ABSTRACT
Cephalopods are renowned for changing the color and pattern of their skin for both camouflage and communication. Yet, we do not fully understand how cephalopods control the pigmented chromatophore organs in their skin and change their body pattern. Although these changes primarily rely on eyesight, we found that light causes chromatophores to expand in excised pieces of Octopus bimaculoides skin. We call this behavior light-activated chromatophore expansion (or LACE). To uncover how octopus skin senses light, we used antibodies against r-opsin phototransduction proteins to identify sensory neurons that express r-opsin in the skin. We hypothesized that octopus LACE relies on the same r-opsin phototransduction cascade found in octopus eyes. By creating an action spectrum for the latency to LACE, we found that LACE occurred most quickly in response to blue light. We fit our action spectrum data to a standard opsin curve template and estimated the λmax of LACE to be 480 nm. Consistent with our hypothesis, the maximum sensitivity of the light sensors underlying LACE closely matches the known spectral sensitivity of opsin from octopus eyes. LACE in isolated preparations suggests that octopus skin is intrinsically light sensitive and that this dispersed light sense might contribute to their unique and novel patterning abilities. Finally, our data suggest that a common molecular mechanism for light detection in eyes may have been co-opted for light sensing in octopus skin and then used for LACE.
KEY WORDS: Behavior, Cephalopod, Dermal light sense
Highlighted Article: Octopus skin senses light independent of the eyes, causing chromatophores in the skin to expand, probably via the same r-opsin phototransduction genes used for vision.
INTRODUCTION
Octopuses, like other coleoid cephalopods, create signals and camouflage themselves by altering the color, pattern and texture of their skin (Holmes, 1940; Hanlon and Messenger, 1988; Packard and Sanders, 1971). While light in the environment influences which body patterns are produced, exactly how cephalopods gather and use environmental light to control their body patterning is still debated (Buresch et al., 2015). In general, body-patterning behaviors in cephalopods depend on three major components: the eyes, the central nervous system (CNS) and pigmented organs called chromatophores embedded in the skin (Messenger, 2001). Chromatophores are an evolutionary novelty because their morphology in coleoid cephalopods is distinct from those found in any other animal taxa, including other mollusks. Cephalopod chromatophores consist of an elastic sac filled with pigment granules and surrounded by radial muscles, which are innervated by nerves that extend directly from the brain (Cloney and Florey, 1968; Young, 1971, 1974). When chromatophore muscles contract, the pigment sac at the center is stretched out, showing the chromatophores' color. Cephalopods seem to use their well-developed, camera-type eyes to gather information about salient features of the light environment, such as brightness, contrast and edges, which strongly influence changes in the appearance of their skin (Messenger, 1979; Chiao and Hanlon, 2001; Zylinski et al., 2009). Chromatophores can be experimentally controlled with electrical stimulation of the eyes or various brain regions (e.g. optic, peduncle and chromatophore lobes), leading to an overall darkening of the skin tone and sometimes even distinct patterns, which also demonstrates the importance of the eyes and CNS in controlling the activity of chromatophores (Messenger, 1967; Boycott, 1961; Young, 1976; Dubas et al., 1986).
Despite the involvement of eyes for detecting light and the CNS for controlling chromatophore activity in cephalopods, several studies suggest that chromatophores might also be controlled locally by the peripheral nervous system. Both Florey (1966) and Packard and Brancato (1993) noted that squid and octopus chromatophores in dissociated or denervated skin seem to expand in response to light, but surprisingly, neither study investigated these observations further. These intriguing notes suggest that cephalopod skin may be intrinsically sensitive to light, and if so, raise the questions of how the skin senses light and to what extent this ability contributes to rapid changes in the color and tone of cephalopod skin.
Recent work on the molecular basis for light sensing in the skin of myriad animals suggest that cephalopod skin could detect light using the same families of proteins that detect light in the eyes of animals, including a subfamily of G-protein-coupled receptor proteins (GPCRs) called opsins. There are at least three major groups of opsins: the r-opsins, c-opsins and Go/RGR (retinal G-protein-coupled receptor) opsins (Porter et al., 2012; Feuda et al., 2012). While c-opsins are typically thought to detect light in vertebrate eyes and r-opsins in invertebrate eyes, various opsins are expressed in the skin of many animals (Ramirez et al., 2011), and opsins have been localized to receptors dispersed across the body of animals from multiple phyla, including cnidarians, echinoderms, annelids and vertebrates (Plachetzki et al., 2012; Raible et al., 2006; Backfisch et al., 2013; Bellono et al., 2013; Fulgione et al., 2014). Because opsins are known to function as light receptors, the cells that express opsin may be dispersed light sensors that could underlie some light-mediated behaviors. While opsins have not been localized to particular cells in the skin of any cephalopods prior to this study, the same r-opsin used to detect light in the eyes of the cuttlefish Sepia officinalis is also expressed in its skin (Mäthger et al., 2010).
The preliminary observations that squid and octopus chromatophores respond directly to light in dissociated skin and the expression of opsin mRNAs in cuttlefish skin suggests: (1) that dispersed light sensitivity in the skin of cephalopods contributes to some chromatophore responses, perhaps separately from eye or CNS input; and (2) that cephalopods use the same r-opsin-based phototransduction genes to detect light with both their eyes and skin. We found that dispersed, dermal light sensitivity contributes to a direct response of Octopus bimaculoides chromatophores to light. We call this chromatophore response light-activated chromatophore expansion (LACE). LACE behavior in isolated octopus skin shows that the skin can sense and respond to light directly. Next, we found multiple r-opsin cascade genes expressed in the skin of O. bimaculoides and localized r-opsin protein expression to ciliated sensory cells in the skin of hatchling octopuses. Finally, like the opsin found in the eyes of Octopus vulgaris, LACE in O. bimaculoides is maximally responsive to blue (470 nm) light. These results are consistent with the hypothesis that r-opsin-based phototransduction underlies LACE behavior in O. bimaculoides.
