Abstract
Background
Tumor recurrence is the most common cause of treatment failure, especially after complete resection of pathological stage N2 non-small cell lung cancer (NSCLC). In this study, we investigated the clinicopathological characteristics in order to identify independent risk factors for postoperative recurrence.
Methods
Between January 2001 and December 2013, 96 patients who underwent surgical resection for pathological N2 NSCLC were retrospectively reviewed. Recurrence-free survival (RFS) was calculated by the Kaplan-Meier method to explore risk factors, while the Cox proportional hazard model was used to assess independent predictors.
Results
The median and five-year RFS rates were 15 months and 27.4%, respectively. Univariate analysis showed a significantly poorer prognosis for non-regional N2 metastasis, more than three metastatic N2 lymph nodes, multiple N2 station, and multiple N2 zone involvement. Multivariate analysis demonstrated that non-regional N2 metastasis (hazard ratio [HR] 1.857, 95% confidence interval [CI] 1.061–3.249, P = 0.030) and more than three metastatic N2 lymph nodes (HR 2.555, 95% CI 1.164–5.606, P = 0.019) were independent risk factors for RFS. Additionally, the incidence of non-regional N2 metastasis was higher in patients with a primary tumor in the left lower (57.1%) or right lower lobe (48.1%), followed by left upper (31.8%), right middle (14.3%) and right upper lobe (7.7%).
Conclusion
The combination of the distribution and number of metastatic N2 lymph nodes provides a more accurate prediction for N2 NSCLC regarding recurrence. Non-regional N2 metastasis could occur with a primary tumor in any lobe, but occurs more frequently in the lower lobe.
Keywords: Mediastinal lymph node, non-small cell lung cancer, recurrence, risk factors, stage N2
Introduction
Completely resected non-small cell lung cancer (NSCLC) with pathologically confirmed N2 disease affects a heterogeneous population, with reported five-year survival rate ranges from 25% to 50%.1–5 Tumor recurrence is the most common cause of treatment failure after surgical resection and the main obstacle for long-term survival. The identification of factors related to recurrence after surgery may help to predict the outcome of N2 disease and, subsequently, to select optimal postoperative therapeutic strategy. One of the reasons for the variance in prognosis may come from differences in the pattern of lymph node metastasis. In the classification of N2 disease by the pattern of mediastinal lymph node (MLN) metastasis, various subgroups may be recognized. Previous studies have reported that prognosis was affected by these subgroups. Therefore, a new classification of N2 disease has been proposed. In our study, the incidence of recurrence was investigated in patients diagnosed with pathological N2 NSCLC, and factors affecting recurrence-free survival (RFS) based on the patterns of MLN metastases were examined.
Patients and methods
Patients
We retrospectively reviewed the records of 96 patients who underwent complete resection for pathological N2 NSCLC between January 2001 and December 2013. Inclusion criteria for the current study were as follows: standard major lung resection was performed including lobectomy, bilobectomy or pneumonectomy; complete resection without either microscopic or macroscopic residual tumor; at least three hilar and interlobar nodes and at least three N2 stations (including the subcarinal station) lymph nodes were completely dissected; and histologically proven NSCLC stage T1-4N2M0 according to the 7th edition of the tumor node metastasis (TNM) classification.6 Patients who received adjuvant therapy were included in the study. Patients who had neoadjuvant therapy (chemotherapy and/or radiation therapy), and those who presented with simultaneous or sequential second primary cancers were excluded. Patients who died of complications within 30 days after surgery were excluded. This study was reviewed and approved by the institutional review board in our hospital.
