Abstract
The ATP-binding cassette (ABC) transporter gene, ABCA4 (ABCR), was characterized in 1997 as the causal gene for autosomal recessive Stargardt disease (STGD1). Shortly thereafter several other phenotypes were associated with mutations in ABCA4, which now have collectively emerged as the most frequent cause of retinal degeneration phenotypes of Mendelian inheritance. ABCA4 functions as an important transporter (or “flippase”) of vitamin A derivatives in the visual cycle. Several ways to alleviate the effects of the defective ABCA4 protein, which cause accumulation of 11-cis and all-trans-retinal in photoreceptors and lipofuscin in the retinal pigment epithelium, have been proposed. Although ABCA4 has proven to be a difficult research target, substantial progress through genetic, functional, and translational studies has allowed major advances in therapeutic applications for ABCA4-associated pathology, which should be available to patients in the (near) future. Here, we summarize the status of the gene therapy-based treatment options of ABCA4-associated diseases.
Mutations in ABCA4 cause Stargardt disease and other inherited forms of retinal degeneration. Several promising gene therapy strategies (both viral and nonviral) for ABCA4-associated diseases are currently being investigated.
Somatic gene therapy has shown success in several animal models of (recessive) retinal degeneration (Campochiaro 2002; Allocca et al. 2006). Most well known have been reports that a recombinant adeno-associated virus (rAAV) carrying wild-type Rpe65 (rAAV-Rpe65) improved visual function in the Briard dog model of childhood blindness (Acland et al. 2001; Narfstrom et al. 2003; Le Meur et al. 2007). Similar successes have been reported for the Rpe65−/− and Lrat−/− mouse models with AAV and lentiviral vectors (Dejneka et al. 2004; Batten et al. 2005; Bemelmans et al. 2006; Yanez-Munoz et al. 2006).
Direct gene replacement represents an attractive therapeutic option also for treatment of all ABCA4-associated diseases, including Stargardt disease (STGD1) (Allikmets et al. 1997; Molday and Zhang 2010). Delivering a normally functioning gene to photoreceptors harboring mutant ABCA4 via gene therapy should therefore be considered as a possible “cure” for ABCA4-associated diseases because: (1) All ABCA4-associated diseases are recessive, so that adding a functional gene could fully restore visual function and, (2) degeneration of the retinal cells in all ABCA4-associated diseases is relatively delayed, allowing a reasonable time window for therapeutic intervention.
Currently, two major approaches for the delivery of the genetic material to the eye are used: one based on viral vectors as delivery vehicles and the other including nonviral gene delivery methods. So far, the identification of an efficient vector for the delivery of the ABCA4 gene to the retina has been hampered by the large size of the ABCA4 coding sequence (6.8 kb) and by the expression of the ABCA4 gene/protein exclusively in photoreceptor (PR) cells, therefore requiring vectors with large cargo capacity and able to transduce PRs efficiently. Most delivery vehicles that accept large cargo capacity, such as lentiviruses, adenoviruses, and nonviral vectors, have been suggested not to able to efficiently transduce PRs, at least in the mouse models. At the same time, vectors with efficient PR transduction capability, for example those based on adeno-associated viruses (AAVs), have a packaging capacity limited to about 4.7 kb (Colella and Auricchio 2012; Lipinski et al. 2013) and therefore would not be able to package the large ABCA4 coding sequence of ∼7 kb. Recently, significant efforts have been directed toward both the development of AAV-based systems, which are able to deliver large genes and the identification of lentiviral and nonviral vectors with higher PR tropism. Here we will review the current status of ABCA4 gene therapy development.