RESULTS
Octopus bimaculoides exhibits LACE in dissociated skin preparations
Chromatophores in skin removed from the funnels of both hatchling and adult Octopus bimaculoides expand dramatically when illuminated by bright white light (absolute irradiance=2.60×1015 photon cm−2 s−1; see Fig. 1 and supplementary material Movie 1). While we observed slow rhythmic contractions of the muscles beneath the skin under red light from an LED (absolute irradiance: 1.36×1014 photon cm−2 s−1), the chromatophores themselves remained in their relaxed position and only expanded in response to either a gentle mechanical stimulus or bright white light. While the light remained on, the chromatophores remained expanded and appeared to pulse rhythmically, but would sometimes contract again after prolonged exposure to white light. When the white light was switched off and the chromatophores were illuminated with only red light, the chromatophores in fresh preparations contracted back to their original state. As preparations aged over the course of 1+ days, their responses to light became erratic: chromatophores would no longer respond to white light, or remain expanded, regardless of whether they were under white or red light. The direction of the response of the chromatophores to light (to increase in size) is consistent across samples (see Fig. 2 and supplementary material Table S1; binomial sign test, N=10, P=0.002).
Fig. 1.
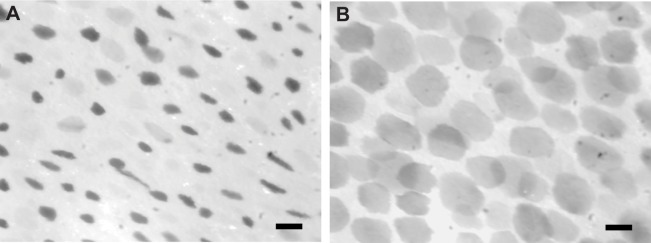
Chromatophores in isolated Octopus bimaculoides skin expand when illuminated. Stills from infrared video of isolated adult O. bimaculoides funnel skin showing LACE (light-activated chromatophore expansion). (A) Chromatophores remain in their contracted state after 3 s of exposure to bright white light. (B) Chromatophores have reached their maximum expansion after 6 s of exposure to bright white light. Scale bars: 100 μm.
Fig. 2.
Chromatophores expand dramatically under bright white light (binomial sign test, N=10, P=0.002). Paired bar plots of mean chromatophore areas (in pixels) before and after LACE. Each bar is the average size of a single chromatophore measured from at least three trials per animal. A1 and A2 are adult samples and H1–H8 are hatchling samples.
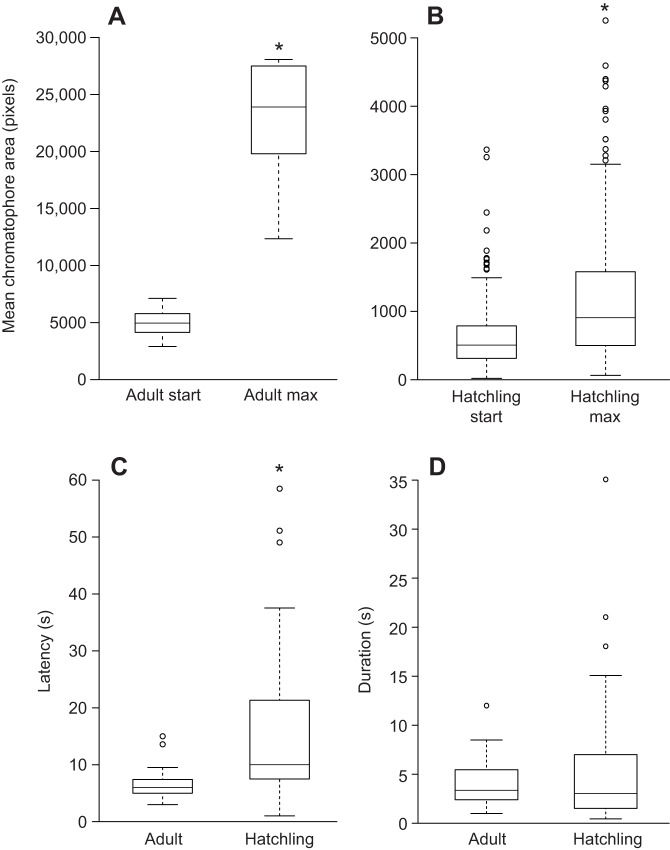
LACE caused a statistically significant increase in the size of chromatophores in both adult and hatchling skin, a five-fold average increase for adults (one-sample t-test on log-ratio, t=8.9246, d.f.=8, P<0.0001; Fig. 3A) and a two-fold average increase for hatchlings (one-sample t-test on log-ratio, t=11.915, d.f.=200, P<0.0001; Fig. 3B). Although both adult and hatchling chromatophores expanded significantly after LACE, the log-ratio of the increase differed significantly between adults and hatchlings (two-sample t-test, t=5.4578, d.f.=9.245, P<0.001). The mean latency of LACE from the beginning of the white light stimulus to the time when maximally expanded was significantly different between adult (6.54±2.42 s) and hatchling (15.37±12.74 s) samples (two sample t-test, t=−5.19, d.f.=64.06, P<0.001; Fig. 3C). Once they began expanding, chromatophores took an average of 4.97±5.1 s to expand fully (Fig. 3). There was no significant difference in the duration of chromatophore expansion between adults and hatchlings (two sample t-test, t=−1.48, d.f.=83.68, P=0.14; Fig. 3D).
Fig. 3.
Box plots of mean adult and hatchling chromatophore size before and after LACE, latency to LACE and duration to maximum chromatophore expansion. (A) The mean size of adult chromatophores at the beginning of LACE and at their maximum expansion. (B) The mean size of hatchling chromatophores at the beginning of LACE and at their maximum. (C) The mean latency to expansion of the chromatophores from the start of the white light stimulus to the beginning of LACE responses (Adult, N=2; hatchling, N=8). (D) The mean length of time from the beginning of LACE to maximum expansion of the chromatophores. (Adult, N=8; hatchling, N=8). Asterisks indicate statistically significant differences (P≤0.05).
