Abstract
A patient presenting with concomitant epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) translocation is rare. We report a non-small cell lung cancer (NSCLC) patient with concomitant ALK rearrangement and exon 19 (E746-A750del) EGFR mutation. The ALK rearrangement was confirmed not only in the primary tumor biopsy specimen, but also in the pleural effusion cell block by reverse transcriptase-polymerase chain reaction (RT-PCR), Ventana ALK immunohistochemistry assay, and fluorescence in situ hybridization. No clinical benefit using chemotherapy or EGFR tyrosine kinase inhibitor gefitinib was obtained in this case.
Keywords: EGFR mutation, EML4–ALK, lung cancer, tyrosine kinase inhibitors
Introduction
Echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) rearrangement is found in 2 to 7% of patients with non-small cell lung cancer (NSCLC) overall.1 ALK gene arrangements are largely mutually exclusive with epidermal growth factor receptor (EGFR) mutations.1–3 Until now, only nine patients harboring EGFR mutation and ALK translocation have been reported with tyrosine kinase inhibitor (TKI)-target therapy in the literature.4–11 Almost all of the abnormal genes of these patients were confirmed by biopsy or resected tissue of the primary tumor or metastatic lymph nodes. Here we present a NSCLC patient with concomitant ALK rearrangement and EGFR mutation, and the ALK rearrangement was confirmed in the primary tumor biopsy specimen and in the pleural effusion cell block by different methods. No clinical benefit using chemotherapy and EGFR-TKI was obtained in this case.
Case presentation
A 47-year-old female non-smoker was admitted to our hospital in June 2012 after X-ray discovery of an abnormal shadow in the right upper lobe field. Based on the Eastern Cooperative Oncology Group (ECOG) scale, performance status was 1. Carcinoembryonic antigen was 39.2 ng/mL in blood serum (normal range: 0.0–5.0 ng/mL). A chest computed tomography (CT) scan revealed a 3.0 × 2.0 cm lobulated nodular lesion in the right upper lobe with multiple metastatic lesions on both sides; enlargement of hilar and mediastinal lymph nodes; and bilateral pleural effusion (Fig 1). The ultrasound showed several enlarged lymph nodes on the right cervical region; the largest one was 3.1 × 1.6 cm with no clear boundary between the cortex and medulla. A brain magnetic resonance imaging scan revealed an abnormal signal in the right side of the saddle pool, which was considered a metastatic lesion. We conducted a CT guided lung biopsy and confirmed pathological diagnosis of adenocarcinoma. We found tumor cells in the pleural effusion, so we prepared a cell block in archives. In conclusion, we made a diagnosis of right upper lobe adenocarcinoma (T4N3M1b stage IV).
Figure 1.
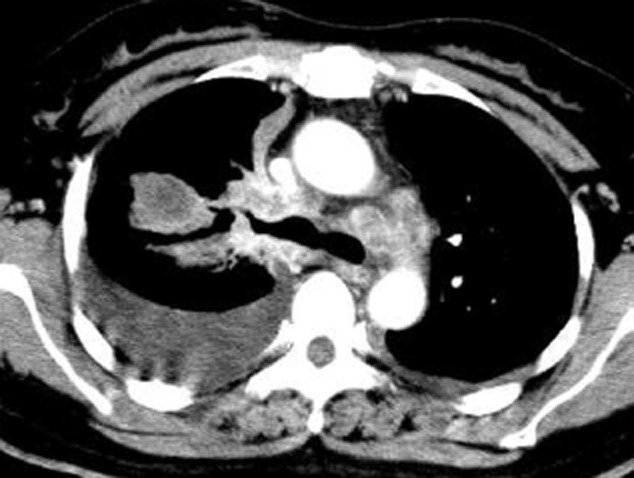
Computed tomography (CT) scan of chest revealed a 3.0 × 2.0 cm lobulated nodular lesion in the right upper lobe with multiple metastatic lesions on both sides; enlargement of hilar and mediastinal lymph nodes; and bilateral pleural effusion.
