Abstract
Drug resistance is a major obstacle in the successful treatment of cancer. Thus, elucidation of the mechanisms responsible is a critical first step in trying to prevent or delay such manifestations of resistance. In this regard, three-dimensional multicellular tumor cell spheroids are intrinsically more resistant to virtually all anticancer cytotoxic drugs than conventional monolayer cultures. We have employed the EMT-6 subline PC5T, which forms highly compact spheroids, and differential display to identify candidate genes whose expression differs between monolayer and spheroids. Approximately 5,000 bands were analyzed, revealing 26 to be differentially expressed. Analysis of EMT-6 tumor variants selected in vivo for acquired resistance to alkylating agents identified eight genes whose expression correlated with drug resistance in tumor spheroids. Four genes (encoding Nop56, the NADH SDAP subunit, and two novel sequences) were found to be down-regulated in EMT-6 spheroids and four (encoding 2-oxoglutarate carrier protein, JTV-1, and two novel sequences) were up-regulated. Analysis of the DNA mismatch repair-associated PMS2 gene, which overlaps at the genomic level with the JTV-1 gene, revealed PMS2 mRNA to be down-regulated in tumor spheroids, which was confirmed at the protein level. Analysis of PMS2−/− mouse embryo fibroblasts confirmed a role for PMS2 in sensitivity to cisplatin, and DNA mismatch repair activity was found to be reduced in EMT-6 spheroids compared to monolayers. Dominant negative PMS2 transfection caused increased resistance to cisplatin in EMT-6 and CHO cells. Our results implicate reduced DNA mismatch repair as a determinant factor of reversible multicellular resistance of tumor cells to alkylating agents.
Of the two major forms of drug resistance encountered during or after anticancer therapies, including chemotherapy, acquired drug resistance has received greater experimental attention. It is considered to have a genetic (mutational) basis and involves (in the case of cytotoxic chemotherapeutic drugs) biochemical alterations operating at the cellular level, such as expression or increased activity of certain DNA repair enzymes, detoxification enzymes, membrane-associated drug efflux molecules (e.g., P glycoprotein or multidrug resistance protein), and antiapoptotic effector molecules such as bcl-2 (12, 16, 22).
Many forms of intrinsic drug resistance, on the other hand, depend on physiologic and/or microenvironmental mechanisms operating at the tissue or multicellular level (14, 29, 37). For example, limited penetration or diffusion of drugs into the deeper layers of a tumor mass can result in a failure to respond, as can lower tumor cell growth fractions due to high cell densities or suboptimal (e.g., low-pH or -oxygen) growth conditions (15). Hypoxia due to factors such as disrupted blood flow in tumors represents another manifestation of such a mechanism, which in many cases can involve changes in gene expression, rather than mutation (19). This multicellular or tissue resistance of solid tumors to anticancer drugs or radiation can be recapitulated in vitro by anchorage-independent growth of tumor cells as small multicellular aggregates, or spheroids (19, 37). In addition, there are some examples of acquired drug resistance which appear to operate primarily at the tissue level (38).
Tissue drug resistance remains a relatively poorly understood phenomenon. The term “multicellular drug resistance” to describe this adhesion-dependent mechanism of resistance was proposed in 1993 (17, 18), when it was observed that the in vivo-selected drug-resistant EMT-6 variants expressed their resistance in vitro as spheroids and formed more compact (or cohesive) spheroids than the parental EMT-6 cell line. Subsequently the rapid acquisition of transient multicellular drug resistance in EMT-6 and human MDA-MB-231 breast cancer cells (which had not previously been exposed to any cytotoxic drugs) following a brief exposure to either cisplatin or 4-hydroperoxycyclophosphamide (4HC) of cell lines grown as spheroids was demonstrated (10). This rapidly induced resistance was not observed when monolayer cell cultures were used. Furthermore, cells exposed to either drug were able to form more compact spheroids, again suggesting a possible correlation between the level of cell-cell adhesion and multicellular drug resistance.
In another study, EMT-6 sublines selected for their ability to form highly compact spheroids were shown to be more resistant to 4HC (when grown as spheroids) than the parental cell line, which forms loose spheroids (35). In addition, treatment with hyaluronidase preparation disrupted EMT-6 spheroids and sensitized the released constituent cells to 4HC. Critically, these experiments were carried out by exposing cell monolayers to 4HC before replating them as monolayers or spheroids, thus excluding drug penetration into the spheroid mass as a factor in the observed multicellular drug resistance. The chemosensitizing effect of hyaluronidase was also observed in vivo when EMT-6 grown as ascites tumors in which the cells grew as small clumps, or spheroids, in the ascites fluid of the intraperitoneal cavity was used. Treatment of tumor-bearing mice with cyclophosphamide and hyaluronidase led to a significant increase in survival (P < 0.005) compared to cyclophosphamide treatment alone (35).
One mechanism contributing to multicellular drug resistance may be the reduced growth fractions of tumor cells, which are typically observed in spheroids, compared to monolayer cell cultures (29). In this regard, it has been found that the cyclin-dependent kinase inhibitor p27Kip1 is up-regulated at the protein level in tumor spheroids, compared to corresponding monolayers, which could contribute to the observed reduced growth fraction (33) in spheroids. Multicellular resistance was also studied in human epithelial tumor cell lines, which were demonstrated to form spheroids as a consequence of E-cadherin cell-cell interactions (36). A monoclonal antibody against E-cadherin (SHE 78-7) was shown to disrupt preformed spheroids of HT29 colon and CaOV3 ovarian cancer cell lines and sensitize these cells to cytotoxic agents, including cisplatin (11, 12).
The aforementioned p27Kip1 results highlight the possibility that changes in expression of a number of genes or proteins mediated by such environmental factors as cell-cell adhesion, high cell density, low pH, or deficient oxygen levels may contribute to multicellular drug resistance. In this regard a number of changes in protein expression (in addition to p27Kip1) in spheroids, e.g., down-regulation of certain integrins (40) or the epidermal growth factor receptor (21) or up-regulation of enzymes such as heme oxygenase (25), have been documented. Thus, to further our understanding of the possible molecular mechanisms associated with multicellular tumor drug resistance, we have employed the differential display (DD) technique (20) to identify changes in gene expression between monolayers and spheroids in the EMT-6 mouse model. We chose a subline of EMT-6 termed PC5T, which forms highly compact spheroids, for our DD comparison and subsequently extended our analysis to EMT-6 drug-resistant variants selected in vivo (38). We now report the identification of eight cDNAs whose expression correlates with spheroid formation in EMT-6 models expressing either intrinsic (PC5T) or acquired (EMT-6 drug-resistant variants) forms of drug resistance. We also show that PMS2, the DNA mismatch repair-associated protein, is down-regulated in tumor spheroids and in EMT-6 tumor variants selected for resistance to alkylating agents. The role of PMS2 in resistance to alkylating agents is shown by testing for sensitivity to cisplatin in mouse embryo fibroblasts (MEFs) derived from wild-type and PMS2 knockout mice, by in vitro colony formation assays. In order to address whether a down-regulation (as opposed to a loss-of-function mutation) of a DNA repair protein is associated with reduced DNA mismatch repair, we employed a genetic assay using a reporter vector (27). EMT-6/P spheroids, in both the presence and absence of cisplatin treatment, showed reduced levels of DNA mismatch repair compared to respective monolayers. Furthermore, transfection of EMT-6/DDP (i.e., cisplatin-resistant) cells with a dominant negative PMS2 expression construct resulted in increased resistance to cisplatin compared to control transfectants. The possible significance of these microenvironmentally induced genetic changes with respect to tumor drug resistance is discussed.
MATERIALS AND METHODS
Cell culture.
The EMT-6 mouse mammary tumor cell line and the EMT-6 variants used in this study were grown in Waymouth's medium. Human HEY (ovarian), LNCaP (prostate), HT-29 (colon), and HBL100 (breast) carcinoma cells and Chinese hamster ovary cells (CHO43 and CHO124; a gift from Morphotek Inc.) were grown in RPMI 1640, and the DU-145 (prostate) and MDA-MB-435 (breast) carcinoma cells, as well as MC5 (wild type) and C18 (PMS2−/−) MEFs, were grown in Dulbecco's modified Eagle medium (8). All media were supplemented with 10% fetal bovine serum and 4 mM l-glutamine. Cells were maintained in a 37°C humidified incubator with an atmosphere of 5% carbon dioxide. Single-cell suspensions were prepared by treatment with trypsin-EDTA (Life Technologies, Inc.) and resuspension in complete medium before monolayer or spheroid cultures were set up.
Spheroid culture.