R-opsin phototransduction cascade genes are expressed in Octopus bimaculoides skin
We searched for the molecular components of r-opsin phototransduction using degenerate PCR. Based on PCR amplification, we found opsin expressed in adult skin samples (N=5) from the dorsal mantle. These sequences are essentially identical to the r-opsin expressed in O. bimaculoides eyes, with only one confirmed nucleotide difference in skin sample 3, indicating that the opsin expressed in the skin is also an r-opsin (GenBank accession no. KR140162; see supplementary material Fig. S1).
Peripheral sensory neurons express r-opsin proteins in hatchling Octopus bimaculoides skin
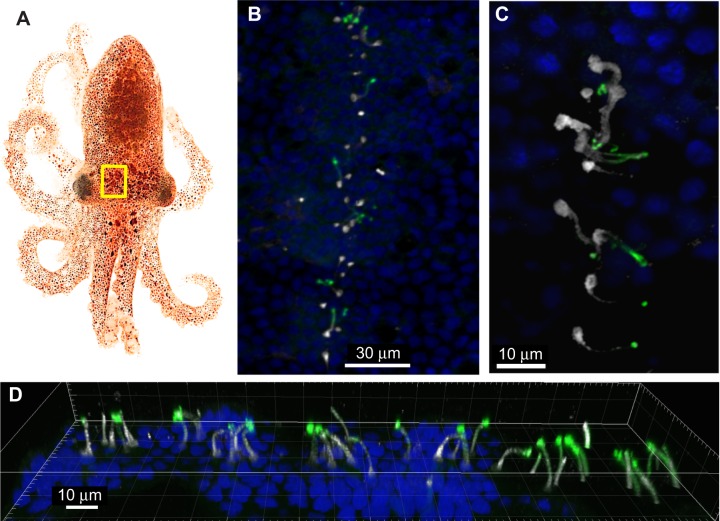
We found that α- and β-tubulin antibodies bind to many multi-ciliated peripheral sensory neurons spread over the entire epidermal surface of the mantle, head and arms. Typically, the cilia of these cells were packaged into bundles, although sometimes the individual cilia were visible. A set of these peripheral sensory neurons form four lines on the head and one on the funnel of the O. bimaculoides hatchlings (Fig. 4).
Fig. 4.
Peripheral sensory neurons in the head and siphon skin of hatchling Octopus bimaculoides express r-opsin proteins. (A) A hatchling O. bimaculoides; the yellow rectangle indicates the region enlarged in B. (B) Fluorescent confocal z-stack of one of four lines of peripheral sensory neurons on the head of a hatchling octopus. (C) Fluorescent confocal z-stack projection of peripheral sensory neurons that comprise the lines found on the head and funnel skin of hatchling octopuses. (D) 3D z-stack projection of r-opsin-expressing peripheral sensory neurons in the head and siphon skin of hatchlings. The cilia bundles attached to sensory neurons embedded in the skin of octopus hatchlings project out onto the skin surface. R-opsin proteins are expressed along the lengths of the cilia bundles and the tops of the cell bodies. Blue, cell nuclei stained with DAPI; green, α- and β-tubulin antibody labeling; white, r-opsin antibody labeling. Hatchling photo credit: Markos Alexandrou.
The octopus r-opsin antibody specifically binds to the cilia of many of the primary sensory neurons on the mantle epidermal surface. When the opsin stain is co-localized with tubulin in these cells (Fig. 4), the length of the cilia binds the opsin antibody, but the tip of each cilium appears to only bind tubulin, not opsin. In some cases, the opsin antibody also bound to the topmost portion of the cell body.
LACE action spectrum
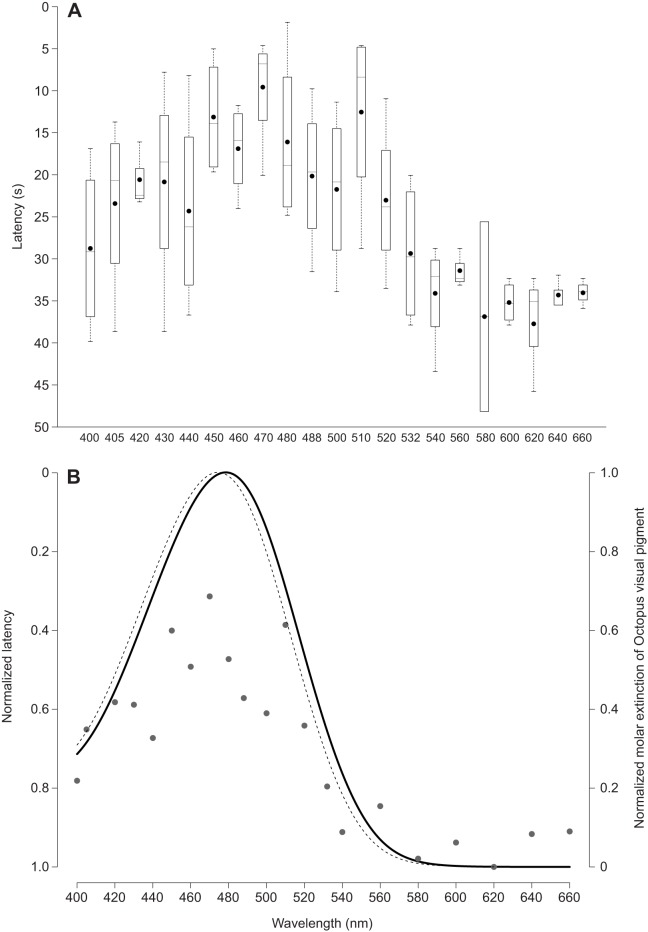
We found that LACE responses occurred more quickly (shorter latency) under blue light (470-480 nm) than other wavelengths of the visible spectrum (Fig. 5A). We estimated the λmax of the LACE response to be 480 nm when fitting to the Govardovskii opsin template (Fig. 5B). The Govardovskii calculated spectral sensitivity of opsin from O. vulgaris eyes using data mined from Brown and Brown (1958) matches what the authors reported for octopus opsin, with a λmax of 474 nm (Fig. 5B).
