Abstract
Mnk1 and Mnk2 are protein kinases that are directly phosphorylated and activated by extracellular signal-regulated kinase (ERK) or p38 mitogen-activated protein (MAP) kinases and implicated in the regulation of protein synthesis through their phosphorylation of eukaryotic translation initiation factor 4E (eIF4E) at Ser209. To investigate their physiological functions, we generated mice lacking the Mnk1 or Mnk2 gene or both; the resulting KO mice were viable, fertile, and developed normally. In embryonic fibroblasts prepared from Mnk1-Mnk2 DKO mice, eIF4E was not detectably phosphorylated at Ser209, even when the ERK and/or p38 MAP kinases were activated. Analysis of embryonic fibroblasts from single KO mice revealed that Mnk1 is responsible for the inducible phosphorylation of eIF4E in response to MAP kinase activation, whereas Mnk2 mainly contributes to eIF4E's basal, constitutive phosphorylation. Lipopolysaccharide (LPS)- or insulin-induced upregulation of eIF4E phosphorylation in the spleen, liver, or skeletal muscle was abolished in Mnk1−/− mice, whereas the basal eIF4E phosphorylation levels were decreased in Mnk2−/− mice. In Mnk1-Mnk2 DKO mice, no phosphorylated eIF4E was detected in any tissue studied, even after LPS or insulin injection. However, neither general protein synthesis nor cap-dependent translation, as assayed by a bicistronic reporter assay system, was affected in Mnk-deficient embryonic fibroblasts, despite the absence of phosphorylated eIF4E. Thus, Mnk1 and Mnk2 are exclusive eIF4E kinases both in cultured fibroblasts and adult tissues, and they regulate inducible and constitutive eIF4E phosphorylation, respectively. These results strongly suggest that eIF4E phosphorylation at Ser209 is not essential for cell growth during development.
Mitogen-activated protein kinases (MAPKs) are activated by various extracellular signals, such as growth factors, stresses, and cytokines, and play crucial roles in the determination of cell fate through proliferation, differentiation, survival, and apoptosis (1, 5, 24, 38). Three classes of MAPK families, the ERK, Jun N-terminal kinase/stress-activated protein kinase, and p38 MAPK, are differentially activated depending on the signaling context and in turn phosphorylate target proteins, which include transcription factors and protein kinases. These proteins can be common targets for subsets of MAPK proteins or specific targets for individual MAPKs. The direct downstream protein kinases, comprehensively called the MAPK-activated protein kinase (MAPKAPK) family, can be categorized into four subclasses, the Rsk, MK, Mnk, and Msk families. The Rsks (Rsk1, Rsk2, and Rsk3) are activated specifically by ERKs, whereas the MKs (MK2/MAPKAPK2, MK3/MAPKAPK3/3pK, and MK5/PRAK) are activated mainly by p38 MAPK in vivo. In contrast, the Mnks (Mnk1 and Mnk2) and Msks (Msk1 and Msk2) are targeted in vivo by both the ERK and p38 MAPK pathways, resulting in the activation of Mnks and Msks by a broad spectrum of extracellular stimuli. Mnk1 (MAPK signal-integrating kinase 1/MAPK-interacting kinase 1) and Mnk2 were identified independently by phosphorylation screening for ERK substrates and in a two-hybrid screen for ERK-binding proteins (12, 60). Both Mnk1 and Mnk2 are directly activated in vitro by the ERK and p38 MAPK, which phosphorylate at least two threonine residues (e.g., Thr197 and Thr202 of the mouse Mnk1) located in the so-called activation loop (12, 46, 60, 61). Studies with cell lines overexpressing Mnk cDNAs have shown that Mnk1 is strongly activated by growth factors, cellular stresses, and inflammatory cytokines via ERK and/or the p38 MAPK, depending on the signaling context, whereas Mnk2 has a relatively high basal activity that is hardly affected by changes in MAPK activity (12, 46, 59, 60).
Although the physiological functions of Mnk1 and Mnk2 remain unknown, several studies have demonstrated that one of the Mnk substrates is eukaryotic translation initiation factor 4E (eIF4E), also known as cap-binding protein. eIF4E specifically binds the 5′ m7GpppN cap structure found in all eukaryotic mRNAs and plays a critical role in cap-dependent translation initiation as a central component of the eIF4F complex, which also contains eIF4A and eIF4G, and this complex facilitates the assembly of the translation initiation complex consisting of mRNA, eIF3, eIF4F itself, and the 40S ribosomal subunit (13). Formation of the eIF4F complex is negatively regulated by 4E-binding proteins (4E-BPs), which bind eIF4E and block its interaction with eIF4G and thus inhibit cap-dependent translation. Phosphorylation of 4E-BP through the mTOR-dependent pathway liberates eIF4E from this inhibition (14). Elevated levels of eIF4E protein are observed in various human cancers (7), and overexpression of eIF4E in cultured cells causes malignant transformation (6, 27). Furthermore, recent studies have shown that overexpression of eIF4E promotes tumor formation in vivo and cooperates with c-Myc in lymphomagenesis, indicating that cellular levels of eIF4E are involved in controlling cell growth, survival, and oncogenesis (44, 62). Previous studies showed that either Mnk1 or Mnk2 can phosphorylate eIF4E at serine 209 in vitro (40, 46, 60, 61) and that the phosphorylation of eIF4E is regulated in response to extracellular signals that activate ERK and/or p38 MAPKs in accordance with the activation spectrum of Mnk1 (12, 33, 59, 60). Furthermore, the overexpression of active Mnk1 or Mnk2 proteins increases the phosphorylation of eIF4E, whereas the expression of a dominant-negative Mnk1 mutant reduces the levels of eIF4E phosphorylation (46, 61). These results collectively established the notion that Mnk1 and Mnk2 are physiological eIF4E kinases that regulate cap-dependent translation initiation in response to extracellular signals (41, 47). This idea was also supported by the observation that Mnk1 and Mnk2 bind to eIF4G in vivo (40, 46, 61).
Serine 209, located near the C terminus, is the major eIF4E phosphorylation site in rabbit reticulocytes and in serum-stimulated hamster CHO or mouse NIH 3T3 cells (11, 18, 63). However, the functional significance of Ser209 phosphorylation in translation initiation remains unclear. Since phosphorylation of eIF4E is upregulated in response to growth factors and cytokines, it has been postulated that eIF4E phosphorylation plays a positive role in cell growth by stimulating translation efficiency. In accordance with this idea, in Drosophila, a point mutation of the eIF4E phosphorylation site (Ser251, corresponding to Ser209 in mammals) results in reduced viability, developmental delay, and a reduction in body size (25). Some other reports, however, do not necessarily support this idea. Increased eIF4E phosphorylation induced by overexpression of active Mnks is reported not to facilitate eIF4F complex formation or general protein synthesis (16, 46). In addition, one study reported that a specific Mnk1 inhibitor, CGP57380, suppresses eIF4E phosphorylation but has no effect on the de novo initiation of translation during the recovery of cells from hypertonic stress (34). In another report, the expression of active Mnk1 or Mnk2 decreased the level of cap-dependent translation relative to cap-independent translation, suggesting a negative role for Mnks in translational regulation (21).
To investigate the physiological functions of Mnk1 and Mnk2, we generated knockout (KO) mice by targeted disruption of their genes. Using these mice, we demonstrated that Mnk1 and Mnk2 are essential for the phosphorylation of eIF4E in embryonic fibroblasts and adult tissues and that they play distinct roles in the regulation of eIF4E phosphorylation. Surprisingly, however, not only the single KO mouse lines but also the Mnk1-Mnk2 double KO (DKO) line were viable and showed no apparent abnormalities or defects in translation. These results strongly suggest that the phosphorylation of eIF4E is not essential for cell growth during development.
MATERIALS AND METHODS
Cells and mice.
Mouse R1 embryonic stem (ES) cells were cultured as described previously (22). Primary embryonic fibroblasts were prepared from day 13.5 mouse embryos. After removing the head and internal organs, the remaining tissues were cut into small pieces, and single cells were liberated by trypsin digestion. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) that was supplemented with 10% fetal calf serum (FCS; Invitrogen). Embryonic fibroblasts at passage 3 or 4 were used throughout this study. Mice were purchased from Charles River Japan, Inc. (Yokohama, Japan) or Nippon SLC, Inc. (Shizuoka, Japan). All mice were housed in a specific-pathogen-free facility at Osaka University Medical School, and all animal experiments were carried out in accordance with protocols approved by the Osaka University Medical School Animal Care and Use Committee.
Construction of targeting vectors and generation of KO mice.
Mnk1 and Mnk2 genomic DNA clones were isolated by plaque hybridization of a mouse 129/Sv genomic DNA library with full-length mouse Mnk1 and Mnk2 cDNA probes, respectively. To construct targeting vectors, a neomycin resistance gene (Neor cassette) was inserted between the MunI site in intron 4 and the AvrII site in intron 6 of the Mnk1 gene or between the NcoI site in intron 4 and the EcoT22I site in intron 9 of the Mnk2 gene. The diphtheria toxin A fragment gene (DTA cassette) for negative selection was ligated to the 3′ end of the 3′ homologous arm.
