Abstract
The INK4 family of cyclin-dependent kinase (CDK) inhibitors negatively regulates cyclin D-dependent CDK4 and CDK6 and induces the growth-suppressive function of Rb family proteins. Mutations in the Cdk4 gene conferring INK4 resistance are associated with familial and sporadic melanoma in humans and result in a wide spectrum of tumors in mice, suggesting that INK4 is a major regulator of CDK4. Mice lacking the Cdk4 gene exhibit various defects in many organs associated with hypocellularity, whereas loss of the p18Ink4c gene results in widespread hyperplasia and organomegaly. To genetically test the notion that the function of INK4 is dependent on CDK4, we generated p18; Cdk4 double-mutant mice and examined the organs and tissues which developed abnormalities when either gene is deleted. We show here that, in all organs we have examined, including pituitary, testis, pancreas, kidney, and adrenal gland, hyperproliferative phenotypes associated with p18 loss were canceled. The double-mutant mice exhibited phenotypes very close to or indistinguishable from that of Cdk4 single-mutant mice. Mice lacking p27Kip1 develop widespread hyperplasia and organomegaly similar to those developed by p18-deficient mice. The p27; Cdk4 double-mutant mice, however, displayed phenotypes intermediate between those of p27 and Cdk4 single-mutant mice. These results provide genetic evidence that in mice p18Ink4c and p27Kip1 mediate the transduction of different cell growth and proliferation signals to CDK4 and that p18Ink4c is functionally dependent on CDK4.
The progression of eukaryotic cells through different phases of mitotic division is controlled primarily by the cyclin-dependent kinase (CDK) whose activity is in turn balanced by its activation by a requisite cyclin subunit and its inhibition by a CDK inhibitor. In mammalian cells, there exist two distinct families of CDK inhibitors. The p21 family includes three related proteins, p21Cip1/Waf1, p27Kip1, and p57Kip2, which evolved from a common ancestor that predates Caenorhabditis elegans and plants. The p16 family consists of four closely related members, p16Ink4a, p15Ink4b, p18Ink4c, and p19Ink4d, and evolved later, after the emergence of vertebrates.
The physiologic significance of evolving a separate family of CDK inhibitors and multiple members within each family in mammalian cells is presumed to meet increasing needs for integrating more intricate and multifaceted cell growth signals, both intracellular and extracellular, into a single cell cycle control machinery. Supporting this notion are the observations that the expression of individual CDK inhibitor genes is activated selectively by different checkpoint pathways, displays distinct temporary and spatial patterns during both in vitro cell differentiation and in vivo embryonic development, and is maintained differentially in different adult and senescent tissues. Further support for different physiologic functions of individual CDK inhibitor genes in vivo comes from the genetic analyses of mutant strains of mice with targeted mutation in each of seven individual CDK inhibitor genes. Various different phenotypes were observed in these mice, ranging from lack of major defects after the loss of the p15 or the p19 gene (27, 55), compromised DNA damage response after inactivation of the p21 gene (4, 8), increase of tumor development resulting from loss of function of p16 (25, 45), and severe developmental defects after p57 mutation (54) to widespread hyperplastic cell proliferation and organomegaly in p18 and p27 null mice (14, 15, 23, 27, 37). Elucidation of the molecular pathways linking various cell growth signals to the expression of individual CDK inhibitor genes, however, remains a major challenge and has only been validated in a few exceptional cases. Two examples are the p53-mediated activation of p21 gene expression following DNA damage (11) and transforming growth factor β-mediated induction of p15Ink4b (17).
Biochemically, CDK inhibitors within each family act almost indistinguishably in binding to and regulating CDK enzymes but differ among the families. The CIP/KIP proteins share a unique N-terminal sequence motif comprising two subdomains for binding cooperatively to and forming ternary complexes with CDK and cyclin subunits. The CIP/KIP proteins diverge in their C-terminal sequences. The INK4 proteins, on the other hand, consist essentially of four or five tandem copies of ankyrin repeats and few additional sequences. Unlike CIP/KIP inhibitors, which are capable of interacting with multiple CDK-cyclin complexes, the only binding partners and functional targets identified thus far for INK4 proteins are two very closely related catalytic CDK subunits, CDK4 and CDK6. Such functional dependency of INK4 on CDK4 or CDK6, however, has not yet been tested genetically.
Ectopic overexpression of individual INK4 genes causes a G1 cell cycle arrest with a correlative dependency on the intact Rb pathway (16), and loss of either Rb function or a combination of p107 and p130 functions effectively canceled the G1 arrest due to INK4 overexpression (3, 24, 35). These findings provide evidence that, at least in cultured cells, the function of INK4, and CDK4 and CDK6 by extension, in controlling the G1-to-S transition is dependent on the presence of both an intact Rb and p107-p130 functions. Hence, there may exist in vivo a linear INK4-CDK4/CDK6 (CDK4/6)-Rb G1 control pathway in mammalian cells. Delineating the pathway(s) for p21 family inhibitors in vivo is more complicated and remains somewhat perplexing; this pathway is attributed largely to their interaction with both the CDK and cyclin subunits and with multiple CDK-cyclin complexes. It was initially observed that p21 levels undergo an increase immediately following mitogenic stimulation of serum-starved human fibroblasts, before declining at the G1-S boundary (29), and that CDK and cyclin can be found in active CDK-cyclin complexes at a one-to-one ratio when expressed at a low concentration (18, 53). Later studies found that the assembly and kinase activity of CDK4-cyclin D correlate concomitantly with the binding of CIP/KIP proteins (26) and were reduced in mouse embryonic fibroblasts (MEFs) lacking p21 and p27 (6). A titration model—cyclin Ds-CDK4/6 complexes act as activators of cyclin Es-CDK2 complexes by titrating CIP/KIP proteins away from, and thus releasing the inhibition of, cyclin Es-CDK2 complexes—was proposed to accommodate these observations, which seemingly contradict the classification of CIP/KIP as a CDK inhibitor. According to this model, CIP/KIP genes can be considered to act genetically downstream of cyclin Ds-CDK4 complexes in an INK4-cyclin Ds/CDK4-CIP/KIP-cyclin Es/CDK2-Rb pathway. Results challenging both notions—that p21 only stoichiometrically inhibits cyclin A-CDK2 and that p21-p27 deficiency reduced cyclin D-CDK4 assembly and activity—were reported (1, 19), leaving the mechanistic role of CIP/KIP proteins in regulating cyclin Ds-CDK4/6 and cyclin Es-CDK2 complexes at an incompletely understood and somewhat confusing state at present.
The p18Ink4c and p27Kip1 genes represent two of the most extensively studied CDK inhibitor genes in mice. Loss of function of either gene resulted in similar and widespread defects in cell proliferation and organ development, providing suitable models for investigating the functional and mechanistic differences between the two families of CDK inhibitors in living animals. In this study, we set forth to test genetically in double-mutant mice the following questions: is the function of INK4 to inhibit CDK4, and how does the loss of Cdk4 and a gene from either CDK inhibitor family affect each other phenotypically in mice?
MATERIALS AND METHODS
Generation of mouse strains.
The generation of p18 and Cdk4 mutant mice has been described previously (15, 49). Both have been backcrossed into and maintained on an enriched C57BL/6 background as previously reported (2, 49). Mice deficient for p18 were bred to the mice heterozygous for Cdk4 to create double-heterozygous mice. Mice heterozygous for p18 and Cdk4 were intercrossed to generate all of the genotypes analyzed in this study. Animals were genotyped by PCR and monitored as described previously (15, 49). Cohorts were housed and analyzed in a common setting, and littermate controls were used for all experiments.