Fig. 5.
LACE behavior is maximally sensitive to blue light. (A) The action spectrum for Octopus bimaculoides LACE shows that the latency for LACE is shortest between 470 and 480 nm. Each box represents four data points for each wavelength, corresponding to the mean latencies across three trials of one skin sample from four adult animals. Black dots are the means at each wavelength average across four animals. (B) Govardovskii opsin models for octopus LACE latency (solid line) and data on the spectral sensitivity of Octopus vulgaris eye opsin mined from Brown and Brown (1958) (dotted line). The predicted λmax for octopus LACE is 480 nm. The Govardovskii predicted λmax for octopus eye opsin is 474 nm, the same λmax reported by Brown and Brown (1958). See supplementary material Table S2 for LACE action spectrum data.
DISCUSSION
Here, we show definitive evidence of dispersed light sensing in octopus skin and document the expression of a candidate light sensor in skin of the same species, Octopus bimaculoides. Two previous studies have speculated that cephalopod skin may be intrinsically sensitive to light, noting that chromatophores in both squid and octopus skin seem to expand when the skin is illuminated, but neither study provided more than preliminary observations (Florey, 1966; Packard and Brancato, 1993). We found that chromatophores in the skin of O. bimaculoides expand significantly and repeatedly when exposed to bright white light, a behavior we call light-activated chromatophore expansion, or LACE. We attribute LACE to light, as we minimized heat reaching the samples by using fiber optics to illuminate the skin, which itself was submerged underwater. LACE responses clearly show that O. bimaculoides skin can detect light by itself, independent of eyes.
While octopus LACE is a robust behavior, we found that some of the parameters of LACE differ from those noted by Packard and Brancato (1993). For instance, they report that chromatophores in denervated Octopus vulgaris skin expand 1 s after a flash of bright white light, which differs from the average 6 s (adults) or 15 s (hatchling) latency for LACE we found in O. bimaculoides. This incongruence in latency may be attributable to differences between the species and/or the preparation itself, as it seems that Packard and Brancato did not isolate skin samples, but denervated portions of skin still attached to the whole animal. We observed a high degree of variation in both the latency of LACE and the time to full expansion of the chromatophores in our preparations and attribute at least some of this variation to differences in the time between dissecting the tissue and running LACE experiments. We also observed differences in LACE between the hatchlings and adults, where adult skin responded more consistently and robustly than the skin from younger animals. We speculate that this could be caused by the presence of more light sensors in adult versus hatchling skin. However, despite these differences from preliminary reports, our data are the clearest demonstration to date that Octopus bimaculoides skin is intrinsically light sensitive, and that light detected by the skin causes the chromatophores to expand.
We hypothesized that r-opsin, a key light sensing protein in the eyes of octopuses and other animals, may also detect light in octopus skin and underlie LACE. To support this hypothesis, we looked for evidence of opsin expression in the skin and determined the action spectrum for LACE. Consistent with our hypothesis, we found that r-opsin is expressed in the skin of O. bimaculoides. This result is similar to Mäthger et al. (2010), who detected r-opsin mRNA from one PCR trial of skin from another cephalopod, the cuttlefish Sepia officinalis, although it is not yet known whether cuttlefish have LACE. Additionally, opsin expression by itself is weak evidence for the ability of skin to detect light. Other essential r-opsin cascade genes, including G-protein α (q) and phospholipase C, are also expressed in the skin of O. bimaculoides, suggesting that the necessary genes for functional opsin-based phototransduction are expressed in octopus skin (Speiser et al., 2014). Finally, the LACE action spectrum is also consistent with our hypothesis. Spectral sensitivity analysis of the opsin from the eyes of another octopus O. vulgaris shows a λmax of 474 nm (Brown and Brown, 1958). If the same opsin found in octopus eyes underlies octopus LACE, then LACE activity should peak close to the known spectral sensitivity of octopus opsin. Indeed, we found that the latency to LACE is shortest in blue light, and fitting the Govordovskii curve to the action spectrum data gives a λmax of 480 nm. Taken together, these data strongly support our hypothesis that opsin phototransduction underlies LACE. Future work should continue to test this hypothesis by manipulating the function of opsin phototransduction proteins and observing how they affect LACE.
Because r-opsin is known to function in light sensing, cells in octopus skin that express opsin are excellent candidates for dispersed light sensors that could underlie LACE. We identified ciliated peripheral sensory neurons in the skin of hatchling O. bimaculoides using α- and β-tubulin antibodies. These cells were similar in morphology and position (Sundermann-Meister, 1978; Sundermann, 1983; Mackie, 2008; Buresi et al., 2014) to cells described as mechanoreceptors in both squid and cuttlefish (Budelmann and Bleckmann, 1988; Bleckmann et al., 1991). It is not yet known whether these peripheral sensory neurons act as mechanoreceptors in the skin of O. bimaculoides. Intriguingly, we localized r-opsin expression to these same peripheral sensory neurons in hatchling skin, raising the possibility that aside from a mechanoreceptive function, these sensory cells may also be dispersed light receptors in octopus and other cephalopods. Unfortunately, the precise connections between candidate dispersed light sensors in octopus skin, the chromatophores and the CNS remain unclear, as does their relationships with LACE and merits further investigation to test the hypothesis that the r-opsin-expressing neurons detect light.
Our finding of opsin expressed in known mechanoreceptors raises the question of whether opsin has a role in mechanoreception, in addition to its well-established role in light detection. While our work is the first description of this opsin expression pattern in mollusks, opsin-expressing mechanoreceptors have been recently described in the annelid Platynereis, zebrafish and Drosophila (Backfisch et al., 2013; Senthilan et al., 2012). From work on mechanoreception in Drosophila antennae, we now know that opsin is required for anntenal mechanoreceptors to detect vibrations, highlighting a previously unknown role for opsin in senses besides light detection (Senthilan et al., 2012). We do not yet know whether the opsin-expressing cells we found in hatchling O. bimaculoides skin function as mechanoreceptors, light sensors or both, or the extent to which opsin is required for detecting either of these stimuli. Still, our results compel future research into the role of opsins in senses other than photoreception. We believe that the phylogenetic spread of opsin expression in mechanoreceptors among vertebrates, annelids, arthropods and now mollusks, suggests that such mechano-sensory roles for opsin could be ancient in animals.