Mouse R1 ES cells were transfected with the Mnk1 or Mnk2 targeting vector by electroporation, and G418-resistant clones were screened for homologous recombination by PCR. ES clones carrying the single Mnk1- or Mnk2-targeted allele were injected into BDF1 blastocysts, which were then implanted into recipient ICR female mice. Chimeric mice with a high ES contribution were crossed to C57BL/6 females. Germ line transmission was identified by coat color and then confirmed by PCR. Single KO mice for either the Mnk1 or Mnk2 gene were generated by crossing the respective heterozygous mice. Mnk1−/− Mnk2−/− DKO mice were generated by crossing Mnk1−/− Mnk2+/− mice with Mnk1+/− Mnk2−/− mice.
PCR and Southern blot analyses.
Genomic DNA for PCR was prepared from tail snips. The genotype of the Mnk1 and Mnk2 genes was determined by PCR with Taq polymerase (Amersham Biosciences) by using the GeneAmp PCR system 9700 (Applied Biosystems). For the wild-type (WT) and mutant alleles of the Mnk1 gene, a sense primer specific for the WT (5′-GACCCAGGAATGACACCTTC-3′) or mutant (5′-GATTCGCAGCGCATCGCCTTCTATCG-3′, a sequence in Neor) allele was used with the Mnk1-specific antisense primer (5′-GCGCAAACCACATGTGCTTT-3′). The WT and mutant alleles of the Mnk2 gene were detected by PCR with the WT (5′-ATTGAGAAGCAGCTGGGCCACATCCGCAGC-3′) or mutant (5′-GATTCGCAGCGCATCGCCTTCTATCG-3′) sense primer and the Mnk2-specific antisense primer (5′-GTCGCAGCGCTTGTCGTAGATGCTGGC-3′).
For Southern hybridization, genomic DNA from an adult liver was digested with XbaI (for Mnk1) or HindIII (for Mnk2), separated by agarose gel electrophoresis, and transferred to a BA85 nitrocellulose filter (Schleicher & Schuell). Hybridization of the Mnk1 gene was carried out by using the 0.68-kb XbaI-PmaCI DNA fragment containing exon 3 as a probe. For Mnk2-specific hybridization, the 2.5-kb HindIII-XbaI fragment including exon 1 was used as a probe.
Stimulation of embryonic fibroblasts and preparation of cell extracts.
Mouse embryonic fibroblasts were seeded at 5 × 105 cells/well in a six-well plate and grown in DMEM with 10% FCS for 24 h. Cells were serum starved in DMEM containing 0.5% calf serum (CS) (starvation medium) for 20 h and then stimulated with 12-O-tetradecanoylphorbol-13-acetate (TPA) (500 nM, 15 min), FCS (25%, 15 min), anisomycin (10 μg/ml, 15 min), UV-C irradiation, tumor necrosis factor alpha (TNF-α) (10 ng/ml, 30 min), interleukin-1β (IL-1β) (10 ng/ml, 15 min), or osmotic shock followed by isotonic recovery. In the experiment with UV-C irradiation, cells in a six-well plate were washed once with phosphate-buffered saline (PBS) prewarmed to 37°C, exposed to UV-C light at 40 J/m2 with a Stratalinker 2400 (Stratagene), and cultured in starvation medium for 20 min. For osmotic shock, serum-starved cells were incubated for 30 min at 37°C in starvation medium supplemented with an additional 0.2 M NaCl and then cultured in normal starvation medium for 30 min at 37°C. After stimulation, the cells were washed with ice-cold PBS and lysed in 1 ml of NP-40 lysis buffer (NLB; 50 mM HEPES-NaOH [pH 7.4], 150 mM NaCl, 1% NP-40, 10% glycerol, 1.5 mM MgCl2, 1 mM EGTA, 20 mM NaF, 20 mM β-glycerophosphate, 0.5 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 U of aprotinin/ml, and 10 μg of leupeptin/ml). The supernatant was recovered after centrifugation for 10 min at 4°C.
For the preparation of tissue extracts, the spleen, liver, femoral muscle, and brain were dissected from 8-week-old mice and a portion of each tissue (0.2 to 0.4 g) was homogenized in 1 ml of extraction buffer (20 mM HEPES-NaOH [pH 7.4], 10% glycerol, 1 mM EGTA, 20 mM NaF, 20 mM β-glycerophosphate, 1 mM PMSF, 10 U of aprotinin/ml, and 10 μg of leupeptin/ml) by using a plastic homogenizer pestle (Kontes) in a microcentrifuge tube. The supernatant was recovered after centrifugation for 30 min at 4°C.
Antibody preparation and immunoblot analyses.
Anti-Mnk1 and anti-Mnk2 antisera were prepared by immunizing rabbits with recombinant mouse Mnk1 (amino acid residues 1 to 415) or Mnk2a (residues 48 to 459) protein that had been cleaved with thrombin from its respective glutathione S-transferase (GST) fusion protein produced in Escherichia coli (BL21). Both antibodies were affinity purified on glutathione-agarose beads that had been covalently coupled to GST-Mnk1 (residues 1 to 415) or GST-Mnk2a (residues 48 to 459) protein, respectively.
For immunoblot analysis, 10 μg of fibroblast extract or 18 μg of tissue extract were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane filter (Immobilon-P; Millipore). For the immunoblotting of Mnk proteins, we used affinity-purified anti-Mnk1 or anti-Mnk2 antibodies. Other antibodies used were an anti-phospho-Mnk1 (Thr197/202) antibody (Cell Signaling), anti-phospho-eIF4E (Ser209) antibody (Cell Signaling), anti-phospho-ERK antibody (anti-active-MAPK; Promega), anti-phospho-p38 antibody (anti-active-p38; Promega), anti-phospho-HSP27(Ser82) antibody (Cell Signaling), and anti-eIF4E monoclonal antibody (clone 87; Transduction Laboratories). Immunoblot detection was carried out with a horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin antibody (Dako) and the enhanced chemiluminescence detection system (PerkinElmer).
Metabolic labeling of eIF4E.
For the 32P in vivo labeling of eIF4E, 5 × 105 embryonic fibroblasts were seeded into 35-mm-diameter dishes and grown in DMEM with 10% FCS for 24 h. Cells were serum starved in phosphate-free DMEM (Invitrogen) supplemented with 0.5% dialyzed CS and 0.6-mCi/ml [32P]orthophosphate (8,500 to 9,120 Ci/mmol; PerkinElmer) for 20 h and then stimulated with 500 nM TPA for 15 min. Cells were washed twice with ice-cold PBS and lysed in 0.9 ml of NLB, and the supernatant was recovered after centrifugation for 10 min at 4°C. For the immunopurification of eIF4E, 1 μg of an anti-eIF4E monoclonal antibody (clone P-2; Santa Cruz) and 20 μl of protein G-Sepharose were added to 0.4 ml of the cell extract and incubated for 1 h at 4°C. For affinity purification, 20 μl of m7GTP-Sepharose (Amersham Biosciences) was added to 0.4 ml of the cell extract and incubated as above. The protein G- or m7GTP-Sepharose resin was washed four times with 1 ml of NLB, suspended in 50 μl of Laemmli's sample loading buffer, and heated for 5 min at 95°C. After centrifugation, 10 μl of the eluted proteins was resolved by SDS-PAGE, electrotransferred to a PVDF membrane, and exposed to X-ray film for 18 h at −80°C with an intensifying screen. After autoradiography, the filter was subjected to immunoblotting with the anti-eIF4E antibody. For enhanced chemiluminescence detection, the filter was exposed to X-ray film for 2 s.
Protein synthesis measurements.
Embryonic fibroblasts (3 × 105 cells) were seeded into 35-mm-diameter dishes, grown in DMEM with 10% FCS for 24 h, and then serum starved for 20 h. Cells were cultured in 0.8 ml of methionine/cysteine-free DMEM (Invitrogen) supplemented with 0.5% dialyzed CS for 30 min, and then 0.2 ml of dialyzed FCS and 20 μl (40 μCi) of a [35S]methionine/[35S]cysteine mixture (redivue Pro-mix l-[35S] in vitro cell labeling mix; Amersham Biosciences) was added. After 90-, 120-, and 240-min incubations, the cells were washed three times with ice-cold PBS and lysed in 0.4 ml of RIPA buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 50 U of aprotinin/ml, and 20 μg of leupeptin/ml). The supernatant was recovered after centrifugation for 10 min at 4°C. Cell extracts (20 μl) were diluted with 230 μl of water containing 10 μg of bovine serum albumin, mixed with 250 μl of 10% (wt/vol) trichloroacetic acid (TCA), and heated for 15 min at 90°C to hydrolyze the aminoacyl-tRNA. After incubation for 30 min on ice, each sample was passed through a glass fiber filter (GF/C; Whatman), and the filter, containing the labeled polypeptides, was washed three times with 5% TCA and once with ethanol and then air-dried. The radioactivity was measured by scintillation counting with Ultima Gold scintillation cocktail (PerkinElmer).
Translation reporter assay.