Multicellular spheroids were generated as previously described (18), using the liquid overlay technique. Briefly, 24-well culture plates (Nunc) were coated with 0.2 ml of 1% SeaPlaque agarose (FMC Bioproducts, Rockland, Maine). Cells from a single-cell suspension were added at 105 per well in a total volume of 1 ml. Spheroids were allowed to form over 24 h for EMT-6 cells (and EMT-6 variants) or 48 h for the human cell lines, as previously described (36).
RNA isolation, Northern blotting, and DD.
Total RNA was isolated with a Trizol kit (Gibco BRL, Grand Island, N.Y.) following the manufacturer's instructions. DD and Northern blotting were carried out as previously described (7). A combination of ethidium bromide staining and ubiquitin mRNA expression was used to reflect equal loading.
DNA sequence analysis.
Plasmid sequencing was carried out manually with a Sequenase II kit (Amersham, Cleveland, Ohio) following the manufacturer's instructions. Database searching was carried out using BLAST.
Reverse transcription-PCR.
DNase I (Novagen)-treated total RNA (10 μg) was reverse transcribed in 20-μl reaction mixtures containing 1× reverse transcription buffer (25 mM Tris-HCl [pH 8.3], 37.6 mM KCl, 1.5 mM MgCl2, 5 mM dithiothreitol), 1 mM deoxynucleoside triphosphates, 10 μM oligo(dT), and 1 μl of 0.1 M dithiothreitol. Mixtures were placed at 65°C for 5 min followed by 5 min at 37°C. Two microliters of Moloney murine leukemia virus reverse transcriptase (50 U/μl; Stratagene) was added, and the reaction mixture was incubated at 37°C for 1 h. Reverse transcriptase was inactivated by incubation at 75°C for 5 min. Five microliters of each reaction mixture was made up to 50 μl of PCR volume containing 1× PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2), 0.1 mM deoxynucleoside triphosphates, 2 μM concentrations of forward and reverse primers, and 1 U of Taq polymerase (Amersham). All PCRs were carried out under the following conditions: 95°C for 3 min, followed by 35 cycles of 95°C for 30 s, 60°C for 1 min, and 72°C for 1 to 2 min (i.e., 1 min per 1 kb of expected product size). A final extension was carried out by treating the reaction mixtures at 72°C for 5 min. Reactions were resolved on a 1% agarose gel, and PCR products were gel purified by using a QiaEx II kit (Qiagen).
5′-RACE.
5′ rapid amplification of cDNA ends (5′-RACE) was carried out with a GeneRacer kit (Invitrogen) following the manufacturer's protocol. Briefly, 5 μg of total RNA (extracted from EMT-6/DDP cells, i.e., cisplatin-resistant spheroids) was dephosphorylated with calf intestinal phosphatase, and the 5′ cap was removed by using tobacco alkaline phosphatase. The decapped mRNA was ligated to a GeneRacer RNA oligonucleotide (Invitrogen) and reverse transcribed with oligo(dT) and avian myeloblastosis virus reverse transcriptase. Hot-start PCR was carried out by using the Advantage cDNA polymerase mix (Clontech) and following the manufacturer's instructions. The PCR and a subsequent nested PCR were carried out with gene-specific primers (5′-GGCCTAGCACCTTTTTATTATTAGGT-3′ for the first reaction and 5′-ACAGAAGCCCGGGCTCCTCCCTA-3′ in the nested reaction for the A3bG product) and 5′ primers specific for the GeneRacer RNA oligonucleotide sequence (Invitrogen). PCR products were resolved on a 1% agarose gel, purified with QiaEx II, cloned into pCR2 (Invitrogen), and sequenced.
Western blotting.
Cells were lysed by the addition of boiling lysis buffer (1% sodium dodecyl sulfate, 1 mM sodium orthovanadate, 10 mM Tris-HCl [pH 7.4]). Tumor tissues were homogenized in boiling lysis buffer, and all lysates were cleared by centrifugation. Protein concentration was evaluated with a Bio-Rad protein assay. Equal amounts of protein were loaded onto a 8% polyacrylamide gel and resolved by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to polyvinylidene difluoride membranes. Membranes were blocked with blocking buffer (5% nonfat dry milk, 10 mM Tris [pH 7.5], 100 mM NaCl, 0.1% Tween-20). Primary antibody was added in blocking buffer (without Tween-20) at concentrations recommended by the manufacturer. Membranes were washed with 10 mM Tris (pH 7.5)-100 mM NaCl-0.1% Tween-20. Rabbit anti-mouse immunoglobulin horseradish peroxidase-conjugated secondary antibody was added in blocking buffer. Membranes were washed and detection was carried out by using ECL (Amersham) and exposure to film.
PCR on genomic DNA.
Mouse genomic DNA was amplified by using the Advantage Taq polymerase mix (Clontech) and following the manufacturer's instructions. Primers used were gmPMS2R (5′-TCG GTT TGC TCC ATG GAT GCA-3′) and gmJTVR (5′-CTT TAC CTG GTA CAT CGG CAT-3′). Conditions were 94°C for 4 min followed by 94°C for 30 s, 60°C for 1 min, and 72°C for 1 min (30 cycles). A final extension step (72°C for 5 min) was applied, and products were resolved on an agarose gel.
In vitro colony formation assays.
Monolayer cultures were exposed to various micromolar concentrations of cisplatin [cis-platinum(II) diammine dichloride; Sigma] for 1 h, after which cells were rinsed, trypsinized, and plated at various dilutions in a colony formation assays (18). Surviving fractions were calculated as plating efficiency of drug-treated cells divided by plating efficiency of the untreated control. Wild type and PMS2−/− MEFs were kindly provided by Michael Liskay (Oregon Health and Science University, Portland).
Genetic analysis of DNA repair.
The pCAR-OF (27) vector (a generous gift from Bert Vogelstein) was transfected into EMT-6/P cells, which were selected in 0.7 mg of hygromycin per ml for 2 weeks. A pooled population of transfected cells was equally split into two plates, of which one was treated with cisplatin (50 μM for 1 h) in phosphate-buffered saline (PBS) and the other was treated with PBS alone. For each treatment, cells were washed in PBS and replated as monolayers and spheroids. Three days after plating, cells were lysed, and beta-galactosidase activity was measured with a high-sensitivity β-galactosidase assay kit (Stratagene), following the manufacturer's instructions.
Analysis of PMS2 dominant negative expression.
CHO cells stably expressing the dominant negative PMS2-134 protein (27) were obtained from Morphotek Inc. The PMS2-134 cDNA (a kind gift from Bert Vogelstein) was cloned into the NheI and NotI sites of the pCEP4 vector (Invitrogen), which allows episomal expression of the transgene. Transfections were carried out with Lipofectamine2000 (Invitrogen) followed by hygromycin selection. The empty pCEP4 vector alone was used for control transfections. Pooled transfected populations of EMT-6/DDP or CHO cells were tested for resistance to cisplatin by colony formation assays.
In vivo tumor growth.
Cells (5 × 105) were injected subcutaneously into syngeneic 6- to 8-week-old female BALB/cJ mice (Jackson Labs). Three weeks later, tumors were excised and immediately placed in RNAlater (Ambion) solution, stored overnight at 4°C, and then placed at −70°C until used.
RESULTS
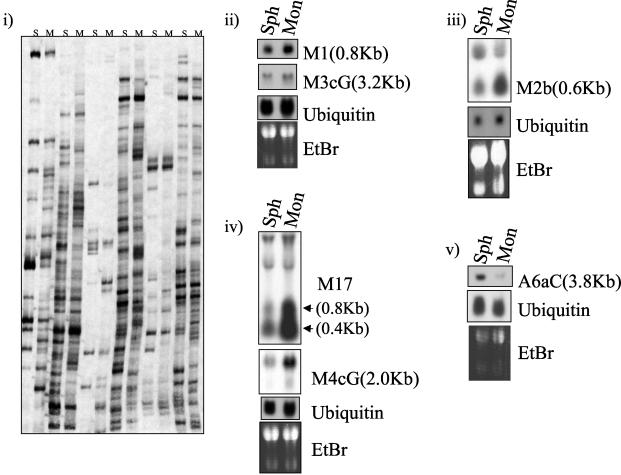
The EMT-6 mammary tumor cell line forms spheroids within 12 to 24 h of growth in suspension (by plating on solidified agarose) (18, 35). Tumor cells within such multicellular aggregates are more resistant to the cytotoxic effects of alkylating agents such as 4-HC than respective monolayer cultures (18). It has also been shown that highly compact or cohesive spheroids are more resistant to alkylating agents than loose spheroids (35). To identify genes that may be involved in this form of intrinsic multicellular drug resistance, we studied a clone of EMT-6 (called PC5T) previously selected for its ability to spontaneously form highly compact spheroids (35). DD analysis (7, 20) was used to compare total RNA extracted from PC5T monolayer (grown to approximately 75% confluence) and spheroid (after 24 h of growth in suspension) cultures. Approximately 5,000 DD bands were analyzed in duplicate experiments, and Northern blotting analysis was used to confirm the differential expression of identified products (Fig. 1). We isolated a total of 26 differentially expressed cDNAs, 17 of which were preferentially expressed in monolayer cultures, compared to spheroids, and 9 of which were up-regulated in spheroids.
