Abstract
PURPOSE
To determine whether the Physical Activity and Lymphedema (PAL) trial weight training program for breast cancer survivors at risk of or with breast cancer related lymphedema provided skeletal benefits.
METHODS
Of the 295 participants in the randomized controlled PAL trial, 258 (weight training; N=128; control: N=130) had complete measures of bone mineral density (BMD (g/cm2) of the proximal femur and lumbar spine and were also categorized by T-scores. Women in the weight training group performed slowly progressive weight training 2 days per week for 12 months compared to women in the control group who maintained their usual physical activities.
RESULTS
There were no significant differences in the rate of BMD change at any skeletal site between weight training and control groups, regardless of menopausal status. Distribution of bone health categories was not significantly different between groups at baseline, but became different at 12 months (p<0.03) among postmenopausal women due to an increase in the percentage of controls who became osteopenic (35% to 44%) compared to stable bone health in weight-lifters.
CONCLUSIONS
The PAL weight training program that increased muscle strength without exacerbating or causing lymphedema among breast cancer survivors was not as efficacious at improving skeletal health. The skeletal loads produced from the PAL program may be insufficient to notably shift BMD, but may have a subtle osteogenic effect.
IMPLICATIONS FOR CANCER SURVIVORS
The safety and efficacy of rigorous weight training programs for improving skeletal health in women at risk for or with breast cancer related lymphedema remains to be determined.
Keywords: Resistance exercise, osteoporosis, fractures, neoplasms, survivorship
INTRODUCTION
Breast cancer survivors are at an increased risk of fractures and poor physical functioning compared to other women who have not been treated for cancer [1]. The risk of hip fracture increases by 55% following a diagnosis of breast cancer (HR = 1.55, CI; 1.13–2.11) [1], while breast cancer survivors are 37% more likely to report a limitation in their physical functioning compared to other women (OR for any functional limitation: 1.37, 95% CI = 1.14, 1.65) [2]. Losses of bone and muscle mass that result directly from the catabolic effects of treatment and indirectly from declines in physical activity likely underpin breast cancer survivors’ elevated risk of fracture and functional limitations [3–7]. Adjuvant chemotherapy has been associated with small, but clinically relevant loss of bone mineral density [8,9] and lean body mass [9,10]. When chemotherapy also results in premature ovarian failure from chemotherapy and/or when women are prescribed an aromatase inhibitor as adjuvant endocrine therapy, bone loss is further accelerated [4,8,12–14]. Breast cancer survivors who become or remain inactive post-treatment may experience additional musculoskeletal declines related to disuse.
Exercise training is a recommended lifestyle approach to preserve musculoskeletal mass and function in adult women and is now being explored as a strategy to reverse bone and muscle loss and functional declines related to cancer treatment [15–20]. Resistance exercise produces an ideal musculoskeletal stimulus for reversing bone and muscle loss associated with cancer treatment. However, concerns over the safety of resistance training for women with or at risk for breast cancer-related lymphedema requires attention to both safety and efficacy in the design of resistance training trials that aim to improve multiple outcomes in women with breast cancer. The Physical Activity and Lymphedema study was a randomized trial to determine whether resistance training (i.e., weight training) was safe for breast cancer survivors with or at risk of lymphedema [21,22]. Following one year of slowly progressive weight training, women with stable lymphedema at baseline had reduced lymphedema symptoms and 50% fewer lymphedema exacerbations than control women [22]. Among women with no lymphedema at baseline, the incidence rates of lymphedema during the trial was similar among weight training and control groups [21]. Among the subset of women with no lymphedema at baseline who had five or more lymph nodes removed, incidence rates of lymphedema were reduced by 70% within the weight training compared to the control group. Women in the weight training group improved their muscle strength and body image significantly more than control women, regardless of baseline lymphedema status [23].
The PAL trial also included secondary measures of body composition by DXA to evaluate the effect of weight training on fat and lean mass. Among women at-risk for breast cancer related lymphedema, weight training significantly reduced the percent of body fat compared to controls [21], but had no similar effect among women with breast cancer related lymphedema [22]. In addition to the assessment of body composition from whole body DXA scans, regional scans of the proximal femur and lumbar spine were conducted to explore any potential benefit of weight training in the PAL trial on bone mineral density (BMD) at these clinically relevant skeletal sites. The purpose of this study is to evaluate whether the PAL program that has shown safety and efficacy for improving lymphedema outcomes, body image, muscle strength and body composition, could also improve hip and spine BMD – a clinically relevant outcome for breast cancer survivors.