The bicistronic translation reporter plasmids, pEF-FFL-IRES-SPL and pEF-SPL-IRES-FFL, were constructed by the sequential subcloning of firefly luciferase (FFL) cDNA from pGL3 (Promega), sea pansy luciferase (SPL, also called Renilla reniformis luciferase) cDNA from pRL-SV40 (Promega), and the internal ribosome entry site (IRES) sequence from pIRES2EGFP (BD Bioscience Clontech) into the pEF-BOS-EX plasmid, which carries the EF-1α enhancer/promoter as an expression driver.
Embryonic fibroblasts were seeded at 5 × 104 cells/well into a 24-well plate, grown for 24 h, and transfected with 1 μg of either pEF-FFL-IRES-SPL or pEF-SPL-IRES-FFL plasmid by the calcium phosphate coprecipitation method. At 5 h after transfection, the medium was changed to fresh DMEM with 10% FCS and further cultured for 36 h. Cell lysates were prepared and assayed for FFL and SPL activities by using the dual-luciferase reporter assay system (Promega). The error bars in the figures indicate standard errors of the mean of the results from two independent transfection experiments.
RESULTS
Generation of Mnk1 and Mnk2 KO mice.
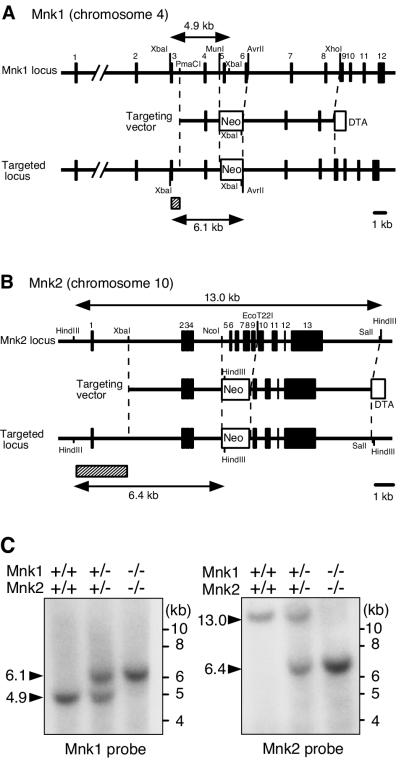
The targeting constructs for the mouse Mnk1 and Mnk2 genes are shown in Fig. 1A and B. By sequence analysis of the mouse Mnk2 gene locus and reverse transcription-PCR analysis of the RNA from embryonic fibroblasts, we found that the mouse Mnk2 gene encodes, in its exons 1 and 2, a 47-amino-acid extension (MVQKRTAELQGFHRSFKGQNPFELAFSLDLAQHRDSDFSPQCEARPD) to the N terminus of the previously published Mnk2 coding sequence (60). The N-terminal extension is highly conserved in the rat and human Mnk2a proteins (50), indicating that the entire coding sequence (459 amino acids), including this extension, represents a mouse Mnk2a homologue (data not shown) (DDBJ/EMBL/GenBank accession number AB164081).
FIG. 1.
Targeted disruption of the mouse Mnk1 and Mnk2 genes. (A and B) Schematic illustration of the exon organization of the Mnk1 (A) and Mnk2 (B) genes and targeting strategy. The exons of each gene are indicated by filled boxes. The targeting vectors were designed to replace exons 5 and 6 of the Mnk1 gene or exons 5 to 9 of the Mnk2 gene with a Neor cassette. The diphtheria toxin A fragment gene (DTA cassette) used for negative selection was placed at the end of the 3′ homologous arm. Probes used for Southern blot analysis are indicated by hatched boxes. (C) Southern blot analysis of genomic DNA from WT, Mnk1+/− Mnk2+/−, and Mnk1−/− Mnk2−/− mice. DNA was digested with XbaI (left panel) or HindIII (right panel) and analyzed by Southern hybridization with Mnk1 and Mnk2 probes, respectively. As indicated in panel A, WT and targeted alleles of the Mnk1 gene were predicted to result in bands at 4.9 and 6.1 kb, respectively, whereas those of the Mnk2 gene were expected to result in 13- and 6.4-kb bands, respectively.
In each targeting construct, exons 5 and 6 of the Mnk1 gene (chromosome 4) or exons 5 to 9 of the Mnk2 gene (chromosome 10) were replaced with a neomycin resistance gene cassette. Mouse ES cell clones containing either the Mnk1- or Mnk2-targeted allele were identified by PCR and Southern blot analyses and used to generate KO mice. Mating of either the Mnk1+/− or Mnk2+/− heterozygous mice yielded healthy littermates of three genotypes (+/+, +/−, and −/−) at Mendelian frequencies (Table 1). Both the Mnk1−/− and Mnk2−/− single KO mice were viable and fertile with no apparent abnormalities for at least 12 months after birth. We then generated mice heterozygous for both Mnk1- and Mnk2-disrupted alleles by mating the Mnk1 and Mnk2 single KOs, and the doubly heterozygous mice were further intercrossed to yield DKO mice. Like the single KO mice, Mnk1−/− Mnk2−/− DKO mice were born normally, were fertile, and did not show developmental abnormalities or morbidity for at least 8 months after birth. The mating of Mnk1+/− Mnk2−/− and Mnk1−/− Mnk2+/− mice yielded littermates of the four genotypes at nearly the same frequencies, indicating independent segregation of the KO alleles with Mendelian inheritance (Table 1). The chromosomal structure of the targeted genes in the KO mice was confirmed by Southern blot analysis of the genomic DNA prepared from WT, doubly heterozygous, and DKO mice (Fig. 1C).
TABLE 1.
Genotype analysis of progeny
| Cross | Genotypes (% of progeny) | Total no. of progeny |
|---|---|---|
| Mnk1+/− × Mnk1+/− | Mnk1+/+ (24), Mnk1+/− (51), Mnk1−/− (25) | 216 |
| Mnk2+/− × Mnk2+/− | Mnk2+/+ (33), Mnk2+/− (40), Mnk2−/− (27) | 119 |
| Mnk1−/− Mnk2+/− × Mnk1+/− Mnk2−/− | Mnk1+/− Mnk2+/− (25), Mnk1+/− Mnk2−/− (23), Mnk1−/− Mnk2+/− (21), Mnk1−/− Mnk2−/− (31) | 459 |
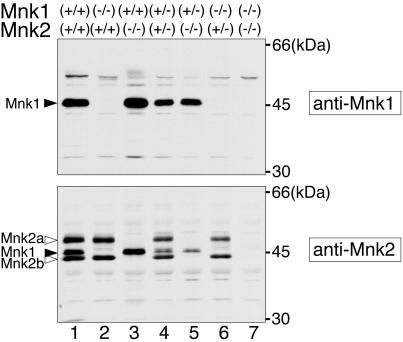
A single Mnk1 protein and two isoforms of Mnk2, Mnk2a and Mnk2b, have been identified in human cells (12, 49, 50), but there has been no report of the characterization of the endogenous protein products of the mouse Mnk1 and Mnk2 genes. To examine the expression of the Mnk1 and Mnk2 proteins in mice, we prepared rabbit antibodies against the mouse proteins and analyzed spleen extracts from WT and KO mice by immunoblotting with these antibodies (Fig. 2). On the blot with the anti-Mnk1 antibody, a single Mnk1 protein of about 47 kDa was detected in the spleen extracts from WT and Mnk2 KO mice (lanes 1 and 3) but not in the extracts from Mnk1 KO or DKO mice (lanes 2 and 7). In contrast, the anti-Mnk2 antibody detected two protein products that were present in the WT and Mnk1 KO extracts (lanes 1 and 2) but absent in the extracts from Mnk2 KO and DKO mice (lanes 3 and 7). The large product (about 51 kDa) is thought to be an Mnk2a product whose calculated molecular size is 51.6 kDa. The small product is likely to be the counterpart of an Mnk2 splice variant, Mnk2b, identified in human cells (49, 50), although we have not been able to find mouse cDNA sequences that would encode the Mnk2b-specific C-terminal region in the DNA sequence databases. The anti-Mnk2 antibody cross-reacted with Mnk1, which was confirmed by the immunoblotting of bacterially produced recombinant Mnk1 and Mnk2 proteins (data not shown). Mice heterozygous for the KO alleles (lanes 4 to 6) showed reduced protein levels of Mnk products, indicating that expression of the Mnk proteins in the spleen is faithfully dependent on the gene dosage.
FIG. 2.
Expression of the Mnk1 and Mnk2 proteins in the spleen. Spleen extracts from WT and KO mice were analyzed by immunoblotting with an anti-Mnk1 (upper panel) or an anti-Mnk2 (lower panel) antibody. Mouse genotypes are indicated at the top.
Mnks are essential for the phosphorylation of eIF4E in embryonic fibroblasts.