Finally, uncovering dispersed light sensitivity in octopus skin raises the question of how it evolved to underlie LACE in octopuses. Our study is the best evidence so far for light-sensitive skin in cephalopods and we hypothesize that LACE may play a role in modulating body patterning for camouflage, alongside the canonical control exerted by the CNS. However, while cephalopods are unique among mollusks for their body-patterning abilities, we know that most other mollusks, especially bivalves, gastropods and chitons, are able to sense light with their skin. There is rich literature describing behaviors like phototaxis or shadow responses and physiology linked to light sensing in the skin of other mollusks (Ramirez et al., 2011). We do not yet know if or how cephalopods use their light-sensing skin for these other more typical molluscan behaviors. However, the widespread distribution of dispersed light sensing and associated behaviors throughout the phylum suggests that dispersed light sensitivity could be an ancestral molluscan trait that has been co-opted in the cephalopod lineage to mediate novel body-patterning behaviors in response to light. Understanding the underlying molecular mechanisms for dispersed light sensing across the mollusk classes would help clarify the evolutionary history of dispersed light sensing and associated behaviors. Our study provides a framework for future comparative work that can integrate already known behavioral data with molecular data for light-detecting components in various mollusks. This work could address the question of whether diverse mollusk behaviors that rely on dispersed light sensing share a common molecular mechanism for light detection, and thus whether dispersed light sensing was present in ancestral mollusks.
MATERIALS AND METHODS
Sample collection
We obtained 11 adult Octopus bimaculoides Pickford and McConnaughey, 1949 from marine collectors at the University of California, Santa Barbara from 2010–2014. We housed the animals in flow-through tanks supplied with filtered seawater. Our hatchling octopuses came from a clutch laid by a captive female in the winter of 2013, and the animals hatched during the following summer. Octopus bimaculoides hatch as fully developed octopuses that are immediately able to hunt and change body patterning. The hatchlings we used for these experiments were between 0 and 4 months old. To kill animals, we first anesthetized them in a seawater solution containing 5% ethanol and 7.5% isotonic MgCl2 until the chromatophores no longer responded to gentle poking and ventilation slowed, followed by quick decerebration (Moltschaniwskyj et al., 2007; Andrews et al., 2013).
LACE behavior under white light
We used insect pins to mount dissected funnels from adult and hatchling octopus (N=10) to Sylgard-lined Petri dishes filled with fresh seawater. To record the activity of the chromatophores on these isolated funnels, we used an infrared CCD camera (LCL902HS, Watec, Newburgh, NY, USA) mounted on a dissecting microscope. We measured the absolute irradiance for our light sources using a spectrophotometer (Jaz, OceanOptics, Dunedin, FL, USA) placed at an equivalent distance from the light source as experienced by the skin samples. We recorded under red LEDs (max intensity: 636 nm, full width at half maximum: 16 nm, absolute irradiance: 1.36×1014 photon cm−2 s−1), which did not stimulate LACE behavior. We allowed skin samples to dark adapt under red light for at least 2 min between trials. The white light stimulus was provided by a fiber optic light source set to maximum brightness (peak intensity: 681 nm, full width at half maximum: 150 nm, absolute irradiance: 2.60×1015 photon cm−2 s−1). The light stimulus lasted until the chromatophores reached maximum expansion or 2 min, whichever was shortest.
We measured multiple aspects of LACE, including the latency of the beginning of LACE from the onset of the stimulus and the time to maximum chromatophore expansion from the video recordings. We also captured individual still images from the video at the beginning of LACE and at the time of maximum chromatophore expansion to measure the change in chromatophore size. These images were processed by thresholding and analyzing particles in FIJI (Schindelin et al., 2012), which allowed us to count the number of pixels of the chromatophore before and after LACE. We performed multiple light trials on each sample, but because the chromatophores do not behave independently, we only measured one randomly selected chromatophore per trial, and averaged the chromatophore area pixel count within each of the 10 samples to get the mean chromatophore size before and after LACE. For all statistical tests, we assumed that similar mechanisms underlie LACE in both adult and hatchling octopuses, but because we found significant differences in the specific values for latency and chromatophore change after LACE, we analyzed the adults and hatchlings separately. To test the hypothesis that exposure to light causes an increase in the size of the chromatophores, we used a binomial sign test (N=10, P=0.5). Because we wanted to compare LACE-dependent changes in chromatophore size between individual animals which also varied in size, we report the log-ratio of the mean area pixel count before and after LACE for each sample. To test whether the change in mean chromatophore size after exposure to light is significant, we used a one-sample t-test of the log-ratio change in chromatophore size after LACE.
Identifying opsin phototransduction cascade gene expression
For long-term storage of dissected skin and eyes prior to RNA extraction, we placed samples in RNAlater (Life Technologies, Carlsbad, CA, USA) and stored them at −20°C. We extracted mRNA from adult eyes and dorsal mantle skin samples stored in RNAlater using the Nucleospin RNA XS kit (Qiagen, Valencia, CA, USA), following the manufacturer's protocol. To make a single-stranded cDNA library for each sample, we used the Superscript II RT reaction kit following the manufacturer's protocol. We stored all cDNA libraries at −20°C, and diluted them (1:200) before using them as PCR templates.
