Abstract
Background
The standard of care for patients with small cell lung cancer (SCLC) is chemotherapy and radiotherapy, even for patients with limited disease. To define the role of surgical resection in patients with limited SCLC, we investigated the outcomes of patients diagnosed with limited-stage disease (LD) SCLC.
Methods
The records of 57 LD SCLC patients who underwent surgical resection from April 1974 to March 2012 were retrospectively analyzed.
Results
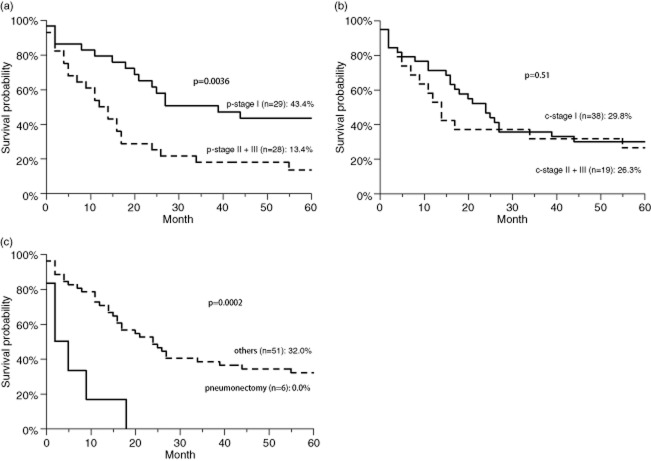
There were six women and 51 men, with a median age of 63.5 years. The overall five-year survival rate was 28.6% (median, 18.2 months). The p-stage II and III patients had a significantly worse survival than the p-stage I patients (13.4% vs. 43.4%, P = 0.0036). However, the c-stage was not found to correlate with survival. Patients who underwent pneumonectomy had a significantly worse outcome than those who underwent other surgical procedures (0.0% vs. 32.0%, P = 0.0002). In a multivariate Cox proportional hazards analysis, p-stage II or III (hazard ratio [HR] 3.040 P = 0.0017) and pneumonectomy (HR 6.177, P = 0.00159) were significant independent predictors of an adverse survival outcome.
Conclusions
Surgical treatment can be considered in SCLC patients with pathologically proven N0 status, although pneumonectomy should be avoided.
Keywords: Limited-stage disease, pneumonectomy, small cell lung cancer
Introduction
Small cell lung cancer (SCLC) is considered to be a systemic disease because it progresses rapidly. Patients with SCLC have a poor prognosis; less than 8% of patients with this type of lung cancer currently survive five years beyond the initial diagnosis.1 The standard of care for patients with SCLC is chemotherapy and radiotherapy, even for patients with limited disease. This recommendation is based on a 1973 study by the British Medical Research Council (MRC), which reported a better survival with radiotherapy compared with surgical intervention.2 This study was conducted in patients with central disease, and most patients did not undergo complete resection. In contrast, the current National Comprehensive Cancer Network (NCCN) guidelines recommend either lobectomy with mediastinal node sampling or dissection, or concurrent chemotherapy and radiotherapy for all patients who have clinical T1-2N0 SCLC with negative mediastinoscopy or mediastinal staging.3
Few randomized trials have so far studied the role of surgical resection for SCLC, and a randomized trial may be difficult to complete because early-stage SCLC is a relatively rare disease. To define the role of surgical resection in patients with limited SCLC, we retrospectively investigated the surgical records of patients diagnosed with limited-stage disease (LD) SCLC in our department.
Patients and methods
A retrospective review of the patients treated from April 1974 to March 2012 identified 57 LD SCLC patients who underwent surgical resection for primary lung cancer at the Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University (Fukuoka, Japan). Preoperative evaluations were performed using chest roentgenography and computed tomography (CT) images, brain magnetic resonance imaging (MRI), bone scintigraphy, and fludeoxyglucose-positron emission tomography (FDG-PET). All patients were staged using the 7th edition of the tumor node metastasis (TNM) classification system of the American Joint Commission on Cancer (AJCC) and the Union for International Cancer Control (UICC).4,5 The medical records of each patient were reviewed to obtain the age, gender, smoking status, clinical stage, pathological stage, nodal status, surgical procedure, and duration of survival. The overall survival was defined as the time from the date of surgery for primary lung cancer. The institutional review board of our university approved this study.
Statistical analyses were carried out using the JMP 9 software program (SAS Institute Inc., Cary, NC, USA). Overall survival was calculated by the Kaplan-Meier method. The differences between survival curves were analyzed using the log-rank test for univariate analyses. A multivariate analysis was performed according to the Cox proportional hazards model to estimate the relative risk with a 95% confidence interval (CI). The results of the analysis were regarded as being statistically significant at a probability value < 0.05.