METHODS
Design
The PAL trial was a randomized controlled trial to assess the safety and efficacy of twice-weekly progressive moderate-intensity weight training on lymphedema outcomes in breast cancer survivors at risk for breast cancer-related lymphedema, or with stable breast cancer-related lymphedema, compared to standard care [24]. Details and primary outcomes of this study are published elsewhere [21,22]. The purpose of this analysis is to assess the effect of this progressive weight training intervention on secondary outcomes relevant to bone health.
Study participants
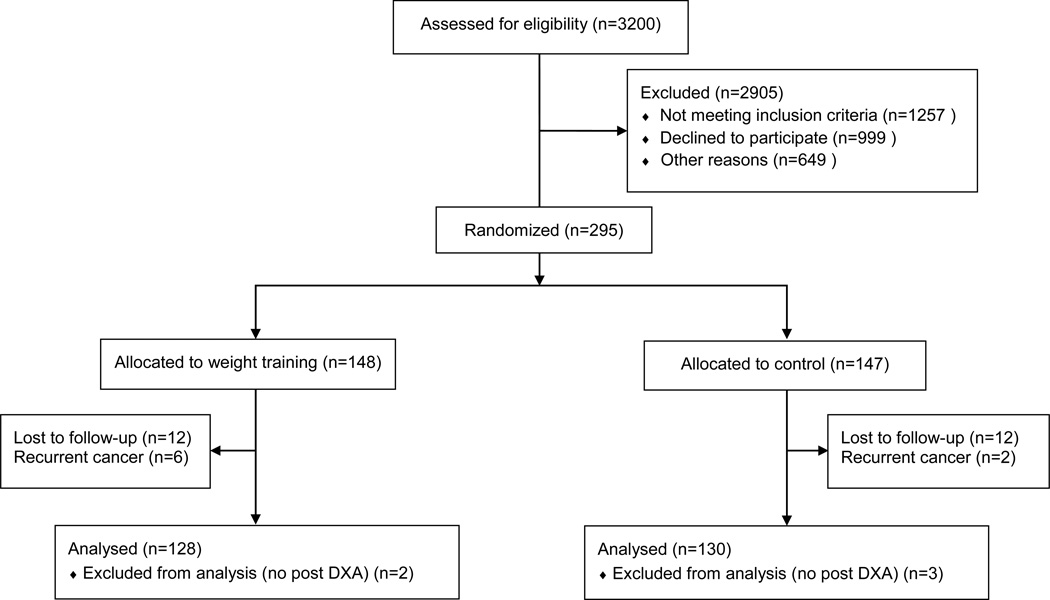
This analysis was conducted from a dataset of 295 breast cancer survivors who participated in the PAL trial (Fig 1). The inclusion and exclusion criteria have been published [24]. Participants were female breast cancer survivors at risk for (n=154) [21] or with stable (n=141) [22] breast cancer-related lymphedema. Additional eligibility criteria included: (1) absence of active disease; (2) minimum of one lymph node removed; (3) no medical conditions or contraindicated medications that would prohibit participation in an exercise program; (4) BMI ≤50 kg/m2; (5) no plans for surgery during the intervention period; (6) no weight training during the previous year; and (7) stable weight and not currently attempting to lose weight. Minimization computer software was used to randomize participants to the intervention or control group. The study protocol was approved by the University of Pennsylvania Institutional Review Board and written consent was obtained from all participants.
Fig 1.
Flow of participants in the PAL trial and availability of DXA data for analysis
Measures
Study measures were completed at baseline and 12 months post-randomization. Demographic characteristics, treatment history and menstrual status were collected through self-report. Cancer staging and number of lymph nodes removed were obtained from surgical pathology reports, state cancer registries, and/or self-report.
Bone health
Whole body and regional (proximal femur (greater trochanter, femoral neck and total hip) and lumbar spine (L1–L4) bone mineral density (BMD, g/cm2) was assessed by dual energy x-ray absorptiometry (DXA; Hologic Discovery, software version 12.4). All scans were performed by the same technician and subsequently analyzed by a different, single technician according to manufacturer protocols. In addition to BMD, we reported bone health as categories based on T-scores according to World Health Organization guidelines [25].
Anthropometry
Height and body weight were assessed with participants in hospital gowns without shoes and at full inhalation using a scale-mounted stadiometer and calibrated digital scale.
Physical activity and dietary intake
Physical activity and dietary intake were measured by self-report. Physical activity outside of the prescribed exercise session was assessed using the long-form self-administered International Physical Activity Questionnaire [26]. Habitual food and nutrient intake was assessed using the Diet History Questionnaire that asks questions about the type and amount of foods consumed in the past 12 months [27]. Of interest for this analysis were total energy intake (kcal/d) and calcium intake (mg/d) so that we could evaluate each as potential confounders of intervention effects.
