Abstract
Small interfering RNA (siRNA)-mediated RNA interference (RNAi) pathways are critical for the detection and inhibition of RNA virus replication in insects. Recent work has also implicated RNAi pathways in the establishment of persistent virus infections and in the control of DNA virus replication. Accumulating evidence suggests that diverse double-stranded RNAs produced by RNA and DNA viruses can trigger RNAi responses yet many viruses have evolved mechanisms to inhibit RNAi defenses. Therefore, an evolutionary arms race exists between host RNAi pathways and invading viral pathogens. Here we review recent advances in our knowledge of how insect RNAi pathways are elicited upon infection, the strategies used by viruses to counter these defenses, and discuss recent evidence implicating Piwi-interacting RNAs in antiviral defense.
Introduction
Central to the survival of all organisms is a competent immune system capable of restricting or eliminating intracellular pathogens such as viruses. Although several innate immunity pathways (e.g. Toll, Imd, JAK-STAT etc.) play virus-specific antiviral roles (reviewed in [1–3]), the RNA interference (RNAi) pathway is the most broadly-acting [4] and robust antiviral pathway in insects (reviewed in [5–8]). RNAi is also a major antiviral system in plants [9] and nematodes [10], and recent evidence suggests that RNAi may also serve an antiviral role in mammals [11,12]. The finding that RNAi inhibitors are encoded by diverse insect RNA [13–25] and DNA [26,27] viruses further emphasizes the importance of RNAi in the evolutionary arms race between virus and host. RNAi pathways restrict virus replication (and also silence cellular gene expression) through the production of small non-coding RNAs called small interfering RNAs (siRNAs). These siRNAs associate with Argonaute (Ago) proteins to seek out and destroy viral (or cellular) single-stranded (ss) RNAs in a sequence-specific manner. Other eukaryotic small RNAs, such as microRNAs (miRNAs) and Piwi-interacting RNAs (piRNAs), which normally regulate cellular gene expression [28] and transposon activity [29], respectively, have also been implicated in antiviral defense recently [8]. These various small RNAs are often defined by their origin, size, interaction with specific Agos, and functions [8]. Here we focus on recent progress in understanding the role of RNAi/siRNAs and piRNAs in mediating antiviral immunity (for a review of miRNA-mediated antiviral defense, see [30,31] and Asgari, this issue). Given the wide availability of genetic tools in Drosophila melanogaster and the importance of other dipterans (e.g. mosquitoes) as vectors for arboviruses (viruses transmitted by arthropods to vertebrates), research in insect antiviral RNAi pathways is most advanced in Diptera. Here we review key aspects of antiviral RNAi in dipterans, but also draw on examples from studies of RNAi-based antiviral immunity in non-dipteran insects.
RNAi, siRNAs, and antiviral defense
Mechanism of the RNAi pathway
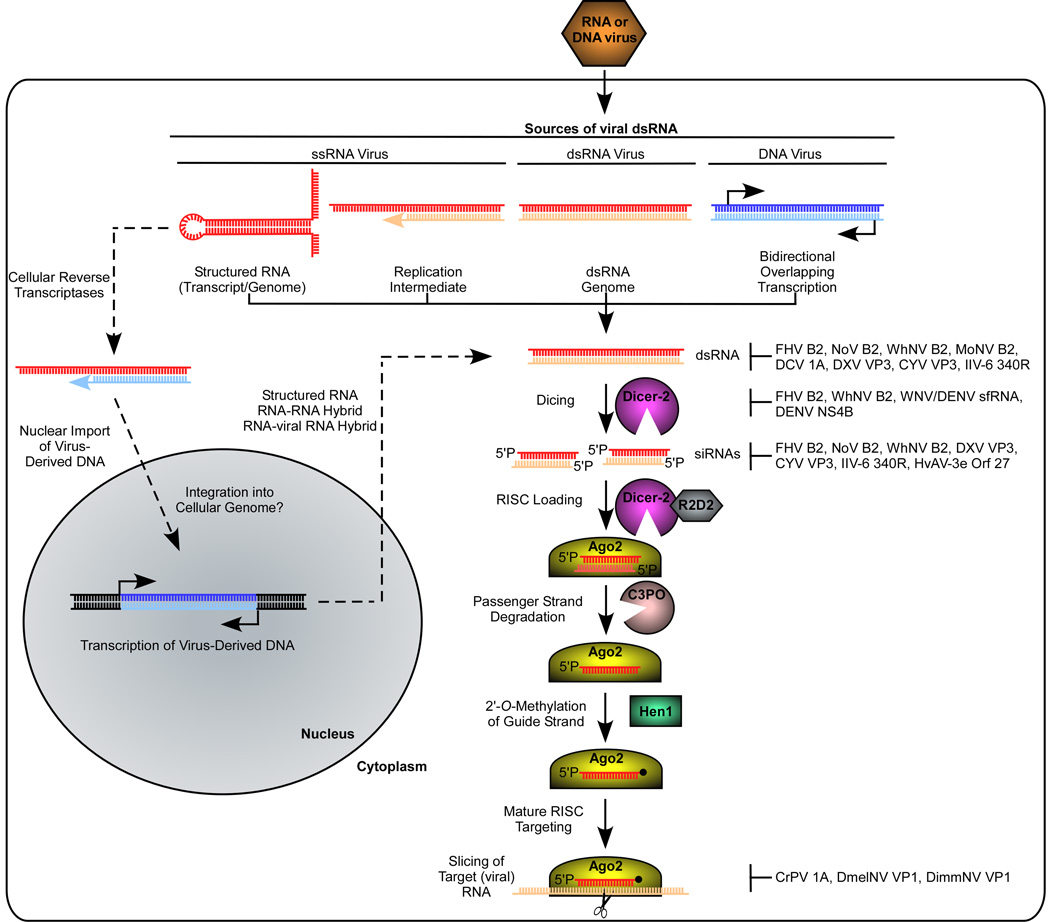
RNAi is initiated upon recognition and cleavage of long double-stranded (ds) RNA by Dicer-2, an RNAse III family dsRNA endonuclease, into ~19–25-nt long siRNA duplexes with characteristic 2 nt 3’ overhangs [32] (Fig. 1). Dicer-2 can recognize dsRNA from endogenous (e.g. cellular transcripts with secondary structures) or exogenous (e.g. experimentally introduced or viral) sources [33,34]. Dicer-2 cleavage of viral dsRNA produces viral siRNAs (vsiRNAs). These siRNAs are then loaded into the Argonaute-2 (Ago2)-containing RNA-induced silencing complex (RISC) [35]. Upon loading into RISC, one of the siRNA strands (the passenger) is degraded in a process dependent upon Ago2 and the endoribonuclease C3PO [36]. The other strand (the guide) remains associated with Ago2 and is 2’-O-methylated on its 3’-terminal nt by the Hen1 methyltransferase, creating an active or mature RISC [37,38]. Base-pairing of the guide strand to a complementary target ssRNA leads to Ago2-mediated cleavage (slicing) of the target. In Drosophila, the biogenesis and loading of siRNAs derived from endogenous and experimentally-introduced dsRNA into RISC require the Dicer-2 cofactors and dsRNA-binding proteins Loquacious PD (Loqs-PD) and R2D2 [39,40]. Only R2D2, however, is required for loading of vsiRNAs into RISC [41]. Thus, invertebrate RNAi systems may recognize or process viral dsRNA differently than other exogenous dsRNAs. In support of this, the antiviral RNAi response in nematodes requires a Dicer-related DEx-H-box protein that is dispensable for the RNAi response to experimentally-introduced dsRNA [42]. Differences in structure or intracellular localization between viral and other exogenous dsRNAs may determine the specific host factors required for their processing [43]. Although RNAi responses initiate in infected cells, studies in dipteran insects have suggested that antiviral RNAi signals (as either vsiRNA or longer viral dsRNA) can travel to uninfected cells [44,45], creating a systemic RNAi response that blocks viral spread [7,44].