Results
The characteristics of the patients at the time of resection of their primary tumor are shown in Table 1. There were six women and 51 men, with a median age of 63.5 years (range, 48 to 86 years). All 57 patients underwent surgical resection, including a pneumonectomy in six patients, bilobectomy in three patients, lobectomy in 42 patients, segmentectomy in three patients and partial resections in three patients. Forty-three patients had no identified clinical lymph node metastasis, eight patients were classified as cN1, and six patients as cN2. Thirty-two patients had no identified pathological lymph node metastasis, 10 patients were classified as pN1, 10 patients as pN2, two patients as pN3, and three patients had an unknown status.
Table 1.
The patient characteristics
| Variable | No. of patients |
|---|---|
| Gender | |
| Female | 51 (89.5%) |
| Male | 6 (10.5%) |
| Age | |
| Median age (range; years) | 63.6 (48–86) |
| Smoking status | |
| Former (pack-year index < 50) | 32 (56.1%) |
| Current | 25 (43.9%) |
| Surgical procedure | |
| Pneumonectomy | 6 (10.5%) |
| Bilobectomy | 3 (5.3%) |
| Lobectomy | 42 (73.7%) |
| Segmentectomy | 3 (5.3%) |
| Partial resection | 3 (5.3%) |
| Clinical stage | |
| IA | 25 (43.9%) |
| IB | 13 (22.8%) |
| IIA | 5 (8.8%) |
| IIB | 7 (12.3%) |
| IIIA | 7 (12.3%) |
| Pathological stage | |
| IA | 20 (35.1%) |
| IB | 9 (15.8%) |
| IIA | 9 (15.8%) |
| IIB | 7 (12.3%) |
| IIIA | 10 (17.5%) |
| IIIB | 2 (3.5%) |
The overall five-year survival rate was 28.6% (median, 18.2 months) (Fig 1). The five-year survival rate according to the p-stage was 43.3% for patients with stage I, 12.5% for patients with stage II, and 12.5% for patients with stage III (Fig 2). The p-stage II and III patients had a significantly worse survival than the p-stage I patients (13.4% vs. 43.4%, P = 0.0036) (Fig 3a). However, the c-stage was not found to correlate with survival (c-stage I, 29.8% vs. c-stage II or III, 26.3%; P = 0.67) (Fig 3b). The cN-positive status was not found to correlate with survival (21.4% vs. 31.1%, P = 0.32). However, patients with pN1 or pN2 status had a significantly worse survival than did the patients with pN0 status (6.8% vs. 41.5%, P = 0.0013). The patients who underwent pneumonectomy had a significantly worse outcome than those who underwent other surgical procedures (0.0% vs. 32.0%, P = 0.0002) (Fig 3c). No other factors (gender, age or smoking status) were correlated with survival. In a multivariate Cox proportional hazards analysis (Table 2), p-stage II or III (hazard ratio [HR] 3.040, P = 0.0017) and pneumonectomy (HR 6.177, P = 0.00159) were significant independent predictors of an adverse survival outcome.
Figure 1.
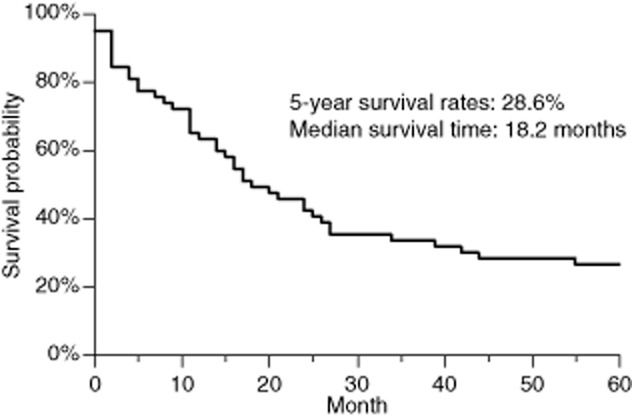
The overall five-year survival rate was 26.3% (median, 17.8 months).
Figure 2.
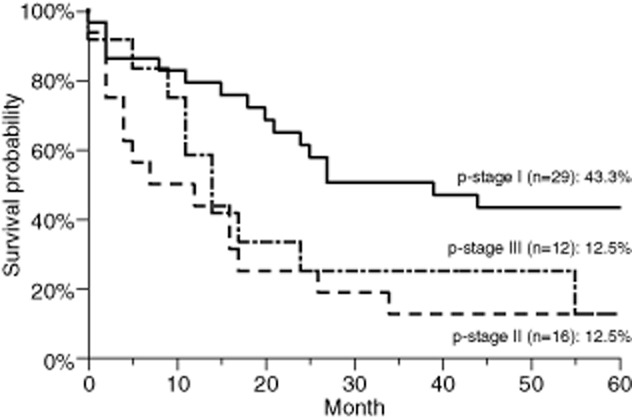
The five-year survival rates according to p-stage.  , p-stage I;
, p-stage I;  , p-stage II;
, p-stage II;  , p-stage III.