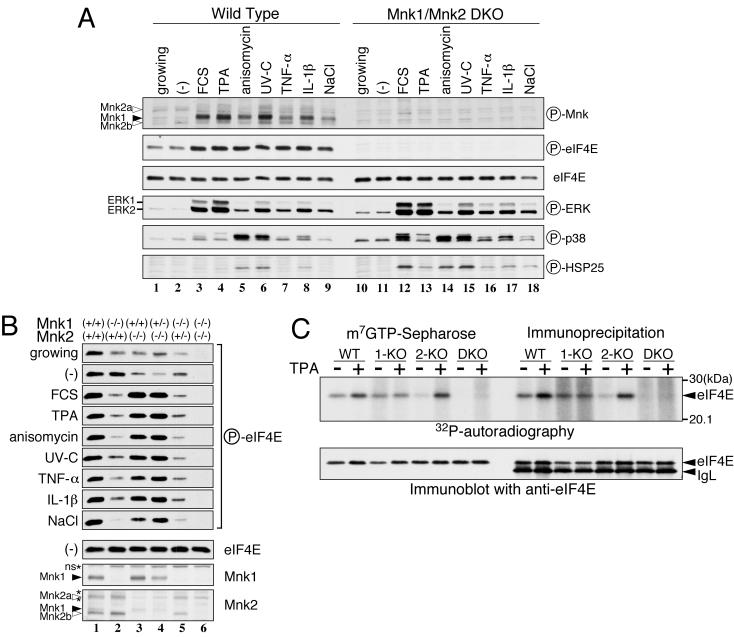
We next investigated the phosphorylation of eIF4E in primary embryonic fibroblasts prepared from embryonic day 13.5 KO embryos in response to MAPK activation. Expression of the single Mnk1 protein and two Mnk2 proteins was detected in the WT mouse embryonic fibroblasts (Fig. 3B). To examine activation of the Mnk proteins, we utilized an anti-phospho-Mnk1 (Thr197/202) antibody, which recognizes the Mnk1 protein phosphorylated at Thr197 and Thr202 and cross-reacts with the phosphorylated Mnk2 protein. As shown in Fig. 3A, Mnk1 was barely phosphorylated in growing or serum-starved fibroblasts (lanes 1 and 2) but was inducibly phosphorylated upon the stimulation of cells with FCS, TPA, anisomycin, UV-C irradiation, TNF-α, IL-1β, and osmotic shock (NaCl) (lanes 3 to 9). In contrast, nearly constant levels of phosphorylated Mnk2 proteins were detected irrespective of stimulation. These bands were absent in the immunoblots of extracts from Mnk1-Mnk2 DKO embryonic fibroblasts (lanes 10 to 18), confirming the specificity of the anti-phospho-Mnk1 antibody. Immunoblotting with anti-phospho-ERK and anti-phospho-p38 antibodies revealed that FCS and TPA preferentially activated ERK, whereas anisomycin and UV strongly activated p38 MAPK and weakly activated ERK. TNF-α, IL-1β, and osmotic shock activated both ERK and p38 modestly. In accordance with results from earlier studies (12, 59, 60), these results demonstrate that, depending on the extracellular stimulus, the ERK and/or p38 MAPKs are differentially activated and in turn phosphorylate Mnk1 in embryonic fibroblasts. Immunoblotting of WT fibroblasts with anti-phospho-eIF4E (Ser209) revealed that eIF4E was weakly phosphorylated in growing and serum-starved cells and became highly phosphorylated in response to all extracellular stimuli tested (lanes 1 to 9). On the other hand, Ser209 phosphorylation was not detected in DKO fibroblasts under any conditions (lanes 10 to 18). Immunoblotting with an anti-eIF4E antibody showed the same expression levels of the eIF4E protein in WT and DKO fibroblasts. Furthermore, the activation spectrum of the ERK and p38 MAPKs in response to extracellular stimulation was similar, and HSP25, a direct target of MAPKAPK2, was properly phosphorylated in the DKO as well as the WT cells, suggesting that the absence of Mnk proteins in the DKO fibroblasts did not have a deleterious effect on MAPK signaling pathways other than on the Mnks themselves.
FIG. 3.
Phosphorylation of eIF4E by Mnk1 and Mnk2 in mouse embryonic fibroblasts. (A) Fibroblasts prepared from WT or DKO embryos were serum starved for 20 h and either left unstimulated (−) or stimulated with FCS (25%, 15 min), TPA (500 nM, 15 min), anisomycin (10 μg/ml, 15 min), UV-C irradiation (40 J/m2, then incubated for 20 min), TNF-α (10 ng/ml, 30 min), IL-1β (10 ng/ml, 15 min), or osmotic shock (additional 0.2 M NaCl, 30 min) followed by isotonic recovery (30 min). Cells growing exponentially in DMEM containing 10% FCS were prepared in parallel (growing). Cell lysates (10 μg protein) were analyzed by immunoblotting with anti-phospho-Mnk1 (Thr197/202), anti-phospho-eIF4E (Ser209), anti-eIF4E, anti-phospho-ERK, anti-phospho-p38, and anti-phospho-HSP27/25 antibodies, as indicated on to the right of the panel. The positions of the phospho-Mnk1 and -Mnk2 proteins are indicated by filled and open arrowheads, respectively. (B) Embryonic fibroblasts of the indicated genotypes were treated as described for panel A, and cell lysates (10 μg of protein) were analyzed by immunoblotting with anti-phospho-eIF4E (Ser209), anti-eIF4E, anti-Mnk1, and anti-Mnk2 antibodies. The asterisks indicate nonspecific signals (ns). (C) Embryonic fibroblasts from WT, Mnk1 KO (1-KO), Mnk2 KO (2-KO), or DKO mice were radiolabeled with [32P]orthophosphate under serum-starved conditions for 20 h and then stimulated with 500 nM TPA for 15 min. eIF4E was purified from cell extracts by either m7GTP-Sepharose affinity resin or immunoprecipitation with an anti-eIF4E monoclonal antibody. Samples were resolved by SDS-PAGE, electrotransferred to a PVDF membrane, and exposed to an X-ray film for autoradiography (upper panel). The filter was then subjected to immunoblotting with the anti-eIF4E antibody (lower panel). IgL, immunoglobulin light chain; circled P, phospho.
Mnk1 and Mnk2 play distinct roles in regulating eIF4E phosphorylation.
Earlier studies showed that Mnk2 has a high basal activity but is relatively insensitive to the activation of ERK and p38 MAPKs (21, 46). Our anti-phospho-Mnk immunoblotting results also suggested that the activation state of Mnk1 is tightly regulated by these MAPKs, whereas Mnk2 is constitutive. To examine whether the two Mnks play distinct roles in phosphorylating eIF4E under various conditions, we analyzed the phosphorylation of eIF4E in embryonic fibroblasts that possessed different combinations of Mnk1 and Mnk2 KO alleles (Fig. 3B). Under the serum-starved condition, eIF4E was phosphorylated at comparable levels in WT and Mnk1−/− Mnk2+/+ fibroblasts (lanes 1 and 2) but at much lower levels in Mnk1+/+ Mnk2−/− fibroblasts (lane 3). In contrast, under conditions in which ERK and/or p38 MAPKs were rapidly activated, the phosphorylation of eIF4E was equally induced in WT, Mnk1+/+ Mnk2−/−, and Mnk1+/− Mnk2−/− fibroblasts (lanes 1, 3, and 4), but no increase in phosphorylation was observed in the Mnk1−/− Mnk2+/+ and Mnk1−/− Mnk2+/− fibroblasts (lanes 2 and 5). These results indicate that Mnk1 plays an essential role in the inducible phosphorylation of eIF4E in response to extracellular signals that are transduced through MAPKs, whereas Mnk2 is involved in the constitutive phosphorylation of eIF4E when MAPK activities are low. Interestingly, in Mnk1−/− Mnk2+/+ and Mnk1−/− Mnk2+/− fibroblasts, eIF4E phosphorylation decreased upon stimulation compared with its phosphorylation under the serum-starved condition (lane 2 and 5). This observation may indicate that the activation of MAPKs promotes the dissociation of Mnk2 from the eIF4G/eIF4E complex, which results in the subsequent dephosphorylation of eIF4E when Mnk1 is absent.
To confirm the results obtained by anti-phospho-eIF4E (Ser209) immunoblotting, we carried out metabolic labeling of eIF4E with [32P]orthophosphate and analyzed the incorporation of radioactivity into eIF4E after stimulation with TPA. As shown in Fig. 3C, we purified eIF4E from cell lysates either with m7GTP-Sepharose or by immunoprecipitation with an anti-eIF4E antibody, which resulted in similar patterns. In the WT fibroblasts, eIF4E was phosphorylated at a basal level after serum starvation and became further phosphorylated upon TPA stimulation. In Mnk1 KO cells, however, eIF4E phosphorylation remained at the basal level, even after stimulation. In clear contrast, Mnk2 KO cells showed a lower level of basal phosphorylation but TPA-dependent induction of eIF4E phosphorylation that was comparable to that in the WT cells. Neither basal nor induced phosphorylation was detected in the DKO fibroblasts. A control immunoblot with the anti-eIF4E antibody showed that the same amount of eIF4E was recovered from every cell extract. These results confirmed the distinct roles of Mnk2 and Mnk1 in the constitutive and inducible phosphorylation of eIF4E, respectively. Furthermore, the absence of 32P radioactivity in eIF4E in DKO cells indicated that Mnk1 and Mnk2 are exclusive eIF4E kinases, at least in embryonic fibroblasts, which is consistent with previous studies demonstrating that Ser209 is the major and perhaps the only phosphorylation site of eIF4E (11, 18, 63).