Analysis of fertility and diabetes.
Mating ability and fertility of mice were determined by keeping each mutant and a wild-type fertile partner together in a cage and checking daily for the presence of a vaginal plug. Females with plugs were separated and monitored throughout pregnancy, delivery, and nursing. Blood glucose level was monitored in the morning (8 to 10 a.m.) by using an automatic glucose monitor (Glucometer Elite; Bayer). Each mouse was analyzed twice for blood glucose on two consecutive days for every time point.
Histopathology.
Tissues of most organs were removed, fixed in 10% neutral buffered formalin, and examined histologically by two pathologists after hematoxylin-eosin staining. Lesions were photographed, and additional sections were taken for immunohistochemical analyses.
Pancreatic islet size determination.
Pancreatic islet size (cells per islet section) was measured as previously reported with minor modifications (33). Briefly, islet size was determined in at least three cut sections from matched pancreatic regions of three animals per genotype at each stage of development. Sections were more than 500 μm from each other to avoid overestimating larger islets in this analysis.
Immunohistochemistry.
To measure proliferating and mitotic cells, sections were blocked with normal goat serum in phosphate-buffered saline (PBS) and incubated with either a polyclonal antibody against mitosis-specific phosphorylated histone H3 (5 μg ml−1; Upstate Biotechnology) or a polyclonal antibody against Ki67 (1:1,000; NCL-Ki67; Novocastra Laboratories) for 1 h and with a biotin-conjugated secondary antibody (Vector Laboratories) for 30 min. Immunocomplexes were detected with the Vectastain ABC alkaline phosphatase kit according to the manufacturer's instructions (Vector Laboratories). For apoptosis assays, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays were carried out using the in situ ApopTag kit (Intergen) according to the manufacturer's protocol.
MEFs and flow cytometry procedures.
Primary MEFs were isolated from embryonic day 13.5 embryos. Early-passage MEFs (younger than passage 4) from individual embryos were plated in 100-mm plates and incubated in Dulbecco's modified Eagle medium (DMEM) plus 10% fetal bovine serum (FBS). For serum starvation, asynchronous cultures at approximately 50% confluence were washed with PBS and serum starved in DMEM containing 0.1% FBS for 72 h. Cells were restimulated by addition of DMEM with 10% FBS. For bromodeoxyuridine (BrdU) labeling, MEFs were grown in media with or without serum containing 10 μM BrdU for 1 h. Nuclei were isolated following trypsinization and fixation with 95% ethanol by incubation in 0.08% pepsin (Sigma)-0.1 N HCl for 20 min at 37°C. Nuclear DNA was denatured by incubation in 2 N HCl for 20 min at 37°C, followed by neutralization with Na2B4O7, pH 8.5. Incorporated BrdU was detected with anti-BrdU-fluorescein isothiocyanate (Becton Dickinson; 1:10 dilution) in 10 mM HEPES (pH 7.3)-150 mM NaCl-4% FBS-0.5% Tween 20. MEFs were then harvested and resuspended in PBS containing 1% FBS and incubated with propidium iodide (5 μg ml−1) and RNase A (0.1 mg ml−1) for 30 min at 37°C. The samples were analyzed with a B-D FACScan (Becton Dickinson), and the data were processed with Summit software (version 3.0; BD Biosciences).
Northern blot analysis.
MEFs at early passage were serum deprived (0.1% FBS) for 3 days and released from quiescence by serum stimulation (10% FBS). Total RNA was prepared from cells at different time points after serum stimulation as well as from an asynchronized cell population. RNA samples were resolved on a 1% agarose gel, transferred to a nitrocellulose filter, and hybridized with a probe derived from full-length mouse p18Ink4c cDNA. The blot was then stripped and rehybridized with a mouse GAPDH (glyceraldehyde-3-phosphate dehydrogenase) probe.
RESULTS
Loss of Cdk4 prevented gigantism of p18 mutant mice but only partially rescued gigantism of p27 mutant mice.
Mutant p18+/−; Cdk4+/−, p18−/−; Cdk4+/−, p18+/−; Cdk4−/−, and p18−/−; Cdk4−/− mice were generated from mating double-heterozygote p18+/−; Cdk4+/− mice. p18−/−; Cdk4−/− mice were born with a ratio of 4.4% (8 of 183), lower than their wild-type (9.3%, 17 of 183) and p18−/−; Cdk4+/+ (10.4%, 19 of 183) siblings, but comparable to Cdk4−/− littermates (3.8%, 7 of 183; Table 1). These results indicate that disruption of Cdk4 may induce a prenatal lethality with incomplete penetrance as previously reported (40) and that deletion of p18 has no significant effect on prenatal lethality caused by Cdk4 deficiency.
TABLE 1.
Incomplete penetrance of prenatal lethality in Cdk4 mutant and double-mutant micea
| Progeny | No (%) of mice with genotype:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| p18+/+; Cdk4+/+ | p18+/+; Cdk4+/− | p18+/+; Cdk4−/− | p18+/−; Cdk4+/+ | p18+/−; Cdk4+/− | p18+/−; Cdk4−/− | p18−/−; Cdk4+/+ | p18−/−; Cdk4+/− | p18−/−; Cdk4−/− | |
| Observed | 17 (9.3%) | 25 (13.7%) | 7 (3.8%) | 27 (14.8%) | 40 (21.9%) | 16 (8.7%) | 19 (10.4%) | 24 (13.1%) | 8 (4.4%) |
| Expected | 11 (6.3%) | 23 (12.5%) | 11 (6.3%) | 23 (12.5%) | 46 (25%) | 23 (12.5%) | 11 (6.3%) | 23 (12.5%) | 11 (6.3%) |
Newborn pups were assessed for viability and genotyped. Mice were derived from p18+/−; Cdk4+/− intercross (183 pups in 19 litters).
At birth, Cdk4−/−, p18−/−, and p18−/−; Cdk4−/− double-mutant mice of various genotypes appeared indistinguishable (data not shown). Soon after birth, however, various growth abnormalities were grossly apparent. While the p18−/− mice lived up to 1 year, at which time they developed both pituitary tumors and lymphomas, the majority of Cdk4−/− mice died early in life (<4 months) as previously reported (40, 49), but 5 to 10% of Cdk4−/− mice survived up to 1 year without diabetes (our unpublished results). The genetic basis for this incomplete penetrance of the diabetes phenotype resulting from Cdk4 loss is not clear at present. Loss of p18 had no significant effect on the postnatal mortality caused by Cdk4 loss or on the development of double-mutant mice. No p18−/−; Cdk4−/− (n = 9) or p18+/−; Cdk4−/− (n = 11) animal lived beyond 5 months.
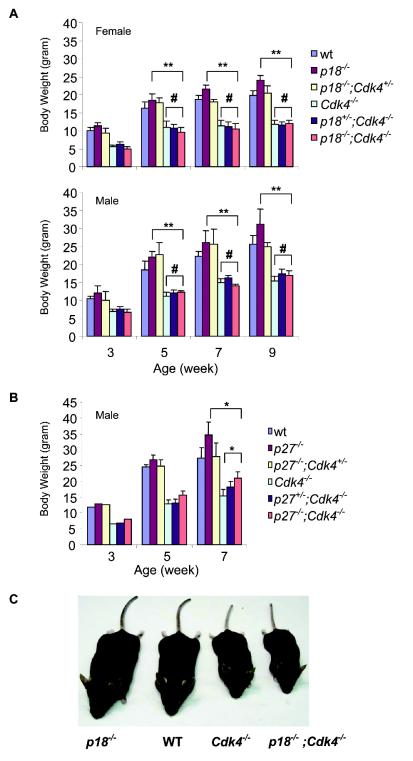
Growth retardation of p18−/−; Cdk4−/− mice became obvious during prepuberty, was persistent through the entire experimental duration of 9 weeks, and was observed in both sexes. The body weights of both p18−/−; Cdk4−/− and p18+/−; Cdk4−/− mice were lower than those of either p18−/− or wild-type mice and were statistically indistinguishable from those of Cdk4−/− single null mice (Fig. 1A and C). Thus, loss of Cdk4 led to a body weight phenotype that was completely dominant over loss of p18.