FIG. 1.
(i) Examples and analysis of identified DD products from PC5T EMT-6 mouse mammary tumor cells grown as monolayers (M) or spheroids (S). (ii to v) Northern blotting was carried out with total RNA (20 μg/lane) extracted from PC5T cells grown as monolayers (Mon) or spheroids (Sph). Probes were labeled with [32P]dCTP. Sizes of bands were approximated by relative migration with respect to 18S and 28S bands. Ethidium bromide (EtBr) and ubiquitin mRNA are shown as loading controls. Grouped blots were generated by stripping and reprobing of the same filter.
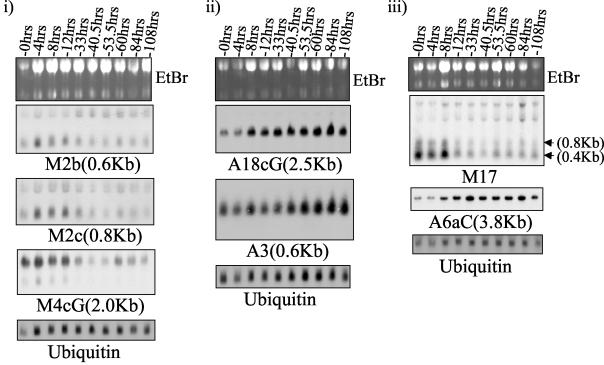
To confirm the altered expression of identified cDNAs as a consequence of spheroid formation, PC5T cells were placed in suspension in a time course experiment (Fig. 2). Changes in mRNA expression for most genes were noted between 8 and 33 h after the cells were removed from monolayers and plated on solidified agarose; thereafter, their relative levels remained stable. One exception was band A3, which continued to increase throughout the period of the experiment (up to 108 h) (Fig. 2). Thus, the identified DD products correspond to genes whose expression correlates with the transition from two-dimensional (monolayer) to three-dimensional (multicellular) growth. However, we cannot exclude the possibility that some of the observed changes are a consequence of cell detachment from tissue culture plates rather than spheroid formation per se.
FIG. 2.
Examples of Northern blotting analysis of DD products in a time course experiment on PC5T spheroid formation. Monolayers were trypsinized and plated on solid agarose for the times indicated. Total RNA was extracted, and approximately 20 μg was loaded per lane. Probes were labeled with [32P]dCTP. Sizes of bands were approximated by relative migration with respect to 18S and 28S bands. Ethidium bromide (EtBr) and ubiquitin mRNA are shown as loading controls. Grouped blots were generated by stripping and reprobing of the same filter.
Spheroid formation, particularly in large (i.e., >2-mm) spheroids, is associated with conditions of hypoxia and stress, as a consequence of limited diffusion of factors such as glucose and oxygen into the core of the spheroid (17, 18). To test whether either of these conditions may contribute to changes in the expression of the identified DD products, PC5T monolayers were grown in low (1%) serum or in the presence of 100 μM cobalt chloride, which acts as a hypoxia mimetic, for 24 h. Total RNA was then extracted and analyzed by Northern blotting. Neither treatment substantially affected the RNA levels of the identified DD products (Fig. 3), with the exception of A6aC, which was found to be up-regulated (twofold) by serum starvation (which also caused a slight down-regulation in M17 and M4cG). Thus, the majority of the 26 identified cDNAs associated with spheroid formation were shown not to be affected by stress (as caused by serum starvation) or hypoxia-like conditions. Presumably the changes detected in gene expression are associated with differences in cell-cell interactions between monolayers and spheroids.
FIG. 3.
Examples of Northern blotting analysis of PC5T EMT-6 monolayers which were serum starved (PC5T/1%FCS) or treated with 100 μM cobalt chloride (PC5T/CoCl2) for 24 h, using isolated DD products. Control PC5T cells were grown in medium containing 10% fetal calf serum (FCS). Total RNA was extracted, and approximately 20 μg was loaded per lane. Probes were labeled with [32P]dCTP. Sizes of bands were approximated by migration relative to 18S and 28S bands. Ethidium bromide (EtBr) and ubiquitin mRNA are shown as loading controls. Grouped blots were generated by stripping and reprobing of the same filter.
It was previously shown that EMT-6 variants selected in vivo for resistance to four alkylating agents (carboplatin, cisplatin, thiotepa, and cyclophosphamide) form more compact spheroids than the parental EMT-6 cell line (18). These variants, however, do not express significant resistance to alkylating agents compared to the parental EMT-6 cells analyzed as monolayer cultures. Initially this was suggestive of the possibility that the drug resistance properties of the variants are operative only in vivo (38). However, we have demonstrated that the EMT-6 variants are significantly more resistant (5- to 10-fold) to the alkylating agent against which they were selected than the parental line, provided that the cells were tested as spheroids (18). We therefore decided to analyze the expression of the identified cDNAs in the EMT-6 drug-resistant variants grown as monolayers and spheroids. By Northern blotting we found that 8 of the 26 identified DD products were differentially expressed in all drug-resistant variants between monolayer and spheroid cultures (Fig. 4). These cDNAs, whose expression was found to correlate in all spheroid models tested, were cloned into pCR2 and sequenced. Four of the eight identified cDNAs were found to be up-regulated in spheroids; these were identified by database search as encoding 2-oxoglutarate carrier protein, JTV-1, and two novel sequences. The four cDNAs identified as being down-regulated in spheroids were found to match Nop56, the NADH SDAP subunit, and two novel sequences.
FIG. 4.
Northern blotting analysis of EMT-6 parental (P) and variants selected in vivo for drug resistance (to cyclophosphamide [CTX], thiotepa [Thio], cisplatin [DDP], and carboplatin [Carbo]) grown as monolayer (Mon) or Spheroid (Sph) cultures for 24 h. Approximately 20 μg of total RNA was loaded per lane. DD products were [32P]dCTP labeled. Sizes of bands were approximated by migration relative to 18S and 28S bands. Ethidium bromide (EtBr) and ubiquitin mRNA are shown as loading controls. Grouped blots (iii and iv) were generated by stripping and reprobing of the same filter.
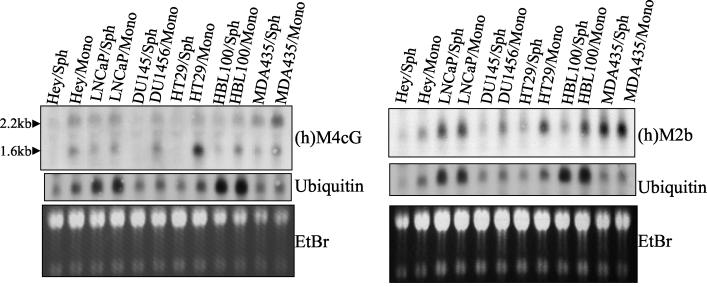
We next decided to test whether the particular changes identified with the EMT-6 tumor system would also differ between monolayers and spheroids of human tumor cell lines. A panel of six human tumor cell lines (HEY [ovarian cancer], LNCaP and DU145 [prostate cancer], HT29 [colorectal cancer], and HBL-100 and MDA-MB-435 [breast cancer]) were grown as monolayers and spheroids, and the corresponding RNA was isolated and analyzed by Northern blotting. We chose the first two products identified in our DD study, which were M4cG (Nop56) and M2b (NADH SDAP), to test the expression of their human homologues in human tumor spheroids. Using the database sequences of the human homologues, primers for reverse transcription-PCR were designed, and the cDNAs were amplified by using HT29 monolayer RNA as a starting material. As shown in Fig. 5 the human Nop56 (M4cG) showed a decrease in mRNA expression in spheroids compared to monolayer cultures in all human tumor cell lines. The human homologue of M2b (NADH SDAP) showed a similar change in expression in three (DU145, HT29, and HBL100) of the six human tumor cell lines tested (Fig. 5). Thus, changes in gene expression identified between EMT-6 monolayer and spheroid cultures show a similar pattern of altered expression in the human tumor cell lines tested.
FIG. 5.
Northern blotting analysis of human ovarian (Hey), prostate (LNCaP and DU145), colon (HT-29), and breast (HBL100 and MDA-435) cancer cell lines grown as monolayers (Mono) or as spheroids (Sph) using [32P]dCTP-labeled probes. Total RNA was loaded at 20 μg per lane. Sizes of bands were approximated by migration relative to 18S and 28S bands. Ethidium bromide (EtBr) and ubiquitin mRNA are shown as loading controls. h, human.