Preoperative evaluation generally included clinical assessment, blood tests, computed tomography (CT) of the chest, CT or ultrasonography of the abdomen, CT or magnetic resonance imaging (MRI) of the brain, whole-body bone scintigraphy in all cases, and positron emission tomography (PET)/PET-CT in recent cases. In this study, patients with MLN enlargement (>1 cm) in the short axis on CT scan were considered to have cN2 lesions; mediastinoscopies or endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) were only performed to exclude N3 disease in cases of suspected contra-lateral mediastinal lymphadenopathy. For patients with pre-operatively diagnosed N2 disease, a multidisciplinary team including experienced thoracic surgeons devised the therapeutic plan; we considered patients with unresectable (particularly bulky, multiple station) N2 disease to have a contraindication for initial surgical resection and recommended induction chemotherapy. We recommended that patients undergo adjuvant chemotherapy based on platinum doublets, based on consideration of the performance status of patients after surgery.
Definition of regional and non-regional N2 metastasis
The lymph node map proposed by the International Association for the Study of Lung Cancer (IASLC) was used for lymph node and N2 zone classification.7 Furthermore, regional N2 metastasis was defined as the involvement of an upper or aortopulmonary (AP) zone for a main tumor located in the left upper lobe, upper zone in the right upper lobe, upper or subcarinal zone in the right middle lobe, and subcarinal or lower zone in both lower lobes; non-regional N2 metastasis was defined as the involvement of the subcarinal or lower zone in both upper lobes, lower zone in the right middle lobe, upper or AP zone in the left lower lobe, or upper zone in the right lower lobe (Table 1).
Table 1.
Categorization of N2 based on the site of primary tumor and N2 zone involved
| Location of primary tumor | Regional N2 | Non-regional N2 |
|---|---|---|
| LUL | Upper zone, AP zone | Subcarinal zone, lower zone |
| LLL | Subcarinal zone, lower zone | Upper zone,AP zone |
| RUL | Upper zone | Subcarinal zone, lower zone |
| RML | Upper zone, subcarinal zone | Lower zone |
| RLL | Subcarinal zone, lower zone | Upper zone |
AP, aortopulmonary; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Follow-up and data collection
Patients were followed up every one to two months and systemically checked every three months after surgery for the first two years and then every six to 12 months thereafter. The postoperative systemic check included clinical assessments, serum levels of tumor marker, chest CT, CT or ultrasonography of the abdomen, CT or MRI of the brain, and whole-body bone scintigraphy. The choices of telephone interviews, mail or direct outpatient clinic visits were offered to each subject. A trained staff member collected information on survival and recurrence during each follow-up session. All of the patients were followed. The follow-up period lasted from the date of surgery to the date of the first recurrence or the last date of follow-up without recurrence. The mean follow-up period was 30 months (range: 6–152 months). Recurrences were diagnosed by physical examination and diagnostic imaging. Histological or cytological confirmation of the recurrence was made when clinically feasible. Second primary lung cancer was differentiated from recurrence according to the criteria proposed by Martini et al.8
To determine prognostic factors, data were recorded and evaluated in statistical analysis on the basis of age, gender, smoking status, tumor location, pathological T factor, histology, adjuvant therapy, and N2-related factors including: distribution of N2 metastases (regional or non-regional), highest MLN status, subcarinal zone status, presence of skip N2 metastasis (N2 nodal metastasis without N1 nodal metastasis), clinical N status (cN0–N1 or cN2), number of N2 stations involved (single or multiple), and number of N2 zones involved (single or multiple).
Statistical methods
All statistical analyses were performed using SPSS version 20 software (IBM Co., Armonk, NY, USA). The RFS was calculated according to the Kaplan-Meier and life-table methods. The significance of comparisons was examined using the log-rank test. Multivariate analyses were performed by means of a Cox proportional hazards model. The mean shrinkage (95% confidence interval) was calculated. Differences were considered statistically significant when P-value <0.05.
Results
During the follow-up period, recurrence was observed in 65 patients: 46 patients had local-regional recurrences; distant metastases were identified in 14 cases; and both local-regional recurrence and distant metastases occurred in five cases. The most common site of local-regional recurrence was the mediastinal lymph node in 30 cases, followed by the lung in 15 cases, pleura in eight cases, and bronchial stump in two cases. The brain was the most common site of distant metastases (8 cases), followed by bone (7 cases), and liver (6 cases). The median RFS and five-year RFS rate for all 96 patients with pN2 status was 15 months and 27.4%, respectively. Univariate analysis showed a significantly poorer prognosis regarding non-regional N2 metastasis (Fig. 1), metastatic N2 lymph nodes >3 (Fig. 2), multiple N2 stations, and multiple N2 zones involved (Table 2).