FIG. 1.
Effect of the loss of Cdk4, p18, and p27 on animal growth. (A) Body weight comparison for male and female mice of different genotypes. Mice from every genotype (four to six each) were weighed from age 3 to 9 weeks. Standard deviation bars are indicated. **, P < 0.001, for comparison of p18−/−; Cdk4−/− mice to p18−/− mice; #, P > 0.05 (no significant difference), for comparison of p18−/−; Cdk4−/− mice to Cdk4−/− mice (Student's t test). (B) Body weight comparison of Cdk4−/−, p27−/−, and p27−/−; Cdk4−/− mutant male mice. Mice from each genotype (three or four each) were weighed from age of 3 to 7 weeks. Standard deviation bars are indicated. *, P < 0.05, for comparison of p27−/−; Cdk4−/− mice to Cdk4−/− or p27−/− mice (Student's t test). (C) p18−/−, wild-type (WT), Cdk4−/−, and p18−/−; Cdk4−/− mice at 4 months of age.
Loss of function of the p27 gene similarly resulted in an increased body weight, as seen in mice with the p18 mutation (14, 23, 37). To determine how Cdk4 and p27 genes interact with each other in controlling the body weight, we analyzed the p27 Cdk4 double-mutant mice. The p27−/−; Cdk4−/− double-mutant mice exhibited a body weight intermediate between those of the single-homozygous mutant mice (Fig. 1B), indicating that loss of Cdk4 and p27 partially rescued the body weight abnormality caused by the loss of the other gene. Together, these results demonstrate that, although p18 and p27 gene disruption resulted in a wide range of similar phenotypes in mice, these two genes do not have the same mechanism for regulation of Cdk4 in vivo. While the phenotype caused by p18 inactivation was completely dependent on CDK4, the function of p27 was only partially dependent on CDK4.
Both male and female p18−/−; Cdk4−/− mice are infertile.
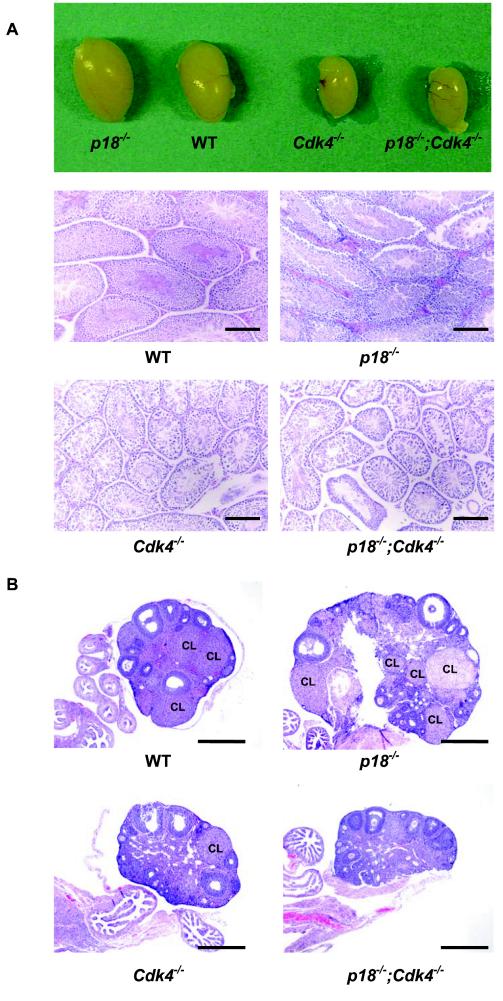
All p18−/−; Cdk4−/− mice, both males (n = 8) and females (n = 10), that we examined are infertile, and most (more than 90%) of Cdk4−/− males and all female mutants are sterile, as reported previously. In contrast to testes enlargement caused by p18 loss, the testes of 5-month-old p18−/−; Cdk4−/− mice were small and had sizes comparable to those of testes of Cdk4−/− mice (Fig. 2A). Histological analysis revealed that, while p18−/− male mice developed Leydig cell hyperplasia, the p18−/−; Cdk4−/− male mouse, like the Cdk4−/− mouse, contains abnormal seminiferous tubules with degeneration of a significant fraction of primary spermatocytes and reduced Leydig cells. Very few normal spermatozoa were found in the lumen of seminiferous tubules of both Cdk4−/− and p18−/−; Cdk4−/− mice at an age of 5 months (Fig. 2A). Hence, the seminiferous tubules in mice lacking both Cdk4 and p18 genes exhibited defects similar to those found in Cdk4−/− mice. Leydig cell proliferation associated with p18 loss was inhibited by Cdk4 loss.
FIG. 2.
Infertility of p18−/−; Cdk4−/− mice. (A) Gross appearance of testes from 5-month-old mice (upper row) and sections of testes stained with hematoxylin and eosin (HE) from mice of different genotypes at 5 months of age. WT, wild type. Bars, 200 μm. (B) Sections of ovary stained with HE from mice of different genotypes from the same litter at 4 months of age. The CL is indicated. Bars, 1 mm.
p18−/− females are fertile and can mate successfully with wild-type or p18−/− males (15). Litter numbers and sizes of mice produced from p18−/− matings were bigger than those for age-matched wild-type mating controls (F. Bai and Y. Xiong, unpublished data). p18−/− ovaries are bigger (twice as big as the wild type), displayed normal antral follicles, and formed many corpus lutea (CL). These results suggest that, despite ovarian enlargement, ovulation from p18−/− mice was not impaired. Cdk4−/− female mice, like p27−/− female mice, are infertile. Adult Cdk4−/− ovaries were 20 to 30% smaller than those of wild-type females and showed some well-developed antral follicles but very few CL (at most one CL in each ovary). Very similar to Cdk4−/− ovaries, p18−/−; Cdk4−/− ovaries were small and rarely developed CL at 4 months of age, when many CL were found in littermate wild-type and p18−/− control ovaries (Fig. 2), indicating that loss of p18 does not alleviate the infertility caused by defective luteal function in Cdk4-deficient mice.
Loss of p27 also resulted in the development of ovarian enlargement but impaired the function of ovulation and caused female sterility (14, 23, 37, 42, 47), indicating that p18 and p27 play different roles in ovulation and granulosa cell proliferation. p27−/−; Cdk4−/− female (as well as male) mice are infertile.
Development of pituitary hyperplasia in p18−/− mice requires CDK4 function.