NONVIRAL GENE DELIVERY IN ABCA4-ASSOCIATED DISEASES
Nonviral vectors offer a number of advantages over viral-based strategies including: (1) reduced toxicity from the vector, (2) lack of an immune response against the vector and possibility to readminister the vector, (3) a large transgene capacity, and (4) simple and relatively inexpensive clinical grade production (Charbel Issa and MacLaren 2012). However, although viruses have evolved to optimize the delivery of the viral gene into the nucleus of the human host cell, “naked” DNA delivered by nonviral vectors needs to overcome several barriers to be expressed, such as: (1) extracellular degradation and immune response mediated by sensor of single stranded or double stranded DNA molecules, such as toll-like receptors, (2) cytoplasmic degradation, and (3) passage through the nuclear envelope during cell division, which is not possible in postmitotic cells, such as PRs. In addition, the presence of physical barriers in the eye, such as the vitreous, inner/outer limiting membranes, the inter-PR matrix, and the high concentrations of glycosaminoglycans present throughout the eye that sequester the DNA, further limit cellular access (Lipinski et al. 2013). As a consequence, many studies have provided evidence of the higher efficiency of viral versus nonviral retinal gene delivery (Andrieu-Soler et al. 2006). In particular, injection of naked DNA via subretinal injection between the PR and retinal pigment epithelium (RPE) (Liang et al. 2000) is highly inefficient (Charbel Issa and MacLaren 2012). Therefore, chemical or physical methods have been used to enhance the efficiency of nonviral vectors gene delivery to the outer retina. Chemical methods, such as liposomes, polymers, and compacted nanoparticles, are based on the conjugation of the DNA with synthetic or natural cationic compound that both protect DNA from nuclease-mediated degradation and allow passage through/into cell membranes via endocytosis and, in some instances, receptor-mediated uptake. Physical methods, such as electroporation or iontophoresis, typically use an electrical stimulus to temporarily permeabilize the membrane and allow the DNA to cross the cellular membranes. To date, however, evidence for effective gene delivery to outer retinal cells remains scarce and the success of the majority of the nonviral delivery technology has been limited to the RPE cell layer (Kachi et al. 2005; Johnson et al. 2008; Souied et al. 2008). However, recently, the polylysine-based compacted DNA nanoparticle (NP) CK30-NP has shown significant improvement in the efficacy of ocular gene transfer in comparison to the previous nonviral methods (Farjo et al. 2006). In the compacted nanoparticles, in particular, the DNA molecule is compacted by polyethylene glycol (PEG)-substituted 30-mer lysine peptides (CK30PEG) (Liu et al. 2003). Their minimal diameter (usually 8–20 nm) is such that allows to readily enter the nucleus (Liu et al. 2003) of dividing and nondividing cells, remaining episomal. Importantly, these particles have no theoretical DNA packaging limitations and have been successfully tested with plasmids up to 20 kb in length (Fink et al. 2006). Notably, subretinal administration of CK30-NPs results in extensive PR transduction, even though with some unexplained patterns of transgene expression (Kumar-Singh 2008) in the absence of detectable immune responses or toxicity (Farjo et al. 2006; Ding et al. 2009). In addition, in contrast with other nonviral approaches, CK30-NPs drive long-term gene expression after subretinal delivery to the mouse eye (Conley and Naash 2010). Given the success of CK30-NPs to mediate phenotypic rescue in rodent models of retinitis pigmentosa (Cai et al. 2009, 2010) and their favorable safety and efficacy profile in a human clinical trial for cystic fibrosis (Konstan et al. 2004). Han et al. (2012) have recently tested CK30-NPs for ABCA4 delivery in the Abca4–/– mouse model of Stargardt disease. This mouse model shows some of the clinical features associated with STGD1, such as accumulation of lipofuscin (A2E) in RPE (Weng et al. 1999; Mata et al. 2001), thickening of the RPE cells (Allocca et al. 2008; Radu et al. 2008; Conley et al. 2012), delayed recovery from light desensitization (Weng et al. 1999; Maiti et al. 2006; Allocca et al. 2008; Radu et al. 2008), and thinning of the outer nuclear (PR cell) layer in albino KO mice (Radu et al. 2008; Wu et al. 2010a). In this mouse model, after subretinal injection of the CK30-NPs carrying the human ABCA4 gene, Han et al. (2012) detected ABCA4 transgene expression for up to 8 months after injection and found both improved recovery of dark adaptation and reduced lipofuscin accumulation. These promising data have provided the first evidence of effective nonviral vector-mediated delivery of large genes, such as ABCA4, to the PR cell layer.