Figure 1.
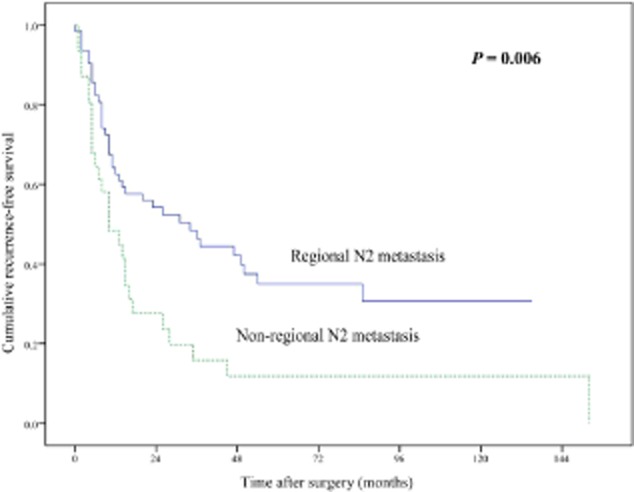
Comparison of recurrence-free survival between patients with regional N2 metastasis and non-regional N2 metastasis (P = 0.006).
Figure 2.
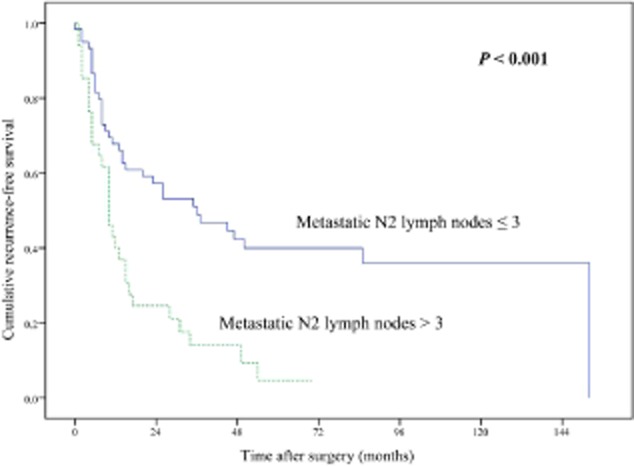
Comparison of recurrence-free survival between patients with metastatic N2 lymph nodes ≤3 and >3 (P < 0.001).
Table 2.
Patient characteristics and comparison of RFS based on variables
| Variable | No. of patients | 5-year RFS (%) | P-value |
|---|---|---|---|
| Age | 0.540 | ||
| <65 | 42 | 33.3 | |
| ≥65 | 54 | 21.6 | |
| Gender | 0.961 | ||
| Male | 63 | 32.7 | |
| Female | 33 | 19.4 | |
| Smoking history | 0.445 | ||
| Yes | 63 | 26.2 | |
| No | 33 | 29.9 | |
| Extent of resection | 0.258 | ||
| Lobectomy | 82 | 29.1 | |
| Bilobectomy | 11 | 18.2 | |
| Pneumonectomy | 3 | 33.3 | |
| Tumour location | 0.103 | ||
| LUL | 22 | 38.6 | |
| LLL | 14 | 24.5 | |
| RUL | 26 | 29.4 | |
| RML | 7 | 42.9 | |
| RLL | 27 | 17.9 | |
| Histology | 0.099 | ||
| Adenocarcinoma | 65 | 28.0 | |
| Others | 31 | 30.1 | |
| Adjuvant therapy | 0.126 | ||
| Chemotherapy | 46 | 32.9 | |
| Others | 50 | 21.8 | |
| pT stage | 0.061 | ||
| T1 | 13 | 44.9 | |
| T2 | 56 | 32.7 | |
| T3 | 23 | 16.8 | |
| T4 | 4 | 0 | |
| Distribution of metastatic N2 | 0.006 | ||
| Regional | 65 | 35.0 | |
| Non-regional | 31 | 11.8 | |
| Highest MLN status | 0.161 | ||
| Negative | 47 | 38.4 | |
| Positive | 49 | 15.8 | |
| Subcarinal zone status | 0.073 | ||
| Negative | 51 | 31.5 | |
| Positive | 45 | 24.2 | |
| Skip N2 metastasis | 0.434 | ||
| Yes | 37 | 29.5 | |
| No | 59 | 26.5 | |
| Clinical N status | 0.505 | ||
| cN0-1 | 60 | 29.5 | |
| cN2 | 36 | 23.8 | |
| No. of metastatic N2 lymph nodes | 0.000 | ||
| ≤3 | 62 | 40.0 | |
| >3 | 34 | 4.7 | |
| N2 station involved | 0.002 | ||
| Single | 51 | 43.8 | |
| Multiple | 45 | 11.3 | |
| N2 zone involved | 0.009 | ||
| Single | 56 | 40.0 | |
| Multiple | 40 | 12.5 |