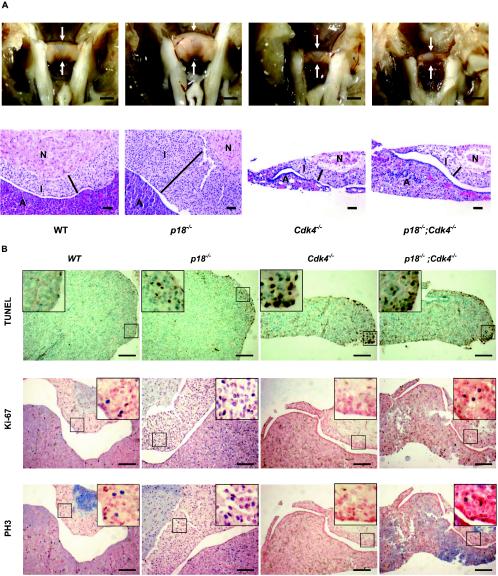
A hallmark defect in mice with reduced or complete loss of Rb function is the hyperplastic phenotype of the pituitary (20, 21, 28, 31, 51). Loss of p18 results in the development of the same hyperplastic phenotype in the intermediate lobe of the pituitary (15, 27) (Fig. 3A) and, conversely, loss of Cdk4 causes a pituitary hypoplasia and lactotroph dysfunction (36), offering an excellent in vivo setting to determine the genetic interaction between these two Rb regulators. We confirmed that pituitaries of Cdk4 null adult mice (4 months old) were significantly smaller and extremely hypoplastic relative to wild-type pituitaries in all three lobes: the intermediate lobe, the anterior lobe, and the neurohypophysis (Fig. 3A). The pituitaries of p18−/−; Cdk4−/− mice were as small as Cdk4 null pituitaries and also showed significant hypoplasia, which might be responsible for the infertility and defect in formation of CL in p18−/−; Cdk4−/− mice. Like those from pituitaries of Cdk4 single-knockout mice, the intermediate lobes from p18−/−; Cdk4−/− pituitaries were hypoplastic, indicating that the development of pituitary hyperplasia in p18−/− mice requires a functional CDK4. Late in life (>9 months), p18−/− mice developed pituitary adenomas in the intermediate lobe. Because of early death of p18−/−; Cdk4−/− mice, we were not able to demonstrate that loss of Cdk4 would prevent the development of pituitary adenomas.
FIG. 3.
Abnormalities of the pituitary in p18 and Cdk4 mutant mice. (A) Pituitary glands (arrows) from mice of different genotypes from the same litter were microscopically examined at 4 months of age either directly (top row) or after hematoxylin and eosin staining (bottom row). The anterior lobe (A), intermediate lobe (I), and neurohypophysis (N) are indicated. The width of the intermediate lobe is shown by the black bars. WT, wild type. Bars, 1 mm (top row) and 50 μm (bottom row) (B) Series of sections of pituitary glands from littermate mice of different genotypes at 4 months of age were examined for apoptotic cell death by TUNEL assay (top), for cell proliferation by Ki-67 staining (middle), and for mitotic activity by immunostaining with an antibody recognizing phosphorylated histone H3 (bottom). Arrows, positive (brown staining or blue staining) cells. Bars, 200 μm.
To determine the cellular basis of functional dependency of p18 on Cdk4, we examined two cellular defects, apoptotic cell death and cell proliferation, often associated with an alteration of genes involved in the Rb pathway. Pituitary glands were isolated from mice of different genotypes. Serial sections were examined by TUNEL assay to determine the level of apoptosis, by Ki-67 staining to determine the level of cell proliferations, and by staining with an antibody against phosphorylated histone H3 to determine mitotic index. Apoptosis in any of the three lobes of the wild-type pituitary was nearly undetectable (Fig. 3B). In the p18−/− pituitary, apoptosis was barely detectable in both the intermediate lobe and neurohypophysis and was very low in the anterior lobe (Fig. 3B), indicating that an abnormal hyperproliferation caused by p18 loss is not associated with an increase of apoptosis and that a net increase of cell number is therefore responsible for the increase in the size of the intermediate lobe. Loss of Cdk4 and p18 did not cause a detectable increase of apoptosis in both the intermediate lobe and neurohypophysis. But loss of Cdk4 substantially increased the apoptotic cell death in the anterior lobe, which might be responsible for defective prolactin production and CL formation. Deletion of the p18 gene did not detectably reduce the apoptosis caused by Cdk4 loss in the anterior lobe of the p18−/−; Cdk4−/− pituitary (Fig. 3B).
Both Ki-67 and phosphor-H3 staining revealed an increase of cell proliferation in the p18−/− pituitary and a decrease of cell proliferation to almost the basal level in the Cdk4−/− pituitary (Fig. 3B). Increase of both Ki-67- and phosphor-H3 positive cells in the p18−/− pituitary is not restricted to the intermediate lobe; it is also evidently in both the anterior lobe and the neurohypophysis (Fig. 3B), revealing a previously unrecognized function of p18 in these parts of the pituitary. Consistent with these results, we also found that p18 is expressed in all three lobes of the pituitary (data not shown). As apoptosis in both cell types was not significantly increased, an increase of cell proliferation, but not the size of both anterior lobe and neurohypophysis, suggests that a separate compensating mechanism may maintain an overall normal tissue size in these parts of pituitary. The numbers of both Ki-67- and phosphor-H3-positive cells in the p18−/−; Cdk4−/− pituitary were similar to those seen in the wild type, indicating that loss of the Cdk4 gene inhibited hyperproliferation caused by p18 loss.
Loss of p18 does not rescue diabetes of Cdk4-deficient mice.
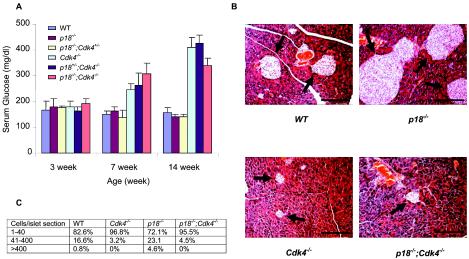
Cdk4-deficient mice displayed normoglycemia at 3 weeks of age and developed diabetes around 7 weeks of age (40, 49) (Fig. 4A). Histological analysis revealed that by the age of 3 weeks the number and size of pancreatic islets were not affected by the loss of CDK4. However, by the ages of 7 and 16 weeks the number of pancreatic islets was significantly decreased. Pancreases of wild-type mice showed 82.6, 16.6, and 0.8% small, medium, and large islets, respectively (Fig. 4B and C). The number of islets from Cdk4−/− mice was 50% less than the number from wild-type littermate mice (Fig. 4B and data not shown). Analysis of the sizes of the islets found in Cdk4−/− pancreases of mice at 16 weeks of age revealed that 96.8% were small (1 to 40 cells/islet section), that only 3.2% were medium sized (41 to 400 cells/islet section), and that there were no large-sized islets (>400 cells/islet section). These results suggest that not only islet number but also the size of the islets were significantly decreased in Cdk4-deficient mice. In contrast, the number of islets from p18−/− mice (16 weeks of age) was approximately 40% higher than the number from wild-type littermate mice. Although most of the islets of p18−/− mice (72.1%) were small, there were significantly more medium-sized (23.1%) and especially large-sized islets (4.6%) than were found in wild-type mice (Fig. 4B). Pancreases of p18−/−; Cdk4−/− mice are very similar to those of Cdk4−/− mice; islets were sparsely located in the p18−/−; Cdk4−/− pancreas, and the number of islets was comparable to the number in Cdk4−/− mice. For the p18−/−; Cdk4−/− islets, 95.5% were small and only 4.5% could grow to medium size. No large-sized islets were found in p18−/−; Cdk4−/− pancreases (Fig. 4C). Consistent with the defects in pancreatic development and function, p18−/−; Cdk4−/− mice also developed diabetes from about 7 weeks of age (Fig. 4A). Loss of p18 did not rescue defective pancreatic islet (β-cell) development. Instead, loss of Cdk4 abolished all hyperplastic growth of pancreatic islet β cells caused by p18 loss.
FIG. 4.