Intervention
Independent of group assignment, all participants were required to attend a 1-hour educational lecture (lymphedema education session) based on material from the National lymphedema Network [28] and led by the study investigator (K.H.S.). The study investigator (K.H.S.) also facilitated a 3-day training session for the certified fitness trainers who delivered the intervention in local YMCAs. Participants attended supervised, small group (N=2–6) strength training sessions during the first 3 months, and then continued to strength train unsupervised for the remaining 9 months. The study team maintained contact with participants via telephone or email during the unsupervised portion. Exercise sessions were 60–90 minutes including a 5–10 minute aerobic warm-up, stretching (beginning and end of session), and core training (for stability). Details of the complete protocol have been published [24]. Briefly, weight training exercises were performed using variable resistance machines and free weights. The prescribed weight training exercises included the bicep curl, triceps pushdown or kickback, lateral or front shoulder raise, seated row or one-arm row, chest press, back extension, and seated leg press, extension and curl. Participants started with no weight or one-pound weights for each upper body exercise. The weight was increased by one-half to one-pound increments per week as long as there were no changes in lymphedema-related symptoms. Participants increased resistance for upper body exercises in the smallest possible increment when a participant lifted the same weight 10–12 times across the 3 sets for 4 consecutive weeks. For each lower body exercise participants lifted a weight they could move 8–10 times, building up to three sets per exercise. There was no upper limit on the maximum weight lifted over 1 year.
Statistical analyses
Descriptive statistics reported for baseline variables include rates for categorical variables and means and standard deviations for continuous variables. Univariate analysis included t-tests for continuous variables and Chi-square test for categorical variables. Mixed models were utilized to estimate the change in BMD using a group by time interaction term. All mixed models controlled for potential confounding by demographic and clinical variables. Model fit was assessed using the Akaike and Bayesian information criterion methods. Sensitivity analysis was conducted using generalized estimating equations with exchangeable and autoregressive correlation matrices; the study findings and conclusions did not differ among methods. Two-sided statistical significance was P < 0.05. All statistical analyses were performed with Stata SE 12.0 software (College Station, TX).
To determine whether the available sample was sufficient to detect significant group × time differences in BMD we used data from a similar controlled trial in breast cancer survivors [16]. A sample size of n=25 would provide power >.90 to detect a 2% difference in BMD change over 12 months between groups with α<0.05.
RESULTS
Among 295 breast cancer survivors in the PAL trial, those who had both a pre and post DXA scan (n=258; weight training; N=128; control: N=130) were included in this ancillary analysis (Table 1). On average, participants were in their mid-fifties, overweight, mostly postmenopausal and most likely to have had stage I or III breast cancer. Baseline bone health and dietary intake did not significantly differ between the weight training and control groups. On average, women had normal skeletal health (T score ≥ −1.0) but reported calcium intakes that were 40% below the current RDI for calcium (1200 mg/d) (Table 2).
Table 1.
Baseline Characteristics of Study Participants for Ancillary Analysis (n=258)
| Characteristic | Total Sample | Weightlifting (n=128) | Control (n=130) |
|---|---|---|---|
| Age — yr | 56.6±8.5 | 56.0±8.3 | 57.2±8.8 |
| Race — no. (%) | |||
| White | 170 (66%) | 79 (62%) | 91 (70%) |
| Black | 79 (31%) | 41 (32%) | 38 (29%) |
| Other | 9 (34%) | 8 (6%) | 1 (1%) |
| Occupation — no. (%) | |||
| Professional | 105 (41%) | 53 (41%) | 52 (40%) |
| Clerical or service | 42 (16%) | 23 (18%) | 19 (15%) |
| Homemaker, student, or unemployed | 23 (9%) | 12 (9%) | 11 (8%) |
| Other or unknown | 24 (9%) | 15 (12%) | 9 (7%) |
| Retired | 64 (25%) | 25 (20%) | 39 (30%) |
| BMI — kg/m2 | 28.9±6.0 | 28.6±6.4 | 29.2±6.4 |
| Stage — no. (%) | |||
| I | 115 (45%) | 61 (48%) | 54 (42%) |
| II | 3 (1%) | 3 (2%) | 0 (0%) |
| III | 82 (32%) | 39 (30%) | 43 (33%) |
| Data not available | 58 (22%) | 25 (20%) | 33 (25%) |
| Time since diagnosis — mo. | 62.0±39.6 | 58.1±37.6 | 65.9±41.3 |
| Treatment(s) — no. (%) | |||
| Chemotherapy | 193 (75%) | 98 (77%) | 95 (73%) |
| Radiation | 201 (78%) | 105 (82%) | 96 (74%) |
| Tamoxifen | 41 (16%) | 25 (19%) | 16 (12%) |
| Aromatase Inhibitor | 2 (1%) | 1 (<1%) | 1 (<1%) |
| Bisphosphonate use | 45 (17%) | 20 (16%) | 25 (19%) |
| Menstrual Status — no. (%) | |||
| Premenopausal | 33 (13%) | 17 (13%) | 16 (12%) |
| Postmenopausal | 225 (87%) | 111 (87%) | 114 (88%) |
Table 2.