LLL, left lower lobe; LUL, left upper lobe; MLN, mediastinal lymph node; RFS, recurrence-free survival; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
The joint effects of these four factors were examined using a Cox proportional hazard model. Multivariate analysis confirmed that non-regional N2 metastasis and metastatic N2 lymph nodes >3 were the independent and significant factors predicting RFS (hazard ratio [HR], 1.857; P = 0.030 and HR, 2.555; P = 0.019, respectively) (Table 3).
Table 3.
Comparison of factors affecting RFS in multivariate analysis using a Cox proportional hazard model
| Variable | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Distribution of metastatic N2 (non-regional/regional) | 1.857 (1.061–3.249) | 0.030 |
| No. of metastatic N2 lymph nodes (>3/≤3) | 2.555 (1.164–5.606) | 0.019 |
| N2 station involved (multiple/single) | 1.647 (0.661–4.102) | 0.284 |
| N2 zone involved (multiple/single) | 0.532 (0.199–1.421) | 0.208 |
CI, confidence interval; RFS, recurrence-free survival.
The distribution and number of metastatic N2 lymph nodes were combined to define an accurate risk classification for predicting prognosis. The patients were stratified into three risk groups: regional N2 metastasis and metastatic N2 lymph nodes 1–3 were placed in the low-risk group; regional N2 metastasis and metastatic N2 lymph nodes >3 or non-regional N2 metastasis and N2 lymph nodes 1–3 were in the medium-risk group; and non-regional N2 metastasis and metastatic N2 lymph nodes >3 were in the high-risk group. There were 48, 31, and 17 patients in the low, medium, and high-risk groups, respectively. The survival data of these groups decreased along with the increased risk, and the differences between each group were statistically significant (Fig. 3) (Table 4).
Figure 3.
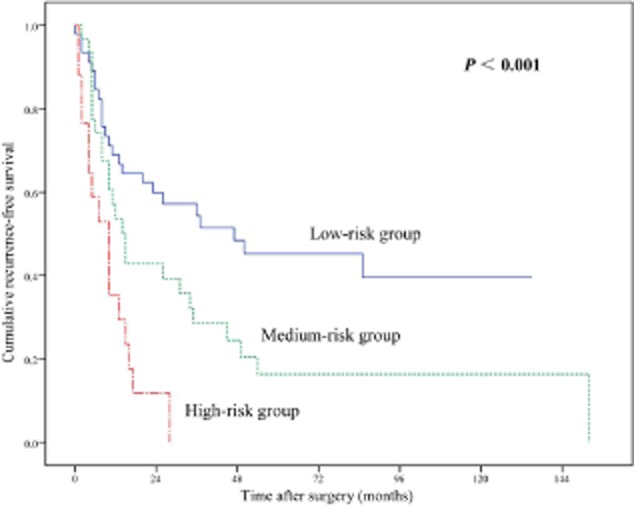
Recurrence-free survival according to risk classification.