Loss of p18 did not rescue diabetes of Cdk4-deficient mice. (A) Glucose levels in serum of 3-, 7-, and 14-month-old mice. Three or four mice from each genotype were examined. Standard deviation bars are indicated. (B) hematoxylin and eosin staining of pancreatic sections obtained from mice of different genotypes from the same litter at 4 months age of. Arrows, islets. WT, wild type. (C) Percentages of islets containing 1 to 40, 41 to 400, or more than 400 cells/section in mice of different genotypes at 4 months of age. Two hundred fifty islets from WT and p18−/− pancreases and 100 islets from Cdk4−/− and p18−/−; Cdk4−/− pancreases were counted. Bars, 200 μm.
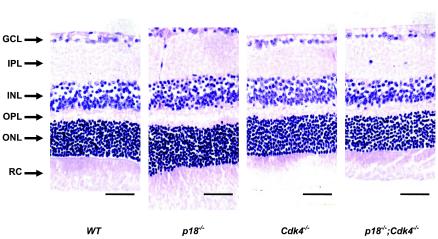
Cdk4 and p18 are dispensable for retinal development.
Rb family proteins play an important role in retinal development. Inactivation of Rb and/or p107 leads to multiple retinal dysplasia (31, 32, 41, 50). Loss of cyclin D1 results in small eyes with thin retinas and a dramatic reduction in cell numbers in all layers of the retina as the result of decreased cell proliferation and increased apoptosis (13, 30, 46), implying a possible role for CDK4 and/or CDK6 and INK4 in normal retinal development through controlling the phosphorylation and activity of Rb proteins. Close examination of retinas of Cdk4−/−, p18−/−, and p18−/−; Cdk4−/− mice did not identify any obvious abnormality (Fig. 5), indicating that CDK4 and p18 are both dispensable in retinal development. These findings suggest that CDK6 is likely to play a prominent role in controlling retinal development. In contrast to cyclin D1-deficient retinas, p27-deficient retinas developed hyperproliferation and displayed disorganized cellular layers and protrusions of the outer photoreceptor cell layer into the rod-and-cone layer (10, 48). Simultaneous deletion of p27 and cyclin D1 rescued hyperproliferation and hypocellularity caused by the deletion of either gene and largely restored normal retinal development (48), providing further evidence for a functional distinction between p18 and p27 in controlling cyclin D-dependent kinases.
FIG. 5.
p18 and Cdk4 are functional dispensable for normal development of the retina. Shown are histologic sections of retinas derived from mice of different genotypes at 5 months of age. The ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), and rod-and-cone layer (RC) are indicated. Bars, 50 μm.
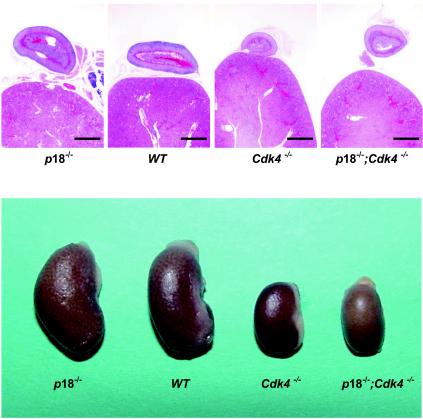
Cdk4 is required for development of kidney, adrenal glands, and seminal vesicles, and deletion of p18 does not restore the developmental defects of these organs in Cdk4 null mice.
Deletion of Cdk4 resulted in small kidney, adrenal glands, and seminal vesicles. These three organs are 55% of the sizes of the wild-type counterparts. Histologically, loss of Cdk4 resulted in severe hypoplasia in these organs (Fig. 6 and data not shown). In contrast, deletion of the p18 gene resulted in increased sizes of kidney, adrenal glands, and seminal vesicles (15) (Fig. 6). Deletion of p18 did not rescue hypoproliferative phenotypes associated with Cdk4-deficient mice. The kidneys, adrenal glands, and seminal vesicles of p18−/−; Cdk4−/− mice are smaller than those of both wild-type and p18−/− mice and are similar to those of Cdk4−/− mice (Fig. 6).
FIG. 6.
Histological analysis of kidneys and adrenal glands of p18 and Cdk4 mutant mice. (Top) Histologic sections of kidneys and adrenal glands derived from mice of different genotypes at 4 months of age were examined microscopically. (Bottom) Gross appearance of kidneys and adrenal glands. WT, wild type. Bars, 1 mm.
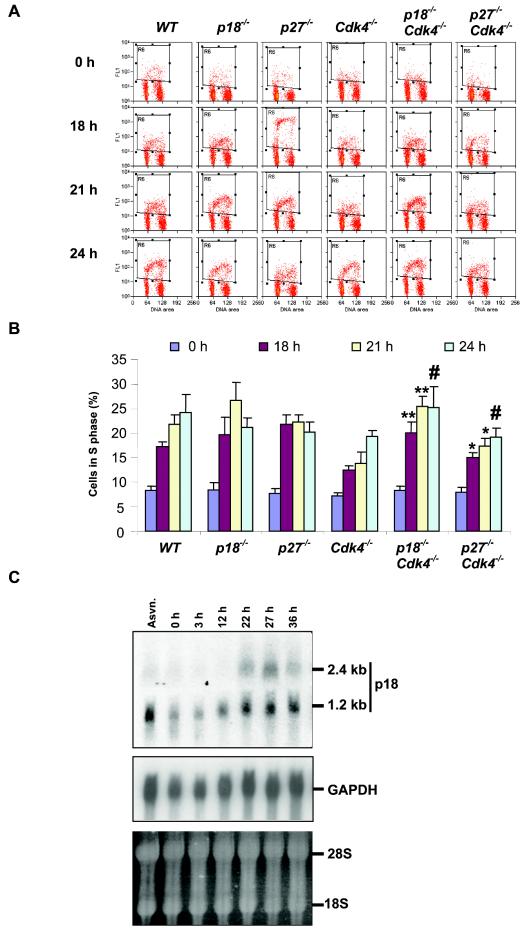
Loss of p18 rescues S phase delay in MEFs lacking Cdk4.
Loss of CDK4 leads to a delay of the initiation of DNA replication of serum-deprived MEFs (49), indicating a role for CDK4 in mediating the exit of MEFs from quiescence. The studies on the signaling pathways regulating CDK4 and CDK6 have been mostly focused on the activation of cyclin D gene expression (34). Whether any INK4 protein plays a role in this regulation has not been reported. To determine the function of p18 in the G0/G1-to-S transition, we derived MEFs from p18-deficient mice and examined their response to serum starvation by flow cytometry. Compared to what was found for wild-type MEFs, deletion of p18 resulted in an accelerated progression through the G1 phase and entry into S phase. While 19.7 and 26.7% of p18−/− MEFs have started DNA replication at 18 and 21 h post-serum stimulation, respectively, only 17.3 and 21.8% of wild-type MEFs were BrdU positive (Fig. 7). Twenty-four hours post-serum stimulation, while more (24.2%) wild-type MEFs continued to enter into S phase, there was a decrease of BrdU-positive p18−/− MEFs, indicating that most of them had completed DNA replication. The cell cycle kinetics of p18−/− MEFs in response to serum deprivation-stimulation is in contrast to that of Cdk4−/− MEFs, which, as previously reported, displayed a delayed entry into S phase relative to wild-type MEFs that was similar to that of p27−/− MEFs, which also had an accelerated progression through the G1 phase.