Figure 1.
Antiviral RNAi pathway in insects (adapted from [90]). Dicer-2 recognizes and cleaves viral dsRNA arising from a variety of sources (see also Table 1), into predominantly 21 or 22 nt long small interfering RNA (siRNA) duplexes. These siRNA duplexes are loaded into the RNA-induced silencing complex (RISC) containing Ago2, the passenger strand is degraded, and the guide strand is 2’-O-methylated at the 3’ end. This mature RISC then targets viral RNA complementary to the guide strand for cleavage (slicing) by Ago2, thereby restricting virus replication. Recent work suggests that cellular reverse transcriptases can convert viral RNA into DNA forms early in infection [66]. Transcription of virus-derived DNA produces dsRNAs containing viral sequences that can enter the RNAi pathway, resulting in siRNA production that serves to dampen virus replication, allowing for the establishment of a persistent infection [66]. Both RNA and DNA viruses encode RNAi suppressors (shown at right) that target the RNAi pathway at one or more steps (see also Table 2). Virus abbreviations: Flock house virus (FHV); Nodamura virus (NoV); Wuhan Nodavirus (WhNV); mosinovirus (MoNV); Drosophila C virus (DCV); Drosophila X virus (DXV); Culex Y virus (CYV); Invertebrate iridescent virus type 6 (IIV-6); West Nile virus (WNV); Dengue virus (DENV); Heliothis virescens ascovirus-3e (HvAV-3e).
Detection and inhibition of virus replication by the RNAi pathway
RNA and DNA virus replication is significantly higher in dipteran cells or animals that are deficient in RNAi pathway components such as Dicer-2, R2D2, and Ago2 [18,19,46–50]. Furthermore, virus infection of RNAi-deficient animals is often associated with higher mortality rates [18,19,47,51]. More recently, we [52] and others [53] have implicated Dicer-2 and Ago2 in the defense against RNA and DNA viruses in lepidopteran hosts [52,53]. These studies illustrate a critical role for insect RNAi pathways in controlling infection by diverse viruses.
Using next generation sequencing to identify vsiRNAs in infected cells and subsequently mapping vsiRNAs to corresponding viral genomes, recent studies have provided insights into the putative viral dsRNAs cleaved by Dicer-2 to generate vsiRNAs [6]. These studies have revealed that, depending on the virus, the source of viral dsRNA processed by Dicer-2 may be from: 1) viral genomes (e.g. for dsRNA viruses); 2) replication intermediates of ssRNA viruses; 3) structured elements in viral ssRNA (genomes or transcripts); and 4) overlapping viral transcripts that base-pair to form dsRNA (Fig. 1 and Table 1). Intriguingly, Dicer-2-mediated recognition of viral dsRNA not only elicits RNAi but can also promote expression of Vago, a secreted protein that activates the antiviral JAK-STAT pathway [54–56]. Therefore, identifying viral RNAs recognized by Dicer-2 may reveal how RNAi and JAK-STAT pathways are triggered during infection.
Table 1.
