Abstract
Background
Thymoma is a cancer with rare incidence, but it is a major malignancy in adult anterior mediastinum, occurring in about 40% of patients with myasthenia gravis. Because of the lack of thymic epithelial tumor cell lines, thymoma has lagged far behind other tumors in cytological studies. It is, therefore, quite necessary to establish a new thymic epithelial tumor cell line from Chinese patients to study the pathogenic mechanism and therapeutic methods.
Methods
Twenty-three samples of tumor tissues were collected from thymoma and thymic carcinoma patients for primary culture by tissue explant, suspension cell culture method, and collagenase digestion. We detected the biological characteristics and origin of the cell line after the establishment of a novel thymoma cell line.
Results
A novel cell line, designed as Thy0517, was established from thymoma type AB with myasthenia gravis patients by tissue explant. As an immortalized cell line, it always has a stable growth cycle, and there is no change in characteristics and morphology after culturing for 18 months and passing 160 generations in vitro. The experimental data demonstrate that the cell line exhibits the growth characteristics of tumor cells, the doubling time of 37 hours, with tumorigenicity in vitro and chromosome abnormality. Immunocytochemistry indicated that the cell line positive expression of CK7, CK8/18, CK19, CK-pan, CD24, BCL-2, P63, Vimentin, epithelial membrane antigen and epidermal growth factor receptor, lymphocyte related antigen CD99, and TdT were negatively expressed.
Conclusions
The newly established thymic epithelial tumor cell line from Chinese patients provides a model in the study of thymoma and molecularly targeted therapies.
Keywords: Cell line, myasthenia gravis, thymoma
Introduction
Thymomas account for 30% of the incidence of adult anterior mediastinal tumors, and are the most common tumor of the anterior mediastinum. Thymomas occur mainly in the 55–60 age group, and prevalence in the Chinese population is higher than in the Western population. Approximately 40% of thymomas are complicated with myasthenia gravis (MG), significantly relating to type AB, B2, and B3.1 The occurrence of a thymoma complicated with MG may be caused by changes in the thymic microenvironment of tumor formation, and the symptoms of complicated myasthenia are usually more serious than mere MG. Although thymomas have a low mortality rate and a low degree of malignancy characteristics, the incidence of second cancers are increased in patients with a thymoma.2 The cause of thymoma is not yet clear, but several studies have indicated that the Epstein–Barr virus3 and genetic aberration4–8 may play an important role in the pathogenesis of thymomas.
From the beginning of the 1950s, many researchers committed to the isolation of human thymic epithelial tumor cell lines in order to study the biological characteristics of the thymoma and evaluate the adaptation of the model in vitro. To our knowledge, only two thymoma cell lines and three thymic carcinoma cell lines were reported in literature until 2013, but no cell lines have been established in the Chinese population. Therefore, we continue to explore the primary culture methods of thymoma tissue according to the basis of existing cell lines, and provide an ideal model for the study of thymoma by the establishment of a novel thymic epithelial tumor cell line.
Methods
Primary culture of human thymoma tissue
The Ethics Committee of the Tianjin Medical University General Hospital approved this study. Tumor tissues were immersed in phosphate buffered saline (PBS) and transferred to the laboratory at low temperatures for culture. Twenty-three samples of tumor tissues, including all pathological types, were primary cultured by tissue explant culture, and cell suspension and collagenase digestion methods.
Tissues were cultured in 5 mL Dulbecco's modified Eagle's medium (DMEM)/high glucose medium without sodium pyruvate (HyClone, Utah, USA), 100 U/mL penicillin, 100 ug/mL streptomycin (Sigma-Aldrich, Missouri, USA) and 10% heat-inactivated fetal bovine serum (FBS) (NQBB, Victoria, Australia) in 5% CO2 incubator (Thermo, California, USA) at 37°C.
Tissue explant culture: blood was washed by PBS, and blood vessels, necrosis, and other connective tissues were removed and placed in a dish. Tissues were cut into 1-2 cm3 with sterile scissors in medium, and these pieces were then divided into 25-cm2 flasks with tweezers, leaving 0.5 cm space in between each flask. After 24 hours, 5 mL medium was gently added.