Baseline and 12 month values of bone mineral density and diet and physical activity habits in weight training and control groups.
| Baseline | 12-months | |||
|---|---|---|---|---|
| Characteristic | Mean±SD | p-value | Mean±SD | p-value |
| Lumbar spine BMD | 0.92 | 0.89 | ||
| Weight training | 0.996±0.126 | 0.989±0.128 | ||
| Control | 0.998±0.152 | 0.992±0.152 | ||
| Total hip BMD | 0.31 | 0.33 | ||
| Weight training | 0.913±0.120 | 0.905±0.125 | ||
| Control | 0.897±0.132 | 0.889±0.132 | ||
| Greater trochanter BMD | 0.35 | 0.30 | ||
| Weight training | 0.678±0.098 | 0.673±0.105 | ||
| Control | 0.666±0.104 | 0.660±0.105 | ||
| Femoral neck BMD | 0.40 | 0.55 | ||
| Weight training | 0.772±0.117 | 0.756±0.121 | ||
| Control | 0.760±0.117 | 0.747±0.111 | ||
| Physical activity (MET-min/wk.) | 0.96 | 0.65 | ||
| Weight training | 3505±3180 | 3823±3297 | ||
| Control | 3485±3871 | 3591±4508 | ||
| Daily energy intake (kcal) | 0.85 | 0.96 | ||
| Weight training | 1713±1232 | 1510±642 | ||
| Control | 1683±1373 | 1505±532 | ||
| Calcium intake (mg/day−1)a | 0.85 | 0.49 | ||
| Weight training | 750±456 | 712±392 | ||
| Control | 764±663 | 671±357 | ||
Calcium intake includes calcium obtained both from dietary sources and from dietary supplements
Adherence to weight training averaged 72% over 12 month study period, but was greater over the first half (82%) than the last half (58%) of the intervention. Reported energy and calcium intakes decreased over the intervention period, but these changes were not significantly different between groups.
BMD T-scores of the hip and spine among post and premenopausal women are shown in Table 3. The distribution of women within bone health categories (normal, osteopenic, and osteoporotic) did not differ between intervention groups at baseline (Table 3). However, at 12 months there was a significant difference between the weight training and control groups for the distribution of postmenopausal women across bone health categories (p=0.03). Eight women in the control group shifted from normal to the osteopenic category, whereas the proportion of women across bone health categories among weightlifters remained the same as baseline.
Table 3.
T-scores and distribution of bone health categories for the femoral neck and lumbar spine at baseline and 12-months in weight training and control groups. Values presented as mean ± standard deviation or n (%)
| Baseline | 12-months | |||||
|---|---|---|---|---|---|---|
| Weight training | Control | p-value | Weight training | Control | p-value | |
| Postmenopausal | ||||||
| Lumbar Spine | ||||||
| T-Score | −0.599±1.090 | −0.541±1.337 | 0.72 | −0.654±1.098 | −0.589±1.345 | 0.69 |
| Normal | 68 (60%) | 70 (63%) | 0.17 | 68 (60%) | 62 (56%) | 0.03 |
| Osteopenia | 40 (35%) | 40 (36%) | 40 (35%) | 49 (44%) | ||
| Osteoporosis | 6 (5%) | 1 (1%) | 6 (5%) | 0 (0%) | ||
| Femoral Neck | ||||||
| T-Score | −0.972±1.001 | −0.843±1.069 | 0.69 | −0.940±1.053 | −0.972±1.001 | 0.81 |
| Normal | 62 (54%) | 66 (59%) | 0.49 | 56 (49%) | 54 (49%) | 0.83 |
| Osteopenia | 48 (42%) | 39 (35%) | 52 (46%) | 49 (44%) | ||
| Osteoporosis | 4 (4%) | 6 (5%) | 6 (5%) | 8 (7%) | ||
| Premenopausal | ||||||
| Lumbar Spine | ||||||
| T-Score | −0.049±1.319 | −0.213±1.587 | 0.75 | −0.102±1.382 | −0.232±1.587 | 0.80 |
| Normal | 13 (81%) | 13 (76%) | 0.44 | 13 (81%) | 12 (71%) | 0.32 |
| Osteopenia | 2 (13%) | 4 (24%) | 2 (13%) | 5 (29%) | ||
| Osteoporosis | 1 (6%) | 0 (0%) | 1 (6%) | 0 (0%) | ||
| Femoral Neck | ||||||
| T-Score | −0.057±1.094 | −0.427±0.911 | 0.23 | −0.193±1.155 | −0.511±0.916 | 0.39 |
| Normal | 12 (75%) | 13 (76%) | 0.92 | 12 (75%) | 12 (71%) | 0.78 |
| Osteopenia | 4 (25%) | 4 (24%) | 4 (25%) | 5 (29%) | ||
| Osteoporosis | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
BMD outcomes among post and premenopausal women in the weight training and control groups were similar at baseline and after the intervention period (Tables 4 and 5). Over the 1-year intervention, BMD decreased at all skeletal sites among postmenopausal women and at the lumbar spine in premenopausal women in both intervention and control groups (Table 4). The direction of changes in BMD within hip sites among premenopausal women was more variable. None of the observed changes were significantly different between groups (p>0.05).