| Virus | Family | Hosta | Viral Genomeb |
Putative Dicer-2 Substratesc |
Refs. |
|---|---|---|---|---|---|
| RNA virus | |||||
| Sindbis virus | Togaviridae | D. melanogaster; Ae. aegypti; Ae. albopictus | (+)ssRNA | dsRNA RIs; structured ssRNA | [4,50,87,88] |
| O’nyong-nyong virus | Togaviridae | An. gambiae | (+)ssRNA | dsRNA RIs; structured ssRNA | [59] |
| Semliki Forest virus | Togaviridae | Ae. albopictus; Ae. aegypti | (+)ssRNA | dsRNA RIs | [60,81] |
| Chikungunya virus | Togaviridae | Ae. aegypti; Ae. albopictus | (+)ssRNA | dsRNA RIs | [78] |
| Drosophila C virus | Dicistroviridae | D. melanogaster | (+)ssRNA | dsRNA RIs; structured ssRNA | [58,62] |
| Homalodisca coagulata virus-1 | Dicistroviridae | H. vitripennis | (+)ssRNA | dsRNA RIs; structured ssRNA | [61] |
| Dengue virus | Flaviviridae | Ae. aegypti; Ae. albopictus | (+)ssRNA | dsRNA RIs | [77,86] |
| West Nile virus | Flaviviridae | D. melanogaster; Ae. albopictus; C. quinquefasciatus | (+)ssRNA | dsRNA RIs; structured ssRNA | [64,87] |
| Cell fusing agent virus | Flaviviridae | Ae. aegypti; Ae. albopictus | (+)ssRNA | dsRNA RIs | [77] |
| Flock House virus | Nodaviridae | D. melanogaster | (+)ssRNA | dsRNA RIs; structured ssRNA | [57,58, 63] |
| American nodavirus | Nodaviridae | D. melanogaster | (+)ssRNA | dsRNA RIs; defective RNAs | [58] |
| Drosophila A virusd | Tetraviridae | D. melanogaster | (+)ssRNA | dsRNA RIs | [58] |
| Nora virus | Unassigned | D. melanogaster | (+)ssRNA | dsRNA RIs | [58] |
| Rift Valley fever virus | Bunyaviridae | D. melanogaster; Ae. aegypti; Ae. albopictus | (−)ssRNA | dsRNA RIs; intergenic RNA hairpin | [62,89] |
| La Crosse virus | Bunyaviridae | D. melanogaster; Ae. albopictus | (−)ssRNA | dsRNA RIs; structured ssRNA | [87] |
| Schmallenberg virus | Bunyaviridae | Ae. aegypti; Cu. sonorensis | (−)ssRNA | dsRNA RIs | [65] |
| Vesicular stomatitis virus | Rhabdoviridae | D. melanogaster | (−)ssRNA | dsRNA RIs; viral genometranscript hybrids | [41,47, 62] |
| Bluetongue virus | Reoviridae | Ae. aegypti; Cu.sonorensis | dsRNA | Genomic dsRNA | [65] |
| Homalodisca vitripennis reovirus | Reoviridae | H. vitripennis | dsRNA | Genomic dsRNA; structured ssRNA | [61] |
| Culex Y viruse | Birnaviridae | C. tarsalis | dsRNA | Genomic dsRNA | [21,85] |
| Drosophila X virus | Birnaviridae | D. melanogaster | dsRNA | Genomic dsRNA | [58] |
| Drosophila birnavirus | Birnaviridae | D. melanogaster | dsRNA | Genomic dsRNA | [58] |
| Drosophila totivirus | Totiviridae | D. melanogaster | dsRNA | Genomic dsRNA | [58] |
| DNA Virus | |||||
| Invertebrate iridescent virus type 6 | Iridoviridae | D. melanogaster | dsDNA | convergent overlapping transcripts | [4,51] |
| Vaccinia virus | Poxviridae | D. melanogaster | dsDNA | Convergent overlapping transcripts; RNA hairpins encoded by genomic termini; structured ssRNA | [62] |
| Helicoverpa armigera single nucleopolyhedrovirus | Baculoviridae | He. armigera | dsDNA | convergent overlapping transcripts; structured ssRNA | [53] |
Either cell line or whole organism.
Genus abbreviations: Ae., Aedes; An., Anopheles; C., Culex; Cu., Culicoides;D., Drosophila; H., Homolodisca; He., Helicoverpa.
Positive and negative polarity ssRNA virus genomes are indicated by (+) and (−), respectively.
RIs, replication intermediates.
Putative Dicer-2 substrates in RNA virus infection
Replication of ssRNA viruses involves the production of an antigenome-an RNA strand of opposite polarity to the genome-that serves as a template for genome synthesis, and vice versa. Consequently, ssRNA virus replication results in dsRNA replication intermediates. Although genomic strands are present at higher levels than antigenome strands during ssRNA virus infection [57], vsiRNAs mapping to genome and antigenome strands are often present at similar levels and are typically distributed across the entire length of the genome or antigenome [41,50,58–60]. These observations suggest that during ssRNA virus replication, dsRNA replication intermediates are major Dicer-2 substrates for vsiRNA production [6]. In contrast, the genomic dsRNA itself is likely the major Dicer-2 substrate during infection with dsRNA viruses [21,58,61].
It is important to note that these general observations are by no means the rule for all RNA virus infections (Table 1). For example, ~87% of the vsiRNAs generated during infection of Drosophila cells with Drosophila C virus, a ssRNA virus, map to the genomic strand [62], suggesting that dsRNA structures within the viral genome are major Dicer-2 substrates. A bias for genome strand vsiRNAs has also been noted during infections of dipteran [48,63,64], and more recently, hemipteran hosts [61] with other ssRNA viruses.
Curiously, although vsiRNAs targeting ssRNA viruses are typically distributed across the entire length of the viral genome or antigenome, they may target certain regions termed “hot spots” more heavily than others (cold spots). Hot spots may occur because those particular regions are more accessible to Dicer-2 or because of highly structured RNA produced at these loci [8]. For example, vsiRNAs targeting Rift Valley fever virus, a tripartite ssRNA virus with three genomic strands (L, M, S), predominantly map in equal numbers to both genome and antigenome strands for L and M segments, but largely map to the antigenomic strand of the S segment in a specific hot spot region that produces an RNA hairpin structure [62]. Hot spots have also been observed during infection with dsRNA viruses from Reoviridae, although it has been suggested that these may result from either differential access of Dicer-2 to regions within genomic dsRNA [65] or because of panhandle structures encoded by reovirus mRNAs [61].
The hot and cold spots detected during vsiRNA profiling may actually reflect a “decoy” mechanism used by viruses to divert host RNAi responses away from targeting essential viral RNAs [8,60]. Indeed, it was hypothesized that the heavily targeted RNA hairpin of the antigenomic S segment of Rift Valley fever virus may in fact act as such a decoy [62]. Furthermore, a prior study found hot spot vsiRNAs targeting the ssRNA virus, Semliki forest virus, to be less effective than vsiRNAs derived from cold spot regions of the viral genome in restricting virus replication [60]. Similarly, hot spot vsiRNAs targeting vesicular stomatitis virus (a ssRNA virus) were found to largely derive from abundant defective interfering particles produced during viral replication and these vsiRNAs were not efficiently loaded into RISC [62]. Therefore, viruses may benefit from the preferential cleavage of abundant decoy RNA transcripts by Dicer-2 because it may prevent processing of more limited viral RNAs needed for replication and because vsiRNAs derived from decoy RNAs may be less competent for loading into RISC.