Table 4.
Comparison of 5-year RFS rateaccording to risk classification
| Subgroup | No. of patients | 5-year RFS rate (%) | Reference | P-value |
|---|---|---|---|---|
| Low risk | 48 | 45.2 | Vs. high risk | 0.000 |
| Medium risk | 31 | 16.3 | Vs. low risk | 0.029 |
| High risk | 17 | 0 | Vs. medium risk | 0.009 |
RFS, recurrence-free survival.
The relationship between the site of the primary tumor and involved N2 zone was investigated: the incidence of non-regional N2 metastasis was higher in patients with a primary tumor in the left lower (57.1%) or right lower lobe (48.1%), followed by left upper (31.8%), right middle (14.3%) and right upper lobe (7.7%) (Table 5).
Table 5.
Relationship between the site of primary tumour and involved N2 zone
| Location of primary tumor | No. of patients | Non-regional N2 metastasis (%) | Upper zone | AP zone | Subcarinal zone | Lower zone |
|---|---|---|---|---|---|---|
| LUL | 22 | 7 (31.8%) | 5 | 21 | 7 | 0 |
| LLL | 14 | 8 (57.1%) | 5 | 4 | 7 | 1 |
| RUL | 26 | 2 (7.7%) | 26 | 0 | 2 | 0 |
| RML | 7 | 1 (14.3%) | 3 | 0 | 6 | 1 |
| RLL | 27 | 13 (48.1%) | 13 | 0 | 23 | 3 |
AP, aortopulmonary; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Discussion
The TNM system remains the most important and principal method for assessing the extent of disease, determining prognosis and affecting treatment strategies. The presence and extent of metastasis to the ipsilateral mediastinal lymph nodes (N2) are important prognostic factors in completely resected NSCLC. However, resectable N2 NSCLC represents a heterogeneous group.9 Many studies have investigated various clinicopathologic factors to identify more accurate predictors for relapse, such as the number of N2 stations or N2 zones involved, the number or rate of N2 lymph nodes involved,10 the presence of a skip metastasis, metastasis to the highest MLN, and metastasis to the subcarinal lymph node.11,12 These factors may help us to select therapeutic strategies for individual patients with the same pathological stage of disease. In our study, the combination of the distribution and number of metastatic N2 lymph nodes provides a more powerful predictor for postoperative recurrence.
As patients with advanced stage NSCLC will receive multidisciplinary treatment after surgical resection, therapeutic strategies including chemotherapy, radiotherapy, and targeted therapy significantly prolong overall survival even after local-regional relapse or distant metastases. Therefore, multiple lines of therapy and non-malignant causes of death potentially diluted overall survival; it may not be the best end point for assessing the efficacy of initial resection based on the patterns of MLN metastases. Additionally, recurrences remain the major causes of treatment failure in completely resected NSCLC. For these reasons, recurrence-free survival was chosen as a valid and reasonable surrogate outcome in our study.
The relation between primary tumor location and N2 involved represents an important risk factor for recurrence-free survival. The concept of lobe-specific nodes was reported to describe the phenomenon that lymphatic drainage generally follows a fixed route. The most frequently metastatic N2 were considered to be the regional nodes nearest to the primary tumor along the mediastinal lymphatic drainage route.13,14 However, metastatic non-regional N2 (i.e. subcarinal metastases from an upper-lobe tumor, or superior mediastinal metastases from a lower-lobe tumor) located far from the primary tumor had been observed, and our study demonstrated that this phenomenon had a poor outcome even when these lymph nodes were systematically dissected. Some previous studies support our results. Asamura et al.14 reported that superior mediastinal metastasis should be recognized as an indicator of a poor prognosis in tumors of both lower lobes. Okada et al.15 reported that patients with subcarinal nodes involved from upper-lobe tumors had a significantly worse prognosis than those patients with metastases only to the upper mediastinal or aortic nodes, and patients with nodal involvement of the upper mediastinum from lower-lobe tumors had a significantly worse survival than those patients with metastases limited to the lower mediastinum. These findings indicated that non-regional N2 metastasis correlated with more aggressive disease and poor prognosis.