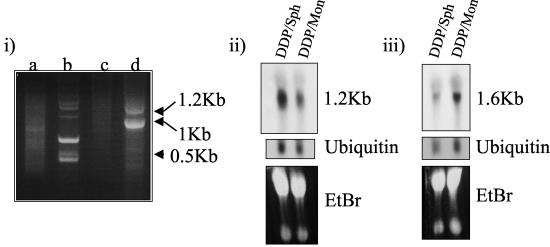
Finally, we decided to attempt to characterize products whose sequence from the DD product did not provide a match with gene database sequences, using a 5′-RACE protocol. Total RNA from EMT-6/DDP cells grown as spheroids was ligated to an RNA oligonucleotide (as described in Materials and Methods). Primers specific to A3bG and the synthetic RNA oligonucleotide were used in a reverse transcription-PCR which generated two bands of 1.2 and 1.0 kb (Fig. 6). The 1.0-kb band was gel purified and shown by Northern blotting to produce the same expression pattern between monolayer and spheroids as observed with A3bG (Fig. 4). Surprisingly, the 1.2-kb band produced a pattern opposite to that expected, i.e., it was down-regulated in spheroids. The 1.0- and 1.2-kb bands were cloned into pCR2 and sequenced. The 1.0-kb band was shown to match the 5′ end of A3bG, as expected, and the 5′ end of this product showed a database match with JTV-1. The 1.2-kb band was found to match transgellin. We do not know how this product was generated in our A3bG-specific 5′-RACE protocol, but since this (fortuitous) gene was found to be down-regulated in spheroids, we included it as an identified product in Table 1.
FIG. 6.
5′-RACE and Northern blotting analysis of products. Total RNA from EMT-6/DDP (cisplatin-resistant) cells grown as spheroids was used in a 5′-RACE reaction to amplify the 5′ end of the A3bG product. (i) 5′-RACE products were resolved on a 1% agarose gel (lane a, primary PCR). Secondary PCRs were carried out with A3bG nested primer alone (lane b) or RNA oligonucleotide-specific nested primer alone (lane c). RACE products were generated with nested primers to A3bG and RNA oligonucleotide (lane d). (ii and iii) The two largest PCR products from the nested PCR (approximately 1.2 and 1 kb) were gel purified and applied to Northern blots of EMT-6/PDD grown as monolayers (Mon) or spheroids (Sph). The 1-kb RACE product hybridized to a message of 1.2 kb (ii), and the 1.2-kb RACE product hybridized to a message of 1.6-kb (iii). Sizes of bands were approximated by using size markers (i) or by migration relative to the 28S and 18S bands. Approximately 20 μg of total RNA was loaded per lane. Probes were labeled with [32P]dCTP. Ethidium bromide (EtBr) and ubiquitin mRNA are shown as loading controls.
TABLE 1.
Sequence analysis of DD products
| DD cDNA | Database search resulta |
|---|---|
| A7eG | 2-Oxoglutarate carrier protein |
| M2b | NADH oxidoreductase SDAP subunit |
| M4cG | Nucloeolar protein 56 |
| A3bG*b | Transgellin (cloned by 5′-RACE) |
| A3bG | EST (corresponding to JTV-1) |
| M3cG | EST |
| M14aG | Novel |
| A7cG | Novel |
| A3 | Ribosomal protein S24 |
| A18cG | EST |
| A6Ac | Guanylate binding protein/gamma interferon inducible protein |
EST, a match was found in the murine expressed sequence tag database; Novel, no match found in any database.
*, additional product identified in the process of cloning A3bG.
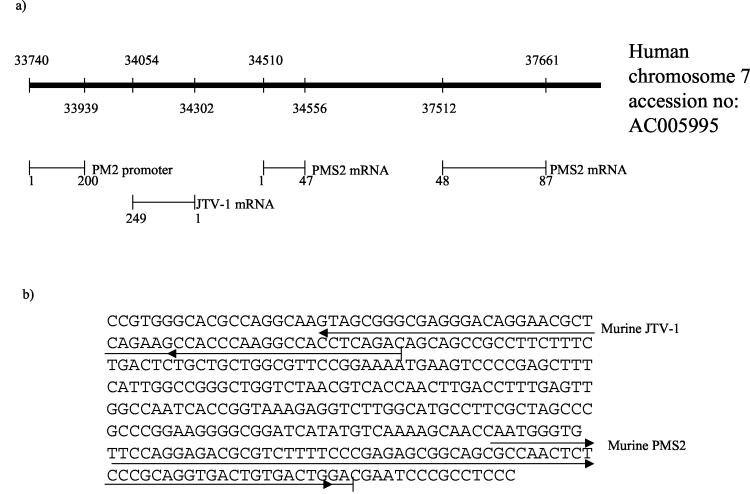
Human JTV-1 was identified as a gene initiating within the human PMS2 promoter, which is transcribed in the orientation opposite to that of PMS2 (26). We therefore decided to test whether PMS2 expression could be affected as a consequence of JTV-1 overexpression in spheroids. By Northern blotting, murine PMS2 was shown to be down-regulated in EMT-6 spheroids compared to monolayers (Fig. 7). Whether this is indeed related to the overexpression of murine JTV-1 is not known, particularly since the murine JTV-1 has not yet been characterized. We decided to test whether, as in the case with the human homologs, the murine JTV-1 and PMS2 genes were separated by a short physical distance (as well as in the opposite orientation) in the mouse genome. A reverse primer from murine JTV-1 and a reverse primer from murine PMS2 were used to amplify EMT-6 genomic DNA. A band of 365 bp was obtained. Sequencing identified regions of JTV-1 and PMS2 at a distance of 185 bp (in humans this distance is approximately 200 bp, according to Nicolaides et al. [26]) from each other (Fig. 8), as detected by BLAST searching.
FIG. 7.
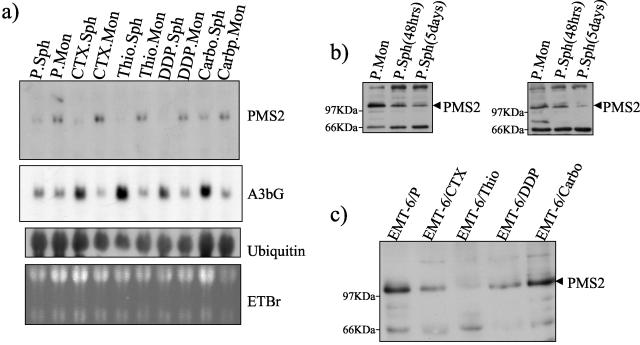
Northern blotting and Western blotting analysis of PMS2. (a) Northern blotting analysis of parental EMT-6 (P) and variants selected in vivo for drug resistance (to cyclophosphamide [CTX], thiotepa [Thio], cisplatin [DDP], and carboplatin [Carbo]) grown as monolayer (Mon) or Spheroid (Sph) cultures for 24 h. Approximately 20 μg of total RNA was loaded per lane. DD products were [32P]dCTP labeled. Band sizes were approximated by migration relative to 18S and 28S bands. Ethidium bromide (EtBr) and ubiquitin mRNA are shown as loading controls. The A3bG blot from Fig. 4 is reproduced here (the same filter was stripped and reprobed for PMS2) for ease of comparison. (b) Western blotting of PMS2 in EMT-6 and EMT-6/DDP showing down-regulation of murine PMS2 in spheroids (Sph) compared to monolayers (Mon). (c) Western blotting of murine PMS2 in lysates of tumors of EMT-6/P and drug-resistant variants. PMS2 is down-regulated in all cells except EMT-6/Carbo compared to parental EMT-6 cells.
FIG. 8.
Identification of a murine genomic 365-bp region containing sequence matches to murine JTV-1 and PMS2 in opposite orientations. (a) The bold line corresponds to a region of human chromosome 7 present in GenBank (accession no. AC005995), with the relative positions of human JTV-1 and human PMS2 shown beneath the line. (b) Sequence of identified mouse genomic DNA showing murine PMS2 and JTV-1 exon regions as identified by BLAST searches against GenBank.
Since antibodies to PMS2 are available, we next decided to test whether PMS2 protein expression is altered in EMT-6 spheroids. By Western blotting, using a mouse anti-human PMS2 monoclonal antibody (Transduction Laboratories), PMS2 was shown to be down-regulated in spheroids compared to monolayers (Fig. 7). PMS2 protein down-regulation was observed after 48 h of growth as spheroid cultures (compared to monolayers) and continued to decrease thereafter.