Putative Dicer-2 substrates in DNA virus infections
Recent studies in Drosophila have demonstrated that dsDNA viruses also elicit vsiRNA production [4,51,62]. These vsiRNAs mostly mapped to hot spots in viral genomes where either convergent overlapping transcription and/or production of a structured transcript was predicted to generate dsRNA (Table 1). Interestingly, infection of the lepidopteran Helicoverpa armigera with Helicoverpa armigera single nucleopolyhedrovirus triggers the production of vsiRNAs that predominantly map to late viral genes required for virus replication and assembly. It has been suggested that preferential targeting of late genes by RNAi may be beneficial to the virus in regulating its own gene expression program and ensuring proper replication prior to host cell lysis [53].
RNAi and Persistent Virus Infection
In insects, arboviruses establish persistent infections in which they are not cleared but are restricted to a level that prevents more pathogenic (and potentially fatal) acute infections. Recently, Goic et al. [66] implicated RNAi in contributing to the establishment of persistent infections. Using Drosophila cells or animals, they showed that during infection with Flock house virus (FHV) endogenous reverse transcriptases copy FHV RNA into complementary DNAs (cDNA) forming FHV-retrotransposon cDNA chimeras. These chimeric cDNAs, which may be incorporated into the cellular genome under certain circumstances, are then transcribed to produce dsRNAs that are processed by Dicer-2 into vsiRNAs that restrict FHV replication. In FHV-infected cells treated with reverse transcriptase inhibitors, FHV-retrotransposon DNA chimeras are not made, persistent FHV infection is blocked, and instead, a more cytopathic FHV infection ensues [66]. These findings suggest that cDNA-derived vsiRNAs contribute to the initial control of viral replication and help to establish a persistent infection. These cDNA-derived vsiRNAs may also serve to amplify the canonical antiviral RNAi response (Fig. 1) in organisms such as D. melanogaster, which lack RNA-directed RNA polymerases (which amplify vsiRNA responses in other organisms such as plants) [7]. Recent studies in arthropods have shown that virus-specific dsRNA immunizations can invoke immunity to subsequent challenge with the corresponding virus, suggesting an RNAi-based immunological memory is created upon viral dsRNA inoculation [44,67,68]. Future studies will be needed to determine if virus-derived DNA chimeras (if integrated into the host genome) could provide a mechanism for RNAi-based immunological memory of virus infection.
Viral countermeasures to antiviral RNAi responses
Given the importance of RNAi in restricting broad classes of viruses, it is not surprising that diverse insect RNA and DNA viruses have evolved strategies to counter RNAi responses. Virus-encoded suppressor of RNAi (VSR) factors can inhibit the RNAi pathway at one or more steps (Fig. 1 and Table 2). For example, B2 proteins encoded by nodaviruses, such as FHV [69], Wuhan nodavirus [15,70], and Nodamura virus, bind both long dsRNAs and siRNAs [14,71], preventing dsRNA processing by Dicer-2 and siRNA loading into RISC. Thus, B2 dsRNA-binding activity may protect viral dsRNA replication intermediates from Dicer-2 cleavage [57]. In contrast, the B2 encoded by mosinovirus (MoNV), a mosquito-specific nodavirus, blocks RNAi triggered by long dsRNA but not by siRNA [16]. However, MoNV-infected mosquito cells are resistant to RNAi triggered by siRNA, suggesting that another MoNV factor suppresses RNAi at a step after siRNA biogenesis [16]. In addition to binding dsRNA, some B2 proteins may also inhibit RNAi through direct interactions with Dicer-2 [70,72], further highlighting the multi-faceted mechanism by which these VSRs inhibit RNAi.
Table 2.
Known/putative RNAi suppressors encoded by insect viruses and arboviruses. Adapted from [6].
| Virus | Family | RNAi suppressor |
Proposed mechanism of RNAi suppressor |
References |
|---|---|---|---|---|
| RNA virus | ||||
| Flock House virus | Nodaviridae | B2 | Binding long dsRNA prevents cleavage by Dicer-2; Binding siRNA prevents incorporation into RISC; Dicer-2 binding | [13,14,57,69, 72] |
| Nodamuravirus | Nodaviridae | B2 | Binding of long dsRNA prevents cleavage by Dicer-2; Binding siRNA prevents incorporation into RISC; inhibition of Dicer-2 activitya | [14,57,71] |
| Wuhan Nodavirus | Nodaviridae | B2 | Binding long dsRNA prevents cleavage by Dicer-2; Binding siRNA prevents incorporation into RISC; Dicer-2 binding | [15,70] |
| Mosinovirus | Nodaviridae | B2 | Binding long dsRNA prevents cleavage by Dicer-2 | [16] |
| Drosophila C virus | Dicistroviridae | 1A | Binding long dsRNA prevents cleavage by Dicer-2 | [17,19] |
| Cricket paralysis virus | Dicistroviridae | 1A | Inhibition of AGO2 slicer (endonuclease) activity | [17,18,22] |
| Drosophila X virus | Birnaviridae | VP3 | Binding long dsRNA prevents cleavage by Dicer-2; Binding siRNA prevents incorporation into RISC | [20,21] |
| Culex Y virus | Birnaviridae | VP3 | Binding long dsRNA prevents cleavage by Dicer-2; Binding siRNA prevents incorporation into RISC | [21] |
| Nora Virus | Unassigned | VP1 | Inhibition of Ago2 slicer (endonuclease) activity | [22,23] |
| Dimm Nora-like virus | Unassigned | VP1 | Inhibition of Ago2 slicer (endonuclease) activity | [23] |
| Dengue virus | Flaviviridae | NS4B | Inhibition of Dicer-2 activity a | [24] |
| West Nile virus | Flaviviridae | sfRNA | Inhibition of Dicer-2 activity a | [25] |
| Dengue virus | Flaviviridae | sfRNA | Inhibition of Dicer-2 activity b | [25] |
| DNA virus | ||||
| Heliothis virescens ascovirus-3e | Ascoviridae | Orf 27 (RNase III) | Degradation of siRNA | [26] |
| Invertebrate iridescent virus type 6 | Iridoviridae | 340R | Binding long dsRNA prevents cleavage by Dicer-2; Binding siRNA prevents incorporation into RISC | [27] |
Experimental data obtained using human Dicer, inhibition of Dicer-2 in insects is presumed.
Presumed function based on similarity to WENV sfRNA and ability to inhibit RNAi in insect cell assays.