FIG. 7.
p18 has a function in serum response in MEFs that is not dependent on Cdk4. (A) Cell cycle analysis by staining with BrdU and propidium iodide (PI). MEFs were starved for 72 h (time zero), followed by stimulation of cells with medium containing 10% serum. Cells were pulse-labeled and harvested at the times indicated after stimulation. Cells were stained with an anti-BrdU antibody and PI and subjected to flow cytometry. BrdU-positive cells are boxed. WT, wild type. (B) The percentage of cells in S phase from each time point was plotted on the basis of flow-cytometric analysis. Results are average values of three independent experiments. Error bars indicate standard deviations of the means. **, P < 0.01, for comparison of p18−/−; Cdk4−/− MEFs to Cdk4−/− MEFs at the same times after serum restimulation; *, P < 0.05, for comparison of p27−/−; Cdk4−/− MEFs to Cdk4−/− MEFs at the same times after serum restimulation; #, P > 0.05, for comparison of p18−/−; Cdk4−/− or p27−/−; Cdk4−/− MEFs to Cdk4−/− MEFs at the same time point (Student's t test). (C) Induction of p18 gene expression during G1 progression. Total RNA was isolated from asynchronously growing (Asyn.) and serum-starved and stimulated MEFs. Twenty micrograms of each RNA sample was resolved on a 1% agarose gel, transferred to a nitrocellulose filter, and hybridized with a probe derived from mouse p18 coding region.
The function of p18 in regulating the cellular response to serum starvation-stimulation has not been previously reported. To confirm this finding, we determined the expression of p18 gene transcripts during G1 progression. Early-passage (younger than passage 4) MEFs were serum starved for 72 h and then stimulated by the addition of 10% FBS. We previously reported that the p18 gene expresses two species of message though promoter switching, a translation-competent short transcript and a translation-attenuated long transcript, due to the presence of a 1.2-kb 5′-untranslated region encoded by exon 1 (39). In serum-starved quiescent cells, the short transcript accumulates to a low level while the long 2.4-kb transcript was barely detectable (Fig. 7C). Following serum stimulation, the levels of both transcripts increased and reached a peak around the G1/S boundary. This result suggests that the expression of p18 is regulated, at least in part, by a transcriptional control and provides a molecular basis for the accelerated G1-to-S progression of p18−/− MEFs.
To determine the functional interaction between CDK4 and its two regulators, p18 and p27, we derived p18−/−; Cdk4−/− and p27−/−; Cdk4−/− double-mutant MEFs and determined their cell cycle kinetics for serum deprivation and stimulation. Consistent with a previous report (49), the p27−/−; Cdk4−/− MEFs displayed cell cycle kinetics intermediate between those of Cdk4−/− and p27−/− MEFs, indicating that deletion of p27 partially restored the delayed S-phase entry caused by Cdk4 loss (Fig. 7A and B). Surprisingly, p18−/−; Cdk4−/− MEFs exhibited kinetics more similar to that of p18−/− MEFs: 20.1 and 25.4% of cells were stained positive for BrdU at 18 and 21 h post-serum stimulation. These results are consistent with the idea that p18 plays a critical role in controlling the serum response in MEFs by regulating not only CDK4 but also another target, likely CDK6, and that delay in S phase entry due to loss of Cdk4 could be compensated by an increase of CDK6 activity.
DISCUSSION
INK4 proteins specifically bind to and negatively regulate the activity of CDK4 and CDK6 (44), and cyclin Ds-CDK4/6 complexes phosphorylate and functionally inactivate Rb family proteins (9, 12, 22). Ectopic overexpression of individual INK4 genes causes a G1 cell cycle arrest with a correlative dependency on the intact Rb (16), and loss of either Rb or a combination of p107 and p130 effectively canceled the G1 arrest caused by INK4 overexpression (3, 24, 35). Together, these findings led to the widely accepted notion that, at least in cultured cells and assays for controlling G1-to-S transition, the primary functional targets of INK4 are CDK4 and CDK6 and the primary functional targets of CDK4 and CDK6 are the pocket proteins. Hence, there may exist in vivo a linear INK4-CDK4/6-Rb G1 control pathway in mammalian cells. The notion that CDK4 and CDK6 are the primary targets of INK4 function, however, has not been tested experimentally in any in vivo setting, due largely to the lack of cells lacking both CDK4 and CDK6. Two additional proteins, orphan steroid receptor Nur77 (5) and the p65RelA subunit of NF-κB (52), were reported to bind with p19Ink4d and p16Ink4a, respectively, although the functional consequence and physiologic significance of both interactions remain unclear. Generation and characterization of p18- and Cdk4-deficient mutant mouse strains and development of a series of consistent phenotypes in these two strains of mice provide an opportunity to determine the functional dependency of INK4 on CDK4 in vivo. In this paper, we provide the first genetic evidence that the developmental function of an INK4 protein is dependent on CDK4.
We have examined in p18−/−, Cdk4−/−, and p18−/−; Cdk4−/− mice body weight and six cell types, tissues, or organs which developed highly penetrant phenotypes because of the loss of p18 alone: testis, ovary, pituitary, endocrine pancreas, kidney, and adrenal gland. In all six cases, simultaneous loss of Cdk4 virtually canceled all p18 loss-induced defects. These results provide direct evidence that the function of p18Ink4c is dependent on CDK4 in vivo. Many of these hyperproliferative defects associated with p18 loss were also developed in p27-deficient mice to a similar extent with a nearly complete penetrance, including body weight increase; pituitary hyperplasia and tumor development; and enlargement of thymus, spleen, testis, and ovary (14, 23, 37). Notably, the body weight increase, or the gigantism phenotype, caused by p27 loss was only partially canceled in p27−/−; Cdk4−/− double-mutant mice (Fig. 1). This result is consistent with and provides genetic support for the model that, while INK4 regulates only CDK4 and CDK6, CIP/KIP proteins have an additional target, CDK2. The role of p27 (and p21) in the regulation of Cdk4 is currently controversial (38), and it has been suggested that p27 has opposing roles as an activator (6) and as an inhibitor (1). Our results that the body weights of p27−/−; Cdk4−/− double-mutant mice and the cell cycle kinetics of p27−/−; Cdk4−/− MEFs exhibited a phenotype intermediate between that of either single mutant are in agreement with an oppose function of these two genes and support the notion that p27 is an inhibitor of Cdk4. In addition to tumor suppression, the Rb pathway controls cell differentiation, tissue development, and organ size. Of various p18 loss-caused defects that were canceled by the simultaneous loss of Cdk4, the body weight increase, pancreatic islet number increase and size enlargement, and ovary enlargement are defects in animal development and organ size regulation, not tumor growth, indicating that the functional dependency of p18 on Cdk4 is not restricted to tumor suppression and may extend into other cellular processes.
The only defect caused by p18 loss that was not significantly alleviated by the loss of Cdk4 is the accelerated G1-to-S progression of MEFs following serum deprivation and stimulation: p18−/−; Cdk4−/− MEFs displayed cell cycle kinetics more similar to that of p18−/− MEFs (Fig. 7). In the same assay, the p27−/−; Cdk4−/− MEFs exhibited intermediate cell cycle kinetics, indicating, again, that deletion of Cdk4 partially reduced accelerated G1-to-S progression caused by p27 loss. The most likely explanation for the lack of significant rescue of p18 loss-caused defects by Cdk4 loss is that in MEFs, unlike other adult tissues and differentiated cells, Cdk6 may play a significant role in regulating G1 progression following serum starvation-stimulation, either in its own right or when induced in a compensatory manner after Cdk4 loss, therefore sensitizing the MEFs to p18 loss. Consistent with this idea, CDK6 is readily detectable in MEFs and CDK6-p27 complexes were noticeably increased in Cdk4 null MEFs (49), suggesting a compensatory regulation of p27, and thus Cdk2, by CDK6 in the absence of CDK4. The in vivo function of CDK6 in the whole animal and how widely CDK6 compensates for CDK4 loss in other cell types and tissues remain unclear. Our results that Cdk4 loss canceled all six defects caused by p18 loss that we have examined suggest that Cdk6 plays a relatively minor role in vivo in mediating the function of p18.
