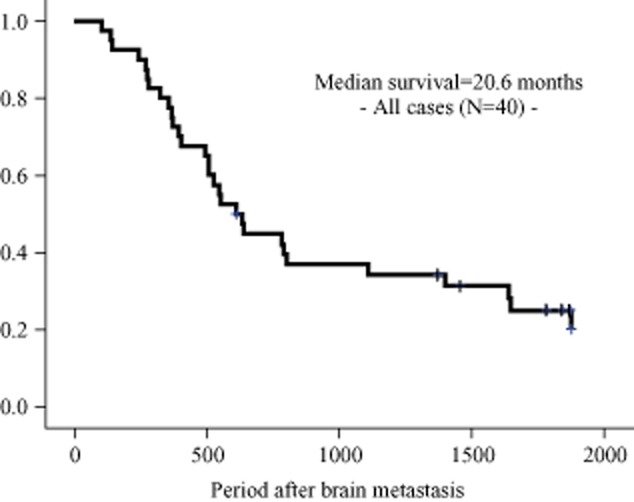
The Kaplan-Meier survival curves for the entire cohort.
Discussion
The majority of postoperative recurrences of NSCLC are distant metastasis.11,12 Brain metastases occur frequently as the initial relapse site during the follow-up period after pulmonary resection for NSCLC.11,13 For lung cancer, some investigators have reported acceptable survival after the resection of distant recurrent lesions, but others have shown data contradicting these conclusions. Abrahams et al. demonstrated a satisfactory outcome in patients with brain metastasis, with a median survival time of 18 months and a five-year survival rate of 28.9%.7 On the contrary, Saitoh et al. conducted 24 brain resections, and noted a five-year survival rate of only 8.3%.5 In this study, the median survival time after recurrence was 20.6 months, and the five-year OS rate after the time of recurrence was 22.5%.
The prognostic factors for survival after the treatment for brain metastasis have not been clarified. In this study, the results of the univariate analyses of the favorable prognostic factors after brain recurrence included normal range of CEA, no extracranial metastasis, no symptoms from the brain metastasis, small metastatic brain tumor, and radical treatment (craniotomy or SRS). The multivariate Cox model identified that a small size of brain metastasis and radical treatment were independent favorable prognostic factors.
Several authors have examined promising prognostic factors in patients with NSCLC who had postoperative brain metastasis. Sakamoto et al. reported that local therapy, such as SRS or craniotomy, is one of the significant prognostic factors for NSCLC patients with brain recurrence.14 In that study, the five-year survival rate after the brain recurrence in patients treated with local therapy was 31.9%, compared to 3.8% for patients without local therapy. In our study, the five-year survival rate of patients treated with local therapy was 31.0%, compared to 0% for patients without local therapy (P < 0.01). Based on these results, the patients who receive radical therapy as cerebral local control could be expected to have a longer survival.
To the best of our knowledge, there have been very few reports concerning the relationship between the size of brain metastasis and the prognosis after treatment of brain metastasis. In our study, the median survival of patients whose brain tumor was less than 2 cm was 43.6 months, compared with 13.3 months for patients whose brain tumor was larger than 2 cm. A statistically significant difference was seen between the two groups (P < 0.05). The reason for this difference may be that metastatic brain tumors larger than 2 cm are very difficult to control, even when using radical treatment. We found that most of the patients whose brain tumors were larger than 2 cm had intracranial recurrence after treatment for brain metastasis.
By univariate analysis, we also identified that a lack of symptoms from the brain metastasis was a favorable prognostic factor for survival. Aoyama et al. showed via multivariate analysis that a good Karnofsky Performance Status (KPS) was a favorable prognostic factor for NSCLC patients with postoperative brain recurrence.15 In that study, patients with a KPS score of 90 or lower had a 1.69-fold increased risk of death compared with patients with a KPS score of 100. In our report, the five-year survival rate after brain recurrence of patients without symptoms from the brain tumor(s) was 42.8%, compared to 11.5% for patients with symptoms (P < 0.01). This difference may be caused by the preservation of the KPS and may be related to the favorable outcome in the patients without symptoms from the brain tumor.
Sakamoto et al. reported that the presence of extracranial metastasis at the time of brain recurrence is one of the unfavorable prognostic factors.14 In our study, a lack of extracranial metastasis at the time of diagnosis for brain metastasis was also a favorable prognostic factor for survival, identified by univariate analysis. However, tumor size of brain metastasis and radical treatment had a greater impact on the patient's survival in our cohort. The patients who had these favorable factors were able to maintain their systemic functions, and as a result, these patients could receive adequate treatment against the extracranial metastasis, including systemic chemotherapy. Therefore, we think that radical treatment for brain tumor(s) might have priority if the patient has brain recurrence with extracranial metastasis.
To date, there have been few prospective studies comparing the survival time after brain metastasis between SRS and craniotomy. Therefore, the evidence based on the current literature is restricted to several retrospective studies, and the results are conflicting. For example, Bindal et al. reported that the OS time in patients undergoing resection + WBRT (16.4 months) was statistically longer than that in patients undergoing SRS + WBRT (7.5 months).16 In contrast, another study revealed a trend toward a longer OS in those receiving SRS + WBRT, but that result did not reach statistical significance.17 In our cohort, there was no statistically significant difference in the OS time after brain recurrence between the patients who underwent craniotomy and those who underwent SRS (median survival time: 20.4 vs. 26.7 months).