Besides nodaviruses, dsRNA-binding VSRs have been identified in other virus families including Dicistroviridae (e.g. Drosophila C virus (DCV) 1A protein [17,19,73]) and, more recently, Birnaviridae (e.g. Culex Y virus and Drosophila X virus VP3 proteins [20,21]) and Iridoviridae (Invertebrate iridescent virus type 6 (IIV-6) 340R protein [27]). Each of these VSRs bind long dsRNA and likely inhibit Dicer-2 cleavage of viral dsRNA. In addition, birnavirus VP3 and IIV-6 340R proteins also bind siRNAs, and may block their loading into RISC. Interestingly, Heliothis virescens ascovirus-3e encodes an RNase III enzyme that may block RNAi initiation by competing with Dicer-2 for dsRNA substrates and/or by degrading siRNAs [26].
Other VSRs, such as Cricket paralysis virus (CrPV) 1A protein, VP1 proteins encoded by Drosophila melanogaster Nora virus (DmelNV) and Drosophila immigrans Nora-like virus (DimmNV), physically interact with Ago2 and haven been shown to block target cleavage by pre-assembled RISC using in vitro slicer assays [17,22,23]. Intriguingly, recombinant DimmNV VP1 protein can interact with D. immigrans but not D. melanogaster Ago2 and thus can antagonize slicer activity in D. immigrans embryo extracts but not in D. melanogaster embryo extracts [23]. In contrast, recombinant DmeINV VP1 proteins interact with Ago2 and antagonize slicer activity when added to either D. melanogaster or D. immigrans embryo extracts [23]. Whether DmeINV can actually infect D. immigrans and, in turn, whether DimmNV can replicate in D. melanogaster is unknown. However, when Sindbis virus (SINV), an arbovirus that lacks a VSR, was engineered to encode DmeINV VP1, the virus replicated to higher levels than the parental virus in both D. melanogaster and D. immigrans animals, whereas recombinant SINV encoding DimmNV VP1 only displayed enhanced replication in D. immigrans and not in D. melanogaster [23]. These results suggest that some VSRs may be host species-specific and must therefore be identified and characterized using a relevant host [23].
Mosquito-borne arboviruses establish a persistent, non-pathogenic infection in mosquitoes despite being targeted by host RNAi machinery [50]. Therefore, it has been unclear whether arboviruses use VSRs to evade RNAi systems or whether they lack VSRs because viral suppression by RNAi ensures that infections remain non-pathogenic to the vector host. Indeed, engineering of Sindbis virus to encode FHV B2 enhances viral pathogenicity in mosquitoes [50]. However, recent evidence suggests that the flaviviruses, West Nile virus and Dengue virus, may produce a structured sub-genomic flavivirus RNA (sfRNA) to act as a decoy for Dicer proteins, preventing the processing of essential dsRNA replication intermediates [25]. Furthermore, the Dengue virus NS4B protein displays VSR activity and can block human Dicer cleavage of dsRNA through an unknown mechanism without binding dsRNA [24]. Future studies are needed to unravel the molecular functions of these flavivirus-encoded VSRs. Given that several insect-restricted viruses establish persistent infections yet encode VSRs, it is likely that multiple virus- and host-specific factors such as virus replication kinetics, VSR potency, and RNAi response efficiencies determine whether persistent infections will be established [74].
Collectively, these findings indicate that diverse insect viruses from diverse families have independently evolved strategies to counter the RNAi pathway. However, because most VSRs have been studied in isolation, future studies with VSR-deficient strains will be needed to determine their effect(s) on the replication and associated pathogenicity of the viruses that encode them.
piRNAs and antiviral defense
Insect piRNAs are 24–30 nts and are defined by their preference for uridine at their 5’ ends (U1 bias), 2’-O-methylation of their 3’ terminal nucleotide, and interaction with Piwi-clade Ago proteins, which include Piwi, Aubergine (Aub), and Ago3 in Drosophila [29]. piRNAs are initially processed from antisense ssRNA precursors transcribed from genomic loci termed piRNA clusters [75]. These “primary” piRNAs associate with Piwi or Aub. Primary piRNAs guide the cleavage of sense-stranded piRNA precursors, generating the 5’ ends of secondary piRNAs that associate with Ago3 and display an adenine bias at position 10 (A10 bias). In turn, the sense-stranded secondary piRNAs guide the Ago3-mediated cleavage of antisense piRNA precursors to generate the 5’ ends of antisense-stranded secondary piRNAs [76]. This self-enforcing loop of secondary piRNA biogenesis is known as the “ping-pong” amplification cycle. piRNAs are abundant in the germline, where they silence transposable elements and protect genomic integrity [29]. However, virus-derived piRNAs (vpiRNAs) were identified in a Drosophila ovarian somatic sheet (OSS) cell line harboring covertly-replicating RNA viruses, suggesting that the piRNA pathway might also function in antiviral defense in somatic cells surrounding ovarian germ cells [58].
vpiRNA-like small RNAs were also found during profiling of small RNAs in Dengue virus type-2- and cell fusing agent virus-infected cells derived from Aedes aegypti and Ae. albopictus. However, like the vpiRNAs observed in the Drosophila OSS cell line, the majority were of positive polarity, and there was no clear evidence for ping-pong amplification [77]. Interestingly, a recent study found vpiRNAs with characteristics of ping-pong amplification (e.g. U1 and A10 biases) in the head and thorax tissues of Aedes albopictus infected with Chikungunya virus [78]. The presence of ping-pong vpiRNAs in the soma of mosquitoes may be due to the broad expression of an amplified family of Piwi clade Agos (including Ago3 and 7–8 Piwi proteins) in mosquito tissues as opposed to Drosophila, where Ago3 and Aub appear to be restricted to the germline [5,78]. Several other recent studies have identified vpiRNAs in mosquitoes and mosquito-derived cell lines infected with RNA viruses (Table 3).
Table 3.