AAV GENE DELIVERY IN ABCA4-ASSOCIATED DISEASES
AAV-derived vectors are currently the most favored vehicles for therapeutic gene delivery to the retina, because they have low immunogenicity, favorable safety profile and support long-term transgene expression after a single administration (Colella and Auricchio 2012; Vandenberghe and Auricchio 2012). AAV is a small (25 nm), nonenveloped virus that packages a linear single-stranded DNA genome of ∼4.7 kb. One of the strengths of the AAV vector platform is the availability of more than 100 different forms called AAV serotypes, which are isolated either as infectious or as molecular clones, and which differ in their external surface capsids proteins. Capsids can be easily exchanged among various AAVs to generate hybrid vectors that contain a genome with the same AAV inverted terminal repeats (ITRs) and the capsid from a different serotype (Auricchio 2003). AAVs obtained through this transcapsidation system are named AAV2/n, where the first number refers to the ITRs and the second to the capsid (Surace and Auricchio 2008). Different capsids confer different tropism and transduction characteristics. Initial AAV vectors were based on AAV serotype 2 (AAV2/2), the most prevalent serotype in humans. However, AAV2/2, although an excellent gene transfer vector for transducing RPE or retinal ganglion cells, is relatively inefficient at transducing other retinal cell types such as PR. Because the majority of mutations causing inherited retinal degeneration, including STGD1, occurs in genes expressed in PRs (Vandenberghe and Auricchio 2012), this concern prompted the search for AAV serotypes able to overcome this limitation. AAV2/5, 2/7, 2/8, and 2/9 vectors have all efficiently transduced PRs, in addition to RPE (Auricchio et al. 2001; Lotery et al. 2003; Allocca et al. 2007), with the AAV2/8 being the most efficient serotype in mice (Auricchio et al. 2001; Lotery et al. 2003; Allocca et al. 2007), pigs (Mussolino et al. 2011), dogs (Stieger et al. 2008), and nonhuman primates (Vandenberghe et al. 2011). The major limitation to the use of AAVs for gene replacement remains their packaging capacity, which is considered to be restricted to the size of the parental genome (4.7 kb), and thus hampers the treatment of certain forms of inherited retinal diseases caused by mutations in genes where cDNA exceeds 5 kb, such as ABCA4. Thus, different strategies to overcome AAV cargo limitations have been investigated. One is based on packaging of oversized genomes, that is, larger than 5 kb (Table 1) (Grieger and Samulski 2005; Allocca et al. 2008; Hirsch et al. 2010). Notably, oversized AAVs have been successfully used to express ABCA4 in the PR of Abca4–/– mice resulting in significant and stable morphological and functional improvement of the Abca4–/– retina (Allocca et al. 2008). However, the mechanism underlying oversized AAV-mediated PR transduction remains elusive as the genomes contained in oversized AAV vectors appear highly heterogeneous in size and are predominantly shorter than expected (Dong et al. 2010; Hirsch et al. 2010; Lai et al. 2010; Wu et al. 2010b; Hirsch et al. 2013), which limits their use in clinical setting.
Table 1.
Schematic representation of AAV-based strategies for large gene transduction
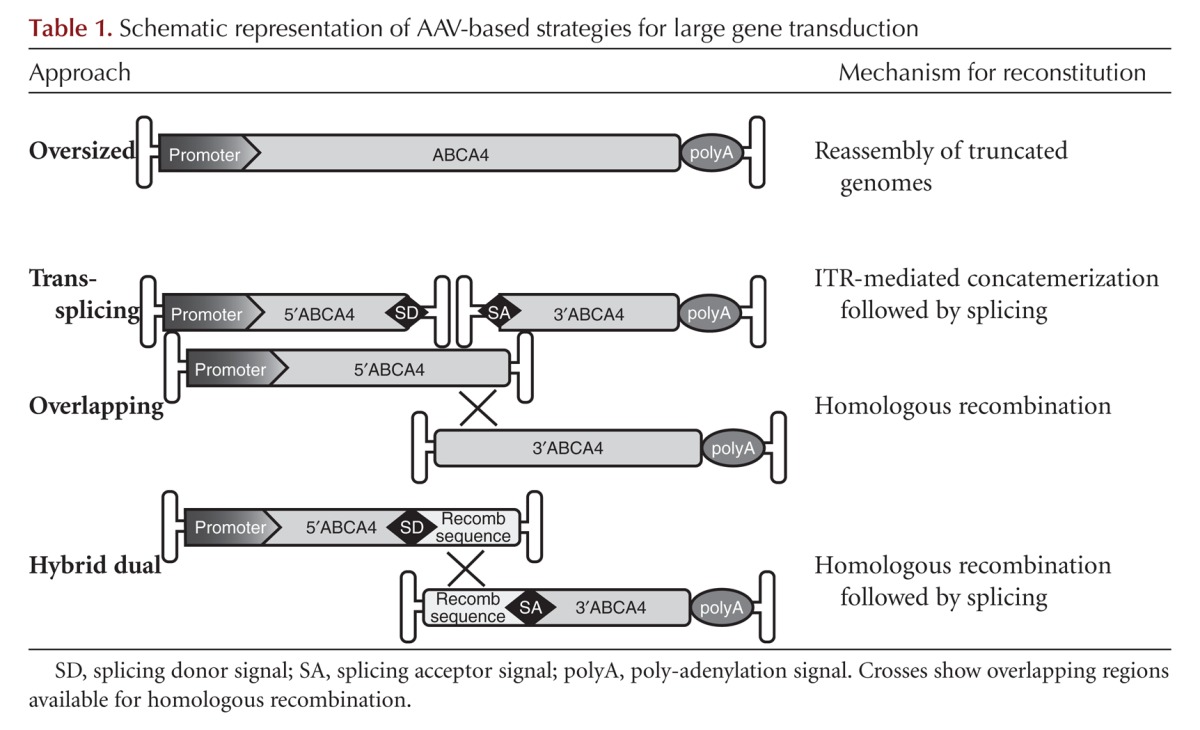