Wild-type MEFs accumulate in either G1 phase of the cell cycle or a quiescence state in response to serum deprivation and then reenter the cell cycle and travel through the G1 phase with consistent kinetics: DNA replication starts approximately 14 to 16 h after serum stimulation. Loss of p18 accelerated G1 progression and initiation of DNA replication (Fig. 7), revealing a previously unrecognized function of p18 in regulation of the serum response in MEFs. Supporting this function of p18 is the observation that p18 mRNA is induced during reentry of serum-deprived MEFs into the cell cycle (Fig. 7). The mechanism responsible for p18 induction during G1 progression remains to be determined but does not appear to be dependent on the function of E2F1. Notably, similar percentages of p18−/− and wild-type MEFs (7 to 8%) were stained positive for BrdU after 72 h of serum starvation (Fig. 7), indicating that p18−/− MEFs were arrested in the G0 quiescent state as efficiently as wild-type MEFs. Likewise, loss of p27, which also accelerated G1 progression following serum stimulation, did not impair the ability of MEFs to enter and maintain a stable quiescence upon serum deprivation. Differently, in response to serum deprivation, the Rb−/−; p107−/−; p130−/− triple-deficient MEFs continue to incorporate BrdU and then undergo apoptotic cell death (7, 43), indicating an essential function of pocket proteins, and by extension implying a function of a CDK inhibitor(s), in causing and/or maintaining G1 arrest under growth-inhibiting conditions. Lack of a significant defect in entering into and maintaining G1 arrest upon serum starvation in p18-deficient MEFs is consistent with its decreased low-level expression in quiescent cells (Fig. 7). It remains to be determined whether, during the cellular response to growth factor deprivation-stimulation, p18 and p27 alone are sufficient to cause and maintain a G1 arrest, but both are required for a proper reentry of quiescent cells into the proliferation cycle. Alternatively, our results suggest that another CDK inhibitor protein separately controls the entry into and maintenance of G1 arrest while p18 and p27 regulate the reentry into the cell cycle.
Acknowledgments
We thank Ned Sharpless, Lishan Su and Stuart Shumway for reading the manuscript and discussion and Y. Joe He for helping with figure preparation.
Y.X. is supported in part by a U.S. Department of Defense Career Development Award (DAMD17-99-1-9574). This study was supported by NIH grants CA100204 to H.K. and CA65572 and CA68377 to Y.X.
REFERENCES
- 1.Bagui, T. K., S. Mohapatra, E. Haura, and W. J. Pledger. 2003. p27Kip1 and p21Cip1 are not required for the formation of active D cyclin-cdk4 complexes. Mol. Cell. Biol. 23:7285-7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, F., X. H. Pei, V. L. Godfrey, and Y. Xiong. 2003. Haploinsufficiency of p18Ink4c sensitizes mice to carcinogen-induced tumorigenesis. Mol. Cell. Biol. 23:1269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce, J. L., R. K. Hurford, M. Clason, J. Koh, and N. Dyson. 2000. Requirements for cell cycle arrest by p16INK4a. Mol. Cell 6:737-742. [DOI] [PubMed] [Google Scholar]
- 4.Brugarolas, J., C. Chandrasekaran, J. I. Gordon, D. Beach, T. Jacks, and G. J. Hannon. 1995. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377:552-557. [DOI] [PubMed] [Google Scholar]
- 5.Chan, F. K. M., L. Zhang, L. Chen, D. N. Shapiro, and A. Winoto. 1995. Identification of human/mouse p19, a novel cdk4/cdk6 inhibitor with homology to p16ink4. Mol. Cell. Biol. 15:2682-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, M., P. Oliver, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21Cip1 and p27Kip1 CDK ′inhibitor' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18:1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dannenberg, J. H., A. van Rossum, L. Schuijff, and H. te Riele. 2000. Ablation of the retinoblastoma gene family deregulates G1 control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev. 14:3051-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng, C., P. Zhang, J. W. Harper, S. J. Elledge, and P. Leder. 1995. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82:675-684. [DOI] [PubMed] [Google Scholar]
- 9.Dowdy, S. F., P. W. Hinds, K. Louie, S. I. Reed, A. Arnold, and R. A. Weinberg. 1993. Physical interaction of the retinoblastoma protein with human D cyclins. Cell 73:499-511. [DOI] [PubMed] [Google Scholar]
- 10.Dyer, M. A., and C. L. Cepko. 2001. p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J. Neurosci. 21:4259-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, D. M. Lin, W. E. Mercer, K. W. V. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817-825. [DOI] [PubMed] [Google Scholar]
- 12.Ewen, M. E., H. K. Sluss, C. J. Sherr, H. Matsushime, J. Kato, and D. M. Livingston. 1993. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell 73:487-497. [DOI] [PubMed] [Google Scholar]
- 13.Fantl, V., G. Stamp, A. Andrews, I. Rosewell, and C. Dickson. 1995. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 9:2364-2372. [DOI] [PubMed] [Google Scholar]
- 14.Fero, M. L., M. Rivkin, M. Tasch, P. Porter, C. E. Carow, E. Firpo, K. Polyak, L.-H. Tsai, V. Broudy, R. M. Perlmutter, K. Kaushansky, and J. M. Roberts. 1996. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell 85:733-744. [DOI] [PubMed] [Google Scholar]
- 15.Franklin, D. S., V. L. Godfrey, H. Lee, G. I. Kovalev, R. Schoonhoven, S. Chen-Kiang, L. Su, and Y. Xiong. 1998. CDK inhibitors p18INK4c and p27KIP1 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 12:2899-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan, K.-L., C. W. Jenkins, Y. Li, M. A. Nichols, X. Wu, C. L. O'Keefe, A. G. Matera, and Y. Xiong. 1994. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 8:2939-2952. [DOI] [PubMed] [Google Scholar]
- 17.Hannon, G. J., and D. Beach. 1994. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature 371:257-261. [DOI] [PubMed] [Google Scholar]
- 18.Harper, J. W., S. J. Elledge, K. Keyomarsi, B. Dynlacht, L.-H. Tsai, P. Zhang, S. Dobrowolski, C. Bai, L. Connell-Crowley, E. Swindell, M. P. Fox, and N. Wei. 1995. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 6:387-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengst, L., U. Gopfert, H. A. Lashuel, and S. I. Reed. 1998. Complete inhibition of Cdk/cyclin by one molecule of p21(Cip1). Genes Dev. 12:3882-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, N., A. Gutsmann, D. C. Herbert, A. Bradley, W.-H. Lee, and E. Y.-H. P. Lee. 1994. Heterozygous Rb-1D20/+ mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene 9:1021-1027. [PubMed] [Google Scholar]
- 21.Jacks, T., A. Fazeli, E. M. Schmitt, R. T. Bronson, M. A. Goodell, and R. A. Weinberg. 1992. Effects of an Rb mutation in the mouse. Nature 359:295-300. [DOI] [PubMed] [Google Scholar]
- 22.Kato, J.-Y., H. Matsushime, S. W. Hiebert, M. Ewen, and C. J. Sherr. 1993. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 7:331-342. [DOI] [PubMed] [Google Scholar]
- 23.Kiyokawa, H., R. D. Kineman, K. O. Manova-Todorova, V. C. Soares, E. S. Hoffman, M. Ono, D. Khanam, A. C. Hayday, L. A. Frohman, and A. Koff. 1996. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell 85:721-732. [DOI] [PubMed] [Google Scholar]