Mnks are essential for steady-state and inducible phosphorylation of eIF4E in vivo.
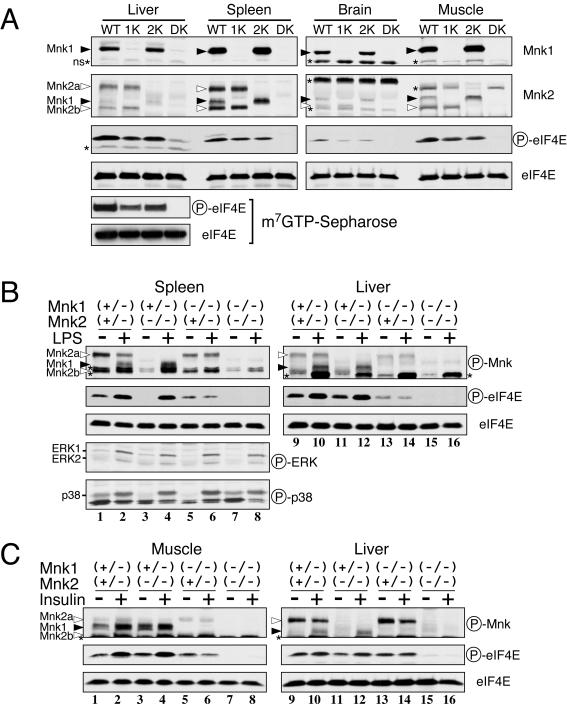
We next examined the expression of Mnk1 and Mnk2 proteins and the phosphorylation of eIF4E in several tissues of the KO mice. As shown in Fig. 4A, immunoblot analysis revealed that both the Mnk1 and Mnk2 proteins were highly expressed in the spleen and modestly expressed in the liver and muscle, although the expression of Mnk2a in muscle could not be determined because of a nonspecific band of the same molecular size. In the brain, relatively low levels of Mnk1 and Mnk2 were expressed, as previously suggested by Northern analysis (60). In accordance with the ubiquitous expression of Mnks, basal levels of eIF4E phosphorylation were detected in all of the WT tissues studied. The phosphorylation levels of eIF4E were partially reduced in the Mnk1 and Mnk2 single KO mice and completely abolished in the DKO mice, indicating that both kinases contribute to the steady-state phosphorylation of eIF4E. Although a band comigrating with eIF4E was seen in the anti-phospho-eIF4E blot of the liver extract from DKO mice, by performing immunoblotting after affinity purification of the eIF4E with m7GTP-Sepharose, we confirmed that this was not phosphorylated eIF4E but rather a nonspecific signal because no eIF4E phosphorylation was detected under these conditions (Fig. 4A, bottom panels).
FIG. 4.
Phosphorylation of eIF4E by Mnk1 and Mnk2 in mouse tissues. (A) Tissue extracts (18 μg of protein) prepared from WT, Mnk1 KO (1K), Mnk2 KO (2K), and DKO (DK) mice were analyzed by immunoblotting with anti-Mnk1, anti-Mnk2, anti-phospho-eIF4E (Ser209), and anti-eIF4E antibodies. eIF4E was also purified from liver extracts with m7GTP-Sepharose and analyzed similarly by immunoblotting (left side, bottom panels). The asterisks indicate nonspecific signals (ns). (B) LPS-stimulated activation of Mnk1 and upregulation of eIF4E phosphorylation. Mnk1-Mnk2 KO mice (8 weeks old) received an intraperitoneal injection of LPS (100 μg/g of body weight, from E. coli O55:B5; Sigma). Sixty minutes after injection, the spleen and liver were dissected, and their extracts were analyzed by immunoblotting with the indicated antibodies. (C) Insulin-stimulated activation of Mnk1 and upregulation of eIF4E phosphorylation. Following an overnight fast, Mnk1-Mnk2 KO mice (8 weeks old) were injected intravenously with human insulin (100 mU/g of body weight, Novolin R; Novo Nordisk). Fifteen minutes after injection, the femoral muscle and liver were dissected, and their extracts were analyzed by immunoblotting with the indicated antibodies. Circled P, phospho.
We next addressed whether the two Mnks play distinct roles in the regulation of eIF4E phosphorylation in adult tissues, as they do in embryonic fibroblasts. For this purpose, Mnk1+/− Mnk2−/− mice were mated with Mnk1−/− Mnk2+/− mice, and the resulting littermates at 8 weeks of age were administered lipopolysaccharide (LPS). LPS treatment induced the activation of ERK and p38 in the spleen 1 h after injection (Fig. 4B, lanes 1 to 8). Accordingly, phosphorylation of Mnk1 and eIF4E was induced in the Mnk1+/− Mnk2+/− and Mnk1+/− Mnk2−/− mice (lanes 1 to 4). In contrast, the basal levels of Mnk2a and eIF4E phosphorylation in Mnk1−/− Mnk2+/− mice did not change after LPS injection (lanes 5 and 6). Phosphorylated eIF4E was undetectable in the Mnk1−/− Mnk2−/− DKO mice, even after LPS treatment (lanes 7 and 8). Similar results were observed with immunoblots of liver extracts (lanes 9 to 16). We also tested the effects of insulin injection on eIF4E phosphorylation (Fig. 4C). Although we could not estimate activation of ERK or p38 in muscle and liver by immunoblotting, because there was a high background of nonspecific bands at around 40 kDa, insulin administration resulted in upregulation of both Mnk1 and eIF4E phosphorylation in these tissues from Mnk1+/− Mnk2+/− and Mnk1+/− Mnk2−/− mice (lanes 1 to 4 and 9 to 12). However, phosphorylation of eIF4E was constant in the Mnk1−/− Mnk2+/− mice and totally absent in the DKO mice (lanes 5 to 8 and 13 to 16). These results collectively indicate that the Mnks are indeed exclusive eIF4E (Ser209) kinases in adult tissues and that Mnk2 and Mnk1 are responsible for the constitutive and inducible phosphorylation, respectively.
Elimination of the Mnks does not affect global protein synthesis in embryonic fibroblasts.
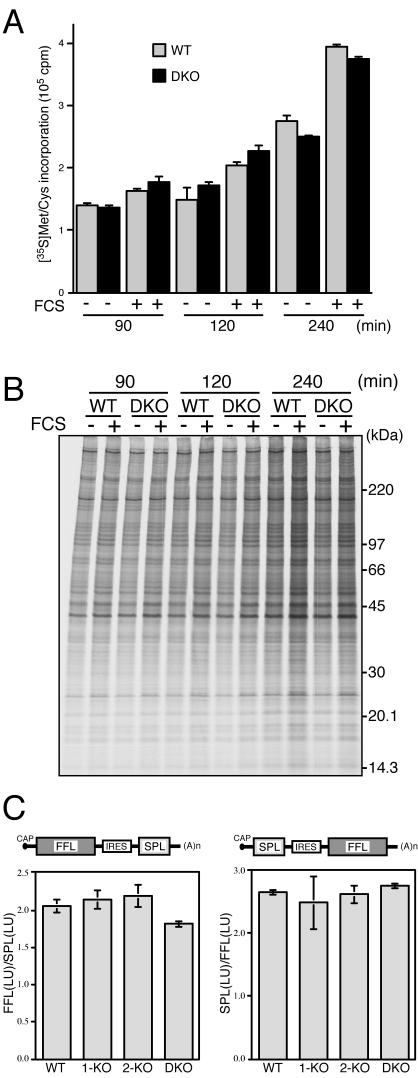
We next examined the effect of Mnk deficiency on protein synthesis. Primary embryonic fibroblasts were serum starved for 20 h and then stimulated with 20% FCS, and the rate of protein synthesis was determined by incorporation of [35S]methionine and [35S]cysteine into the TCA-insoluble polypeptide fraction. As shown in Fig. 5A, the time course of 35S incorporation revealed that the basal level of protein synthesis under the serum-starved condition was similar in the WT and DKO fibroblasts. Stimulation of cells with FCS enhanced the 35S incorporation into polypeptides to a similar extent in these fibroblasts. These data show that the absence of Mnk1 and Mnk2 does not significantly affect the basal or serum-stimulated global protein synthesis. SDS-PAGE analysis of proteins from 35S-labeled embryonic fibroblasts showed a general increase in the intensity of radiolabeled proteins after FCS stimulation and no detectable differences in the synthesis of any individual proteins between the WT and DKO fibroblasts (Fig. 5B).
FIG. 5.
General protein synthesis is not impaired in Mnk1-Mnk2-deficient fibroblasts. (A) WT and DKO embryonic fibroblasts were serum starved for 20 h. Cells were left unstimulated (−) or stimulated with 20% FCS (+) and cultured in labeling medium containing 35S-labeled amino acids for 90, 120, and 240 min at 37°C. After the cells were lysed in RIPA buffer, the incorporation of 35S-labeled amino acids into the TCA-insoluble fraction was measured. (B) The RIPA lysates analyzed in panel A were resolved by SDS-PAGE and subjected to fluorography with ENLIGHTNING (PerkinElmer). (C) WT, Mnk1 KO (1-KO), Mnk2 KO (2-KO), and DKO embryonic fibroblasts were transfected with the bicistronic reporter plasmid pEF-FFL-IRES-SPL, and after incubation for 36 h at 37°C, the FFL and SPL activities were measured. The ratio of FFL light units to SPL light units in each sample was calculated, and the averages of the results from two independent experiments are shown (left panel). The same reporter assay was carried out with the reciprocal construct, pEF-SPL-IRES-FFL, and the results are shown as the ratio of SPL light units to FFL light units (right panel).