Alternatively, the ability of heterologous AAV genomes to form intermolecular concatemers in the nucleus of target cells (Duan et al. 1998) can be exploited to express large genes, which can be split into two halves, independently packaged in two different (dual) AAV vectors. Various dual AAVs strategies have been described (referred to as trans-splicing, overlapping, and hybrid dual-vector strategies, Table 1) and have been used to efficiently delivery large genes to different tissues. In the trans-splicing approach the 5′-half vector has a splice donor (SD) signal at the 3′ end, whereas the 3′-half vector carries a splice acceptor (SA) signal at the 5′end that allow trans-splicing of a single large mRNA molecule following head-to-tail concatemerization of the two AAVs (Yan et al. 2000). In the overlapping approach, the dual AAV genomes share overlapping sequences, thus the reconstitution of the large gene expression cassette relies on homologous recombination (Duan et al. 2001). The third dual AAV approach (hybrid dual) is a combination of the two previous approaches and it is based on the addition of a highly recombinogenic exogenous sequence to the trans-splicing vectors, to increase their recombination efficiency (Ghosh et al. 2008, 2011; Trapani et al. 2014). This recombinogenic sequence is placed downstream from the SD signal in the 5′-half vector and upstream of the SA signal in the 3′-half vector, so that, after recombination, it is spliced out from the mature RNA (Table 1). The recombinogenic sequences used so far to induce the recombination between dual AAV hybrid vectors derive from regions of either the alkaline phosphatase gene (AP) (Ghosh et al. 2008, 2011) or the F1 phage genome (AK) (Trapani et al. 2014). We recently compared side-by-side the efficiency of the various oversized, dual AAV overlapping, trans-splicing, and hybrid strategies for AAV-mediated large gene transduction in the mouse and pig retina (Trapani et al. 2014), and found that dual AAV trans-splicing and hybrid AK vectors efficiently reconstitute the large ABCA4 gene in mouse PRs. Notably, subretinal administration of dual AAV trans-splicing and hybrid AK vectors improved the phenotype of a mouse model of STGD1, providing evidence for the efficacy of these strategies for ABCA4 gene therapy. Dozens of successful proofs of concept of the efficacy of AAV mediated gene therapy for recessive and dominant inherited retinal diseases have been generated in small and large animal models (Stieger et al. 2010) and have paved the way to the first clinical trials using AAV in patients with Leber congenital amaurosis, a severe form of childhood blindness (Bainbridge et al. 2008; Maguire et al. 2008, 2009; Cideciyan et al. 2009; Simonelli et al. 2010). The results from these initial trials suggest that retinal gene therapy with AAV is safe in humans, that vision can be improved in patients that have suffered from severe impairment of visual function, in some cases for decades, and that readministration of AAV to the subretinal space is feasible, effective, and safe. This, as well as the recent evidence of the efficacy of dual AAV-mediated ABCA4 delivery in mice (Trapani et al. 2014), strongly supports further investigation using AAV for treatment of diseases caused by ABCA4 mutation.
LENTIVIRAL GENE DELIVERY IN ABCA4-ASSOCIATED DISEASES
Lentiviral vectors offer several advantages for gene therapy applications in general, as well as in the specific case of ABCA4-associated diseases. First and foremost, lentiviruses are capable of delivering genes stably and permanently into the genome of transduced cells in vivo (Lois et al. 2002; Gouze et al. 2003; Kostic et al. 2003). Second, they can transduce nondividing cells, a crucial requirement for terminally differentiated cells such as photoreceptors (Kingsman 2003). Third, they can carry relatively large expression cassettes, which is essential in this case because the human ABCA4 cDNA is almost 7 kb, which exceeds the capacity of the commonly used AAV vectors. However, two major problems associated with lentiviral-based gene therapy are, as suggested, the relatively low PR tropism of lentiviral vectors in mouse models and possible tumorigenic side effects resulting from uncontrolled, random integration of lentiviruses to the host genome.