Table 4.
Baseline and 12 month bone mineral density values by menopausal status in weight training and control groups.
| Postmenopausal | |||||||
| Weight training (n=111) | Control (n=114) | ||||||
| Regional BMD (g/cm2) | Baseline Mean (SD) | 12 months Mean (SD) | % Change | Baseline Mean (SD) | 12 months Mean (SD) | % Change | p-valuea |
| Spine (L1–L4) | 0.987 (0.122) | 0.981 (0.123) | −0.57 | 0.993 (0.149) | 0.988 (0.149) | −0.54 | 0.80 |
| Total hip | 0.904 (0.113) | 0.895 (0.117) | −1.05 | 0.894 (0.133) | 0.884 (0.131) | −1.00 | 0.84 |
| Greater trochanter | 0.671 (0.091) | 0.665 (0.095) | −0.87 | 0.662 (0.104) | 0.655 (0.105) | −1.09 | 0.88 |
| Femoral neck | 0.761 (0.114) | 0.745 (0.117) | −2.22 | 0.755 (0.119) | 0.741 (0.111) | −1.72 | 0.40 |
| Premenopausal | |||||||
| Weight training (n=17) | Control (n=16) | ||||||
| Regional BMD (g/cm2) | Baseline Mean (SD) | 12 months Mean (SD) | % Change | Baseline Mean (SD) | 12 months Mean (SD) | % Change | p-valuea |
| Spine (L1–L4) | 1.047 (0.147) | 1.040 (0.154) | −0.69 | 1.028 (0.174) | 1.025 (0.175) | −0.19 | 0.18 |
| Total hip | 0.969 (0.156) | 0.968 (0.159) | −0.20 | 0.923 (0.124) | 0.929 (0.134) | 0.59 | 0.41 |
| Greater trochanter | 0.720 (0.133) | 0.723 (0.136) | 0.38 | 0.691 (0.102) | 0.696 (0.105) | 0.61 | 0.95 |
| Femoral neck | 0.843 (0.131) | 0.827 (0.128) | −1.89 | 0.795 (0.101) | 0.792 (0.102) | −0.34 | 0.17 |
p-value from mixed model for the time by group interaction that included baseline and 12-month time points and controlled for the following: age, time since diagnosis, current adjuvant hormone therapy use, baseline calcium intake, and current bisphosphonate use. p-values are observed and not corrected for multiple testing.
DISCUSSION
The Physical Activity and Lymphedema (PAL) Trial demonstrated that breast cancer survivors with or at risk of breast cancer related lymphedema could safely weight train at a rate and level sufficient to increase muscle strength, improve body image and lymphedema outcomes, and that among women at risk of lymphedema only, weight training also reduced the percentage of body fat [21,22]. When considering additional outcomes reflecting skeletal health in this analysis, the PAL training program did not have a significant effect on BMD of the hip and spine. However, subtle changes in BMD were enough to cause a shift in the distribution of bone health categories that favored the postmenopausal trainees. Over the one-year study period more women in the control group moved from being normal to being osteopenic at the spine compared to women in the weight training group whose status did not change (Table 3). There was no notable shift in the number of women classified as osteoporotic between groups at either skeletal site.