The patient initially received two chemotherapy regimens of GP (gemcitabine 1250 mg/m2 D1, 8; cisplatin 75 mg/m2 divided into D1-2) in June 2012 and July 2012. Treatment was then altered to gefitinib because the EGFR mutation test in the lung biopsy tissue by amplification refractory mutation system (ARMS) PCR showed (E746-A750del) deletion in exon 19 (Fig 2c), but not T790M in exon 20, which was also confirmed by pyrosequencing assay based on PCR (Fig 2d). Unfortunately, no clinical benefit was obtained and the efficacy evaluation showed an increase in bilateral pleural effusion and an enlarged tumor size after two months of gefitinib therapy. The therapy response was assessed as progressive disease (PD) according to Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). The patient died of tumor progression in October 2012.
Figure 2.
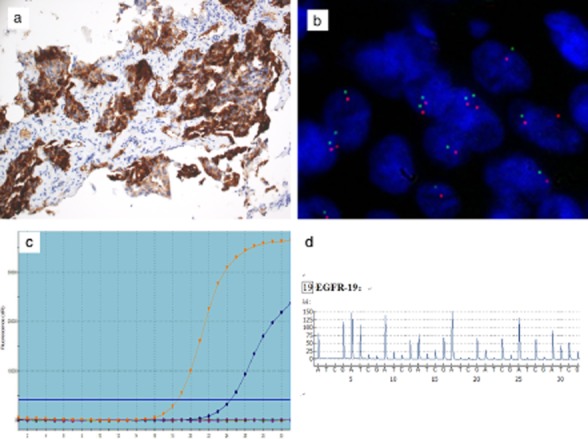
Computed tomography (CT) guided lung biopsy specimen presented as anaplastic lymphoma kinase (ALK) positive by: (a) Ventana immunohistochemistry (×200); (b) fluorescence in situ hybridization (FISH) (×1000); epidermal growth factor receptor (EGFR) gene mutation (Exon 19 E746-A750del) positive by (c) amplification refractory mutation system (ARMS) polymerase chain reaction (PCR); (d) pyrosequencing assay based on PCR.
We retrospectively analyzed the patient's ALK gene rearrangements. ALK rearrangements were confirmed as positive in the biopsy tissue by fully automated immunohistochemistry (IHC) assay (Ventana pre-diluted ALK D5F3 antibody with the Optiview DAB IHC detection kit) (Fig 2a), and by fluorescence in situ hybridization (FISH) using the Vysis LSI ALK Dual Color, Break Apart Rearrangement Probe (Abbott Molecular, Abbott Park, IL, USA) (Fig 2b). The cell block of pleural effusion failed in ALK FISH assessment because of a limited tumor cell count (minimum of 50 cell nuclei); however, it was positive by Ventana ALK IHC (Fig 3a) and by reverse transcriptase polymerase chain reaction (RT-PCR) using the ADx EML4-ALK Fusion Gene Diagnostic Kit (Amoy Diagnostics Company Ltd, Xiamen, China) (Fig 3b). An EGFR mutation test was negative in the cell block by ARMS PCR (Fig 3c).
Figure 3.
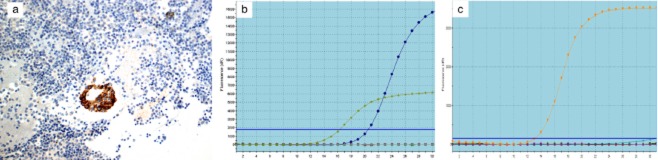
Cell block specimen made from the pleural effusion presented as anaplastic lymphoma kinase (ALK) positive by: (a) Ventana immunohistochemistry; and (b) reverse transcription-polymerase chain reaction (RT-PCR). Epidermal growth factor receptor (EGFR) gene mutation negative by (c) amplification refractory mutation system (ARMS) polymerase chain reaction (PCR).
Discussion
We experienced a rare case with concomitant ALK rearrangement and EGFR mutation. It is noteworthy that in our case EGFR mutation was confirmed by ARMS and pyrosequencing assay, and ALK rearrangement by FISH in the biopsy tissue; and ALK positive by Ventana IHC and RT-PCR in the cell block of pleural effusion.