Cell suspension method: After cutting tissues into small pieces, 10 mL of PBS was added. The tissues were thoroughly shaken in order to isolate and aggregate small pieces of tissues and cells. After standing, cell suspension was carefully removed to the centrifuge tube and centrifuge was performed at 1000 rpm for five minutes. The precipitate was then washed with 10 mL PBS and centrifuged again. Medium was used to re-suspend the cell mass. Cells were transferred into 25-cm2 flasks after counting.
Collagenase digestion method: After cutting tissues into small pieces, 20 mL 0.3% collagenase digestion was added and shaken at 37°C for two hours until the tissues dispersed into single cells. After 200 mesh filters, the collagenase solution was centrifuged at 1000 rpm for five minutes, then the precipitate was washed with 10 mL PBS and centrifuged again. The cell mass was re-suspended in medium and cells transferred into 25-cm2 flasks after counting.
The medium should be replaced once every two to three days depending on the change of colour.
Establishment of thymoma cell line and materials of the thymoma patient
Although 23 thymoma tissues were cultured by three methods, 73.9% (17/23) of the cases saw cells climbing out of the tissue explant method, and non-adherent cells were shown by the cell suspension and collagenase digestion method. Nevertheless, the establishment of a cell line only succeeded in one thymoma patient (1/23). The cell line, designed as Thy0517, was established from an anterior mediastinal tumor of 5 cm in diameter (Fig 1a,b) in a 50-year-old male patient with MG(IIb) which was confirmed by neostigmine test and neurological examination. He did not have any history of family genetics or radiotherapy. The tumor was found during surgery located next to the ascending aorta in violation of the pleura and phrenic nerve (Masaoka Stage IIb). Pathological diagnosis revealed an AB type thymoma (Fig 1c) according to the World Health Organization (2004) classification. Two weeks later, the patient was discharged with improved clinical symptoms.
Figure 1.
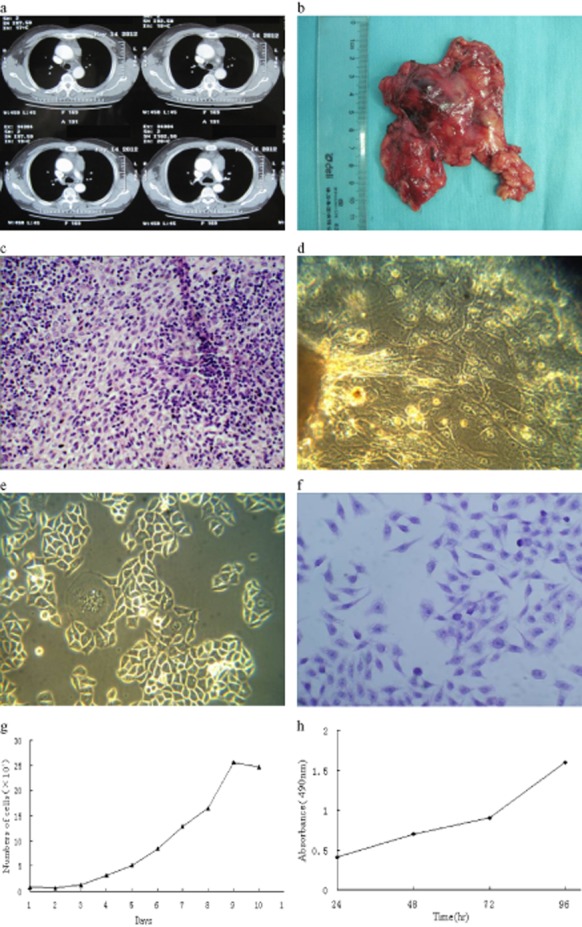
Anterior mediastinum image of the patient and morphology feature of Thy0517 cell line in vitro. (a) The enhanced computed tomography (CT) report of the patient; (b) the picture of the original tumor; (c) hematoxylin and eosin (H&E) staining of the original tumor; (d) epithelial cells climb out after nine days of primary culture (×200); (e) morphology of the cell line at passage 30 (×200); (f) H&E staining of the cultured cell line under microscope (×200); (g,h) the growth curve of the cell line shown by cell count and MTT assay.