We next decided to test whether the down-regulation of PMS2 protein could be detected in EMT-6 sublines selected for acquired drug resistance, compared to the parental cell lines. EMT-6 and drug-resistant tumors were grown in BALB/c mice and excised, and protein lysates were prepared. By Western blotting, down-regulation of PMS-2 protein was observed in EMT-6 variants resistant to cyclophosphamide, cisplatin, and thiotepa (but not in carboplatin-resistant variants), compared to the parental line (Fig. 7). Thus, PMS2 is down-regulated not only in EMT-6 spheroids compared to monolayer cultures but also in three EMT-6 drug-resistant variants grown in vivo compared to the parental EMT-6 tumor.
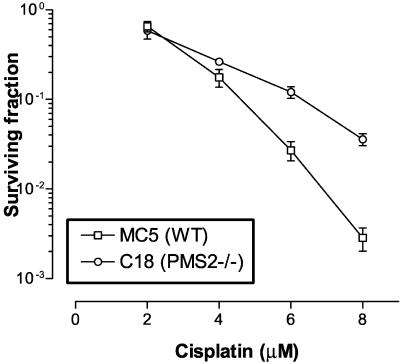
To test the role of PMS2 in resistance to alkylating agents, we compared, as proof of concept, the sensitivity to cisplatin between wild-type MEFs and MEFs derived from PMS2 knockout mice (31). At higher concentrations of cisplatin (10 to 20 μM), no surviving colonies were observed for wild-type MC5 fibroblasts even at high plating cell numbers (>106), whereas colonies were noted for C18 (PMS2−/−) fibroblasts (data not shown). At lower concentrations of drug (2 to 8 μM), C18 MEFs were also shown to be more resistant (e.g., approximately 10-fold at 8 μM cisplatin) than MC5 cells (Fig. 9).
FIG. 9.
In vitro survival of MC5 (wild-type [WT]) and C18 (PMS2−/−) MEFs treated with cisplatin. Monolayer cultures exposed to cisplatin for 1 h were dispersed and replated in a colony formation assay. Values are averages ± standard deviations.
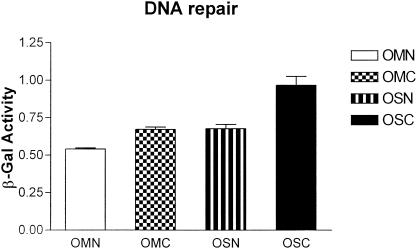
We next decided to measure DNA mismatch repair activity between EMT-6 monolayers and spheroids. Since biochemical assays for this process typically require 109 mammalian cells (5), it was not feasible to use this approach to analyze spheroid cultures (each spheroid is composed of approximately 105 cells). We therefore decided to use a genetic approach developed by Nicolaides and colleagues (27). pCAR-OF (out of frame) is an episomal vector containing a hygromycin resistance gene and a beta-galactosidase gene with an upstream 58-bp out-of-frame poly(CA) tract; this vector can only generate beta-galactosidase activity as a consequence of a frame-restoring mutation (i.e., insertion or deletion) following transfection. Beta-galactosidase activity can therefore be used to measure the rate of such mutations (27). EMT-6/P cells selected for stable transfection with pCAR-OF were plated as monolayers and spheroids. In a parallel experiment, EMT-6 transfectants were first treated with cisplatin (50 μM for 1 h) and then replated as monolayers and spheroids. Three days after plating, cells were analyzed for beta-galactosidase activity. In both experiments, beta-galactosidase activity was found to be higher in spheroids than in monolayers (Fig. 10).
FIG. 10.
Analysis of beta-galactosidase activity in EMT-6/P cells transfected with a DNA mismatch repair reporter vector (pCAR-OF). EMT-6/P cells transfected with pCAR-OF were plated after cisplatin treatment (50 μM for 1 h) as monolayers (OMC) and spheroids (OSC) or after no drug treatment as monolayers (OMN) or spheroids (OSN). After 3 days of plating, beta-galactosidase (β-Gal) activity (units per milligram of lysate) was measured; error bars indicate standard deviations.
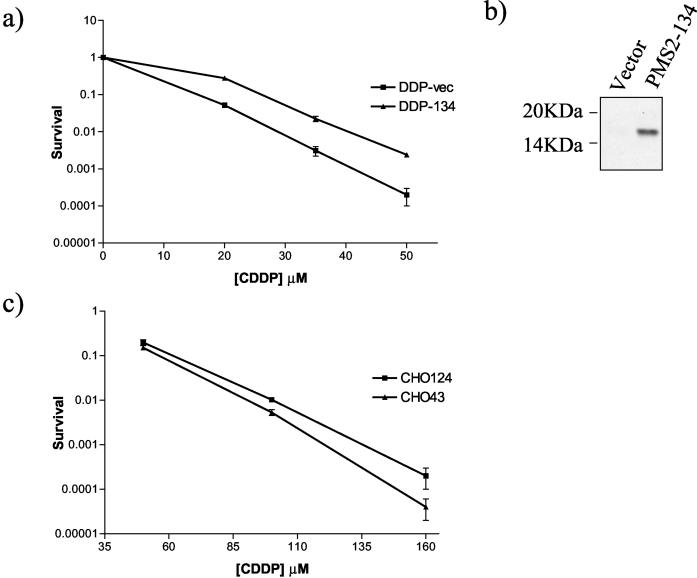
To determine the role of PMS2 down-regulation in EMT-6 spheroids, we attempted to overexpress wild-type PMS2 in spheroids or to down-regulate this gene product (in monolayers) using RNAi interference. Despite repeated attempts, we were unable to reproducibly alter PMS2 expression by either method. We therefore sought to make use of a dominant negative PMS2 (PMS2-134) cDNA, which was previously shown to inhibit endogenous PMS2 and impair DNA mismatch repair (27), transfected in Chinese hamster ovary cells (provided by Morphotek). In addition we transfected PMS2-134 into EMT-6/DDP using an episomal (pCEP4) vector. Western blotting analysis for PMS2-134 (Fig. 11b) was carried out with the anti-hPMS2 E-19 antibody (Santa Cruz Biotechnology), which recognizes the N terminus of human PMS2. For both CHO and EMT-6/DDP, pooled transfectant populations were used to avoid artifacts due to clonal variance. By colony formation assays, we found EMT-6/DDP cells transfected with PMS2-134 to be more resistant to cisplatin than control transfected EMT-6/DDP cells (Fig. 11a). A similar result was observed with Chinese hamster ovary cells transfected with the dominant negative (27) PMS2 compared to controls (Fig. 11c).
FIG. 11.
(a) In vitro survival of EMT-6/DDP cells transfected with dominant negative PMS2 (DDP-134) or vector only (DDP-vec), following cisplatin treatment. (b) Western blot analysis of dominant negative PMS2 (PMS2-134) expression in transfected EMT-6/DDP cells and vector-only transfectants, showing a band of approximately 17 kDa. (c) In vitro survival of CHO cells transfected with vector (CHO43) or dominant negative PMS2 (CHO124) following cisplatin treatment. Monolayer cultures were exposed to cisplatin (CDDP) for 1 h, dispersed, and replated into colony formation assays. Values are averages ± standard deviations.
DISCUSSION
Studies of drug resistance mechanisms in cancer have been dominated by the use of mutant or variant cell lines of drug-resistant cells, usually selected in vitro. Typically, a cancer cell line is serially exposed to increasing concentrations of a cytotoxic drug over a prolonged period, and the selected variant subpopulation is then compared to the parental, drug-sensitive cell line. It is not unusual, by selection in monolayer cultures, to generate resistant cell line variants which exhibit 10- to 100-fold-increased resistance to a particular drug (17). This approach has revealed a number of biochemical mechanisms, such as increased drug efflux, increased DNA enzyme repair activity, and increased resistance to apoptosis, among others (1, 4). Such mechanisms, which typically result from genetic mutations leading to loss of function or overexpression of a particular gene, are generally assumed to be involved in in vivo situations when tumors relapse and stop responding to chemotherapy. For example, P glycoprotein (1) became a major target for circumvention of tumor cell multidrug resistance. However, while P-glycoprotein antagonists, such as calcium channel blockers, cyclosporine or cyclosporine analogues, and verapamil (2, 6), are remarkably effective in reversing the multidrug-resistant phenotype in monolayer cultures, the same is not necessarily true for multicellular spheroids (32, 34) or other multicellular systems (39). This may help explain results from clinical trials of P-glycoprotein reversal agents in patients with P-glycoprotein-expressing adult solid tumors, as they have shown little, if any, efficacy thus far, despite over a decade of clinical testing (6, 13).