Currently, there are three main methods for ALK detection: FISH, IHC, and RT-PCR. Despite the high cost and long turnaround time, FISH is regarded as the gold standard for ALK detection.1,12 IHC for ALK protein overexpression is a promising screening modality.12 Several recent studies have confirmed that a relatively new ALK clone, D5F3, 5A4, could accurately identify ALK-rearranged lung cancer, with the sensitivity and the specificity of 90–100%.12 In China, Ventana IHC is one of the recommended methods of the China Food and Drug Administration. Our result in which Ventana IHC and RT-PCR were positive, while FISH results were uncertain in the cell block, may be the result of an insufficent tumor cell sample in the FISH test. Tumor heterogeneity or relatively insufficient tumor cells in the cell block may have caused the conflicting results of EGFR mutation in the biopsy tissue and cell block. Lindeman et al. reported that EGFR mutations can be detected from cytologic specimens, particularly if cell blocks are available. But the possibility of false-negative results of cytologic specimens remains an important problem.1
There is no consensus on the treatment of patients with coexsiting ALK rearrangement and EGFR mutation. In current literature (Table 1),4–11 four patients had a good response with progression-free survival (PFS) ranging from five to nine months. One patient even obtained a partial response for two years after gefitinib treatment. Five patients, including the present case, experienced progressive disease and showed resistance to EGFR-TKI. On the contrary, Maemondo et al. demonstrated that the median PFS after gefitinib treatment in EGFR mutated patients was 10.8 months.13 These results suggest that EGFR-TKI treatment in EGFR mutated patients is better than in patients with co-existing EGFR mutation and ALK rearrangement. Lee et al. reported two ALK-positive and EGFR-mutant NSCLC patients who did not respond to EGFR-TKI, but achieved a durable partial response to ALK inhibitors.4 Because ALK positive was presented at a frequency of 8.4% in unselected lung adenocarcinoma,14 it might be necessary for EGFR mutated patients to take an ALK test as soon as possible when expected effects are not received after targeted treatment.
Table 1.
Clinic-pathological characteristics and treatment outcome of NSCLC with concomitant EGFR mutation and ALK rearrangement
| Patient | Age | Gender | Histology | TNM Stage | Smoker | EGFR mutation | ALK translocation | Response to EGFR TKI | Response to ALK TKI |
|---|---|---|---|---|---|---|---|---|---|
| J.K. Lee et al.4 | 73 | M | ACA | IV | Yes | (Exon19)L747-E749(A750P) | FISH+ | PD | PR, 9 months |
| Miyanaga et al.5 | 55 | F | ACA | IV | No | (Exon19)L747-A750 | FISH+ | PD | SD, 4 months |
| Chen et al.6 | 56 | M | ACA | IV | Yes | (Exon19)E746-A750 | FISH+, RT-PCR+ | SD, 8 months | CR, >22 months |
| Chiari et al.7 | 67 | F | ACA | IV | No | (Exon21)L858R | FISH+ | PR,24 months | PR, 25 months |
| Kuo et al.8 | 72 | F | ACA | IV | No | (Exon19)E746-A750 | RT-PCR+ | PR 7 months | NA |
| Tiseo et al.9 | 48 | M | Adenosq. | IV | No | (Exon19)E746-A750 | FISH+ | PD | NA |
| Tanaka et al.10 | 39 | M | ACA | UK | Yes | (Exon21)L858R | RT-PCR+ | PD | NA |
| Sasaki et al.11 | UK | UK | UK | UK | UK | (Exon21)L858R | FISH+ | PR, 9months | NA |
| Sasaki et al.11 | UK | UK | UK | UK | UK | (Exon19)E746-A750 | FISH+ | PR, 5months | NA |
| our case | 47 | F | ACA | IV | No | (Exon19)E746-A750 | FISH+ | PD | NA |
ACA, adenocarcinoma; Adenosq, adenosquamous carcinoma; ALK, anaplastic lymphoma kinase; CR, complete response; EGFR, epidermal growth factor receptor; F, female; FISH, fluorescence in situ hybridization; M, male; NA, not applicable; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; RT-PCR, reverse transcription-polymerase chain reaction; SD, stable disease; TKI, tyrosine kinase inhibitor; TNM, tumor node metastasis; UK, Unknown.