Thy0517 growth properties
Cell growth ability was assessed by cell count, MTT assay, and soft agar assay. Cells at passage 30 were plated at 8000 cells/well in two 24-well plates or 2000 cells/well in four 96-well plates, and allowed to attach overnight. The cells were counted every two days after trypsin digestion in 24-well plate and the doubling time of the cell line was calculated. Twenty uL MTT reagent (ATCC, Virginia, USA) was added to a 96-well plate after 24, 48, 72, and 96 hours, and incubated for four hours until purple precipitate was visible. The absorbance at 490 nm after crystals were dissolved in 150 uL DMSO (ATCC) was recorded. In order to appraise the soft agar colony formation, the cell line was suspended in a top layer of DMEM/high glucose containing 10% FBS and 0.3% agar at 5000 cells per well in 6-well plates, plated on a bottom layer of DMEM/high glucose containing 0.5% agar. Plants were transferred into a 5% CO2 incubator after agar coagulation.
Immunohistochemistry of human tumor and phenotype of thymoma cell line
Paraffin sections of human thymoma tissue were cut and deparaffinized in xylene and rehydrated with graded ethanol. The sections were autoclaved for three minutes in Tris/ethylenediaminetetraacetic acid buffer (pH 9.0) for antigen retrieval before incubation with primary antibodies.
Cell lines were cultured on sterile coverslip in a dish. Cells were fixed with 95% ethanol for 20 minutes, until they reached 90% confluency. The fixed coverslip can be used in immunocytochemistry after washing with PBS.
Hematoxylin and eosin staining should be carried out to observe the morphology of the tumor and cell line before detecting surface antigens. Several anti-human monoclonal primary antibodies (Table 1) were incubated overnight at 4°C in paraffin slides and a fixed coverslip. Secondary horseradish peroxidase (HRP) conjugated against mouse and rabbit immunoglobulin G was obtained from ZSGB-BIO (Beijing, China). Immunoreaction of signal amplification was detected by labeled streptavidin biotin (LSAB) and the color reaction was controlled by 3, 3′-diaminobenzidine under the microscope, followed by counterstaining with hematoxylin. After the sections and coverslip were cleared in xylene and dehydrated with graded ethanol, they were fixed by neutral balsam and the staining was viewed under the microscope.
Table 1.
Primary antibodies for immunohistochemistry and immunocytochemistry
| Antibody | Type | Source | Dilution | Specificity |
|---|---|---|---|---|
| CK7 | M | ZSGB-BIO | 1:200 | Epithelial cells |
| CK5/6 | M | ZSGB-BIO | 1:200 | Epithelial cells |
| CK8/18 | M | ZSGB-BIO | 1:200 | Epithelial cells |
| CK19 | M | ZSGB-BIO | 1:200 | Epithelial cells |
| CK-Pan(AE1/3) | M | ZSGB-BIO | 1:200 | Epithelial cells |
| CD20 | R | ZSGB-BIO | 1:200 | B cells |
| CD24 | M | ZSGB-BIO | 1:200 | B cells, Multiple neoplasms |
| CD99 | R | ZSGB-BIO | 1:200 | Premature lymphocytes |
| CD117 | R | ZSGB-BIO | 1:200 | Multiple neoplasms |
| BCL-2 | R | ZSGB-BIO | 1:200 | Neoplastic cell |
| P63 | M | ZSGB-BIO | 1:200 | Epithelial cells, basal cells |
| Vimentin | R | ZSGB-BIO | 1:200 | Mesenchymal cell |
| EMA | M | ZSGB-BIO | 1:200 | Epithelial cells |
| COX2* | R | ABCAM | 1:500 | Multiple neoplasms |
| EGFR | R | ZSGB-BIO | 1:200 | Multiple epithelial neoplasms |
| TDT | M | ZSGB-BIO | 1:200 | B cell, T cell |
Polyclonal antibody. EGFR, epidermal growth factor receptor; EMA, epithelial membrane antigen; M, monoclonal (mouse); R, monoclonal (rabbit).
Chromosome analyses
Thy0517 cell line at passage 30 was tested for chromosome analyses. For the purpose of obtaining metaphase cells, 0.1 ug/mL colchicine (Sigma, USA) was added when the cells were at 70% confluence. The cells were harvested and their karyotypes were analyzed according to the G-banding technique.