Viruses for which vpiRNA-like small RNAs have been rep orted in insect host infections.
| Virus | Family | Viral Genomea |
Insect Host (cell line or in vivo) |
References |
|---|---|---|---|---|
| Drosophila X virus | Birnaviridae | dsRNA | D. melanogaster (cell line) | [58] |
| Drosophila birnavirus | Birnaviridae | dsRNA | D. melanogaster (cell line) | [58] |
| American nodavirus | Nodaviridae | (+)ssRNA | D. melanogaster (cell line) | [58] |
| Drosophila A virusb | Tetraviridae | (+)ssRNA | D. melanogaster (cell line) | [58] |
| Nora virus | Unassigned | (+)ssRNA | D. melanogaster (cell line) | [58] |
| Drosophila C virus | Dicistroviridae | (+)ssRNA | D. melanogaster (cell line) | [58] |
| Dengue virus | Flaviviridae | (+)ssRNA | Ae. aegypti (cell line and in vivo); Ae. albopictus (cell line) | [77,86] |
| Cell fusing agent virus | Flaviviridae | (+)ssRNA | Ae. aegypti (cell line); Ae. albopictus (cell line) | [77] |
| Sindbis virus | Togaviridae | (+)ssRNA | Ae. aegypti (cell line); Ae. albopictus (cell line) | [87,88] |
| Chikungunya virus | Togaviridae | (+)ssRNA | Ae. albopictus (cell line and in vivo); Ae. aegypti (cell line and in vivo) | [78] |
| Semliki Forest virus | Togaviridae | (+)ssRNA | Ae. albopictus (cell line); Ae. aegypti (cell line) | [81] |
| La Crosse virus | Bunyaviridae | (−)ssRNA | Ae. albopictus (cell line); Ae. aegypti (cell line) | [87,88] |
| Schmallenberg virus | Bunyaviridae | (−)ssRNA | Ae. aegypti (cell line) | [65] |
| Rift Valley fever virus | Bunyaviridae | (−)ssRNA | Ae. albopictus (cell line); Ae. aegypti (cell line) | [89] |
How viral transcripts enter the piRNA biogenesis pathway and a definitive role for piRNAs in antiviral defense remain to be determined. The strongest evidence implicating piRNAs in antiviral defense comes from studies showing increased susceptibility of dipteran hosts to infection upon inactivation or knockdown of Piwi clade Agos [48,79,80]. More recently, Schnettler et al. [81] have shown that knockdown of Ago2 or Piwi4 enhances Semliki Forest virus replication in Aedes aegypti-derived cells, suggesting roles for both RNAi and piRNA pathways in restricting virus replication. The use of genome-editing tools to inactivate Ago genes should help sort out the specific contributions of RNAi and piRNA pathways to antiviral defense in mosquitoes.
Concluding remarks
The findings that: 1) antiviral RNAi genes are among the most rapidly evolving immunity genes in insects [82,83]; 2) RNAi-deficient animals are hypersensitive to virus infection; and 3) divergent viruses encode VSRs, all point to a central role for RNAi in the evolutionary arms race between viruses and insect hosts. Recent profiling of vsiRNAs has provided insights into the viral signatures recognized by Dicer-2, but the relative effectiveness of different vsiRNAs in restricting virus replication is still largely unknown. This is an important point because vsiRNAs generated in hot spot regions, although more abundant, can be less effective than vsiRNAs from cold spot regions of the viral genome in restricting virus replication [60]. Furthermore, because vsiRNA and endogenous small RNA profiles might be altered by VSR activities [63,73,84], it will be important to both identify and characterize VSRs to understand how they modulate RNAi processes and contribute to viral pathogenesis. Important challenges for the future will be to understand the relative contribution of the siRNA, miRNA, and piRNA pathways in antiviral immunity and to characterize the potential effects of virus infection and virus-encoded factors on each pathway. Identifying viral factors that specifically inhibit the piRNA pathway would help to solidify a role for piRNAs in antiviral defense and may also provide new tools for answering many questions that remain regarding their biogenesis.
Highlights.
Insect RNA interference (RNAi) pathways detect and restrict viruses
Dicer-2 detects diverse viral dsRNA signatures and initiates the RNAi pathway
RNAi may play a role in establishing persistent, non-pathogenic virus infections
Both RNA and DNA viruses encode inhibitors of RNAi
The RNAi-related piRNA pathway may also restrict virus replication in insects
Acknowledgements
The authors thank Dr. Darryl Conte and members of the Mello Lab for their constructive comments on the manuscript. Work in the Mello Lab is supported by an NIH grant (GM058800) to C.C.M. C.C.M. is a Howard Hughes Medical Institute Investigator. Funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of this review; and in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Kingsolver MB, Huang Z, Hardy RW. Insect antiviral innate immunity: pathways, effectors, and connections. J Mol Biol. 2013;425:4921–4936. doi: 10.1016/j.jmb.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merkling SH, van Rij RP. Beyond RNAi: antiviral defense strategies in Drosophila and mosquito. J Insect Physiol. 2013;59:159–170. doi: 10.1016/j.jinsphys.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Cherry S. Viruses and antiviral immunity in Drosophila. Dev Comp Immunol. 2014;42:67–84. doi: 10.1016/j.dci.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kemp C, Mueller S, Goto A, Barbier V, Paro S, Bonnay F, Dostert C, Troxler L, Hetru C, Meignin C, et al. Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J Immunol. 2013;190:650–658. doi: 10.4049/jimmunol.1102486. •Using adult flies deficient in either the RNAi or JAK-STAT pathway, the authors demonstrate the broad antiviral activity of RNAi against six RNA viruses and one DNA virus and the virus-specific antiviral activity of the JAK-STAT pathway against certain RNA viruses.