The absence of a significant training effect from the PAL weight training program on BMD is congruent with other studies in women cancer survivors that used resistance training programs of similar intensity and nature of mechanical loading. In women undergoing chemotherapy for cancer, Schwartz (2007) used a home-based program of resistance bands that apply low to moderate loads with exercises that did not specifically use musculature with attachments to the spine (i.e., leg extensions, bicep curls) and found no training effect on spine BMD [29]. Similarly, Waltman reported that among postmenopausal breast cancer survivors initiating bisphosphonate therapy, no additional gains in BMD were achieved from 24-months of resistance exercise that was low to moderate intensity, used mostly seated resistance machines, and included only two exercises that engaged muscles with bony attachments at the hip or spine [30]. Across these trials, the resistance training programs effectively improved muscle strength suggesting that an adequate muscular stimulus was achieved and importantly, that the level of training could be performed without initiating or exacerbating lymphedema in women with breast cancer. However, the absence of any measurable effect on BMD from these training programs most likely reflects the adaptive nature of bone, that is distinct from muscle, and requires sufficiently high loads delivered in specific loading patterns to produce significant BMD change.
Numerous systematic reviews and controlled trials in women without cancer have identified the key elements of osteogenic exercise training programs [31–41]. Those key elements are that moderate to high bone loading forces are applied through impact, resistance and weight-bearing endurance activities and that loads are applied in a fashion that specifically loads a target bone [9,42]. The PAL weight training program was designed to start at a low intensity and progress very gradually so that strength could be increased over 12 months without risk of causing or worsening lymphedema, and thus the magnitude of loading may have been too low to significantly shift BMD. The program was also designed to increase total body strength of both central and peripheral musculature so exercises targeted a variety of muscle groups rather than targeting muscles with bony attachments to the proximal femur or lumbar spine, limiting loading at these clinically relevant sites. Lower body exercises were largely performed in the seated position which reduces to the total forces applied to the hip compared to standing lower body programs [9]. Other controlled trials in breast cancer survivors, where training programs were specifically designed to provide targeted loading to the hip and spine and followed training principles recommended for skeletal adaptations, have demonstrated that combined loading programs of resistance + impact or aerobic + impact exercise can significantly increase and/or preserve BMD at the hip and spine [15–17]. Thus, the innate adaptive capacity of the skeleton may remain relatively unchanged from cancer treatment, but still requires that exercise generate sufficient levels of loading delivered to specific skeletal sites in order to be significantly osteogenic.
While the PAL training program did not significantly change BMD after 12 months of training, there were apparently subtle enough effects of training among a proportion of participants to affect the clinical status of bone health at the spine. Among postmenopausal trainees a higher proportion of women with lumbar spine t-scores in the normal category retained this status over 12 months compared to women in the control group where more women became osteopenic over time. In contrast to comparison of group mean changes in BMD over time that may mask positive intervention effects among individuals, analysis of the distribution of bone health categories can better illustrate the effect of training on subgroups of participants. To that end, it appears that the PAL intervention may have been able to slow the shift from normal to osteopenia at the spine in postmenopausal women. The clinical relevance of this finding is unclear since the change in t-scores among the control group is below that which would indicate clinical recommendations or intervention [43]. While our observations are encouraging, the number of comparisons performed raises the chance for a Type I error and thus additional studies are warranted to confirm our findings.
This ancillary analysis on the potential additional skeletal effects of a weight training program designed to safely improve muscle strength in breast cancer survivors at risk or with breast cancer related lymphedema capitalized on the opportunity to evaluate the multiple benefits of physical activity in a large, controlled trial. The sample size in the study was sufficiently large and the training program of sufficient length to evaluate changes in BMD from exercise [18]. Additional analysis evaluating pre and postmenopausal women separately and accounting for changes in calcium intake and physical activity outside the intervention help ensure that potential moderators of the bone response to exercise were considered. The PAL weight training intervention was not specifically designed to meet the recommended training principles for the design of programs targeting skeletal health, rather it was designed to evaluate the safety of slowly progressive resistance exercise for women with or at risk of breast cancer related lymphedema and the efficacy of this program to improve muscle strength. Though the program did not significantly change BMD among breast cancer survivors, preservation of the clinical status of bone health at the spine among trainees suggests that future studies with similar programs and/or that evaluate the effectiveness of PAL in community settings continue to consider a potential benefit of long-term participation on skeletal health.
Acknowledgments
Funding
The work was supported by grants from the National Cancer Institute (R01-CA106851, to Dr. Schmitz) and the National Center for Research Resources (UL1RR024134, to the University of Pennsylvania). BSN Medical provided custom-fitted compression garments, and the fitness centers where the weight training sessions took place (YMCA of Philadelphia and Vicinity, Sisters in Shape, and the Family YMCA of Burlington County, NJ) provided discounted membership fees for study participants.
Footnotes
Conflict of Interest
The authors have no disclosures to report.
