Abstract
While 9-O-acetylation of sialic acids has been reported in some mammalian tissues, the distribution of this modification on specific cell types and molecules is largely unknown. The influenza C virus hemagglutinin-esterase is a membrane-bound glycoprotein that binds specifically to 9-O-acetylated sialic acids (hemagglutinin activity) and then hydrolyzes the O-acetyl group (receptor-destroying activity). A recombinant soluble form of influenza C virus hemagglutinin-esterase wherein the C-terminal transmembrane and cytoplasmic domains are replaced by the Fc portion of human IgG retains both its recognition and enzymatic functions. The latter activity can selectively remove 9-O-acetyl groups from bound or free sialic acids and, under specific conditions, 7-O-acetyl groups as well. Irreversible inactivation of the esterase unmasks stable recognition activity, giving a molecule that binds specifically to 9-O-acetylated sialic acids. These probes demonstrate widespread but selective expression of 9-O-acetylated sialic acids in certain cell types of rat tissues. Patterns of polarized or gradient expression further demonstrate the regulated nature of this modification. Direct probing of blots and thin-layer plates shows selective expression of 9-O-acetylation on certain glycoproteins and glycolipids in such tissues. Thus, 9-O-acetylation is more widespread than previously thought and occurs on specific molecules and cell types.
Full text
PDF


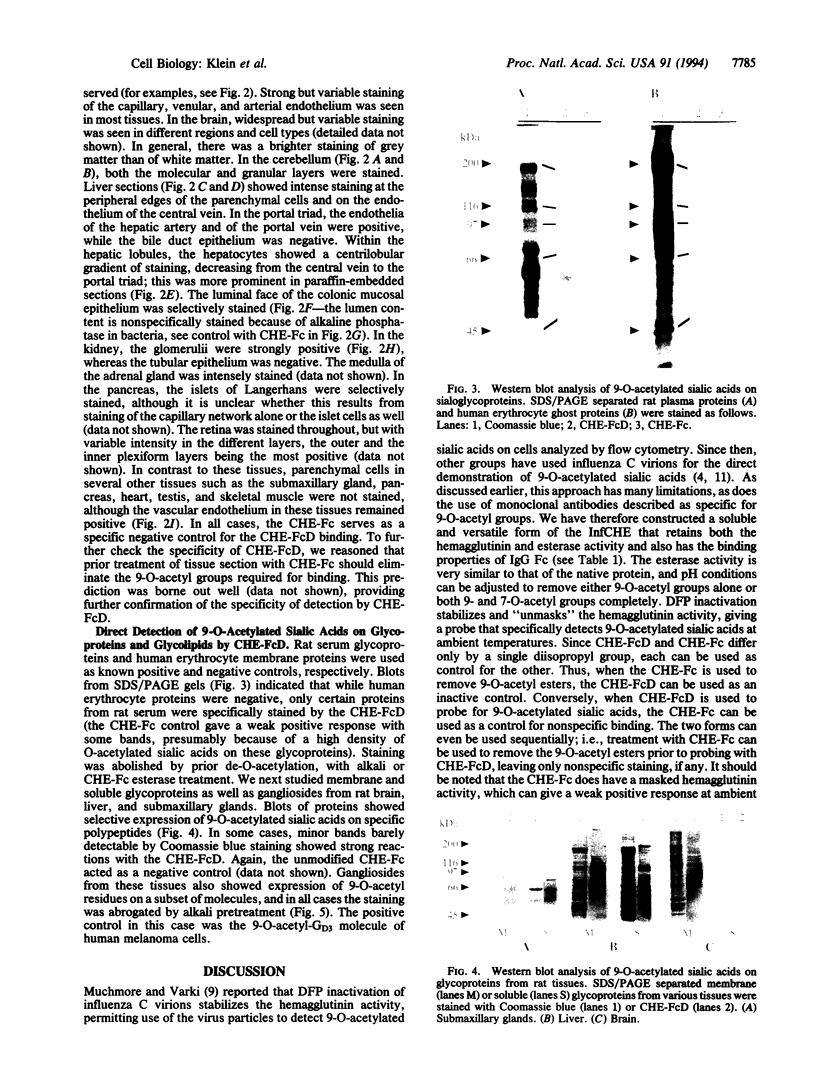

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Butor C., Diaz S., Varki A. High level O-acetylation of sialic acids on N-linked oligosaccharides of rat liver membranes. Differential subcellular distribution of 7- and 9-O-acetyl groups and of enzymes involved in their regulation. J Biol Chem. 1993 May 15;268(14):10197–10206. [PubMed] [Google Scholar]
- Corfield A. P., Wagner S. A., O'Donnell L. J., Durdey P., Mountford R. A., Clamp J. R. The roles of enteric bacterial sialidase, sialate O-acetyl esterase and glycosulfatase in the degradation of human colonic mucin. Glycoconj J. 1993 Feb;10(1):72–81. doi: 10.1007/BF00731190. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A., Villar E., Manuguerra J. C., Hannoun C., Cabezas J. A. Activity of influenza C virus O-acetylesterase with O-acetyl-containing compounds. Biochem J. 1991 Jan 15;273(Pt 2):435–441. doi: 10.1042/bj2730435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrler G., Rott R., Klenk H. D., Müller H. P., Shukla A. K., Schauer R. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J. 1985 Jun;4(6):1503–1506. doi: 10.1002/j.1460-2075.1985.tb03809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa H. H., Manzi A., Varki A. O-acetylation and de-O-acetylation of sialic acids. Purification, characterization, and properties of a glycosylated rat liver esterase specific for 9-O-acetylated sialic acids. J Biol Chem. 1989 Nov 15;264(32):19435–19442. [PubMed] [Google Scholar]
- Kamerling J. P., Schauer R., Shukla A. K., Stoll S., Van Halbeek H., Vliegenthart J. F. Migration of O-acetyl groups in N,O-acetylneuraminic acids. Eur J Biochem. 1987 Feb 2;162(3):601–607. doi: 10.1111/j.1432-1033.1987.tb10681.x. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Manuguerra J. C., DuBois C., Hannoun C. Analytical detection of 9(4)-O-acetylated sialoglycoproteins and gangliosides using influenza C virus. Anal Biochem. 1991 May 1;194(2):425–432. doi: 10.1016/0003-2697(91)90252-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzi A. E., Diaz S., Varki A. High-pressure liquid chromatography of sialic acids on a pellicular resin anion-exchange column with pulsed amperometric detection: a comparison with six other systems. Anal Biochem. 1990 Jul;188(1):20–32. doi: 10.1016/0003-2697(90)90523-c. [DOI] [PubMed] [Google Scholar]