Knauf et al. showed that expression of constitutively active mutants of Mnk1 and Mnk2 decreased the cap-dependent translation relative to cap-independent translation by using a bicistronic luciferase reporter system (21). To examine whether the absence of eIF4E phosphorylation affected the cap-dependent translation efficiency, we employed a similar reporter system, in which two reporter cistrons, FFL and SPL, are separated by an IRES (21, 39). In the pEF-FFL-IRES-SPL construct, translation of the FFL cistron is cap dependent, whereas the SPL cistron is translated by the cap-independent, IRES-directed initiation (Fig. 5C, left). Transfection of WT fibroblasts with this plasmid resulted in an average ratio (2.05) of relative luciferase light units of FFL and SPL. All of the other fibroblasts, Mnk1 KO, Mnk2 KO, and DKO cells, showed similar ratios of FFL and SPL activities. When the reporter assay was carried out with the reciprocal construct, pEF-SPL-IRES-FFL, the average ratio of SPL light units to FFL light units was 2.62 ± 0.13 in all fibroblasts tested (Fig. 5C, right). These results suggest that the elimination of eIF4E phosphorylation does not affect the cap-dependent translation relative to cap-independent translation in embryonic fibroblasts.
DISCUSSION
A previous study isolated mouse Mnk2 cDNA encoding a 412-amino-acid protein (60), but later, the identification of human Mnk2a cDNA suggested that the reported mouse Mnk2 clone might be a partial cDNA or a splice variant lacking the N-terminal region (49, 50). In this report, we have identified a cDNA sequence and chromosomal exons for mouse Mnk2a that include the N-terminal 47-amino-acid extension. By immunoblot analyses of tissues from WT and KO mice, we showed that, in addition to Mnk2a, a small Mnk2 product of about 44 kDa is expressed in various tissues and embryonic fibroblasts. Although the small product is likely to be a mouse counterpart of human Mnk2b that is produced by an alternative usage of the last exon (exon 13), encoding the C-terminal region (49, 50), we were not able to find a DNA sequence that would carry the Mnk2b-specific coding region in the Mnk2 gene locus we have sequenced or in databases for the mouse DNA sequence, and therefore, we cannot exclude the possibility that the smaller product is generated by some other splicing.
Previous studies have demonstrated that Mnk1 and Mnk2 phosphorylate eIF4E at Ser209 in vivo (21, 46, 49, 61). However, it has not been clear whether other protein kinases are also involved in the phosphorylation of eIF4E. It was previously reported that protein kinase C and MK3/MAPKAPK3/3pK, both of which are activated by extracellular stimuli, could phosphorylate eIF4E at least in vitro (33, 61, 63). Here, we unequivocally demonstrate that both Mnk1 and Mnk2 are physiological eIF4E kinases, not only in cultured fibroblasts but also in mouse tissues such as spleen, liver, muscle, and brain. We did not detect even a trace amount of phosphorylated eIF4E in embryonic fibroblasts or tissues from DKO mice by immunoblot analysis with an anti-phospho-4E (Ser209) antibody. The metabolic labeling of eIF4E with [32P]orthophosphate showed that the phosphorylation level of eIF4E in DKO fibroblasts was very low, if there was any, by comparison with WT and single KO cells. The latter result confirmed the findings from the immunoblot analysis and also supported the previous conclusion that Ser209 is the major and perhaps the only phosphorylation site of eIF4E in vivo (11, 18, 63). Therefore, it is likely that Mnks are the only physiological protein kinases that phosphorylate eIF4E.
Analyses of the single KO mice indicated that the protein kinase activities of Mnk1 and Mnk2 are differentially regulated by changes in MAPK signaling. In Mnk2 KO fibroblasts and tissues, the basal level of eIF4E phosphorylation was very low, but the induction of eIF4E phosphorylation was comparable to that of WT cells. In contrast, the inducible phosphorylation of eIF4E was completely lost, but the basal phosphorylation was not impaired in Mnk1 KO cells. These results indicated that the dynamic regulation of Mnk1 activity by MAPKs determines the magnitude of the changes in the phosphorylation state of eIF4E, whereas the basal phosphorylation of eIF4E is directed primarily by Mnk2, whose activity is relatively constant even when MAPK activities are perturbed. This is consistent with an earlier report that Mnk2 has a high level of basal activity and is less sensitive to changes in the MAPK signaling status than Mnk1 (46). Considering that phosphorylation of at least three threonine sites by MAPKs is likely to be essential for Mnk2 activity (46), these results suggest that Mnk2 is more susceptible than Mnk1 to low levels of MAPK activity and/or is less sensitive to the protein phosphatase(s) that inactivates Mnks.
The biological significance of eIF4E phosphorylation is not well understood. Recently, a positive function of eIF4E phosphorylation in cell growth was suggested by the finding that, in Drosophila melanogaster, a point mutation of the phosphorylation site (Ser251) of eIF4E resulted in reduced viability, developmental delay, and reduction in adult body size (25). In the present study, however, DKO mice, which showed no detectable eIF4E phosphorylation, were born normally and were fertile. We found no apparent differences in the morphology or size of the tissues between WT and DKO mice. Furthermore, primary fibroblasts from DKO embryos grew normally and showed no difference in general protein synthesis compared with WT fibroblasts. These facts strongly suggest that, at least in mammals, the phosphorylation of eIF4E is not required for cell growth and ontogenic development.
Previous studies have not reached a consensus on the physiological function of eIF4E phosphorylation in the regulation of protein synthesis. On one hand, it has been suggested that the phosphorylation of eIF4E is likely to play a role in enhancing the eIF4F assembly and subsequent translation initiation. The activation of cap-dependent mRNA translation in response to growth-stimulatory signals correlates with increased eIF4E phosphorylation, whereas the inhibition of protein synthesis resulting from heat shock, osmotic stress, or viral infection correlates with the dephosphorylation of eIF4E (3, 4, 9, 10, 13, 20, 32, 33, 59). On the other hand, recent studies have suggested that the phosphorylation of eIF4E is not sufficient or required for the upregulation of general protein synthesis (30). The expression of active Mnk1 or Mnk2 increased eIF4E phosphorylation but did not enhance the overall rate of protein synthesis (16, 21, 45); rather, it decreased the cap-dependent translation relative to the cap-independent translation (21). Furthermore, treatment of cells with an Mnk-specific inhibitor, CGP57380, blocked the eIF4E phosphorylation but did not impair the mitogen-stimulated translational activation or de novo protein synthesis following recovery from hypertonic stress (21, 34). An initial study reported that the phosphorylation of eIF4E increased its affinity for cap analogs (31), but more recent studies demonstrated that the phosphorylation of eIF4E markedly reduces its affinity for capped mRNA (48, 64). Based on these studies and the cocrystal structure of eIF4E-cap analog complexes (29, 53, 54), Scheper and Proud proposed two models for the physiological role of eIF4E phosphorylation, in which the phosphorylation of eIF4E by Mnks may stimulate either the rapid and sequential loading of multiple initiation complexes and ribosomes to the same mRNA or the rapid recycling of the initiation factors from one mRNA to another, depending on the time point at which the eIF4E phosphorylation occurs during the initiation process (47). According to these models, the phosphorylation of eIF4E may play a role in reprogramming the protein synthesis pattern by promoting polysome assembly on a limited group of mRNAs or the redistribution of translational machineries from preexisting mRNAs to newly synthesized mRNAs. In any case, it is likely that the mitogen-stimulated activation of general protein synthesis is regulated primarily by other mechanisms, such as mTOR-dependent phosphorylation of 4E-BP1 and S6 kinase, and that the phosphorylation of eIF4E may instead play a role in the fine control of limited aspects of mRNA translation, stabilization, or processing. A recent report showed that overexpression of Mnk1 in adult cardiocytes increased the translational efficiency of a reporter mRNA that has pronounced secondary structure in its 5′ untranslated region (57). Another report has indicated that phosphorylation of eIF4E by Mnk1 is critical for the viral protein synthesis and replication of herpes simplex virus type 1 in quiescent cells (58). These studies suggest that the eIF4E phosphorylation is involved in the translational control of specific groups of mRNAs.
The strong expression of both Mnk1 and Mnk2 in the spleen suggests that they play a role in the immune response, such as in the activation of lymphocytes or phagocytes, antibody production, or cytokine production. MK2/MAPKAPK2 has been shown to regulate the LPS-induced production of TNF-α and IL-6 in splenocytes by posttranscriptional mechanisms (23, 35). Although eIF4E is unlikely to be the target of MK2 in this process, Mnk-dependent eIF4E phosphorylation may regulate acute-phase cytokine production directly or indirectly at the translational level. In this regard, it has been suggested that Mnk1 regulates the production of the chemokine RANTES through translational control of RFLAT-1, a transcription factor for RANTES gene expression in T cells (36).