The reported low efficiency of PR transduction with lentiviruses in (adult) rodents has been considered a major limiting factor for gene therapy of eye diseases with lentiviral vectors. Although the early experiments with rats were successful (Miyoshi et al. 1997; Takahashi et al. 1999), they were not confirmed subsequently, especially in adult rodent models (Kostic et al. 2003; Gruter et al. 2005). The best efficacy of PR transduction in mice (5%–15%) was achieved after subretinal injection of mice at postnatal 4 and 5 d (Kong et al. 2008). In adult mice, the efficacy rarely exceeded 5%, even after chemical modification of the subretinal space by neuraminidase X or chondroitinase ABC (Gruter et al. 2005). It had been noted, however, that the PR transduction by lentiviruses was more efficient in other animals, such as chicken and nonhuman primates (Lotery et al. 2002; Williams et al. 2006). Subsequently, we were able to show a more efficient PR transduction of the NHP retina by EIAV lentivectors using either GFP (Binley et al. 2013) or LacZ (data not shown) as reporters under the constitutive CMV promoter. Transduction of PRs, in addition to RPE, in the injected area of adult macaques suggests that lentiviral vectors can more efficiently transduce PRs in adult NHPs than in mice, thus raising hope for gene therapy in humans, especially for delivering large genes such as ABCA4. Additional studies with PR-specific promoters should unequivocally address the PR transduction efficiency issue for lentiviral vectors in NHPs.
However, Abca4−/− mice are still the only animal model for STGD1 disease, therefore all preclinical models have used this mouse strain. An efficient amelioration of a major disease marker, A2E accumulation, was successfully achieved in studies utilizing lentiviral vectors in the Abca4−/− mouse model (Kong et al. 2008). After demonstrating safety and acceptable biodistribution of EIAV vectors carrying the human ABCA4 gene under the constitutive CMV promoter, phase I/II clinical trials for patients with STGD1 were started in 2012 by Oxford BioMedica (NCT01367444; see http://www.clinicaltrials.gov).
In addition to the suggested low PR transduction efficiency by lentiviruses in mice, two other concerns have slowed the development of the lentiviral platform. First, despite greatly improved biosafety, psychological concerns regarding using vectors derived from primate lentiviruses HIV-1, HIV-2, and simian immunodeficiency virus (SIV) have not been completely overcome, despite highly remote possibility of generating replication-competent lentivirus in clinical application. These concerns have been alleviated by using nonprimate lentiviral vectors, which cannot replicate in human cells (Maury 1998), such as those derived from equine infectious anemia virus (EIAV), bovine immunodeficiency virus (BIV), or feline immunodeficiency viruses (FIV) (Olsen 2001). Second, serious complications in early gene therapy clinical trials for X-linked SCID3 using retroviral vectors raised ethical and safety concerns about the use of retrovirus-based therapies in humans because of uncontrolled integration into genome (Kohn et al. 2003). These concerns are being addressed by creating either integration-deficient lentiviral vectors or vectors with targeted, clinically safe integration (Wanisch and Yanez-Munoz 2009).
CONCLUDING REMARKS
Several gene therapy applications are being used for ABCA4-associated diseases. Although currently only lentivirus-based therapy is in clinical trials, those based on AAV or nonviral vectors are close to follow. Another delivery vehicles not discussed above, but which are tried in preclinical studies are adenoviral (Ad) vectors. Although the Ad vectors, which are nonintegrating and transduce both dividing and nondividing cells, used to elicit immune responses that limited the duration of transgene expression, the current, helper-dependent Ad vectors, can substantially extend the duration of the expression of the transgene (Lamartina et al. 2007). Comparison of all methods will reveal which one is the most efficient and safe for patients; however, regardless of the final outcome, it is clear that efficient gene therapy will soon be available to patients affected with all ABCA4-associated diseases.
ACKNOWLEDGMENTS
Supported, in part, by grants from the National Eye Institute (NIH) EY021163, EY019861, and EY019007 (Core Support for Vision Research); Foundation Fighting Blindness (Owings Mills, Maryland), and unrestricted funds from Research to Prevent Blindness (New York, NY) to the Department of Ophthalmology, Columbia University.