We created species-specific PCR primers using the coding sequence for O. bimaculoides eye opsin found in Genbank (accession no. AY545172.1). The forward primer sequence was: GCGGCATCAAGAAAATGTCC; and the reverse primer sequence was: TGCAAGAAGAGCGATGATGG. These primers amplify an approximate 340 bp region of the opsin cDNA. The PCR thermocycler program was as follows, repeated for 40 cycles: denaturation, 94°C for 15 s; annealing, 55°C for 30 s; extension, 72°C for 120 s. After 40 cycles, there was a 7 min hold at 72°C for final elongation. To sequence the PCR products, we cloned them into TOP 10 cells (Invitrogen, Carlsbad, CA, USA), extracted the product and sent to UC Berkeley for Sanger sequencing. We used MUSCLE (Edgar and Sjolander, 2004) in Seaview (Gouy et al., 2010) to align the sequences. We amplified and sequenced opsin products from the eyes (n=2) and dorsal mantle skin (n=5) of adult animals.
Antibody staining
We fixed samples for antibody staining in 4% formaldehyde in phosphate buffered saline (PBS) overnight at room temperature. We washed them with PBS, dehydrated them step-wise into 100% methanol and stored them at −20°C. We rehydrated formaldehyde-fixed samples into 100% PBS, and dissected as necessary. The samples were then blocked (4% donkey serum, 10% bovine serum albumin in PBS/0.1% Tween-20) for at least 1 h. Next, we incubated the samples in primary antibody solution (1:2000 μl antibody in blocking solution) using anti-octopus rhodopsin (LSL- LB-5509, Cosmo Bio USA, Carlsbad, CA, USA) and α- and β-tubulin as neural markers (α-tubulin: T7451, Sigma-Aldrich, St Louis, MO, USA; β-tubulin: E7, Developmental Studies Hybridoma Bank, Iowa City, IA, USA) for 4 h at room temperature or 24 h at 4°C. We then washed in PBS three times for 5 min, and transferred them to the secondary antibody solution (1:250 goat anti-mouse Cy3: A10521; goat anti-rabbit Cy5: A10523, Life Technologies, Carlsbad, CA, USA) to stain for 2 h at room temperature. Samples were then washed in 100% PBS twice for 5 min each, then transferred to PBS containing DAPI (0.5:1000 μl) for at least 10 min before two more washes in 100% PBS. Samples were mounted in glycerol and visualized using a confocal microscope (Fluoview 1000 Spectral Confocal, Olympus America Inc., Center Valley, PA, USA).
Creating the LACE latency action spectrum
To generate an action spectrum, we collected 6-mm-diameter skin punches from the distal surface of adult O. bimaculoides funnels (N=4). See supplementary material Fig. S2 for our experimental setup. We used the same white light source, red LED light, video camera, digital converter and computer software as described for the initial LACE trials. Briefly, each skin sample was visualized using a dissecting scope and infrared CCD camera. We lit the samples with narrow-bandwidth light through a 1 mm fiber optic cable that we positioned just above the surface of the water with a micromanipulator, such that it illuminated the area of skin captured by the video camera. The fiber optic cable was not moved from this position during a trial, although the exact position differed slightly between trials and samples. On the other side of an opaque partition, we used a white scope light (LG-PS2, Olympus America Inc., Center Valley, PA, USA) as the initial light source and lenses (Qioptic, Fairport, NY, USA) directed white light through one of two color filter wheels (Thorlabs, Newton, New Jersey, USA) before the filtered light was directed into the fiber optic cable. Each filter wheel contained 11 colored filters. Together the filters spanned the visible spectrum from 375 to 660 nm (375, 400, 405, 420, 430, 440, 450, 460, 470, 480, 488, 500, 510, 520, 532, 540, 560, 580, 600, 620, 640 and 660 nm).
To ensure that each skin sample within a trial was exposed to similar photon counts for each wavelength of light, we measured the photon counts for each filter at maximum intensity of the white light source. Within the range of the spectrum we expected to find the peak sensitivity of the LACE behavior (between 440 and 660 nm), we adjusted the power of the light source such that photon counts for each filter were equivalent to the max photon count at the wavelength with the least power in the spectrum. For our particular white light source, we calibrated the photon counts for filters between 440 and 660 nm to the photon count at 510 nm. To do this, we used a spectrophotometer (Jaz, Ocean Optics, Dunedin, FL, USA) to measure the absolute irradiance (in photons cm−2 s−1) in the position of the sample, and another spectrophotometer (SM700, Milwaukee Instruments Inc., Rocky Mount, NC, USA) to monitor the overall output of the white light coming from the back of the scope light. For each colored filter, we adjusted the power of the light source until the photon counts matched those measured at 510 nm, and recorded the output of the monitor white light.
During the experimental trials, we used these lux values to adjust the power of the white light to standardize photon counts that the skin received. Our white light source was less powerful for wavelengths under 440 nm, and so photon counts for 375–430 nm consistently fell below the 510 nm count standard. For these wavelengths, we used the maximum power allowed by the scope light to maximize the photon counts for the filters. Receiving fewer photons potentially increased the latency of the LACE response at these shorter wavelengths, but in doing so we maximized the likelihood of LACE occurring at the wavelengths surrounding our predicted λmax, based on previous reports of λmax of opsin from the eyes of Octopus vulgaris (Brown and Brown, 1958).
To induce LACE, we illuminated the samples with light through each filter for 45 s, then allowed them to sit under red light for 90 s as a dark adapt period before trying another wavelength. We recorded the activity of the chromatophores continuously within a trial, and performed three trials for all but sample 3, which only had two trials for technical reasons. Each trial consisted of 22 light stimuli, presented to the skin in randomized order. From the video we recorded the start time of the light stimulus and the time when a LACE event began (marked by first noticeable expansion of the chromatophores) after the start of the stimulus. This duration is the latency of LACE for that particular wavelength. We excluded LACE data at 375 nm from our analyses because we were unable to measure the photon count at 375 nm.
Quantifying the spectral sensitivity of the light-sensing protein
We estimated the λmax for the octopus LACE action spectrum using the Govardovskii model to fit an opsin specific template (Govardovskii et al., 2000) to the data by minimizing the sums of squares using the optimize function in R (R Core Team, 2013). We extracted data on the spectral sensitivity of O. vulgaris eye opsin using Data Thief (Tummers, 2006) from Brown and Brown (1958).