Thymoma xenograft model
Five three-week-old BALBC nude mice (purchased from the Chinese Academy of Military Medical Sciences, License No.: SCXK-military-2007-004) were inoculated in the back with 2 × 106 Thy0517 cells. Tumor generation was observed and measured by vernier caliper, and the volume was calculated using the equation (L × W2/2). The sections of nude mice tumor were stained by hematoxylin and eosin staining for the purpose of histomorphological observation and immunohistochemistry detected several epithelial membrane antigens and cytokeratin.
Results
Morphology and growth ability of Thy0517
Single cells started to grow out from the tumor tissue after nine days (Fig 1d). In the course of primary culture, we removed fibroblasts by several methods, including 5% FBS, low concentrations of trypsin, and mechanical scraping. When cells reached 90% confluence after 35 days, the first passage was commenced. Cell culture medium was replaced once every two days, passage every four to five days. So far, the cell line has been cultured in the 160th passage for 18 months in vitro.
Thy0517 are polygonal adherent cell lines, complying with tumor cell morphology and with apparent nuclear membrane and nucleolus by microscopic observation (Fig 1e), excluding contact-inhibition. The nuclear-cytoplasmic ratio imbalance and giant multinucleate cells can be observed by hematoxylin and eosin staining (Fig 1f). Cell count, MTT assay (Fig 1g,h), and soft agar assay were used for the purpose of acquiring growth feature and doubling time of Thy0517 in vitro. The cell growth curve indicated that the cell lines had a logarithmic phase of six days and the doubling time was 37 hours. Three weeks later, about 82 colonies were observed by invert microscope; all of the colonies were larger than 90 um in diameter and the cloning efficiency was approximately 1.6%.
Tumorigenicity in vitro
White protrusions at 2–4 mm in diameter were formed on five nude mice two weeks following injection. Twelve weeks later, the tumor diameter was between 26 mm to 32 mm (Fig 2a,b) and the average volume was 14.4 cm3. The results indicate that the Thy0517 cell line possesses tumorigenic characteristics (NOD/SCID, 5/5). Hematoxylin and eosin staining showed that the xenograft cells are generally large and the nucleus is of varied shape and tightly packed together. Cells display more varied and irregular shapes and some may exhibit abnormal mitotic phases, with the characteristics of tumor cells (Fig 3i).
Figure 2.
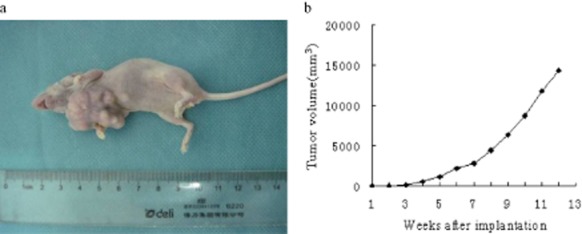
The tumorigenicity test of the Thy0517 cell line in nude mice. (a) The state of the tumor after xenotransplantation of 12 weeks; (b) tumor growth of nude mice after Thy0517 cell line implantation.
Figure 3.
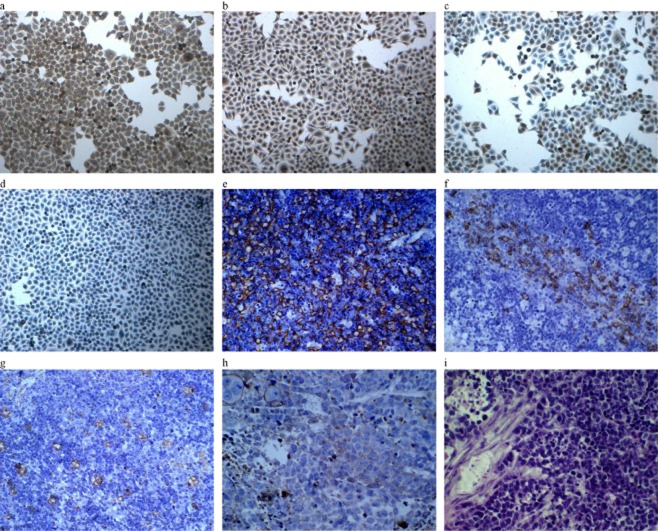
Immunocytochemistry and immunohistochemistry analysis for surface expression (high-power magnification × 20). (a) Immunocytochemical staining with positivity for CK-19; (b) immunocytochemical staining with positivity for epidermal growth factor receptor (EGFR); (c) immunocytochemical staining with positivity for CD24; (d) immunocytochemical staining with nuclear positivity for P63; (e) immunohistochemical staining with positivity for CK19 of the original tumor; (f) immunohistochemical staining with positivity for CD20 of the original tumor; (g) immunohistochemical staining with positivity for Vimentin of the original tumor; (h) immunohistochemical staining with positivity for CK19 of nude mice tumor; (i) hematoxylin and eosin staining of nude mice tumor.