Footnotes
Editors: Eric A. Pierce, Richard H. Masland, and Joan W. Miller
Additional Perspectives on Retinal Disorders: Genetic Approaches to Diagnosis and Treatment available at www.perspectivesinmedicine.org
REFERENCES
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, et al. 2001. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 28: 92–95. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, et al. 1997. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 15: 236–246. [DOI] [PubMed] [Google Scholar]
- Allocca M, Tessitore A, Cotugno G, Auricchio A. 2006. AAV-mediated gene transfer for retinal diseases. Expert Opin Biol Ther 6: 1279–1294. [DOI] [PubMed] [Google Scholar]
- Allocca M, Mussolino C, Garcia-Hoyos M, Sanges D, Iodice C, Petrillo M, Vandenberghe LH, Wilson JM, Marigo V, Surace EM, et al. 2007. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol 81: 11372–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D, Kim SR, Maguire A, Rex TS, Di Vicino U, et al. 2008. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest 118: 1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieu-Soler C, Bejjani RA, de Bizemont T, Normand N, BenEzra D, Behar-Cohen F. 2006. Ocular gene therapy: A review of nonviral strategies. Mol Vis 12: 1334–1347. [PubMed] [Google Scholar]
- Auricchio A. 2003. Pseudotyped AAV vectors for constitutive and regulated gene expression in the eye. Vision Res 43: 913–918. [DOI] [PubMed] [Google Scholar]
- Auricchio A, Kobinger G, Anand V, Hildinger M, O’Connor E, Maguire AM, Wilson JM, Bennett J. 2001. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: The retina as a model. Hum Mol Genet 10: 3075–3081. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, et al. 2008. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med 358: 2231–2239. [DOI] [PubMed] [Google Scholar]
- Batten ML, Imanishi Y, Tu DC, Doan T, Zhu L, Pang J, Glushakova L, Moise AR, Baehr W, Van Gelder RN, et al. 2005. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med 2: e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemelmans AP, Kostic C, Crippa SV, Hauswirth WW, Lem J, Munier FL, Seeliger MW, Wenzel A, Arsenijevic Y. 2006. Lentiviral gene transfer of Rpe65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med 3: e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley K, Widdowson P, Loader J, Kelleher M, Iqball S, Ferrige G, de Belin J, Carlucci M, Angell-Manning D, Hurst F, et al. 2013. Transduction of photoreceptors with equine infectious anemia virus lentiviral vectors: Safety and biodistribution of StarGen for Stargardt disease. Invest Ophthalmol Vis Sci 54: 4061–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Nash Z, Conley SM, Fliesler SJ, Cooper MJ, Naash MI. 2009. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS ONE 4: e5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Conley SM, Nash Z, Fliesler SJ, Cooper MJ, Naash MI. 2010. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J 24: 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA. 2002. Gene therapy for retinal and choroidal diseases. Expert Opin Biol Ther 2: 537–544. [DOI] [PubMed] [Google Scholar]
- Charbel Issa P, MacLaren RE. 2012. Non-viral retinal gene therapy: A review. Clin Experiment Ophthalmol 40: 39–47. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Roman AJ, et al. 2009. Vision 1 year after gene therapy for Leber’s congenital amaurosis. N Engl J Med 361: 725–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella P, Auricchio A. 2012. Gene therapy of inherited retinopathies: A long and successful road from viral vectors to patients. Hum Gene Ther 23: 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley SM, Naash MI. 2010. Nanoparticles for retinal gene therapy. Prog Retin Eye Res 29: 376–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley SM, Cai X, Makkia R, Wu Y, Sparrow JR, Naash MI. 2012. Increased cone sensitivity to ABCA4 deficiency provides insight into macular vision loss in Stargardt’s dystrophy. Biochim Biophys Acta 1822: 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejneka NS, Surace EM, Aleman TS, Cideciyan AV, Lyubarsky A, Savchenko A, Redmond TM, Tang W, Wei Z, Rex TS, et al. 2004. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther 9: 182–188. [DOI] [PubMed] [Google Scholar]
- Ding XQ, Quiambao AB, Fitzgerald JB, Cooper MJ, Conley SM, Naash MI. 2009. Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PLoS ONE 4: e7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Nakai H, Xiao W. 2010. Characterization of genome integrity for oversized recombinant AAV vector. Mol Ther 18: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y, Fisher KJ, Engelhardt JF. 1998. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol 72: 8568–8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Yue Y, Engelhardt JF. 2001. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: A quantitative comparison. Mol Ther 4: 383–391. [DOI] [PubMed] [Google Scholar]
- Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. 2006. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE 1: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink TL, Klepcyk PJ, Oette SM, Gedeon CR, Hyatt SL, Kowalczyk TH, Moen RC, Cooper MJ. 2006. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther 13: 1048–1051. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Yue Y, Lai Y, Duan D. 2008. A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol Ther 16: 124–130. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Yue Y, Duan D. 2011. Efficient transgene reconstitution with hybrid dual AAV vectors carrying the minimized bridging sequences. Hum Gene Ther 22: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouze E, Pawliuk R, Gouze JN, Pilapil C, Fleet C, Palmer GD, Evans CH, Leboulch P, Ghivizzani SC. 2003. Lentiviral-mediated gene delivery to synovium: Potent intra-articular expression with amplification by inflammation. Mol Ther 7: 460–466. [DOI] [PubMed] [Google Scholar]
- Grieger JC, Samulski RJ. 2005. Packaging capacity of adeno-associated virus serotypes: Impact of larger genomes on infectivity and postentry steps. J Virol 79: 9933–9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruter O, Kostic C, Crippa SV, Perez MT, Zografos L, Schorderet DF, Munier FL, Arsenijevic Y. 2005. Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Ther 12: 942–947. [DOI] [PubMed] [Google Scholar]
- Han Z, Conley SM, Makkia RS, Cooper MJ, Naash MI. 2012. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J Clin Invest 122: 3221–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch ML, Agbandje-McKenna M, Samulski RJ. 2010. Little vector, big gene transduction: Fragmented genome reassembly of adeno-associated virus. Mol Ther 18: 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch ML, Li C, Bellon I, Yin C, Chavala S, Pryadkina M, Richard I, Samulski RJ. 2013. Oversized AAV transductifon is mediated via a DNA-PKcs-independent, Rad51C-dependent repair pathway. Mol Ther 21: 2205–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CJ, Berglin L, Chrenek MA, Redmond TM, Boatright JH, Nickerson JM. 2008. Technical brief: Subretinal injection and electroporation into adult mouse eyes. Mol Vis 14: 2211–2226. [PMC free article] [PubMed] [Google Scholar]
- Kachi S, Oshima Y, Esumi N, Kachi M, Rogers B, Zack DJ, Campochiaro PA. 2005. Nonviral ocular gene transfer. Gene Ther 12: 843–851. [DOI] [PubMed] [Google Scholar]
- Kingsman SM. 2003. Lentivirus: A vector for nervous system applications. Ernst Schering Res Found Workshop 43: 179–207. [DOI] [PubMed] [Google Scholar]
- Kohn DB, Sadelain M, Glorioso JC. 2003. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat Rev Cancer 3: 477–488. [DOI] [PubMed] [Google Scholar]
- Kong J, Kim SR, Binley K, Pata I, Doi K, Mannik J, Zernant-Rajang J, Kan O, Iqball S, Naylor S, et al. 2008. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther 15: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstan MW, Davis PB, Wagener JS, Hilliard KA, Stern RC, Milgram LJ, Kowalczyk TH, Hyatt SL, Fink TL, Gedeon CR, et al. 2004. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther 15: 1255–1269. [DOI] [PubMed] [Google Scholar]
- Kostic C, Chiodini F, Salmon P, Wiznerowicz M, Deglon N, Hornfeld D, Trono D, Aebischer P, Schorderet DF, Munier FL, et al. 2003. Activity analysis of housekeeping promoters using self-inactivating lentiviral vector delivery into the mouse retina. Gene Ther 10: 818–821. [DOI] [PubMed] [Google Scholar]
- Kumar-Singh R. 2008. Barriers for retinal gene therapy: Separating fact from fiction. Vision Res 48: 1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Yue Y, Duan D. 2010. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome≥8.2 kb. Mol Ther 18: 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamartina S, Cimino M, Roscilli G, Dammassa E, Lazzaro D, Rota R, Ciliberto G, Toniatti C. 2007. Helper-dependent adenovirus for the gene therapy of proliferative retinopathies: Stable gene transfer, regulated gene expression and therapeutic efficacy. J Gene Med 9: 862–874. [DOI] [PubMed] [Google Scholar]
- Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, Mendes-Madeira A, Provost N, Pereon Y, Cherel Y, et al. 2007. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther 14: 292–303. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Anand V, Maguire AM, Bennett J. 2000. Intraocular delivery of recombinant virus. Humana, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- Lipinski DM, Thake M, MacLaren RE. 2013. Clinical applications of retinal gene therapy. Prog Retin Eye Res 32: 22–47. [DOI] [PubMed] [Google Scholar]
- Liu G, Li D, Pasumarthy MK, Kowalczyk TH, Gedeon CR, Hyatt SL, Payne JM, Miller TJ, Brunovskis P, Fink TL, et al. 2003. Nanoparticles of compacted DNA transfect postmitotic cells. J Biol Chem 278: 32578–32586. [DOI] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. 2002. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295: 868–872. [DOI] [PubMed] [Google Scholar]
- Lotery AJ, Derksen TA, Russell SR, Mullins RF, Sauter S, Affatigato LM, Stone EM, Davidson BL. 2002. Gene transfer to the nonhuman primate retina with recombinant feline immunodeficiency virus vectors. Hum Gene Ther 13: 689–696. [DOI] [PubMed] [Google Scholar]