The number of metastatic lymph nodes is prognostically significant in many forms of cancer including NSCLC.16–19 Furthermore, in the current TNM staging system, the number of metastatic lymph nodes is included in the definition of pN factors in stomach, esophageal, colorectal, and breast cancers, but has not yet been incorporated in lung cancer.20 There is increasing evidence that the number of metastatic lymph nodes may be stronger prognostic indicators than current nodal classification. Fukui et al.16 classified nodal categories according to the number of metastatic lymph nodes as 1–3, 4–6, and >6, and Lee et al.9 reviewed 1478 patients who were divided into four subgroups according to the number of metastatic lymph nodes; both determined that the new category was a significant prognostic indicator. In our study, the number of metastatic N2 lymph nodes provides an independent risk factor for postoperative recurrence.
Considering that a combination of the two independent risk factors may provide us with a more accurate method of prediction, we divided the study population into three categories according to the distribution and number of metastatic N2 lymph nodes. Survival data showed significant differences between each subgroup. The new risk stratification system provides a more powerful risk factor for postoperative recurrence. The prognosis of patients with non-regional N2 metastasis and metastatic N2 lymph nodes >3 was extremely poor.
We investigated the ratio of non-regional N2 metastasis for each tumor-bearing lobe and observed that the incidence of non-regional N2 metastasis was higher in both lower lobes than in the upper and middle lobes. Watanabe et al.21 noted that about half of the right middle/lower lobe NSCLC had nodal metastases to the upper mediastinum; nodal metastases to the lower mediastinum from upper lobe NSCLC were found in one-third of cases. Saeteng et al.22 also reported that 40% of lower lobes had nodal metastasis to the upper mediastinal lymph nodes, while 20% of upper lobes had nodal metastasis to the lower mediastinal lymph nodes. On the basis of these findings and our present data, systemic nodal dissection is strongly recommended for stage N2 NSCLC, especially for lower lobe lesions, not only to obtain an accurate staging of the disease, but also to eradicate intrathoracic disease. The method of selective lymph node dissection or lobe-specific nodal removal is insufficient for cancers of advanced stages.
Conclusion
The distribution (regional vs. non-regional) and number (>3 vs. ≤3) of metastatic N2 lymph nodes were found to influence the RFS of patients with completely resected stage N2 NSCLC. Risk classification based on the two factors can be a useful tool to identify patients at high risk for recurrence. Therefore, it can contribute to the adequate selection of an optimal postoperative therapeutic strategy. However, we acknowledge that as a single-institution and retrospective analysis, our sample size was limited; therefore, validity of the new N2 stratification method needs to be further explored.
Acknowledgments
This work was supported by a grant from the National Key Clinical Speciality Construction Program [2011]873.
Disclosure
No authors report any conflict of interest.