Immunohistochemistry of original tumor, in vitro and in vivo
As shown by immunohistochemistry and immunocytochemistry, the cell lines, human thymoma, and nude mice tumors were positive for expressions of cytokeratin CK7, CK8/18, CK19, CK-pan, CD24, BCL-2, P63, Vimentin, epithelial membrane antigen (EMA), and COX2 (Fig 3a–j). However, the human thymoma tissue was positive in CK5/6, CD20, CD99, TdT, while nude mice tumor and cell lines were negative. It is worth noting that the expression of epidermal growth factor receptor (EGFR) may be found in cell lines and mice tumors (Table 2).
Table 2.
Immunochemistry reactivity of Thy0517, in vitro and in vivo, including the original tumor
| Thy0517 cell lines | |||
|---|---|---|---|
| Antibody | In vitro | In vivo | Original tumor |
| CK7 | +++ | +++ | +++ |
| CK5/6 | − | − | ++ |
| CK8/18 | +++ | + | + |
| CK19 | +++ | +++ | +++ |
| CK-pan | +++ | +++ | +++ |
| CD20 | − | − | ++ |
| CD24 | ++ | +++ | + |
| CD99 | − | − | +++ |
| CD117 | − | − | − |
| BCL-2 | ++ | +++ | ++ |
| P63 | + | + | + |
| Vimentin | +++ | +++ | + |
| EMA | +++ | +++ | ++ |
| COX2 | + | + | + |
| EGFR | +++ | + | − |
| TDT | − | − | +++ |
Notes: +++, >70% positive cells; ++, 30–70% positive cells; +, <30% positive cells; −, negative reaction.
EGFR, epidermal growth factor receptor; EMA, epithelial membrane antigen.
Chromosome aberrations of Thy0517 cell lines
In the cytogenetic analysis of Thy0517 cells, 15 metaphases were investigated using a G-banding technique. Chromosome aberrations detected in the Thy0517 cell line were: 65–70, XY, +X, +1, der(1;?)(q10;?), −3, +der(5;?)(q10;?), +der(5;?)(p10;?) × 2, +7, +7 × 2, +8, +8 × 2, i(9)(q10), +12, +15, +17, +19, +20, +12∼13Mar (CP15) (Fig 4).
Figure 4.
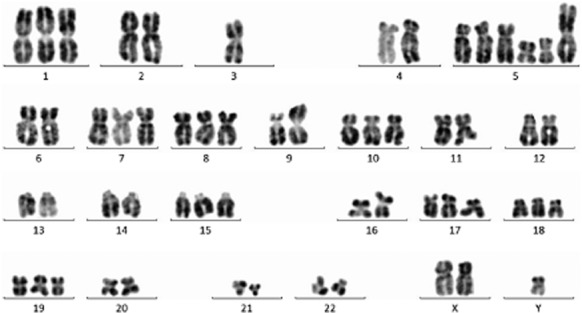
Chromosome analyses of Thy0517 cell line.