Most eIF4E is present in the cytoplasm, but a fraction of it localizes to the nucleus, especially to speckled structures called promyelocytic leukemia (PML) nuclear bodies (8, 26, 28). Recent studies have suggested that the nuclear eIF4E regulates the nucleocytoplasmic transport of cyclin D1 mRNA in conjunction with PML protein (2, 42, 43, 55, 56). Likewise, the Mnk1 and Mnk2a proteins are present mainly in the cytoplasm, but recent studies have shown that both Mnk1 and Mnk2 possess a potential nuclear localization signal and that a fraction of human Mnk2b localizes to the PML nuclear bodies, suggesting that the Mnks have some nuclear function (37, 49). Although we have not determined the subcellular distribution of the phosphorylated eIF4E, it would be intriguing to examine whether the nuclear function of eIF4E is regulated by Mnk-dependent phosphorylation at Ser209.
Accumulating evidence has shown that local protein synthesis at the synaptic area in dendrites plays a critical role in the control of neuronal plasticity, including the control of long-term potentiation and long-term depression (17, 52). A recent study showed that brain-derived neurotrophic factor induces the translocation of eIF4E to the so-called mRNA granules, cytoskeleton-associated granules for mRNA transport in dendrites (51). A more recent study showed that conditional inhibition of ERK activity in the postnatal murine forebrain results in a reduction in hippocampus-dependent memory retention, translation-dependent long-term potentiation, neuronal activity-induced translation, and phosphorylation of eIF4E, 4E-BP1, and S6 kinase in hippocampal neurons, suggesting that synaptic plasticity is regulated by ERK-dependent translational control (19). Analysis of the neural activities of Mnk KOs would elucidate whether eIF4E phosphorylation is involved in the local protein synthesis and synaptic plasticity.
Besides eIF4E, no physiological targets for Mnk1 or Mnk2 have been identified except for cytosolic phospholipase A2 (15), which catalyzes the first step of the arachidonate cascade for eicosanoid synthesis. Mnk1 and Mnk2 KO mice and their cultured cells would be useful systems for investigating the physiological significance of eIF4E phosphorylation in the fine control of mRNA translation, as speculated above, and to explore other Mnk functions that are executed through unidentified targets of these protein kinases.
Acknowledgments
We are grateful to Kohki Kawane for help in the generation and analysis of KO mice. Special thanks go to Tony Hunter for critical reading of the manuscript.
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 2.Cohen, N., M. Sharma, A. Kentsis, J. M. Perez, S. Strudwick, and K. L. Borden. 2001. PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. EMBO J. 20:4547-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connor, J. H., and D. S. Lyles. 2002. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J. Virol. 76:10177-10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuesta, R., Q. Xi, and R. J. Schneider. 2000. Adenovirus-specific translation by displacement of kinase Mnk1 from cap-initiation complex eIF4F. EMBO J. 19:3465-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 6.De Benedetti, A., and R. E. Rhoads. 1990. Overexpression of eukaryotic protein synthesis initiation factor 4E in HeLa cells results in aberrant growth and morphology. Proc. Natl. Acad. Sci. USA 87:8212-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Benedetti, A., and A. L. Harris. 1999. eIF4E expression in tumors: its possible role in progression of malignancies. Int. J. Biochem. Cell Biol. 31:59-72. [DOI] [PubMed] [Google Scholar]
- 8.Dostie, J., F. Lejbkowicz, and N. Sonenberg. 2000. Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles. J. Cell Biol. 148:239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan, R. F., D. R. Cavener, and S. Qu. 1995. Heat shock effects on phosphorylation of protein synthesis initiation factor proteins eIF-4E and eIF-2 alpha in Drosophila. Biochemistry (Moscow) 34:2985-2997. [DOI] [PubMed] [Google Scholar]
- 10.Duncan, R. F., H. Peterson, C. H. Hagedorn, and A. Sevanian. 2003. Oxidative stress increases eukaryotic initiation factor 4E phosphorylation in vascular cells. Biochem. J. 369:213-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn, A., and C. G. Proud. 1995. Serine 209, not serine 53, is the major site of phosphorylation in initiation factor eIF-4E in serum-treated Chinese hamster ovary cells. J. Biol. Chem. 270:21684-21688. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga, R., and T. Hunter. 1997. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 16:1921-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gingras, A. C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913-963. [DOI] [PubMed] [Google Scholar]
- 14.Gingras, A. C., B. Raught, and N. Sonenberg. 2001. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15:807-826. [DOI] [PubMed] [Google Scholar]
- 15.Hefner, Y., A. G. Borsch-Haubold, M. Murakami, J. I. Wilde, S. Pasquet, D. Schieltz, F. Ghomashchi, J. R. Yates III, C. G. Armstrong, A. Paterson, P. Cohen, R. Fukunaga, T. Hunter, I. Kudo, S. P. Watson, and M. H. Gelb. 2000. Serine 727 phosphorylation and activation of cytosolic phospholipase A2 by MNK1-related protein kinases. J. Biol. Chem. 275:37542-37551. [DOI] [PubMed] [Google Scholar]
- 16.Herbert, T. P., G. R. Kilhams, I. H. Batty, and C. G. Proud. 2000. Distinct signalling pathways mediate insulin and phorbol ester-stimulated eukaryotic initiation factor 4F assembly and protein synthesis in HEK 293 cells. J. Biol. Chem. 275:11249-11256. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, C., and E. M. Schuman. 2002. Regulation and function of local protein synthesis in neuronal dendrites. Trends Biochem. Sci. 27:506-513. [DOI] [PubMed] [Google Scholar]
- 18.Joshi, B., A. L. Cai, B. D. Keiper, W. B. Minich, R. Mendez, C. M. Beach, J. Stepinski, R. Stolarski, E. Darzynkiewicz, and R. E. Rhoads. 1995. Phosphorylation of eukaryotic protein synthesis initiation factor 4E at Ser-209. J. Biol. Chem. 270:14597-14603. [DOI] [PubMed] [Google Scholar]
- 19.Kelleher, R. J., III, A. Govindarajan, H.-Y. Jung, H. Kang, and S. Tonegawa. 2004. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116:467-479. [DOI] [PubMed] [Google Scholar]
- 20.Kleijn, M., G. C. Scheper, H. O. Voorma, and A. A. Thomas. 1998. Regulation of translation initiation factors by signal transduction. Eur. J. Biochem. 253:531-544. [DOI] [PubMed] [Google Scholar]
- 21.Knauf, U., C. Tschopp, and H. Gram. 2001. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol. Cell. Biol. 21:5500-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondoh, G., Y. Yamamoto, K. Yoshida, Y. Suzuki, S. Osuka, Y. Nakano, T. Morita, and J. Takeda. 1999. Easy assessment of ES cell clone potency for chimeric development and germ-line competency by an optimized aggregation method. J. Biochem. Biophys. Methods 39:137-142. [DOI] [PubMed] [Google Scholar]
- 23.Kotlyarov, A., A. Neininger, C. Schubert, R. Eckert, C. Birchmeier, H. D. Volk, and M. Gaestel. 1999. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat. Cell Biol. 1:94-97. [DOI] [PubMed] [Google Scholar]
- 24.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 25.Lachance, P. E., M. Miron, B. Raught, N. Sonenberg, and P. Lasko. 2002. Phosphorylation of eukaryotic translation initiation factor 4E is critical for growth. Mol. Cell. Biol. 22:1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai, H. K., and K. L. Borden. 2000. The promyelocytic leukemia (PML) protein suppresses cyclin D1 protein production by altering the nuclear cytoplasmic distribution of cyclin D1 mRNA. Oncogene 19:1623-1634. [DOI] [PubMed] [Google Scholar]
- 27.Lazaris-Karatzas, A., K. S. Montine, and N. Sonenberg. 1990. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature 345:544-547. [DOI] [PubMed] [Google Scholar]
- 28.Lejbkowicz, F., C. Goyer, A. Darveau, S. Neron, R. Lemieux, and N. Sonenberg. 1992. A fraction of the mRNA 5′ cap-binding protein, eukaryotic initiation factor 4E, localizes to the nucleus. Proc. Natl. Acad. Sci. USA 89:9612-9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcotrigiano, J., A. C. Gingras, N. Sonenberg, and S. K. Burley. 1997. Cocrystal structure of the messenger RNA 5′ cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell 89:951-961. [DOI] [PubMed] [Google Scholar]
- 30.McKendrick, L., S. J. Morley, V. M. Pain, R. Jagus, and B. Joshi. 2001. Phosphorylation of eukaryotic initiation factor 4E (eIF4E) at Ser209 is not required for protein synthesis in vitro and in vivo. Eur. J. Biochem. 268:5375-5385. [DOI] [PubMed] [Google Scholar]