- 24.Koh, J., G. H. Enders, B. D. Dynlacht, and E. Harlow. 1995. Tumor-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature 375:506-510. [DOI] [PubMed] [Google Scholar]
- 25.Krimpenfort, P., K. C. Quon, W. J. Mooi, A. Loonstra, and A. Berns. 2001. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413:83-86. [DOI] [PubMed] [Google Scholar]
- 26.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Sligerland, C. Sandhu, H. S. Cjou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 27.Latres, E., M. Malumbres, R. Sotillo, J. Martin, S. Ortega, J. Martin-Caballero, J. M. Flores, C. Cordon-Cardo, and M. Barbacid. 2000. Limited overlapping roles of p15INK4b and p18INK4c cell cycle inhibitors in proliferation and tumorigenesis. EMBO J. 19:3496-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, Y.-H. P., C.-Y. Chang, N. Hu, Y.-C. J. Wang, C.-C. Lai, K. Herrup, W.-H. Lee, and A. Bradley. 1992. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359:288-294. [DOI] [PubMed] [Google Scholar]
- 29.Li, Y., C. W. Jenkins, M. A. Nichols, and Y. Xiong. 1994. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene 9:2261-2268. [PubMed] [Google Scholar]
- 30.Ma, C., D. Papermaster, and C. L. Cepko. 1998. A unique pattern of photoreceptor degeneration in cyclin D1 mutant mice. Proc. Natl. Acad. Sci. USA 95:9938-9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maandag, E. C., M. Van Der Valk, M. Vlaar, C. Feltkamp, J. O'Brien, M. Van Roon, N. Van Der Lugt, A. Berns, and H. Te Riele. 1994. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 13:4260-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacPherson, D., J. Sage, D. Crowley, A. Trumpp, R. T. Bronson, and T. Jacks. 2003. Conditional mutation of Rb causes cell cycle defects without apoptosis in the central nervous system. Mol. Cell. Biol. 23:1044-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin, J., S. L. Hunt, P. Dubus, R. Sotillo, F. Nehme-Pelluard, M. A. Magnuson, A. F. Parlow, M. Malumbres, S. Ortega, and M. Barbacid. 2003. Genetic rescue of Cdk4 null mice restores pancreatic beta-cell proliferation but not homeostatic cell number. Oncogene 22:5261-5269. [DOI] [PubMed] [Google Scholar]
- 34.Matsushime, H., M. F. Roussel, R. A. Ashmum, and C. J. Sherr. 1991. Colony-stimulating factor 1 regulates a novel gene (CYL1) during the G1 phase of the cell cycle. Cell 65:701-713. [DOI] [PubMed] [Google Scholar]
- 35.Medema, R. H., R. E. Herrera, F. Lam, and R. A. Weinberg. 1995. Growth suppression by p16INK4 requires functional retinoblastoma protein. Proc. Natl. Acad. Sci. USA 92:6289-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moons, D. S., S. Jirawatnotai, A. F. Parlow, G. Gibori, R. D. Kineman, and H. Kiyokawa. 2002. Pituitary hypoplasia and lactotroph dysfunction in mice deficient for cyclin-dependent kinase-4. Endocrinology 143:3001-3008. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama, K., N. Ishida, M. Shirane, A. Inomata, T. Inoue, N. Shishido, I. Horii, D. Y. Loh, and K. Nakayama. 1996. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707-720. [DOI] [PubMed] [Google Scholar]
- 38.Olashaw, N., T. K. Bagui, and W. J. Pledger. 2004. Cell cycle control: a complex issue. Cell Cycle 3:263-264. [DOI] [PubMed] [Google Scholar]
- 39.Phelps, D., K.-M. Hsiao, Y. Li, N. Hu, D. S. Franklin, E. Westphal, E. Y.-H. P. Lee, and Y. Xiong. 1998. Coupled transcriptional and translational control of the CDK inhibitor p18Ink4c's expression during myogenesis. Mol. Cell. Biol. 18:2334-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rane, S. G., P. Dubus, R. Mettus, E. J. Galvreath, G. Boden, E. P. Reddy, and M. Barbacid. 1999. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat. Genet. 22:44-52. [DOI] [PubMed] [Google Scholar]
- 41.Robanus-Maandag, E., M. Dekker, M. van der Valk, M. L. Carrozza, J. C. Jeanny, J. H. Dannenberg, A. Berns, and H. te Riele. 1998. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes Dev. 12:1599-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robker, R. L., and J. S. Richards. 1998. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol. Endocrinol. 12:924-940. [DOI] [PubMed] [Google Scholar]
- 43.Sage, J., G. J. Mulligan, L. D. Attardi, A. Miller, S. Chen, B. Williams, E. Theodorou, and T. Jacks. 2000. Targeted disruption of the three Rb-related genes leads to loss of G1 control and immortalization. Genes Dev. 14:3037-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serrano, M., G. J. Hannon, and D. Beach. 1993. A new regulatory motif in cell cycle control causing specific inhibition of cyclin D/CDK4. Nature 366:704-707. [DOI] [PubMed] [Google Scholar]
- 45.Sharpless, N. E., N. Bardeesy, K. H. Lee, D. Carrasco, D. H. Castrillon, A. J. Aguirre, E. A. Wu, J. W. Horner, and R. A. DePinho. 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413:86-91. [DOI] [PubMed] [Google Scholar]
- 46.Sicinski, P., J. L. Donaher, S. B. Parker, T. Li, A. Fazeli, H. Gardner, S. Z. Haslam, R. T. Bronson, S. J. Elledge, and R. A. Weinberg. 1995. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82:621-630. [DOI] [PubMed] [Google Scholar]
- 47.Tong, W., H. Kiyokawa, T. J. Soos, M. S. Park, V. C. Soares, K. Manova, J. W. Pollard, and A. Koff. 1998. The absence of p27Kip1, an inhibitor of G1 cyclin-dependent kinases, uncouples differentiation and growth arrest during the granulosa→luteal transition. Cell Growth Differ. 9:787-794. [PubMed] [Google Scholar]
- 48.Tong, W., and J. W. Pollard. 2001. Genetic evidence for the interactions of cyclin D1 and p27Kip1 in mice. Mol. Cell. Biol. 21:1319-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsutsui, T., B. Hesabi, D. S. Moons, P. A. Pandolfi, K. S. Hansel, A. Koff, and H. Kiyokawa. 1999. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27Kip1 activity. Mol. Cell. Biol. 19:7011-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams, B. O., L. Remington, D. M. Albert, S. Mukai, R. T. Bronson, and T. Jacks. 1994. Cooperative tumorigenic effects of germline mutations in Rb and p53. Nat. Genet. 7:480-484. [DOI] [PubMed] [Google Scholar]
- 51.Williams, B. O., E. M. Schmitt, L. Remington, R. T. Bronson, D. M. Albert, R. A. Weinberg, and T. Jacks. 1994. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 13:4251-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolff, B., and M. Naumann. 1999. INK4 cell cycle inhibitors direct transcriptional inactivation of NF-κB. Oncogene 18:2663-2666. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, H., G. J. Hannon, and D. Beach. 1994. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 8:1750-1758. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, P., N. Liegeois, C. Wong, M. Finegold, H. Hou, J. C. Thompson, A. Silverman, J. W. Harper, R. A. DePinho, and S. J. Elledge. 1997. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature 387:151-158. [DOI] [PubMed] [Google Scholar]
- 55.Zindy, F., J. van Deursen, G. Grosveld, C. J. Sherr, and M. F. Roussel. 2000. INK4d-deficient mice are fertile despite testicular atrophy. Mol. Cell. Biol. 20:372-378. [DOI] [PMC free article] [PubMed] [Google Scholar]