Discussion
In recent decades, a large number of human tumor cell lines were isolated and used for long-term culture. These cell lines have become an important tool in the study of the biological characteristics of tumors. However, because of the low incidence of thymoma and the lack of available and effective tumor tissue specimens, which greatly restrict the cytological studies of thymoma, no human thymic epithelial tumor cell lines have been analysed in recent times. Five different strategies, using serum free medium,9–11 culturing in serum-containing medium with D-valine,12,13 removing fibroblasts and preparation of feeder layers,14 adding complement activating cytotoxic antibodies,15 isolating thymic epithelial cells and culturing alone,16,17 have been applied in non-neoplastic thymic epithelial cell culture, but these techniques have not been compared systematically in neoplastic thymic epithelial cells. Because tumor tissue comes from adults, long-term thymic epithelial tumor cell cultures are difficult to establish. Some scholars have experimentally confirmed that thymic epithelial tumor cell culture in vitro can survive up to 45 days, and the proliferation ability of tumor cells decline over this period.18 In spite of this, microsatellite instability was analyzed in type AB and B2 thymoma by limited short-term primary culture.5
Even though the difficulties in developing thymoma cell lines have been well reported, researchers have still been committed to the establishment of thymic epithelial tumor cell lines. To date, five thymic neoplasm cell lines were established in the US, Japan, and Germany, but have not been widely utilized. The successfully established cell lines used the tissue explant method or culturing epithelial cells from pleural effusion in DMEM/high glucose medium, containing 5–20% FBS, with or without 10% human cord serum.
In 1992, a cell line named Ty-82 from a 22-year-old female with undifferentiated thymic carcinoma and t(15;19)(q12;p13) chromosome translocation was established.19 Although Ty-82 may not represent an ideal model for study of thymoma development, the translocation in this type of thymic carcinoma should be concerned. ThyL-6 derived from a 57-year-old male patient with undifferentiated thymic carcinoma was established in 2008.20 ThyL-6 produces multiple inflammatory cytokines and provides a tool for the study of cytokine-chemokine network system in thymic carcinoma. In the same year, a cell line from type B1 thymoma and a cell line from poorly differentiated thymic carcinoma were established by tissue explant method as the first definitive human thymic neoplasm cell lines. They were cultured in passage 29 and 85, but no evidence has shown their tumorigenicity in vitro.21 A novel thymoma cell line, named IU-TAB-1, was announced in 2012 as the latest thymoma cell line, established from a 53-year-old male patient with type AB thymoma.22
All of the pathology types of thymoma were cultured by tissue explant, cell suspension, and collagenase digestion methods in our work, but only one case by tissue explant method succeeded. The mean survival time of thymic epithelial tumor cells is 20.75 days in abortive primary culture, except for the successfully established cell line, Thy0517. The Thy0517 cell line was established from thymoma type AB with MG by tissue explant method and the detailed characterization and bionomics of the novel cell line were detected. The cell line as an immortalized thymoma cell line and grows in DMEM/high glucose medium with 10% FBS, which is similar to the five thymic neoplasm cell line culture medium. Thy0517 cell line as an adherent monolayer did not appear to display any changes in morphological and growth characteristics following repeated passages. The doubling time of Thy0517 (37 hours) is greater than thymoma cell line IU-TAB-1 (48 hours), but lower than thymic carcinoma cell line ThyL-6 (26.3 hours).
IU-TAB-1 and Thy0517 are derived from type AB thymoma, but the supplier of IU-TAB-1 did not have any samples of autoimmune diseases. The IU-TAB-1 cell line exhibits epithelial markers (pan-cytokeratin), CD29, CD9, CD58, CD24, P63, and epithelial cell adhesion molecule (EpCAM) without showing C-Kit (CD117) and EGFR markers. In contrast, Thy0517 cell line expresses p63, EGFR, BCL-2, Vimentin, and several epithelial markers including pan-cytokeratin, CK7, CK8/18, CK19, EMA, but no expression of lymphocyte antigen CD99 and TdT. Because the cell line expresses EGFR, this serves as a powerful model for the study of EGFR mutation in thymoma. However, the cell line expresses CD24 scattered, which has been considered as a kind of cancer stem cell marker in malignant mesothelioma,23 colon,24,25 ovarian,26 gastric27 and hepatic cancers.28 The human tumor of Thy0517, but not the cell line, was focally positive for CK5/6 and CD20.