- Mehansho H., Carlson D. M. Induction of protein and glycoprotein synthesis in rat submandibular glands by isoproterenol. J Biol Chem. 1983 May 25;258(10):6616–6620. [PubMed] [Google Scholar]
- Muchmore E. A., Varki A. Selective inactivation of influenza C esterase: a probe for detecting 9-O-acetylated sialic acids. Science. 1987 Jun 5;236(4806):1293–1295. doi: 10.1126/science.3589663. [DOI] [PubMed] [Google Scholar]
- Muchmore E. A., Varki N. M., Fukuda M., Varki A. Developmental regulation of sialic acid modifications in rat and human colon. FASEB J. 1987 Sep;1(3):229–235. doi: 10.1096/fasebj.1.3.3623000. [DOI] [PubMed] [Google Scholar]
- Ohuchi M., Ohuchi R., Mifune K. Demonstration of hemolytic and fusion activities of influenza C virus. J Virol. 1982 Jun;42(3):1076–1079. doi: 10.1128/jvi.42.3.1076-1079.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reivinen J., Holthöfer H., Miettinen A. A cell-type specific ganglioside of glomerular podocytes in rat kidney: an O-acetylated GD3. Kidney Int. 1992 Sep;42(3):624–631. doi: 10.1038/ki.1992.327. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Herrler G., Paulson J. C., Klenk H. D. Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J Biol Chem. 1986 May 5;261(13):5947–5951. [PubMed] [Google Scholar]
- Sarris A. H., Palade G. E. The sialoglycoproteins of murine erythrocyte ghosts. A modified periodic acid-Schiff stain procedure staining nonsubstituted and O-acetylated sialyl residues on glycopeptides. J Biol Chem. 1979 Jul 25;254(14):6724–6731. [PubMed] [Google Scholar]
- Sjoberg E. R., Varki A. Kinetic and spatial interrelationships between ganglioside glycosyltransferases and O-acetyltransferase(s) in human melanoma cells. J Biol Chem. 1993 May 15;268(14):10185–10196. [PubMed] [Google Scholar]
- Troy F. A., 2nd Polysialylation: from bacteria to brains. Glycobiology. 1992 Feb;2(1):5–23. doi: 10.1093/glycob/2.1.5. [DOI] [PubMed] [Google Scholar]
- Varki A., Diaz S. The release and purification of sialic acids from glycoconjugates: methods to minimize the loss and migration of O-acetyl groups. Anal Biochem. 1984 Feb;137(1):236–247. doi: 10.1016/0003-2697(84)90377-4. [DOI] [PubMed] [Google Scholar]
- Varki A. Diversity in the sialic acids. Glycobiology. 1992 Feb;2(1):25–40. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A., Hooshmand F., Diaz S., Varki N. M., Hedrick S. M. Developmental abnormalities in transgenic mice expressing a sialic acid-specific 9-O-acetylesterase. Cell. 1991 Apr 5;65(1):65–74. doi: 10.1016/0092-8674(91)90408-q. [DOI] [PubMed] [Google Scholar]
- Vlasak R., Krystal M., Nacht M., Palese P. The influenza C virus glycoprotein (HE) exhibits receptor-binding (hemagglutinin) and receptor-destroying (esterase) activities. Virology. 1987 Oct;160(2):419–425. doi: 10.1016/0042-6822(87)90013-4. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Zimmer G., Reuter G., Schauer R. Use of influenza C virus for detection of 9-O-acetylated sialic acids on immobilized glycoconjugates by esterase activity. Eur J Biochem. 1992 Feb 15;204(1):209–215. doi: 10.1111/j.1432-1033.1992.tb16626.x. [DOI] [PubMed] [Google Scholar]