, p-stage III.
Figure 3.
The results of the Kaplan-Meier survival analysis. (a) The survival curves of patients with p-stage II or III versus p-stage I disease.  , p-stage I;
, p-stage I;  , p-stage II + III. (b) The survival curves of patients with c-stage II or III versus c-stage I disease.
, p-stage II + III. (b) The survival curves of patients with c-stage II or III versus c-stage I disease.  , c-stage I;
, c-stage I;  , c-stage II + III. (c) Pneumonectomy versus other surgical procedures.
, c-stage II + III. (c) Pneumonectomy versus other surgical procedures.  , pneumonectomy;
, pneumonectomy;  , others.
, others.
Table 2.
The results of the multivariate analysis of the patient characteristics predicting survival (Cox proportional hazards analysis)
| Variables | Hazard ratio | 95% Cl | P-value | |
|---|---|---|---|---|
| Age | >70 years | 1.145 | 0.649–2.012 | 0.64 |
| Gender | Male | 0.545 | 0.230–1.524 | 0.23 |
| Smoking status | Current | 1.019 | 0.546–1.888 | 0.95 |
| Clinical stage | II or III | 0.546 | 0.258–1.145 | 0.11 |
| Pathological stage | II or III | 3.101 | 1.543–6.216 | 0.0016 |
| Surgical procedure | Pneumonectomy | 6.345 | 2.127–17.02 | 0.0017 |
CI, confidence interval.
Discussion
As noted above, the standard of care for patients with SCLC is chemotherapy and radiotherapy, even for patients with limited disease. However, only a few randomized trials provide support for the current recommendations. The British MRC trial from 19732 randomized patients with central SCLC to surgery or radiotherapy. In that study, most patients did not undergo complete resection. Therefore, the results of that study may not be applicable to the clinical situation of a patient with a peripheral early-stage SCLC. Evidence of benefits of surgery in patients with LD-SCLC has not been reported so far, although some studies have suggested that surgical resection plus chemotherapy in LD-SCLC is of more benefit than nonsurgical management.6–8 According to the NCCN guidelines, patients with disease in a stage higher than T1-2 with N0 do not benefit from surgery.9 That study retrospectively reviewed patients treated during a very long interval, from 1974 to 2012. However, no significant difference in survival was observed between the patients treated before 2000, compared to those treated after 2000. A variety of surgical cases were included, and the preoperative diagnosis was not always known for all cases. In particular, seven clinical stage IIIA patients underwent surgery. Five c-stage IIIA cases were treated before 1990 and for the other two cases, the preoperative diagnosis was not known, because the tumor size was small.
Surgery is a modality that can reduce the incidence of local relapse.10 A study by Meyer et al. advocated the inclusion of surgery in multimodality therapy for SCLC.11 In various series of LD-SCLC cases managed by surgery plus perioperative chemotherapy, the five-year OS ranged from 32% to 60%.6–8,12,13 In our study, the five-year survival rate was 28.6%, which was worse than that of previous reports. One reason for this was that 12 patients with p-stage III were included in our study, while the other studies included few stage III patients. The five-year survival rate of patients with p-stage I disease was 43.4%. This was similar to the previously reported rates. In our study, perioperative chemotherapy was not considered. However, radiotherapy and chemotherapy have evolved, and combined modality therapy is now generally indicated.
The preoperatively established clinical stage may not correctly represent the postoperatively confirmed pathological stage. There is agreement between a diagnosis of clinical stage I disease and pathological stage I disease in only about 50–60% of cases.14 Our data showed the correspondence rates for clinical stage IA and IB to be 76% and 46%, respectively. Lim et al. reported that some patients with clinical II or III disease may have a pathological stage I classification and could benefit from surgery.15 In our study, among the 14 patients who were diagnosed with clinical lymph node metastasis, three patients had no pathological lymph node metastasis (21.4%). In recent years, FDG-PET has been demonstrated to be superior to CT in the staging of the lymph nodes. Therefore, the preoperative stage might have changed if PET had been performed. However, FDG-PET is also not perfect. Therefore, we suggest that patients who are diagnosed with clinical lymph node metastasis should undergo mediastinoscopy or another type of surgical mediastinal staging to rule out false-positive findings. The lymph node stage is an important factor that affects survival, most likely by properly assigning patients to a true pathological stage, and, therefore, to appropriate treatment.