References
- 1.Chen ZMM, Aragaki AK, Mouton C, Arendell L, Lopez AM, Bassford T, Chlebowski RT. Fracture risk increases after diagnosis of breast or other cancers in postmenopausal women: results from the Women's Health Initiative. Osteoporos Int. 2009;20(4):527–536. doi: 10.1007/s00198-008-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional Limitations in Elderly Female Cancer Survivors. J Natl Cancer Inst. 2006;98(8):521–529. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 3.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obesity Rev. 2011;12(4):282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 4.Santen RJ. Effect of Endocrine Therapies on Bone in Breast Cancer Patients. J Clin Endocrinol Metab. 2011;96(2):308–319. doi: 10.1210/jc.2010-1679. [DOI] [PubMed] [Google Scholar]
- 5.Cameron D, Douglas S, Brown J, Anderson R. Bone mineral density loss during adjuvant chemotherapy in pre-menopausal women with early breast cancer: is it dependent on oestrogen deficiency? Breast Cancer Res Treat. 2010;23(3):805–814. doi: 10.1007/s10549-010-0899-7. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong MEG, Spencer EA, Cairns BJ, Banks E, Pirie K, Green J, Wright FL, Reeves GK, Beral V for the Million Women Study C. Body mass index and physical activity in relation to the incidence of hip fracture in postmenopausal women. J Bone Min Res. 2011;26(6):1330–1338. doi: 10.1002/jbmr.315. [DOI] [PubMed] [Google Scholar]
- 7.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, Pritchard KI. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008;26(33):5465–5476. doi: 10.1200/JCO.2008.18.4184. [DOI] [PubMed] [Google Scholar]
- 9.Kelley G, Kelley K, Kohrt W. Effects of ground and joint reaction force exercise on lumbar spine and femoral neck bone mineral density in postmenopausal women: a meta-analysis of randomized controlled trials. BMC Musculosk Dis. 2012;13(1):177. doi: 10.1186/1471-2474-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, Blackwell K, Rimer BK. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19(9):2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 11.Freedman RJ, Aziz N, Albanes D, Hartman T, Danforth D, Hill S, Sebring N, Reynolds JC, Yanovski JA. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab. 2004;89(5):2248–2253. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- 12.Vehmanen L, Saarto T, Elomaa I, Makela P, Valimaki M, Blomqvist C. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. Eur J Cancer. 2001;37(18):2373–2378. doi: 10.1016/s0959-8049(01)00317-3. [DOI] [PubMed] [Google Scholar]
- 13.Saarto T, Blomqvist C, Valimaki M, Makela P, Sarna S, Elomaa I. Clodronate improves bone mineral density in post-menopausal breast cancer patients treated with adjuvant antioestrogens. Br J Cancer. 1997;75(4):602–605. doi: 10.1038/bjc.1997.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19(14):3306–3311. doi: 10.1200/JCO.2001.19.14.3306. [DOI] [PubMed] [Google Scholar]
- 15.Saarto T, Sievanen H, Kellokumpu-Lehtinen P, Nikander R, Vehmanen L, Huovinen R, Kautiainen H, Jarvenpaa S, Penttinen HM, Utriainen M, Jaaskelainen AS, Elme A, Ruohola J, Palva T, Vertio H, Rautalahti M, Fogelholm M, Luoto R, Blomqvist C. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int. 2012;23(5):1601–1612. doi: 10.1007/s00198-011-1761-4. [DOI] [PubMed] [Google Scholar]
- 16.Winters-Stone K, Dobek J, Nail L, Bennett JA, Naik A, Schwartz A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2011;27(2):447–456. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winters-Stone KM, Dobek J, Nail LM, Bennett JA, Leo MC, Torgrimson-Ojerio B, Luoh SW, Schwartz A. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int. 2013;24(5):1637–46. doi: 10.1007/s00198-012-2143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winters-Stone KM, Schwartz A, Nail LM. A review of exercise interventions to improve bone health in adult cancer survivors. J Cancer Surviv. 2010;4(3):187–201. doi: 10.1007/s11764-010-0122-1. [DOI] [PubMed] [Google Scholar]
- 19.Saarto T, Penttinen HM, Sievanen H, Kellokumpu-Lehtinen PL, Hakamies-Blomqvist L, Nikander R, Huovinen R, Luoto R, Kautiainen H, Jarvenpaa S, Idman I, Utriainen M, Vehmanen L, Jaaskelainen AS, Elme A, Ruohola J, Palva T, Vertio H, Rautalahti M, Fogelholm M, Blomqvist C, Luoma ML. Effectiveness of a 12-month exercise program on physical performance and quality of life of breast cancer survivors. Anticancer Res. 2012;32(9):3875–3884. [PubMed] [Google Scholar]