Several authors have recently reported the efficacy of EGFR-TKIs for brain metastasis in NSCLC patients harboring an activating EGFR mutation.18,19 Park et al. revealed that EGFR-TKIs provided high disease control rates, but did not have a significant impact on patient survival. In our cohort, we did not observe any significant difference in the survival time between the patients with and without EGFR mutations (data not shown). However, we did not perform a mutation analysis for all patients because we started the analysis in 2005. Furthermore, the number of patients harboring EGFR mutations was small compared with that of patients without mutations. These factors might have had an impact on the results of the present study. Further accumulation of cases analyzed for mutations will be necessary to perform an adequately powered study of the impact of EGFR mutations and EGFR-TKIs.
Conclusion
In this study, small tumor size of brain metastasis and radical treatment were important favorable predictors for survival in patients with postoperative brain recurrence. Intracranial recurrence tends to have a greater impact on the patient's systemic activity compared with other sites of recurrence. Therefore, to provide adequate intracranial disease control and to maintain the patient's activities of daily living, it is necessary to detect brain metastases while they are still small during the follow-up period, and patients should undergo radical therapy prior to the development of symptoms from the brain metastasis. To achieve these purposes, the patients' symptoms should be carefully observed, and periodic postoperative follow-up examinations should be performed to detect brain metastases before they become larger than 2 cm.
Disclosure
No authors report any conflict of interest.
References
- Sørensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474–1480. doi: 10.1200/JCO.1988.6.9.1474. [DOI] [PubMed] [Google Scholar]
- Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795–803. doi: 10.1016/s0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- Rodrigus P, de Brouwer P, Raaymakers E. Brain metastases and non-small cell lung cancer. Prognostic factors and correlation with survival after irradiation. Lung Cancer. 2001;32:129–136. doi: 10.1016/s0169-5002(00)00227-0. [DOI] [PubMed] [Google Scholar]
- Mamon HJ, Yeap BY, Jänne PA, et al. High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol. 2005;23:1530–1537. doi: 10.1200/JCO.2005.04.123. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Fujisawa T, Shiba M, et al. Prognostic factors in surgical treatment of solitary brain metastasis after resection of non-small-cell lung cancer. Lung Cancer. 1999;24:99–106. doi: 10.1016/s0169-5002(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Granone P, Margaritora S, D'Andrilli A, Cesario A, Kawamukai K, Meacci E. Non-small cell lung cancer with single brain metastasis: the role of surgical treatment. Eur J Cardiothorac Surg. 2001;20:361–366. doi: 10.1016/s1010-7940(01)00744-8. [DOI] [PubMed] [Google Scholar]
- Abrahams JM, Torchia M, Putt M, Kaiser LR, Judy KD. Risk factors affecting survival after brain metastases from non-small cell lung carcinoma: a follow-up study of 70 patients. J Neurosurg. 2001;95:595–600. doi: 10.3171/jns.2001.95.4.0595. [DOI] [PubMed] [Google Scholar]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- Porta R, Sánchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37:624–631. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- Yano T, Yokoyama H, Inoue T, et al. The first site of recurrence after complete resection in non-small-cell carcinoma of the lung. Comparison between pN0 disease and pN2 disease. J Thorac Cardiovasc Surg. 1994;108:680–683. [PubMed] [Google Scholar]
- Yano T, Yokoyama H, Inoue T, Asoh H, Tayama K, Ichinose Y. Surgical results and prognostic factors of pathologic N1 disease in non-small-cell carcinoma of the lung. Significance of N1 level: lobar or hilar nodes. J Thorac Cardiovasc Surg. 1994;107:1398–1402. [PubMed] [Google Scholar]
- Figlin RA, Piantadosi S, Feld R. Intracranial recurrence of carcinoma after complete surgical resection of stage I, II, and III non-small-cell lung cancer. N Engl J Med. 1988;318:1300–1305. doi: 10.1056/NEJM198805193182004. [DOI] [PubMed] [Google Scholar]
- Sakamoto J, Sonobe M, Kobayashi M, et al. Prognostic factors for patients in postoperative brain metastases from surgically resected non-small cell lung cancer. Int J Clin Oncol. 2014;19:50–56. doi: 10.1007/s10147-012-0503-8. [DOI] [PubMed] [Google Scholar]
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- Bindal AK, Bindal RK, Hess KR, et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84:748–754. doi: 10.3171/jns.1996.84.5.0748. [DOI] [PubMed] [Google Scholar]
- Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien) 2000;142:621–626. doi: 10.1007/s007010070104. [DOI] [PubMed] [Google Scholar]
- Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77:556–560. doi: 10.1016/j.lungcan.2012.05.092. [DOI] [PubMed] [Google Scholar]
- Bartolotti M, Franceschi E, Brandes AA. EGF receptor tyrosine kinase inhibitors in the treatment of brain metastases from non-small-cell lung cancer. Expert Rev Anticancer Ther. 2012;12:1429–1435. doi: 10.1586/era.12.121. [DOI] [PubMed] [Google Scholar]