Conclusion
In summary, although the treatment strategy of patients concomitant with both genes has not reached consensus, once expected effects have not been received in EGFR mutated patients after EGFR targeted treatment, detection of ALK rearrangement is necessary. For suspected cases, dual diagnostic testing should be considered to accurately identify lung adenocarcinoma molecular typing.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81101768) and the State Scholarship Fund of China (No. 201308330145).
Disclosure
No authors report any conflict of interest.
References
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8:823–859. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YR, Park HY, Jeon K, et al. EGFR and KRAS mutation analyses from specimens obtained by bronchoscopy and EBUS-TBNA. Thorac Cancer. 2013;4:264–272. doi: 10.1111/1759-7714.12006. [DOI] [PubMed] [Google Scholar]
- Boland JM, Jang JS, Li J, et al. MET and EGFR mutations identified in ALK-rearranged pulmonary adenocarcinoma: molecular analysis of 25 ALK-positive cases. J Thorac Oncol. 2013;8:574–581. doi: 10.1097/JTO.0b013e318287c395. [DOI] [PubMed] [Google Scholar]
- Lee JK, Kim TM, Koh Y, et al. Differential sensitivities to tyrosine kinase inhibitors in NSCLC harboring EGFR mutation and ALK translocation. Lung Cancer. 2012;77:460–463. doi: 10.1016/j.lungcan.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Miyanaga A, Shimizu K, Noro R, et al. Activity of EGFR- tyrosine kinase and ALK inhibitors for EML4-ALK- rearranged non-small-cell lung cancer harbored coexisting EGFR mutation. BMC Cancer. 2013;13:262. doi: 10.1186/1471-2407-13-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang J, Hu Q, Li X, Zhou C. A case of lung adenocarcinoma harboring exon 19 EGFR deletion and EML4-ALK fusion gene. Lung Cancer. 2013;81:308–310. doi: 10.1016/j.lungcan.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Chiari R, Duranti S, Ludovini V, et al. Long-term response to gefitinib and crizotinib in lung adenocarcinoma harboring both epidermal growth factor receptor mutation and EML4-ALK fusion gene. J Clin Oncol. 2014;32:e30–32. doi: 10.1200/JCO.2012.47.7141. [DOI] [PubMed] [Google Scholar]
- Kuo YW, Wu SG, Ho CC, Shih JY. Good response to gefitinib in lung adenocarcinoma harboring coexisting EML4-ALK fusion gene and EGFR mutation. J Thorac Oncol. 2010;5:2039–2040. doi: 10.1097/JTO.0b013e3181f43274. [DOI] [PubMed] [Google Scholar]
- Tiseo M, Gelsomino F, Boggiani D, et al. EGFR and EML4-ALK gene mutations in NSCLC: a case report of erlotinib-resistant patient with both concomitant mutations. Lung Cancer. 2011;71:241–243. doi: 10.1016/j.lungcan.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Hayashi A, Morimoto T, et al. A case of lung adenocarcinoma harboring EGFR mutation and EML4-ALK fusion gene. BMC Cancer. 2012;12:558. doi: 10.1186/1471-2407-12-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Koivunen J, Ogino A, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71:6051–6060. doi: 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuononen K, Sarhadi VK, Wirtanen A, et al. Targeted resequencing reveals ALK fusion in non-small cell carcinomas detected by FISH, immunohistochemistry, and real-time RT-PCR: a comparison of four methods. Biomed Res Int. 2013;2013:757490. doi: 10.1155/2013/757490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhao J, Sun K, et al. Accurate and economical detection of ALK positive lung adenocarcinoma with semiquantitative immunohistochemical screening. PLoS ONE. 2014;9:e92828. doi: 10.1371/journal.pone.0092828. [DOI] [PMC free article] [PubMed] [Google Scholar]