One intriguing finding that may help explain the differences between monolayer and in vivo tumors with regard to drug resistance comes from the work of Teicher et al. (38), who showed that acquired drug resistance generated in vivo by the EMT-6 tumor was not expressed in monolayer cultures. This is but one example of monolayer cell culture systems providing what may be overly simplistic unicellular mechanisms of drug resistance, leading to an underappreciation of tissue- or multicellular aggregate-mediated mechanisms. Since the drug-resistant phenotypes were previously re-expressed by growing the EMT-6 variants generated by Teicher et al. as spheroids in vitro, a mechanism that is cell-cell or cell contact dependent, termed multicellular resistance, was proposed (4, 18). It is noteworthy that the typical magnitude of resistance generated by spheroid cancer cell line models (17) is in the range of 2- to 10-fold greater than that generated by controls (or parent cell line), i.e., less dramatic than what can be obtained by using monolayer culture models, and it remains to be established whether unicellular or multicellular mechanisms are most relevant in the clinical setting.
In an attempt to begin to elucidate possible molecular mechanisms responsible for multicellular drug resistance, we analyzed an EMT-6 subline capable of forming compact spheroids using the DD method to identify changes in gene expression between monolayers and spheroids. Having isolated 26 differentially expressed cDNAs, we then used the EMT-6 variants generated in vivo by Teicher et al. (38) to find genes that were consistently differentially expressed between monolayers and spheroids. In this manner we detected four genes that were up-regulated in spheroids and four that were down-regulated compared to monolayer cultures. We are attempting to characterize the four novel sequences identified as differentially expressed between monolayers and spheroids.
Nature of differentially expressed genes.
The M4cG DD product, which is down-regulated in spheroids, was found to correspond to the murine homologue of the human nucleolar protein 56 (Nop56p/SIK1). This gene was originally reported as SIK1, for “suppressor of IκB”; SIK1 was isolated as a high-copy-number suppressor in yeast of the growth-inhibitory phenotype induced by an IκB-GAL4 fusion protein (24).
The M2b cDNA, which is also down-regulated in spheroids, was found to match the NADH SDAP subunit, which is a component of respiratory chain complex I in mitochondria. This complex consists of 34 different subunits, though the effect of deregulation of the SDAP subunit on the complex and its activity is unknown. The A7eG cDNA, which is up-regulated in spheroids, was found to match the 2-oxoglutarate/malate carrier protein. It is of interest that two of the identified genes are involved in mitochondrial metabolism and redox reactions. In that respect, a recent study demonstrated that an increase in reactive oxygen species increases proliferation in spheroids and decreases P-glycoprotein-mediated drug resistance (41). Furthermore the expression of another protein involved in redox reactions, DT-diaphorase, has been correlated with drug resistance in HT29 spheroids (30). Further work will be needed to determine how mitochondrial metabolism impacts proliferation (and drug resistance) in tumor spheroids.
The JTV-1 gene was identified by 5′-RACE of the A3bG DD product, which was found to be up-regulated in EMT-6 spheroids compared to monolayers. JTV-1 was originally identified by Nicolaides and colleagues (26) as a transcript originating within the PMS2 gene region, but on the opposite strand. It is not known what interactions these two genes may have, e.g., whether the expression of one affects the other as a consequence of their juxtaposition.
Nature of differentially expressed genes: PMS2 and the possible impact of suppressed DNA mismatch repair in multicellular drug resistance.
Although the murine genomic region of PMS2 has not been characterized to the point of determining the nearby presence or absence of a murine JTV-1 homolog, we decided nevertheless to evaluate the relative expression of murine PMS2 in EMT-6 monolayer and spheroids. This was based on the possibility of a murine juxtaposition of these two genes that would be similar to the situation in human genomic DNA, and that transcription of one gene could interfere with that of the other. Our interest was driven by the fact that JTV1 was closely associated (at the genomic level) with a human gene (PMS2), loss of expression of which has already been implicated in resistance to DNA-damaging drugs. In this regard, our findings that PMS2 is indeed differentially expressed between monolayers and spheroids prompted us to test whether these two genes were physically in close proximity in mouse genomic DNA. By using the database murine PMS2 sequence and our 5′-RACE sequence of murine JTV-1, two reverse primers were generated, one corresponding to the 5′ end of each gene. These primers were used in a PCR to amplify mouse genomic DNA. Our results suggest that the murine JTV-1 is oriented in the opposite direction and 185 bp from the murine PMS2. We do not know, however, whether this physical proximity is related to the fact that both genes are differentially expressed between monolayers and spheroids (JTV-1 is up-regulated in spheroids, whereas PMS2 is down-regulated).
The PMS2 gene is mutated in a subset of hereditary nonpolyposis colorectal cancers (28), which is involved in DNA mismatch repair. In this regard, it is of interest that mismatch repair deficiency has been shown to confer resistance to DNA alkylating agents such as N-methyl-N′-nitro-N-nitrosoguanidine and N-methyl-N-nitrosourea (3), as well as the chemotherapeutic drugs cisplatin and doxorubicin (5). It has been suggested that defects in this DNA mismatch repair can lead to tolerance to DNA mutations of the kind induced by alkylating agents. For example, efficient DNA mismatch repair may lead to a signal to induce apoptosis if cellular DNA damage cannot be repaired adequately; thus, mismatch repair deficiency may allow tolerance and accumulation of mutations and failure to undergo apoptosis (5, 27). We do not know yet whether down-regulation of PMS2, as we have observed in tumor spheroids, is sufficient to mimic the effect of PMS2 loss or mutation that others have described in terms of drug resistance. Our current working hypothesis is that down-regulation of PMS2 in tumor spheroids can lead to resistance to alkylating agents and certain other DNA damaging agents via reduced (or impaired) DNA mismatch repair.
There is already some evidence that alterations in PMS2 activity can affect DNA mismatch repair (and presumably drug resistance): Nicolaides and colleagues (27) have described a mutant PMS2 sequence (PMS2-134) derived from a patient with hereditary nonpolyposis colorectal cancer syndrome which appears to act in a dominant negative fashion when transfected into DNA mismatch repair-proficient cells, reducing their ability to carry out repair of different lesions (e.g., G/T heteroduplexes and heteroduplexes containing 2- to 4-bp loops) (27). To test our hypothesis, we used the same genetic approach used by Nicolaides et al. (27). Using the pCAR-OF vector transfected into EMT-6/P cells we observed a decrease in DNA mismatch repair activity in spheroids compared to monolayers (Fig. 10) after 3 days of culture, in both the presence and absence of cisplatin treatment. There were a number of reasons for choosing such a time point and protocol. First, spheroids are typically cultured for 24 to 72 h to prevent spheroids from reaching a size (i.e., >2 mm) at which hypoxia becomes a factor. Second, we chose the longest possible time to increase the sensitivity of the assay, since previous applications of the pCAR-OF reporter vector involved transfectants being cultured for at least 1 to 2 weeks (27). Third, to further increase sensitivity, a DNA-damaging agent (cisplatin) was also included. Fourth, since EMT-6/P monolayers proliferate faster than spheroids (13), a short time had to be chosen to minimize the possible artifact of DNA mutations in monolayer cultures being a consequence of a higher rate of DNA replication than in spheroids. Finally, since cisplatin-induced cell death (which could complicate the interpretation of the results) is typically observed after 3 to 4 days in EMT-6 cells, the assay was carried out for 3 days.
Given the above limitations, it is remarkable that after 3 days in culture a 1.5-fold difference in beta-galactosidase activity was noted between cisplatin-treated EMT-6/P spheroids and monolayers (Fig. 10). A similar result, discussed below, was recently observed by Mihaylova et al. (23) in EMT-6 monolayer cells grown under hypoxia for 2 days. The change we observed (Fig. 10) is unlikely to be merely a consequence of drug treatment, since an increase in DNA mismatch repair in spheroids was also observed in the absence of cisplatin treatment (Fig. 10). A direct comparison (between drug-treated and non-drug-treated cells) is complicated by the higher compaction in spheroids that results from exposure to alkylating agents, which is associated with higher levels of drug resistance (10, 18) (data not shown) and lower levels of PMS2. It is probable that the observed decrease in DNA mismatch repair in spheroids is an underestimate, as a consequence of reduced cell cycle kinetics in EMT6 spheroids compared to monolayers (17, 33). Nor can we exclude the possibility that the reduced growth fraction in spheroids (compared to monolayers) directly affects PMS2 expression. Nevertheless, our results indicate that a contributing factor in increased resistance to alkylating agents in tumor spheroids (compared to monolayers) is a decrease in the level of DNA mismatch repair. Our result stands in contrast to other types of DNA repair enzymes whose activity is frequently increased in tumor cells selected for resistance to DNA-damaging agents, e.g., enzymes involved in nucleotide excision repair (42).