- 31.Minich, W. B., M. L. Balasta, D. J. Goss, and R. E. Rhoads. 1994. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc. Natl. Acad. Sci. USA 91:7668-7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morley, S. J. 1997. Intracellular signalling pathways regulating initiation factor eIF4E phosphorylation during the activation of cell growth. Biochem. Soc. Trans. 25:503-509. [DOI] [PubMed] [Google Scholar]
- 33.Morley, S. J., and L. McKendrick. 1997. Involvement of stress-activated protein kinase and p38/RK mitogen-activated protein kinase signaling pathways in the enhanced phosphorylation of initiation factor 4E in NIH 3T3 cells. J. Biol. Chem. 272:17887-17893. [DOI] [PubMed] [Google Scholar]
- 34.Morley, S. J., and S. Naegele. 2002. Phosphorylation of eukaryotic initiation factor (eIF) 4E is not required for de novo protein synthesis following recovery from hypertonic stress in human kidney cells. J. Biol. Chem. 277:32855-32859. [DOI] [PubMed] [Google Scholar]
- 35.Neininger, A., D. Kontoyiannis, A. Kotlyarov, R. Winzen, R. Eckert, H. D. Volk, H. Holtmann, G. Kollias, and M. Gaestel. 2002. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J. Biol. Chem. 277:3065-3068. [DOI] [PubMed] [Google Scholar]
- 36.Nikolcheva, T., S. Pyronnet, S. Y. Chou, N. Sonenberg, A. Song, C. Clayberger, and A. M. Krensky. 2002. A translational rheostat for RFLAT-1 regulates RANTES expression in T lymphocytes. J. Clin. Investig. 110:119-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parra-Palau, J. L., G. C. Scheper, M. L. Wilson, and C. G. Proud. 2003. Features in the N and C termini of the MAPK-interacting kinase Mnk1 mediate its nucleocytoplasmic shuttling. J. Biol. Chem. 278:44197-44204. [DOI] [PubMed] [Google Scholar]
- 38.Pearson, G., F. Robinson, T. Beers Gibson, B. E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 39.Poulin, F., A. C. Gingras, H. Olsen, S. Chevalier, and N. Sonenberg. 1998. 4E-BP3, a new member of the eukaryotic initiation factor 4E-binding protein family. J. Biol. Chem. 273:14002-14007. [DOI] [PubMed] [Google Scholar]
- 40.Pyronnet, S., H. Imataka, A. C. Gingras, R. Fukunaga, T. Hunter, and N. Sonenberg. 1999. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 18:270-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pyronnet, S. 2000. Phosphorylation of the cap-binding protein eIF4E by the MAPK-activated protein kinase Mnk1. Biochem. Pharmacol. 60:1237-1243. [DOI] [PubMed] [Google Scholar]
- 42.Rosenwald, I. B., R. Kaspar, D. Rousseau, L. Gehrke, P. Leboulch, J. J. Chen, E. V. Schmidt, N. Sonenberg, and I. M. London. 1995. Eukaryotic translation initiation factor 4E regulates expression of cyclin D1 at transcriptional and post-transcriptional levels. J. Biol. Chem. 270:21176-21180. [DOI] [PubMed] [Google Scholar]
- 43.Rousseau, D., R. Kaspar, I. Rosenwald, L. Gehrke, and N. Sonenberg. 1996. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc. Natl. Acad. Sci. USA 93:1065-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruggero, D., L. Montanaro, L. Ma, W. Xu, P. Londei, C. Cordon-Cardo, and P. P. Pandolfi. 2004. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat. Med. 10:484-486. (First published 18 April 2004; 10.1038/nm1042.) [DOI] [PubMed]
- 45.Saghir, A. N., W. J. Tuxworth, Jr., C. H. Hagedorn, and P. J. McDermott. 2001. Modifications of eukaryotic initiation factor 4F (eIF4F) in adult cardiocytes by adenoviral gene transfer: differential effects on eIF4F activity and total protein synthesis rates. Biochem. J. 356:557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheper, G. C., N. A. Morrice, M. Kleijn, and C. G. Proud. 2001. The mitogen-activated protein kinase signal-integrating kinase Mnk2 is a eukaryotic initiation factor 4E kinase with high levels of basal activity in mammalian cells. Mol. Cell. Biol. 21:743-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheper, G. C., and C. G. Proud. 2002. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur. J. Biochem. 269:5350-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheper, G. C., B. van Kollenburg, J. Hu, Y. Luo, D. J. Goss, and C. G. Proud. 2002. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J. Biol. Chem. 277:3303-3309. [DOI] [PubMed] [Google Scholar]
- 49.Scheper, G. C., J. L. Parra, M. Wilson, B. Van Kollenburg, A. C. Vertegaal, Z. G. Han, and C. G. Proud. 2003. The N and C termini of the splice variants of the human mitogen-activated protein kinase-interacting kinase Mnk2 determine activity and localization. Mol. Cell. Biol. 23:5692-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slentz-Kesler, K., J. T. Moore, M. Lombard, J. Zhang, R. Hollingsworth, and M. P. Weiner. 2000. Identification of the human Mnk2 gene (MKNK2) through protein interaction with estrogen receptor beta. Genomics 69:63-71. [DOI] [PubMed] [Google Scholar]
- 51.Smart, F. M., G. M. Edelman, and P. W. Vanderklish. 2003. BDNF induces translocation of initiation factor 4E to mRNA granules: evidence for a role of synaptic microfilaments and integrins. Proc. Natl. Acad. Sci. USA 100:14403-14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steward, O., and E. M. Schuman. 2001. Protein synthesis at synaptic sites on dendrites. Annu. Rev. Neurosci. 24:299-325. [DOI] [PubMed] [Google Scholar]
- 53.Tomoo, K., X. Shen, K. Okabe, Y. Nozoe, S. Fukuhara, S. Morino, T. Ishida, T. Taniguchi, H. Hasegawa, A. Terashima, M. Sasaki, Y. Katsuya, K. Kitamura, H. Miyoshi, M. Ishikawa, and K. Miura. 2002. Crystal structures of 7-methylguanosine 5′-triphosphate (m7GTP)- and P1-7-methylguanosine-P3-adenosine-5′,5′-triphosphate (m7GpppA)-bound human full-length eukaryotic initiation factor 4E: biological importance of the C-terminal flexible region. Biochem. J. 362:539-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomoo, K., X. Shen, K. Okabe, Y. Nozoe, S. Fukuhara, S. Morino, M. Sasaki, T. Taniguchi, H. Miyagawa, K. Kitamura, K. Miura, and T. Ishida. 2003. Structural features of human initiation factor 4E, studied by X-ray crystal analyses and molecular dynamics simulations. J. Mol. Biol. 328:365-383. [DOI] [PubMed] [Google Scholar]
- 55.Topisirovic, I., A. D. Capili, and K. L. Borden. 2002. Gamma interferon and cadmium treatments modulate eukaryotic initiation factor 4E-dependent mRNA transport of cyclin D1 in a PML-dependent manner. Mol. Cell. Biol. 22:6183-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Topisirovic, I., B. Culjkovic, N. Cohen, J. M. Perez, L. Skrabanek, and K. L. Borden. 2003. The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. EMBO J. 22:689-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuxworth, W. J., Jr., A. N. Saghir, L. S. Spruill, D. R. Menick, and P. J. McDermott. 2004. Regulation of protein synthesis by eIF4E phosphorylation in adult cardiocytes: the consequence of secondary structure in the 5′-untranslated region of mRNA. Biochem. J. 378:73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walsh, D., and I. Mohr. 2004. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 18:660-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, X., A. Flynn, A. J. Waskiewicz, B. L. Webb, R. G. Vries, I. A. Baines, J. A. Cooper, and C. G. Proud. 1998. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J. Biol. Chem. 273:9373-9377. [DOI] [PubMed] [Google Scholar]
- 60.Waskiewicz, A. J., A. Flynn, C. G. Proud, and J. A. Cooper. 1997. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 16:1909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waskiewicz, A. J., J. C. Johnson, B. Penn, M. Mahalingam, S. R. Kimball, and J. A. Cooper. 1999. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol. Cell. Biol. 19:1871-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wendel, H. G., E. De Stanchina, J. S. Fridman, A. Malina, S. Ray, S. Kogan, C. Cordon-Cardo, J. Pelletier, and S. W. Lowe. 2004. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 428:332-337. [DOI] [PubMed] [Google Scholar]
- 63.Whalen, S. G., A. C. Gingras, L. Amankwa, S. Mader, P. E. Branton, R. Aebersold, and N. Sonenberg. 1996. Phosphorylation of eIF-4E on serine 209 by protein kinase C is inhibited by the translational repressors, 4E-binding proteins. J. Biol. Chem. 271:11831-11837. [DOI] [PubMed] [Google Scholar]
- 64.Zuberek, J., A. Wyslouch-Cieszynska, A. Niedzwiecka, M. Dadlez, J. Stepinski, W. Augustyniak, A. C. Gingras, Z. Zhang, S. K. Burley, N. Sonenberg, R. Stolarski, and E. Darzynkiewicz. 2003. Phosphorylation of eIF4E attenuates its interaction with mRNA 5′ cap analogs by electrostatic repulsion: intein-mediated protein ligation strategy to obtain phosphorylated protein. RNA 9:52-61. [DOI] [PMC free article] [PubMed] [Google Scholar]