Lobectomy is the standard of care for resectable NSCLC, providing superior survival and a lower risk of local recurrence than segmentectomy or wedge resection.16 Varlotto et al. reported that lobectomy or more extensive surgery led to superior overall and cancer-specific survival in node-negative SCLC patients.17 However, there was no difference in the prognosis of the patients who underwent lobectomy or greater and the patients who underwent segmentectomy or wedge resection in our study. Mediastinal lymph node dissection may not improve survival, but it does provide useful pathological information for predicting survival; in our study, patients with pN1 or pN2 status had a significantly worse survival rate than patients with pN0 status, as previously reported.16
On the contrary, patients who underwent pneumonectomy had a significantly worse outcome than those with metastases who underwent other surgical procedures in our study. All pneumonectomy cases were treated before 1990, and the possibility of curability by complete resection could not be ruled out. It is possible that adequate chemotherapy after surgery may not have been administered to the patients who underwent pneumonectomy, because of a worsening of their general status.
Conclusion
In conclusion, the present study suggests that surgical treatment can be considered in patients with pathological N0 status, although pneumonectomy should be avoided. Patients who are clinically diagnosed to have lymph node metastases should undergo a pathological examination of the lymph nodes by mediastinoscopy or other surgical mediastinal staging to rule out false-positive findings.
Disclosure
No authors report any conflict of interest.
References
- Lassen U, Osterlind K, Hansen M, Domebernowsky P, Bergman B, Hansen HH. Long-term survival in small cell lung cancer: post-treatment characteristics in patients surviving 5 to 18+ years – an analysis of 1714 consecutive patients. J Clin Oncol. 1995;13:1215–1220. doi: 10.1200/JCO.1995.13.5.1215. [DOI] [PubMed] [Google Scholar]
- Johnson BE, Crawford J, Downey RJ, et al. Small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:602–622. doi: 10.6004/jnccn.2006.0050. [DOI] [PubMed] [Google Scholar]
- Fox W, Scandding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet. 1973;2:63–65. doi: 10.1016/s0140-6736(73)93260-1. [DOI] [PubMed] [Google Scholar]
- Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th edn. New York: Springer; 2010. [Google Scholar]
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th edn. Oxford: Wiley-Blackwell; 2009. [Google Scholar]
- Hara N, Ohta M, Ichinose Y, et al. Influence of surgical resection before and after chemotherapy on survival in small cell lung cancer. J Surg Oncol. 1991;47:53–61. doi: 10.1002/jso.2930470112. [DOI] [PubMed] [Google Scholar]
- Badzio A, Kurowski K, Karnicka-Mlodkowska H, Jassem J. A retrospective comparative study of surgery followed by chemotherapy vs. non-surgical management in limited-disease small cell lung cancer. Eur J Cardiothorac Surg. 2004;26:183–188. doi: 10.1016/j.ejcts.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg. 2000;70:1615–1619. doi: 10.1016/s0003-4975(00)01401-6. [DOI] [PubMed] [Google Scholar]
- Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw. 2011;9:1086–1113. doi: 10.6004/jnccn.2011.0092. [DOI] [PubMed] [Google Scholar]
- Kohman LJ. Is there a place for surgery in central small cell lung cancer? Chect Surg Clin N Am. 1997;7:105–112. [PubMed] [Google Scholar]
- Meyer J, Comis R, Ginsberg SJ, et al. Phase II trial of extended indications for resection in small cell carcinoma of the lung. J Thorac Cardiovasc Surg. 1982;83:12–19. [PubMed] [Google Scholar]
- Osterlind K, Hansen M, Hansen HH, Dombernowsky P. Influence of surgical resection prior to chemotherapy on the long term results in small cell lung cancer. A study of 150 operable patients. Eur J Cancer Clin Oncol. 1986;22:589–593. doi: 10.1016/0277-5379(86)90048-9. [DOI] [PubMed] [Google Scholar]
- Shields TW, Higgins GA, Jr, Matthews MJ, Keehn RJ. Surgical resection in the management of small cell carcinoma of the lung. J Thorac Cardiovasc Surg. 1982;84:481–488. [PubMed] [Google Scholar]
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:1049–1059. doi: 10.1097/JTO.0b013e3181b27799. [DOI] [PubMed] [Google Scholar]
- Lim E, Belcher E, Yap YK, Nicholson AG, Goldstraw P. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol. 2008;3:1267–1271. doi: 10.1097/JTO.0b013e318189a860. [DOI] [PubMed] [Google Scholar]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–623. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- Varlotto JM, Recht A, Flickinger JC, Medford-Davis LN, Dyer AM, DeCamp MM. Lobectomy leads to optimal survival in early-stage small cell lung cancer: a retrospective analysis. J Thorac Cardiovasc Surg. 2011;142:538–546. doi: 10.1016/j.jtcvs.2010.11.062. [DOI] [PubMed] [Google Scholar]