- 5.Nayak A, Tassetto M, Kunitomi M, Andino R. RNA interference-mediated intrinsic antiviral immunity in invertebrates. Curr Top Microbiol Immunol. 2013;371:183–200. doi: 10.1007/978-3-642-37765-5_7. [DOI] [PubMed] [Google Scholar]
- 6.Bronkhorst AW, van Rij RP. The long and short of antiviral defense: small RNA-based immunity in insects. Curr Opin Virol. 2014;7:19–28. doi: 10.1016/j.coviro.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Karlikow M, Goic B, Saleh MC. RNAi and antiviral defense in Drosophila: setting up a systemic immune response. Dev Comp Immunol. 2014;42:85–92. doi: 10.1016/j.dci.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Vijayendran D, Airs PM, Dolezal K, Bonning BC. Arthropod viruses and small RNAs. J Invertebr Pathol. 2013;114:186–195. doi: 10.1016/j.jip.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Szittya G, Burgyan J. RNA interference-mediated intrinsic antiviral immunity in plants. Curr Top Microbiol Immunol. 2013;371:153–181. doi: 10.1007/978-3-642-37765-5_6. [DOI] [PubMed] [Google Scholar]
- 10.Sarkies P, Miska EA. RNAi pathways in the recognition of foreign RNA: antiviral responses and host-parasite interactions in nematodes. Biochem Soc Trans. 2013;41:876–880. doi: 10.1042/BST20130021. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Lu J, Han Y, Fan X, Ding SW. RNA interference functions as an antiviral immunity mechanism in mammals. Science. 2013;342:231–234. doi: 10.1126/science.1241911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maillard PV, Ciaudo C, Marchais A, Li Y, Jay F, Ding SW, Voinnet O. Antiviral RNA interference in mammalian cells. Science. 2013;342:235–238. doi: 10.1126/science.1241930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Li WX, Ding SW. Induction and suppression of RNA silencing by an animal virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- 14.Li WX, Li H, Lu R, Li F, Dus M, Atkinson P, Brydon EW, Johnson KL, Garcia-Sastre A, Ball LA, et al. Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. Proc Natl Acad Sci U S A. 2004;101:1350–1355. doi: 10.1073/pnas.0308308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi N, Cai D, Qiu Y, Xie J, Wang Z, Si J, Zhang J, Zhou X, Hu Y. RNA binding by a novel helical fold of b2 protein from wuhan nodavirus mediates the suppression of RNA interference and promotes b2 dimerization. J Virol. 2011;85:9543–9554. doi: 10.1128/JVI.00785-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster S, Zirkel F, Kurth A, van Cleef KW, Drosten C, van Rij RP, Junglen S. A unique nodavirus with novel features: mosinovirus expresses two subgenomic RNAs, a capsid gene of unknown origin, and a suppressor of the antiviral RNA interference pathway. J Virol. 2014;88:13447–13459. doi: 10.1128/JVI.02144-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayak A, Berry B, Tassetto M, Kunitomi M, Acevedo A, Deng C, Krutchinsky A, Gross J, Antoniewski C, Andino R. Cricket paralysis virus antagonizes Argonaute 2 to modulate antiviral defense in Drosophila. Nat Struct Mol Biol. 2010;17:547–554. doi: 10.1038/nsmb.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang XH, Aliyari R, Li WX, Li HW, Kim K, Carthew R, Atkinson P, Ding SW. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Rij RP, Saleh MC, Berry B, Foo C, Houk A, Antoniewski C, Andino R. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006;20:2985–2995. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valli A, Busnadiego I, Maliogka V, Ferrero D, Caston JR, Rodriguez JF, Garcia JA. The VP3 factor from viruses of Birnaviridae family suppresses RNA silencing by binding both long and small RNA duplexes. PLoS One. 2012;7:e45957. doi: 10.1371/journal.pone.0045957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Cleef KW, van Mierlo JT, Miesen P, Overheul GJ, Fros JJ, Schuster S, Marklewitz M, Pijlman GP, Junglen S, van Rij RP. Mosquito and Drosophila entomobirnaviruses suppress dsRNA- and siRNA-induced RNAi. Nucleic Acids Res. 2014;42:8732–8744. doi: 10.1093/nar/gku528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Mierlo JT, Bronkhorst AW, Overheul GJ, Sadanandan SA, Ekstrom JO, Heestermans M, Hultmark D, Antoniewski C, van Rij RP. Convergent evolution of argonaute-2 slicer antagonism in two distinct insect RNA viruses. PLoS Pathog. 2012;8:e1002872. doi: 10.1371/journal.ppat.1002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Mierlo JT, Overheul GJ, Obadia B, van Cleef KW, Webster CL, Saleh MC, Obbard DJ, van Rij RP. Novel Drosophila viruses encode host-specific suppressors of RNAi. PLoS Pathog. 2014;10:e1004256. doi: 10.1371/journal.ppat.1004256. •The authors identify novel Nora-like viruses from different Drosophila species and demonstrate that Nora-like virus VP1 VSRs inhibit host Ago2 slicer activity in a host species-specific manner, illustrating that VSRs may be host-specificity factors.
- 24. Kakumani PK, Ponia SS, S RK, Sood V, Chinnappan M, Banerjea AC, Medigeshi GR, Malhotra P, Mukherjee SK, Bhatnagar RK. Role of RNA interference (RNAi) in dengue virus replication and identification of NS4B as an RNAi suppressor. J Virol. 2013;87:8870–8883. doi: 10.1128/JVI.02774-12. •The authors describe the first flavivirus-encoded VSR protein, NS4B, that inhibits both siRNA and miRNA pathways by interfering with Dicer activity through a mechanism independent of dsRNA-binding.
- 25. Schnettler E, Sterken MG, Leung JY, Metz SW, Geertsema C, Goldbach RW, Vlak JM, Kohl A, Khromykh AA, Pijlman GP. Noncoding flavivirus RNA displays RNA interference suppressor activity in insect and Mammalian cells. J Virol. 2012;86:13486–13500. doi: 10.1128/JVI.01104-12. •The authors describe the first flavivirus-encoded VSR- a subgenomic flavivirus RNA. This structural RNA could inhibit both siRNA and miRNA pathways.
- 26.Hussain M, Abraham AM, Asgari S. An Ascovirus-encoded RNase III autoregulates its expression and suppresses RNA interference-mediated gene silencing. J Virol. 2010;84:3624–3630. doi: 10.1128/JVI.02362-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bronkhorst AW, van Cleef KW, Venselaar H, van Rij RP. A dsRNA-binding protein of a complex invertebrate DNA virus suppresses the Drosophila RNAi response. Nucleic Acids Res. 2014;42:12237–12248. doi: 10.1093/nar/gku910. •The authors show that an insect DNA virus encodes a VSR that binds long dsRNA and siRNAs, akin to the VSRs encoded by multiple RNA viruses, suggesting that DNA viruses have independently evolved similar strategies to counter RNAi.