References
- Inoue M, Sawabata N, Takeda S, Ohta M, Ohno Y, Maeda H. Results of surgical intervention for p-stage IIIA (N2) non-small cell lung cancer: acceptable prognosis predicted by complete resection in patients with single N2 disease with primary tumor in the upper lobe. J Thorac Cardiovasc Surg. 2004;127:1100–1106. doi: 10.1016/j.jtcvs.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Saito M, Kato H. Prognostic factors in patients with pathological and N2 non-small cell lung cancer. Ann Thorac Cardiovasc Surg. 2008;14:1–2. [PubMed] [Google Scholar]
- Nakagiri T, Sawabata N, Funaki S, et al. Validation of pN2 sub-classifications in patients with pathological stage IIIA N2 non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2011;12:733–738. doi: 10.1510/icvts.2010.249896. [DOI] [PubMed] [Google Scholar]
- Tanaka F, Yanagihara K, Otake Y, et al. Prognostic factors in resected pathologic (p-) stage IIIA-N2, non-small-cell lung cancer. Ann Surg Oncol. 2004;11:612–618. doi: 10.1245/ASO.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Cerfolio RJ, Maniscalco L, Bryant AS. The treatment of patients with stage IIIa non-small cell lung cancer from N2 disease: who returns to the surgical arena and who survives. Ann Thorac Surg. 2008;86:912–920. doi: 10.1016/j.athoracsur.2008.04.073. [DOI] [PubMed] [Google Scholar]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–129. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg. 2008;85:211–215. doi: 10.1016/j.athoracsur.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Ito M, Yamashita Y, Tsutani Y, et al. Classifications of n2 non-small-cell lung cancer based on the number and rate of metastatic mediastinal lymph nodes. Clin Lung Cancer. 2013;14:651–657. doi: 10.1016/j.cllc.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Sakao Y, Miyamoto H, Yamazaki A, et al. Prognostic significance of metastasis to the highest mediastinal lymph node in non-small lung cancer. Ann Thorac Surg. 2006;81:292–297. doi: 10.1016/j.athoracsur.2005.06.077. [DOI] [PubMed] [Google Scholar]
- Aokage K, Yoshida J, Ishii G, Hishida T, Nishimura M, Nagai K. Subcarinal lymph node in upper lobe non-small cell lung cancer patients: is selective lymph node dissection valid? Lung Cancer. 2010;70:163–167. doi: 10.1016/j.lungcan.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Terashima M, Koike T, Akamatsu H, Kurita Y, Yokoyama A. Mediastinal lymph node metastasis in patients with clinical stage I peripheral non-small-cell lung cancer. J Thorac Cardiovasc Surg. 1997;113:248–252. doi: 10.1016/S0022-5223(97)70320-9. [DOI] [PubMed] [Google Scholar]
- Asamura H, Nakayama H, Kondo H, Tsuchiya R, Naruke T. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg. 1999;117:1102–1111. doi: 10.1016/s0022-5223(99)70246-1. [DOI] [PubMed] [Google Scholar]
- Okada M, Tsubota N, Yoshimura M, Miyamoto Y, Matsuoka H. Prognosis of completely resected pN2 non-small cell lung carcinomas: what is the significant node that affects survival? J Thorac Cardiovasc Surg. 1999;118:270–275. doi: 10.1016/S0022-5223(99)70217-5. [DOI] [PubMed] [Google Scholar]
- Fukui T, Mori S, Yokoi K, Mitsudomi T. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol. 2006;1:120–125. [PubMed] [Google Scholar]
- Tepper JE, O'Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19:157–163. doi: 10.1200/JCO.2001.19.1.157. [DOI] [PubMed] [Google Scholar]
- Weir L, Speers C, D'yachkova Y, Olivotto IA. Prognostic significance of the number of axillary lymph nodes removed in patients with node-negative breast cancer. J Clin Oncol. 2002;20:1793–1799. doi: 10.1200/JCO.2002.07.112. [DOI] [PubMed] [Google Scholar]
- Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002;167:1295–1298. [PubMed] [Google Scholar]
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. 7th edn. Oxford: Wiley-Blackwell; 2010. [Google Scholar]
- Watanabe Y, Shimizu J, Tsubota M, Iwa T. Mediastinal spread of metastatic lymph nodes in bronchogenic carcinoma. Mediastinal nodal metastases in lung cancer. Chest. 1990;97:1059–1065. doi: 10.1378/chest.97.5.1059. [DOI] [PubMed] [Google Scholar]
- Saeteng S, Tantraworasin A, Euathrongchit J, Lertprasertsuke N, Wannasopha Y. Nodal involvement pattern in resectable lung cancer according to tumor location. Cancer Manag Res. 2012;4:151–158. doi: 10.2147/CMAR.S30526. [DOI] [PMC free article] [PubMed] [Google Scholar]