- 20.Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. J Cancer Surviv. 2012;6(2):189–199. doi: 10.1007/s11764-011-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis-Grant L, Smith R, Bryan CJ, Williams-Smith CT, Chittams J. Weight Lifting for Women at Risk for Breast Cancer–Related Lymphedema. JAMA. 2010;304(24):2699–2705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, Bryan CJ, Williams-Smith CT, Greene QP. Weight Lifting in Women with Breast-Cancer–Related Lymphedema. New Engl J Med. 2009;361(7):664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 23.Speck RM, Gross CR, Hormes JM, Ahmed RL, Lytle LA, Hwang WT, Schmitz KH. Changes in the Body Image and Relationship Scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res Treat. 2010;121(2):421–430. doi: 10.1007/s10549-009-0550-7. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz KH, Troxel AB, Cheville A, Grant LL, Bryan CJ, Gross CR, Lytle LA, Ahmed RL. Physical activity and lymphedema (the PAL trial): Assessing the safety of progressive strength training in breast cancer survivors. Contemp Clin Trials. 2009;30(3):233–245. doi: 10.1016/j.cct.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baim S, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Lewiecki EM, Silverman S. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom. 2008;11(1):75–91. doi: 10.1016/j.jocd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 27.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 28.National Lymphedema Network Medical Advisory Committee. Position Statement of the National Lymphedema Network; 2011. Available at http://www.lymphnet.org/pdfDocs/nlnexercise.pdf, 2012. [Google Scholar]
- 29.Schwartz AL, Winters-Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 2007;34(3):627–633. doi: 10.1188/07.ONF.627-633. [DOI] [PubMed] [Google Scholar]
- 30.Waltman NL, Twiss JJ, Ott CD, Gross GJ, Lindsey AM, Moore TE, Berg K, Kupzyk K. The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: a 24-month randomized controlled trial. Osteoporos Int. 2010;21(8):1361–1369. doi: 10.1007/s00198-009-1083-y. [DOI] [PubMed] [Google Scholar]
- 31.Kelley G. Aerobic exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis. J Am Geriatr Soc. 1998;46(2):143–152. doi: 10.1111/j.1532-5415.1998.tb02530.x. [DOI] [PubMed] [Google Scholar]
- 32.Kelley GA. Aerobic Exercise and Bone Density at the Hip in Postmenopausal Women: A Meta-Analysis. Preventive Medicine. 1998;27(6):798–807. doi: 10.1006/pmed.1998.0360. [DOI] [PubMed] [Google Scholar]
- 33.Kelley GA, Kelley KS. Efficacy of Resistance Exercise on Lumbar Spine and Femoral Neck Bone Mineral Density in Premenopausal Women: A Meta-Analysis of Individual Patient Data. J Women Health. 2004;13(3):293–300. doi: 10.1089/154099904323016455. [DOI] [PubMed] [Google Scholar]
- 34.Kelley GA, Kelley KS. Exercise and bone mineral density at the femoral neck in postmenopausal women: a meta-analysis of controlled clinical trials with individual patient data. Am J Obstet Gynecol. 2006;194(3):760–767. doi: 10.1016/j.ajog.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Kelley GA, Kelley KS, Tran ZV. Exercise and bone mineral density in men: a meta-analysis. J Appl Physiol. 2000;88(5):1730–1736. doi: 10.1152/jappl.2000.88.5.1730. [DOI] [PubMed] [Google Scholar]
- 36.Kelley GADA, Kelley KSM, Tran ZVP. Resistance Training and Bone Mineral Density in Women: A Meta-Analysis of Controlled Trials. Am J Phys Rehabil. 2001;80(1):65–77. doi: 10.1097/00002060-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Martyn-St James M, Carroll S. High-intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int. 2006;17(8):1225–1240. doi: 10.1007/s00198-006-0083-4. [DOI] [PubMed] [Google Scholar]
- 38.Martyn-St James M, Carroll S. Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone. 2008;43(3):521–531. doi: 10.1016/j.bone.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. 2009;43(12):898–908. doi: 10.1136/bjsm.2008.052704. [DOI] [PubMed] [Google Scholar]
- 40.Wolff I, van Croonenborg J, Kemper H, Kostense P, Twisk J. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int. 1999;9(1):1–12. doi: 10.1007/s001980050109. [DOI] [PubMed] [Google Scholar]
- 41.Berard A, Bravo G, Gauthier P. Meta-analysis of the effectiveness of physical activity for the prevention of bone loss in postmenopausal women. Osteoporos Int. 1997;7(4):331–337. doi: 10.1007/BF01623773. [DOI] [PubMed] [Google Scholar]
- 42.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36(11):1985–1996. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 43.Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(1):25–54. doi: 10.1097/gme.0b013e3181c617e6. [DOI] [PubMed] [Google Scholar]