We have attempted to express PMS2 de novo in EMT-6 cells as a means of overexpressing PMS2 in spheroids and also tried RNA interference oligonucleotides to down-regulate PMS2 in monolayers. We were, however, unable to obtain high expression of PMS2 by transfection, and RNA interference experiments in EMT-6 proved futile in our hands. In cases where RNA interference oligonucleotides caused down-regulation of PMS2, the effect was not readily reproducible, or the conditions employed (e.g., increasing the concentration of interfering oligonucleotides) resulted in the marked down-regulation of nonspecific genes (e.g., GAPDH) (unpublished observation). We therefore sought to inhibit PMS2 function in EMT-6/DDP monolayers using a dominant negative PMS2 approach (27). Transfection of PMS2-134 into EMT-6/DDP cells resulted in increased resistance to cisplatin compared to controls, which was also observed in Chinese hamster ovary cells transfected with the dominant negative PMS2. Our results therefore suggest that both loss (as observed with PMS2 knockout cells) and inhibition (using a dominant negative approach) of PMS2 result in increased resistance to cisplatin.
The PMS2 protein has been shown to be down-regulated in a number of tumors in vivo and in drug-resistant variants of ovarian cancer cell lines (5). We have also shown that the PMS2 protein is down-regulated in three of four EMT-6 drug-resistant variants compared to the parental EMT-6 tumor. Using MEFs derived from wild-type and PMS2 knockout mice (31), we have shown that loss of PMS2 leads to increased resistance to cisplatin by in vitro colony formation assays (Fig. 9) (Michael Liskay, personal communication). These results are in agreement with those of Fritzell et al. (9), who similarly showed increased resistance to gamma irradiation in PMS2-deficient cells compared to the wild type. Taken together, our results suggest that a down-regulation in PMS2 is associated with a decrease in DNA mismatch repair activity, which can contribute to increased resistance to DNA-damaging drugs and, furthermore, that this change and relationship are of particular importance in multicellular resistance to such agents.
It is of interest that no difference in PMS2 levels could be detected, by Northern blotting, between EMT-6/P and drug-resistant variants when these were analyzed as monolayers. This is consistent with our previous observations of little or no difference in drug resistance between these cell line variants when grown under these conditions (18). Furthermore, it is highly unlikely that down-regulation of PMS2 can explain part of the drug resistance mechanisms in all tumor spheroid models. For example, the EMT-6 variant resistant to carboplatin does not show down-regulation in PMS2 in vivo compared to the parental tumor (Fig. 7). This variant cell line may down-regulate other genes involved in DNA mismatch repair mechanisms (such as the PMS2-associating protein MLH1) when grown in vivo (or as spheroids) or by an entirely different mechanism (e.g., resistance to apoptosis). We have not yet established whether other mismatch repair proteins are involved in multicellular drug resistance, although we have begun to analyze MLH1 levels in murine and human spheroids. Our preliminary analysis found that although MLH1 is down-regulated in some cell lines (e.g., EMT-6/Thio and the MDA435 human breast cancer cell line), we cannot correlate these changes with the down-regulation of PMS2. With regard to MLH1, changes in the levels of this protein may be a consequence of promoter methylation (a process which is not reported to regulate PMS2 expression). Recently, Mihaylova et al. (23) reported the down-regulation of MLH1, and PMS2, in the same cell line system we used (EMT-6) when the cells were grown as monolayers under hypoxic conditions. Although their results are strikingly similar to ours (e.g., with respect to decreased levels of DNA mismatch repair function in EMT-6 cells that down-regulate PMS2), they reported PMS2 mRNA to be unchanged in EMT-6 cells grown under hypoxic conditions. This is in sharp contrast to our observation that in spheroids PMS2 mRNA is down-regulated (compared to monolayers). Mihaylova et al. suggest that under hypoxia MLH1 protein is down-regulated and that in turn leads to decreased stability of the PMS2 protein. Regardless of the mechanism involved, our results and those of Mihaylova et al. suggest that tumors can down-regulate DNA mismatch repair in response to environmental factors (e.g., hypoxic conditions or increased cell-cell adhesion), which results in increased resistance to alkylating agents.
Resistance to DNA-damaging drugs through changes in DNA mismatch repair has typically been described and thought of as a consequence of an irreversible loss of or mutation in one or more DNA mismatch repair genes (27, 42). Our results, however, suggest that modest changes in these repair processes may occur as a consequence of deregulation (e.g., down-regulation of PMS2) of associated proteins, presumably by cell-cell adhesion mechanisms and/or tumor microenvironmental factors. Furthermore, the extent of these reversible changes can be selected in vivo by exposing tumors to alkylating agents (three of four EMT-6 drug resistant variants, compared to parent EMT-6, showed greater down-regulation of PMS2 both in vivo as solid tumors and in vitro as tumor spheroids). Therefore, the inhibition of this selectable (but reversible) phenotype, for example, using antiadhesives such as hyaluronidase (35) or anti-E-cadherin antibodies (12), is a new rationale for the development of chemosensitizers for the treatment of solid tumors.
In summary, using the EMT-6 mouse tumor model and DD, we have identified eight cDNAs whose expression correlates positively or inversely with tumor spheroid formation. To the best of our knowledge, this is the first attempt to systematically identify novel genetic changes associated with tumor spheroid formation, in particular with respect to multicellular drug resistance. The identified genes suggest a possible role of mitochondrial metabolism and a homologue of the yeast suppressor of IκB-mediated growth inhibition. Perhaps most interestingly, PMS2 was found to be down-regulated in spheroids compared to monolayers and in three of four EMT-6 selected drug-resistant variants compared to the parental tumor. Analysis of PMS2-deficient fibroblasts treated with cisplatin provided further evidence for the role of this gene in sensitivity to alkylating agents, and a decrease in DNA mismatch repair was observed in spheroid compared to monolayer cultures. The effect of cell-cell adhesion on the expression of mismatch repair genes and, in turn, their contribution to resistance to chemotherapeutic drugs provide an additional contributing factor in a “reversible” form of drug resistance observed in spheroids and in vivo. Further studies of the genetic changes observed in this study should also lead to a better understanding of other mechanisms involved in multicellular tumor drug resistance.
Acknowledgments
We are extremely grateful to Michael Liskay for providing PMS2−/− cells and discussing unpublished results. We are grateful for the excellent secretarial assistance of Cassandra Cheng and Lynda Woodcock. Thanks are extended to Guido Bocci, Ralph Durand, Bert Vogelstein, and Shane Green for review of the manuscript and to the anonymous reviewers for their suggestions.
Giulio Francia was funded by the Sunnybrook and Women's College Health Sciences Centre Trust Fellowship. This work was supported by a grant to R. S. Kerbel from the National Cancer Institute of Canada.
REFERENCES
- 1.Borst, P. 1991. Genetic mechanisms of drug resistance. Rev. Oncol. 4:87-105. [DOI] [PubMed] [Google Scholar]
- 2.Bradley, G., P. F. Juranka, and V. Ling. 1988. Mechanisms of multidrug resistance. Biochem. Biophys. Acta 948:87-128. [DOI] [PubMed] [Google Scholar]
- 3.Branch, P., G. Aquilina, M. Bignami, and P. Karran. 1993. Defective mismatch binding and a mutator phenotype in cells tolerant to DNA damage. Nature 362:652-654. [DOI] [PubMed] [Google Scholar]
- 4.Desoize, B., and J. Jardillier. 2000. Multicellular resistance: a paradigm for clinical resistance? Crit. Rev. Oncol. Hematol. 36:193-207. [DOI] [PubMed] [Google Scholar]
- 5.Drummond, J. T., A. Anthoney, R. Brown, and P. Modrich. 1996. Cisplatin and adriamycin resistance are associated with MutLα and mismatch repair deficiency in an ovarian tumor cell line. J. Biol. Chem. 271:19645-19648. [DOI] [PubMed] [Google Scholar]
- 6.Fan, D. 1994. Reversal of multidrug resistance, p. 93-125. In J. A. Kellen (ed.), Reversal of multidrug resistance in cancer. CRC Press, Boca Raton, Fla.