Supplementary Material
Acknowledgements
We would like to thank Christine Castillo and James Peniston for their assistance with video analysis and Gordon Fain for use of the CCD camera. We would also like to thank Mary Raven and the NRI-MCDB Microscopy Facility at UCSB for assistance with confocal microscopy. Finally, we thank Dan Speiser for critiques and comments on this manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
M.D.R. and T.H.O. designed the study and wrote the paper. M.D.R. performed the experiments, imaging and statistical analysis. T.H.O. created the R code for the Govardovskii opsin curve.
Funding
The confocal microscope was funded by a National Institutes of Health Shared Instrumentation grant [1 S10 OD010610-01A1] to Mary Raven at the NRI Microscopy Facility at UCSB. This project was funded by National Science Foundation grants to THO [IOS-1045257 and DEB-1146337]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.110908/-/DC1
References
- Andrews P. L. R., Darmaillacq A.-S., Dennison N., Gleadall I. G., Hawkins P., Messenger J. B., Osorio D., Smith V. J. and Smith J. A. (2013). The identification and management of pain, suffering and distress in cephalopods, including anaesthesia, analgesia and humane killing. J. Exp. Mar. Biol. Ecol. 447, 46-64. 10.1016/j.jembe.2013.02.010 [DOI] [Google Scholar]
- Backfisch B., Veedin Rajan V. B., Fischer R. M., Lohs C., Arboleda E., Tessmar-Raible K. and Raible F. (2013). Stable transgenesis in the marine annelid Platynereis dumerilii sheds new light on photoreceptor evolution. Proc. Natl. Acad. Sci. USA 110, 193-198. 10.1073/pnas.1209657109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellono N. W., Kammel L. G., Zimmerman A. L. and Oancea E. (2013). UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc. Natl. Acad. Sci. USA 110, 2383-2388. 10.1073/pnas.1215555110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckmann H., Budelmann B. U. and Bullock T. H. (1991). Peripheral and central nervous responses evoked by small water movements in a cephalopod. J. Comp. Physiol. A 168, 247-257. 10.1007/BF00218417 [DOI] [PubMed] [Google Scholar]
- Boycott B. B. (1961). The functional organization of the brain of the cuttlefish Sepia officinalis. Proc. R. Soc. B Biol. Sci. 153, 503-534. 10.1098/rspb.1961.0015 [DOI] [Google Scholar]
- Brown P. K. and Brown P. S. (1958). Visual pigments of the octopus and cuttlefish. Nature 182, 1288-1290. 10.1038/1821288a0 [DOI] [PubMed] [Google Scholar]
- Budelmann B. U. and Bleckmann H. (1988). A lateral line analogue in cephalopods: water waves generate microphonic potentials in the epidermal head lines of Sepia and Lolliguncula. J. Comp. Physiol. A 164, 1-5. 10.1007/BF00612711 [DOI] [PubMed] [Google Scholar]
- Buresch K. C., Ulmer K. M., Akkaynak D., Allen J. J., Mäthger L. M., Nakamura M. and Hanlon R. T. (2015). Cuttlefish adjust body pattern intensity with respect to substrate intensity to aid camouflage, but do not camouflage in extremely low light. J. Exp. Mar. Biol. Ecol. 462, 121-126. 10.1016/j.jembe.2014.10.017 [DOI] [Google Scholar]
- Buresi A., Croll R. P., Tiozzo S., Bonnaud L. and Baratte S. (2014). Emergence of sensory structures in the developing epidermis in Sepia officinalis and other coleoid cephalopods. J. Comp. Neurol. 522, 3004-3019. 10.1002/cne.23562 [DOI] [PubMed] [Google Scholar]
- Chiao C. C. and Hanlon R. T. (2001). Cuttlefish camouflage: visual perception of size, contrast and number of white squares on artificial checkerboard substrata initiates disruptive coloration. J. Exp. Biol. 204, 2119-2125. [DOI] [PubMed] [Google Scholar]
- Cloney R. A. and Florey E. (1968). Ultrastructure of cephalopod chromatophore organs. Z. Zellforsch. Mikrosk. Anat. 89, 250-280. 10.1007/BF00347297 [DOI] [PubMed] [Google Scholar]
- Dubas F., Hanlon R. T., Ferguson G. P. and Pinsker H. M. (1986). Localization and stimulation of chromatophore motoneurones in the brain of the squid, Lolliguncula brevis. J. Exp. Biol. 121, 1-25. [DOI] [PubMed] [Google Scholar]
- Edgar R. C. and Sjolander K. (2004). A comparison of scoring functions for protein sequence profile alignment. Bioinformatics 20, 1301-1308. 10.1093/bioinformatics/bth090 [DOI] [PubMed] [Google Scholar]
- Feuda R., Hamilton S. C., McInerney J. O. and Pisani D. (2012). Metazoan opsin evolution reveals a simple route to animal vision. Proc. Natl. Acad. Sci. USA 109, 18868-18872. 10.1073/pnas.1204609109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florey E. (1966). Nervous control and spontaneous activity of the chromatophores of a cephalopod, Loligo opalescens. Comp. Biochem. Physiol. 18, 305-324. 10.1016/0010-406X(66)90189-7 [DOI] [PubMed] [Google Scholar]
- Fulgione D., Trapanese M., Maselli V., Rippa D., Itri F., Avallone B., Van Damme R., Monti D. M. and Raia P. (2014). Seeing through the skin: dermal light sensitivity provides cryptism in moorish gecko. J. Zool. 294, 122-128. 10.1111/jzo.12159 [DOI] [Google Scholar]
- Gouy M., Guindon S. and Gascuel O. (2010). SeaView Version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221-224. 10.1093/molbev/msp259 [DOI] [PubMed] [Google Scholar]