Thy0517 cell line exhibits some aberrations reported in type AB thymomas, including 1, 3, 7, 8 and 17.5,6 The abnormal chromosome numbers and karyotypes increase the malignant level of the Thy0517 cell line. Compared with IU-TAB-1, the cell line possesses stronger tumorigenicity in vitro and the growth velocity of a nude mice tumor is faster during a corresponding time period.
Conclusion
We have established a novel thymoma cell line from patients with thymoma with MG by primary culture, which is tumorigenic in immunodeficient mice, and will be an ideal model for the understanding of thymoma and the development of molecularly targeted therapies.
Acknowledgments
This work was sponsored by the Specialized Research Fund for the Doctoral Program of Higher Education (No.20121202110009).
Disclosure
No authors report any conflict of interest.
References
- Chen YP, Wang W, Wang ZK, Dou YK, Wei DN. The clinical characteristics of patients with thymoma-associated myasthenia gravis. Zhonghua Nei Ke Za Zhi. 2012;51:623–625. (In Chinese.) [PubMed] [Google Scholar]
- Kadota Y, Utsumi T, Inoue M, Sawabata N, Minami M, Okumura M. Radiation-induced osteosarcoma 17 years after mediastinal irradiation following surgical removal of thymoma. Gen Thorac Cardiovasc Surg. 2010;58:651–653. doi: 10.1007/s11748-010-0587-x. [DOI] [PubMed] [Google Scholar]
- Guibert N, Brouchet L, Rouquette I, et al. Thymoma and solid-organ transplantation. Lung Cancer. 2012;77:232–234. doi: 10.1016/j.lungcan.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Girard N, Shen R, Guo T, et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin Cancer Res. 2009;15:6790–6799. doi: 10.1158/1078-0432.CCR-09-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Starostik P, Zettl A, et al. Correlating genetic aberrations with World Health Organization-defined histology and stage across the spectrum of thymomas. Cancer Res. 2003;63:3708–3715. [PubMed] [Google Scholar]
- Penzel R, Hoegel J, Schmitz W, et al. Clusters of chromosomal imbalances in thymic epithelial tumours are associated with the WHO classification and the staging system according to Masaoka. Int J Cancer. 2003;105:494–498. doi: 10.1002/ijc.11101. [DOI] [PubMed] [Google Scholar]
- Lee GY, Yang WI, Jeung HC, et al. Genome-wide genetic aberrations of thymoma using cDNA microarray based comparative genomic hybridization. BMC Genomics. 2007;8:305. doi: 10.1186/1471-2164-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettl A, Ströbel P, Wagner K, et al. Recurrent genetic aberrations in thymoma and thymic carcinoma. Am J Pathol. 2000;157:257–266. doi: 10.1016/S0002-9440(10)64536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L, Eshel I, Meilin A, Sharabi Y, Shoham J. Analysis of thymic stromal cell subpopulations grown in vitro on extracellular matrix in defined medium. III. Growth conditions of human thymic epithelial cells and immunomodulatory activities in their culture supernatant. Immunology. 1991;74:621–629. [PMC free article] [PubMed] [Google Scholar]
- Meilin A, Sharabi Y, Shoham J. Analysis of thymic stromal cell subpopulations grown in vitro on extracellular matrix in defined medium – V. Proliferation regulating activities in supernatants of human thymic epithelial cell cultures. Int J Immunopharmacol. 1997;19:39–47. doi: 10.1016/s0192-0561(96)00042-2. [DOI] [PubMed] [Google Scholar]
- Meilin A, Shoham J, Schreiber L, Sharabi Y. The role of thymocytes in regulating thymic epithelial cell growth and function. Scand J Immunol. 1995;42:185–190. doi: 10.1111/j.1365-3083.1995.tb03644.x. [DOI] [PubMed] [Google Scholar]
- Dalloul AH, Arock M, Fourcade C, et al. Human thymic epithelial cells produce interleukin-3. Blood. 1991;77:69–74. [PubMed] [Google Scholar]