- 7.Francia, G., S. D. Mitchell, S. E. Moss, A. M. Hanby, J. F. Marshall, and I. R. Hart. 1996. Identification by differential display of annexin-VI, a gene differentially expressed during melanoma progression. Cancer Res. 56:3855-3858. [PubMed] [Google Scholar]
- 8.Frankel, A., S. Man, P. Elliott, J. Adams, and R. S. Kerbel. 2000. Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341. Clin. Cancer Res. 6:3719-3728. [PubMed] [Google Scholar]
- 9.Fritzell, J. A., L. Narayanan, S. M. Baker, C. E. Bronner, S. E. Andrew, T. A. Prolla, A. Bradley, F. R. Jirik, R. M. Liskay, and P. M. Glazer. 1997. Role of DNA mismatch repair in the cytotoxicity of ionizing radiation. Cancer Res. 57:5143-5147. [PubMed] [Google Scholar]
- 10.Graham, C. H., H. Kobayashi, K. S. Stankiewicz, S. Man, S. J. Kapitain, and R. S. Kerbel. 1994. Rapid acquisition of multicellular drug resistance after a single transient exposure of mammary tumor cells to alkylating agents. J. Natl. Cancer Inst. 86:975-982. [DOI] [PubMed] [Google Scholar]
- 11.Green, S. K., G. Francia, C. Isidoro, and R. S. Kerbel. 2004. Anti-adhesive antibodies targeting e-cadherin sensitize multicellular tumor spheroids to chemotherapy in vitro. Mol. Cancer Ther. 3:149-159. [PubMed]
- 12.Green, S. K., A. Frankel, and R. S. Kerbel. 1999. Adhesion-dependent multicellular drug resistance. Anti-Cancer Drug Design 14:153-168. [PubMed] [Google Scholar]
- 13.Houghton, P. J., and S. B. Kaye. 1994. Multidrug resistance is not an important factor in therapeutic outcome in human malignancies. J. NIH Res. 6:55. [Google Scholar]
- 14.Jain, R. K. 1989. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J. Natl. Cancer Inst. 81:570-576. [DOI] [PubMed] [Google Scholar]
- 15.Jeltsch, M., a. Kaipainen, V. Joukov, X. Meng, M. Lakso, H. Rauvala, M. Swartz, D. Fukumura, R. K. Jain, and K. Alitalo. 1997. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276:1423-1425. [DOI] [PubMed] [Google Scholar]
- 16.Kartner, N., J. R. Riordan, and V. Ling. 1983. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science 221:1285-1288. [DOI] [PubMed] [Google Scholar]
- 17.Kerbel, R. S., J. Rak, H. Kobayashi, M. S. Man, B. St. Croix, and C. H. Graham. 1994. Multicellular resistance: a new paradigm to explain aspects of acquired drug resistance of solid tumors. Cold Spring Harbor Symp. Quant. Biol. 59:661-672. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi, H., S. Man, S. J. Kapitain, B. A. Teicher, and R. S. Kerbel. 1993. Acquired multicellular-mediated resistance to alkylating agents in cancer. Proc. Natl. Acad. Sci. USA 90:3294-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lal, A., H. Peters, B. St Croix, Z. A. Harpoon, M. W. Dewhirst, R. L. Strausberg, J. H. Kaanders, A. J. van der Kogel, and G. J. Riggins. 2001. Transcriptional response to hypoxia in human tumors. J. Natl. Cancer Inst. 93:1337-1343. [DOI] [PubMed] [Google Scholar]
- 20.Liang, P., and A. B. Pardee. 1992. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 257:967-971. [DOI] [PubMed] [Google Scholar]
- 21.Mansbridge, J. N., W. A. Ausserer, M. A. Knapp, and R. M. Sutherland. 1994. Adaptation of EGF receptor signal transduction to three-dimensional culture conditions: changes in surface receptor expression and protein tyrosine phosphorylation. J. Cell Physiol. 161:374-382. [DOI] [PubMed] [Google Scholar]
- 22.Martin, S. J., and D. R. Green. 1994. Apoptosis as a goal of cancer therapy. Curr. Opin. Cell Biol. 6:616-621. [DOI] [PubMed] [Google Scholar]
- 23.Mihaylova, V. T., R. S. Bindra, J. Yuan, D. Campisi, L. Narayanan, R. Jensen, F. Giordano, R. S. Johnson, S. Rockwell, and P. M. Glazer. 2003. Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol. Cell. Biol. 23:3265-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morin, P. J., J. A. Downs, A. M. Snodgrass, and T. D. Gilmore. 1995. Genetic analysis of growth inhibition by GAL4-L κ B-α in Saccharomyces cerevisiae. Cell Growth Differ. 6:789-798. [PubMed] [Google Scholar]
- 25.Murphy, B. J., K. R. Laderoute, H. J. Vreman, T. D. Grant, N. S. Gill, D. K. Stevenson, and R. M. Sutherland. 1993. Enhancement of heme oxygenase expression and activity in A431 squamous carcinoma multicellular tumor spheroids. Cancer Res. 53:2700-2703. [PubMed] [Google Scholar]
- 26.Nicolaides, N. C., K. W. Kinzler, and B. Vogelstein. 1995. Analysis of the 5′ region of PMS2 reveals heterogeneous transcripts and a novel overlapping gene. Genomics 29:329-334. [DOI] [PubMed] [Google Scholar]
- 27.Nicolaides, N. C., S. J. Littman, P. Modrich, K. W. Kinzler, and B. Vogelstein. 1998. A naturally occurring hPMS2 mutation can confer a dominant negative mutator phenotype. Mol. Cell. Biol. 18:1635-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolaides, N. C., N. Papadopoulos, B. Liu, Y. F. Wei, K. C. Carter, S. M. Ruben, C. A. Rosen, W. A. Haseltine, R. D. Fleischmann, and C. M. Fraser. 1994. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 371:75-80. [DOI] [PubMed] [Google Scholar]
- 29.Olive, P. L., and R. E. Durand. 1994. Drug and radiation resistance in spheroids: cell contact and kinetics. Cancer Metastasis Rev. 13:121-138. [DOI] [PubMed] [Google Scholar]
- 30.Phillips, R. M., A. de la Cruz, R. D. Traver, and N. W. Gibson. 1994. Increased activity and expression of NAD(P)H: quinone acceptor oxidoreductase in confluent cell cultures and within multicellular spheroids. Cancer Res. 54:3766-3771. [PubMed] [Google Scholar]
- 31.Prolla, T. A., S. M. Baker, A. C. Harris, J. L. Tsao, X. Yao, C. E. Bronner, B. Zheng, M. Gordon, J. Reneker, N. Arnheim, D. Shibata, A. Bradley, and R. M. Liskay. 1998. Tumour susceptibility and spontaneous mutation in mice deficient in Mlh1, Pms1 and Pms2 DNA mismatch repair. Nat. Genet. 18:276-279. [DOI] [PubMed] [Google Scholar]
- 32.Sakata, K., T. T. Kwok, G. R. Gordon, N. S. Waleh, and R. M. Sutherland. 1994. Resistance to verapamil sensitization of multidrug resistant cells grown as multicellular spheroids. Int. J. Cancer 59:282-286. [DOI] [PubMed] [Google Scholar]
- 33.St. Croix, B., V. A. Florenes, J. W. Rak, M. Flanagan, N. Bhattacharya, J. M. Slingerland, and R. S. Kerbel. 1996. Impact of the cyclin dependent kinase inhibitor p27Kip1 on adhesion-dependent resistance of tumor cells to anticancer agents. Nat. Med. 2:1204-1210. [DOI] [PubMed] [Google Scholar]
- 34.St. Croix, B., and R. S. Kerbel. 1997. Cell adhesion and drug resistance in cancer. Curr. Opin. Cell Biol. 9:549-556. [DOI] [PubMed] [Google Scholar]
- 35.St. Croix, B., J. W. Rak, S. Kapitain, C. Sheehan, C. H. Graham, and R. S. Kerbel. 1996. Reversal by hyaluronidase of adhesion-dependent multicellular drug resistance in mammary carcinoma cells. J. Natl. Cancer Inst. 88:1285-1296. [DOI] [PubMed] [Google Scholar]
- 36.St. Croix, B., C. Sheehan, J. W. Rak, V. A. Florenes, J. M. Slingerland, and R. S. Kerbel. 1998. E-cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27KIP1. J. Cell Biol. 142:557-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland, R. M. 1988. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 240:177-184. [DOI] [PubMed] [Google Scholar]
- 38.Teicher, B. A., T. S. Herman, S. A. Holden, Y. Wang, M. R. Pfeffer, J. W. Crawford, and E. Frei III. 1990. Tumor resistance to alkylating agents conferred by mechanisms operative only in vivo. Science 247:1457-1461. [DOI] [PubMed] [Google Scholar]
- 39.Tunggal, J. K., T. Melo, J. R. Ballinger, and I. F. Tannock. 2000. The influence of expression of P-glycoprotein on the penetration of anticancer drugs through multicellular layers. Int. J. Cancer 86:101-107. [DOI] [PubMed] [Google Scholar]
- 40.Waleh, N. S., J. Gallo, T. D. Grant, B. J. Murphy, R. H. Kramer, and R. M. Sutherland. 1994. Selective down-regulation of integrin receptors in spheroids of squamous cell carcinoma. Cancer Res. 54:838-843. [PubMed] [Google Scholar]
- 41.Wartenberg, M., K. Fischer, J. Hescheler, and H. Sauer. 2000. Redox regulation of P-glycoprotein-mediated multidrug resistance in multicellular prostate tumor spheroids. Int. J. Cancer 85:267-274. [PubMed] [Google Scholar]
- 42.Wood, R. D., M. Mitchell, J. Sgouros, and T. Lindahl. 2001. Human DNA repair genes. Science 291:1284-1289. [DOI] [PubMed] [Google Scholar]