- Govardovskii V. I., Fyhrquist N., Reuter T., Kuzmin D. G. and Donner K. (2000). In search of the visual pigment template. Vis. Neurosci. 17, 509-528. 10.1017/S0952523800174036 [DOI] [PubMed] [Google Scholar]
- Hanlon R. T. and Messenger J. B. (1988). Adaptive coloration in young cuttlefish (Sepia officinalis L.): the morphology and development of body patterns and their relation to behaviour. Philos. Trans. R. Soc. Lond. B Biol. Sci. 320, 437-487. 10.1098/rstb.1988.0087 [DOI] [Google Scholar]
- Holmes W. (1940). The colour changes and colour patterns of Sepia officinalis L. Proc. Zool. Soc. Lond. A110, 17-35. 10.1111/j.1469-7998.1940.tb08457.x [DOI] [Google Scholar]
- Mackie G. O. (2008). Immunostaining of peripheral nerves and other tissues in whole mount preparations from hatchling cephalopods. Tissue Cell 40, 21-29. 10.1016/j.tice.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Mäthger L. M., Roberts S. B. and Hanlon R. T. (2010). Evidence for distributed light sensing in the skin of cuttlefish, Sepia officinalis. Biol. Lett. 6, 600-603. 10.1098/rsbl.2010.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger J. B. (1967). The peduncle lobe: a visuo-motor centre in octopus. Proc. R. Soc. Lond. B Biol. Sci. 167, 225-251. 10.1098/rspb.1967.0025 [DOI] [PubMed] [Google Scholar]
- Messenger J. B. (1979). Eyes and skin of octopus: compensating for sensory deficiencies. Endeavour 3, 92-98. 10.1016/0160-9327(79)90096-6 [DOI] [Google Scholar]
- Messenger J. B. (2001). Cephalopod chromatophores: neurobiology and natural history. Biol. Rev. Camb. Philos. Soc. 76, 473-528. 10.1017/S1464793101005772 [DOI] [PubMed] [Google Scholar]
- Moltschaniwskyj N. A., Hall K., Lipinski M. R., Marian J. E. A. R., Nishiguchi M., Sakai M., Shulman D. J., Sinclair B., Sinn D. L., Staudinger M. et al. (2007). Ethical and welfare considerations when using cephalopods as experimental animals. Rev. Fish Biol. Fisheries 17, 455-476. 10.1007/s11160-007-9056-8 [DOI] [Google Scholar]
- Packard A. and Brancato D. (1993). Some responses of Octopus chromatophores to light. J. Physiol. 459, 429P. [Google Scholar]
- Packard A. and Sanders G. D. (1971). Body patterns of Octopus vulgaris and maturation of the response to disturbance. Anim. Behav. 19, 780-790. 10.1016/S0003-3472(71)80181-1 [DOI] [Google Scholar]
- Plachetzki D. C., Fong C. R. and Oakley T. H. (2012). Cnidocyte discharge is regulated by light and opsin-mediated phototransduction. BMC Biol. 10, 17 10.1186/1741-7007-10-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M. L., Blasic J. R., Bok M. J., Cameron E. G., Pringle T., Cronin T. W. and Robinson P. R. (2012). Shedding new light on opsin evolution. Proc. R. Soc. Lond. B Biol. Sci. 279, 3-14. 10.1098/rspb.2011.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; Available at: http://www.R-project.org/. [Google Scholar]
- Raible F., Tessmar-Raible K., Arboleda E., Kaller T., Bork P., Arendt D. and Arnone M. I. (2006). Opsins and clusters of sensory G-protein-coupled receptors in the sea urchin genome. Dev. Biol. 300, 461-475. 10.1016/j.ydbio.2006.08.070 [DOI] [PubMed] [Google Scholar]
- Ramirez M. D., Speiser D. I., Pankey M. S. and Oakley T. H. (2011). Understanding the dermal light sense in the context of integrative photoreceptor cell biology. Vis. Neurosci. 28, 265-279. 10.1017/S0952523811000150 [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilan P. R., Piepenbrock D., Ovezmyradov G., Nadrowski B., Bechstedt S., Pauls S., Winkler M., Möbius W., Howard J. and Göpfert M. C. (2012). Drosophila auditory organ genes and genetic hearing defects. Cell 150, 1042-1054. 10.1016/j.cell.2012.06.043 [DOI] [PubMed] [Google Scholar]
- Speiser D. I., Pankey M. S., Zaharoff A. K., Battelle B. A., Bracken-Grissom H. D., Breinholt J. W., Bybee S. M., Cronin T. W., Garm A., Lindgren A. R. et al. (2014). Using phylogenetically-informed annotation (PIA) to search for light-interacting genes in transcriptomes from non-model organisms. BMC Bioinformatics 15, 350 10.1186/s12859-014-0350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann G. (1983). The fine structure of epidermal lines on arms and head of postembryonic Sepia officinalis and Loligo vulgaris (Mollusca, Cephalopoda). Cell Tissue Res. 232, 669-677. 10.1007/BF00216437 [DOI] [PubMed] [Google Scholar]
- Sundermann-Meister G. (1978). Ein neuer Typ von Cilienzellen in der Haut von spätembryonalen und juvenilen Loligo vulgaris (Mollusca, Cephalopoda). Zool. Jb. Anat. 99, 493-499. [Google Scholar]
- Tummers B. (2006). DataThief III. http://datathief.org/.
- Young J. Z. (1971). The Anatomy of the Nervous System of Octopus Vulgaris. Oxford, UK: Clarendon Press. [Google Scholar]
- Young J. Z. (1974). The central nervous system of Loligo I. The optic lobe. Philos. Trans. R. Soc. Lond. B Biol. Sci. 267, 263-302. 10.1098/rstb.1974.0002 [DOI] [PubMed] [Google Scholar]
- Young J. Z. (1976). The nervous system of Loligo. II. Suboesophageal centres. Philos. Trans. R. Soc. Lond. B Biol. Sci. 274, 101-167. 10.1098/rstb.1976.0041 [DOI] [PubMed] [Google Scholar]
- Zylinski S., Osorio D. and Shohet A. J. (2009). Cuttlefish camouflage: context-dependent body pattern use during motion. Proc. R. Soc. B Biol. Sci. 276, 3963-3969. 10.1098/rspb.2009.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.