- Fernández E, Vicente A, Zapata A, et al. Establishment and characterization of cloned human thymic epithelial cell lines. Analysis of adhesion molecule expression and cytokine production. Blood. 1994;83:3245–3254. [PubMed] [Google Scholar]
- Singer KH, Harden EA, Robertson AL, Lobach DF, Haynes BF. In vitro growth and phenotypic characterization of mesodermal-derived and epithelial components of normal and abnormal human thymus. Hum Immunol. 1985;13:161–176. doi: 10.1016/0198-8859(85)90009-6. [DOI] [PubMed] [Google Scholar]
- Singer KH, Le PT, Denning SM, Whichard LP, Haynes BF. The role of adhesion molecules in epithelial-T-cell interactions in thymus and skin. J Invest Dermatol. 1990;94(6 Suppl):85S–90S. doi: 10.1111/1523-1747.ep12876038. [DOI] [PubMed] [Google Scholar]
- Oosterwegel MA, Haks MC, Jeffry U, Murray R, Kruisbeek AM. Induction of TCR gene rearrangements in uncommitted stem cells by a subset of IL-7 producing, MHC class-II-expressing thymic stromal cells. Immunity. 1997;6:351–360. doi: 10.1016/s1074-7613(00)80337-4. [DOI] [PubMed] [Google Scholar]
- Anderson G, Jenkinson E. Piecing together the thymic puzzle. Immunol Today. 1997;18:363–364. doi: 10.1016/s0167-5699(97)01099-2. [DOI] [PubMed] [Google Scholar]
- Papadopoulos T, Kirchner T, Marx A, Müller-Hermelink HK. Primary cultures of human thymic epithelial tumors morphological and immunocytochemical characterization. Virchows Arch B Cell Pathol. 1989;56:363–370. doi: 10.1007/BF02890038. [DOI] [PubMed] [Google Scholar]
- Kuzume T, Kubonishi I, Takeuchi S, et al. Establishment and characterization of a thymic carcinoma cell line (Ty-82) carrying t(15;19)(q15;p13) chromosome abnormality. Int J Cancer. 1992;50:259–264. doi: 10.1002/ijc.2910500216. [DOI] [PubMed] [Google Scholar]
- Inai K, Takagi K, Takimoto N, et al. Multiple inflammatory cytokine-productive ThyL-6 cell line established from a patient with thymic carcinoma. Cancer Sci. 2008;99:1778–1784. doi: 10.1111/j.1349-7006.2008.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehemann V, Kern MA, Breinig M, et al. Establishment, characterization and drug sensitivity testing in primary cultures of human thymoma and thymic carcinoma. Int J Cancer. 2008;122:2719–2725. doi: 10.1002/ijc.23335. [DOI] [PubMed] [Google Scholar]
- Gökmen-Polar Y, Sanders KL, Goswami CP, et al. Establishment and characterization of a novel cell line derived from human thymoma AB tumor. Lab Invest. 2012;92:1564–73. doi: 10.1038/labinvest.2012.115. [DOI] [PubMed] [Google Scholar]
- Ghani FI, Yamazaki H, Iwata S, et al. Identification of cancer stem cell markers in human malignant mesothelioma cells. Biochem Biophys Res Commun. 2011;404:735–742. doi: 10.1016/j.bbrc.2010.12.054. [DOI] [PubMed] [Google Scholar]
- Ke J, Wu X, Wu X, et al. A subpopulation of CD24+ cells in colon cancer cell lines possess stem cell characteristics. Neoplasma. 2012;59:282–288. doi: 10.4149/neo_2012_036. [DOI] [PubMed] [Google Scholar]
- Yeung TM, Gandhi SC, Wilding JL, Muschel R, Bodmer WF. Cancer stem cells from colorectal cancer-derived cell lines. Proc Natl Acad Sci U S A. 2010;107:3722–3727. doi: 10.1073/pnas.0915135107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao MQ, Choi YP, Kang S, Youn JH, Cho NH. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29:2672–2680. doi: 10.1038/onc.2010.35. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li C, He F, Cai Y, Yang H. Identification of CD44+CD24+ gastric cancer stem cells. J Cancer Res Clin Oncol. 2011;137:1679–1686. doi: 10.1007/s00432-011-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hou J, Lin Z, et al. Attenuated Listeria monocytogenes as a cancer vaccine vector for the delivery of CD24, a biomarker for hepatic cancer stem cells. Cell Mol Immunol. 2014;11:184–196. doi: 10.1038/cmi.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]