- Lotery AJ, Yang GS, Mullins RF, Russell SR, Schmidt M, Stone EM, Lindbloom JD, Chiorini JA, Kotin RM, Davidson BL. 2003. Adeno-associated virus type 5: Transduction efficiency and cell-type specificity in the primate retina. Hum Gene Ther 14: 1663–1671. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, et al. 2008. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 358: 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, et al. 2009. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet 374: 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti P, Kong J, Kim SR, Sparrow JR, Allikmets R, Rando RR. 2006. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochemistry 45: 852–860. [DOI] [PubMed] [Google Scholar]
- Mata NL, Tzekov RT, Liu X, Weng J, Birch DG, Travis GH. 2001. Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: Implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci 42: 1685–1690. [PubMed] [Google Scholar]
- Maury W. 1998. Regulation of equine infectious anemia virus expression. J Biomed Sci 5: 11–23. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Takahashi M, Gage FH, Verma IM. 1997. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci 94: 10319–10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, Zhang K. 2010. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog Lipid Res 49: 476–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussolino C, della Corte M, Rossi S, Viola F, Di Vicino U, Marrocco E, Neglia S, Doria M, Testa F, Giovannoni R, et al. 2011. AAV-mediated photoreceptor transduction of the pig cone-enriched retina. Gene Ther 18: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narfstrom K, Katz ML, Bragadottir R, Seeliger M, Boulanger A, Redmond TM, Caro L, Lai CM, Rakoczy PE. 2003. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci 44: 1663–1672. [DOI] [PubMed] [Google Scholar]
- Olsen JC. 2001. EIAV, CAEV and other lentivirus vector systems. Somat Cell Mol Genet 26: 131–145. [DOI] [PubMed] [Google Scholar]
- Radu RA, Yuan Q, Hu J, Peng JH, Lloyd M, Nusinowitz S, Bok D, Travis GH. 2008. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Invest Ophthalmol Vis Sci 49: 3821–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, et al. 2010. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 18: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souied EH, Reid SN, Piri NI, Lerner LE, Nusinowitz S, Farber DB. 2008. Non-invasive gene transfer by iontophoresis for therapy of an inherited retinal degeneration. Exp Eye Res 87: 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger K, Colle MA, Dubreil L, Mendes-Madeira A, Weber M, Le Meur G, Deschamps JY, Provost N, Nivard D, Cherel Y, et al. 2008. Subretinal delivery of recombinant AAV serotype 8 vector in dogs results in gene transfer to neurons in the brain. Mol Ther 16: 916–923. [DOI] [PubMed] [Google Scholar]
- Stieger K, Chauveau C, Rolling F. 2010. Preclinical studies on specific gene therapy for recessive retinal degenerative diseases. Curr Gene Ther 10: 389–403. [DOI] [PubMed] [Google Scholar]
- Surace EM, Auricchio A. 2008. Versatility of AAV vectors for retinal gene transfer. Vision Res 48: 353–359. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Miyoshi H, Verma IM, Gage FH. 1999. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol 73: 7812–7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani I, Colella P, Sommella A, Iodice C, Cesi G, De Simone S, Marrocco E, Rossi S, Giunti M, Palfi A, et al. 2014. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol Med 6: 194–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Auricchio A. 2012. Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther 19: 162–168. [DOI] [PubMed] [Google Scholar]
- Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R, Wang L, Castle MJ, Maguire AC, Grant R, et al. 2011. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med 3: 88ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanisch K, Yanez-Munoz RJ. 2009. Integration-deficient lentiviral vectors: A slow coming of age. Mol Ther 17: 1316–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. 1999. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell 98: 13–23. [DOI] [PubMed] [Google Scholar]
- Williams ML, Coleman JE, Haire SE, Aleman TS, Cideciyan AV, Sokal I, Palczewski K, Jacobson SG, Semple-Rowland SL. 2006. Lentiviral expression of retinal guanylate cyclase-1 (RetGC1) restores vision in an avian model of childhood blindness. PLoS Med 3: e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Nagasaki T, Sparrow JR. 2010a. Photoreceptor cell degeneration in Abcr –/– mice. Adv Exp Med Biol 664: 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yang H, Colosi P. 2010b. Effect of genome size on AAV vector packaging. Mol Ther 18: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhang Y, Duan D, Engelhardt JF. 2000. Trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc Natl Acad Sci 97: 6716–6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, Buch P, MacLaren RE, Anderson PN, Barker SE, et al. 2006. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med 12: 348–353. [DOI] [PubMed] [Google Scholar]