- 28.Asgari S. MicroRNA functions in insects. Insect Biochem Mol Biol. 2013;43:388–397. doi: 10.1016/j.ibmb.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Weick EM, Miska EA. piRNAs: from biogenesis to function. Development. 2014;141:3458–3471. doi: 10.1242/dev.094037. [DOI] [PubMed] [Google Scholar]
- 30.Asgari S. Role of microRNAs in arbovirus/vector interactions. Viruses. 2014;6:3514–3534. doi: 10.3390/v6093514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucas KJ, Myles KM, Raikhel AS. Small RNAs: a new frontier in mosquito biology. Trends Parasitol. 2013;29:295–303. doi: 10.1016/j.pt.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 33.Okamura K, Balla S, Martin R, Liu N, Lai EC. Two distinct mechanisms generate endogenous siRNAs from bidirectional transcription in Drosophila melanogaster. Nat Struct Mol Biol. 2008;15:998. doi: 10.1038/nsmb0908-998c. [DOI] [PubMed] [Google Scholar]
- 34.Ghildiyal M, Seitz H, Horwich MD, Li C, Du T, Lee S, Xu J, Kittler EL, Zapp ML, Weng Z, et al. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science. 2008;320:1077–1081. doi: 10.1126/science.1157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Ye X, Jiang F, Liang C, Chen D, Peng J, Kinch LN, Grishin NV, Liu Q. C3PO, an endoribonuclease that promotes RNAi by facilitating RISC activation. Science. 2009;325:750–753. doi: 10.1126/science.1176325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horwich MD, Li C, Matranga C, Vagin V, Farley G, Wang P, Zamore PD. The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr Biol. 2007;17:1265–1272. doi: 10.1016/j.cub.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 38.Saito K, Sakaguchi Y, Suzuki T, Siomi H, Siomi MC. Pimet, the Drosophila homolog of HEN1, mediates 2'-O-methylation of Piwi- interacting RNAs at their 3' ends. Genes Dev. 2007;21:1603–1608. doi: 10.1101/gad.1563607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyoshi K, Miyoshi T, Hartig JV, Siomi H, Siomi MC. Molecular mechanisms that funnel RNA precursors into endogenous small-interfering RNA and microRNA biogenesis pathways in Drosophila. RNA. 2010;16:506–515. doi: 10.1261/rna.1952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marques JT, Kim K, Wu PH, Alleyne TM, Jafari N, Carthew RW. Loqs and R2D2 act sequentially in the siRNA pathway in Drosophila. Nat Struct Mol Biol. 2010;17:24–30. doi: 10.1038/nsmb.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marques JT, Wang JP, Wang X, de Oliveira KP, Gao C, Aguiar ER, Jafari N, Carthew RW. Functional specialization of the small interfering RNA pathway in response to virus infection. PLoS Pathog. 2013;9:e1003579. doi: 10.1371/journal.ppat.1003579. ••The authors show that, in Drosophila, vsiRNA biogenesis and loading into RISC is independent of Loqs-PD, which is required for the biogenesis of siRNAs derived from endogenous or experimentally-introduced dsRNA. Their results suggest that vsiRNA generation occurs through a separate pathway than siRNAs derived from endogenous or experimentally-introduced dsRNA.
- 42.Lu R, Yigit E, Li WX, Ding SW. An RIG-I-Like RNA helicase mediates antiviral RNAi downstream of viral siRNA biogenesis in Caenorhabditis elegans. PLoS Pathog. 2009;5:e1000286. doi: 10.1371/journal.ppat.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soares ZG, Goncalves AN, de Oliveira KP, Marques JT. Viral RNA recognition by the Drosophila small interfering RNA pathway. Microbes Infect. 2014;16:1013–1021. doi: 10.1016/j.micinf.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Saleh MC, Tassetto M, van Rij RP, Goic B, Gausson V, Berry B, Jacquier C, Antoniewski C, Andino R. Antiviral immunity in Drosophila requires systemic RNA interference spread. Nature. 2009;458:346–350. doi: 10.1038/nature07712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Attarzadeh-Yazdi G, Fragkoudis R, Chi Y, Siu RW, Ulper L, Barry G, Rodriguez-Andres J, Nash AA, Bouloy M, Merits A, et al. Cell-to-cell spread of the RNA interference response suppresses Semliki Forest virus (SFV) infection of mosquito cell cultures and cannot be antagonized by SFV. J Virol. 2009;83:5735–5748. doi: 10.1128/JVI.02440-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell CL, Keene KM, Brackney DE, Olson KE, Blair CD, Wilusz J, Foy BD. Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC Microbiol. 2008;8:47. doi: 10.1186/1471-2180-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller S, Gausson V, Vodovar N, Deddouche S, Troxler L, Perot J, Pfeffer S, Hoffmann JA, Saleh MC, Imler JL. RNAi-mediated immunity provides strong protection against the negative-strand RNA vesicular stomatitis virus in Drosophila. Proc Natl Acad Sci U S A. 2010;107:19390–19395. doi: 10.1073/pnas.1014378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O'nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci U S A. 2004;101:17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez-Vargas I, Scott JC, Poole-Smith BK, Franz AW, Barbosa-Solomieu V, Wilusz J, Olson KE, Blair CD. Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito's RNA interference pathway. PLoS Pathog. 2009;5:e1000299. doi: 10.1371/journal.ppat.1000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myles KM, Wiley MR, Morazzani EM, Adelman ZN. Alphavirus-derived small RNAs modulate pathogenesis in disease vector mosquitoes. Proc Natl Acad Sci U S A. 2008;105:19938–19943. doi: 10.1073/pnas.0803408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bronkhorst AW, van Cleef KW, Vodovar N, Ince IA, Blanc H, Vlak JM, Saleh MC, van Rij RP. The DNA virus Invertebrate iridescent virus 6 is a target of the Drosophila RNAi machinery. Proc Natl Acad Sci U S A. 2012;109:E3604–E3613. doi: 10.1073/pnas.1207213109. •One of the first reports indicating that DNA viruses are inhibited by the RNAi pathway.
- 52. Gammon DB, Duraffour S, Rozelle DK, Hehnly H, Sharma R, Sparks ME, West CC, Chen Y, Moresco JJ, Andrei G, et al. A single vertebrate DNA virus protein disarms invertebrate immunity to RNA virus infection. Elife. 2014;3 doi: 10.7554/eLife.02910. •The first report indicating that RNA viruses are inhibited by the RNAi pathway in lepidopteran insects.
- 53. Jayachandran B, Hussain M, Asgari S. RNA interference as a cellular defense mechanism against the DNA virus baculovirus. J Virol. 2012;86:13729–13734. doi: 10.1128/JVI.02041-12. •One of the first reports indicating that DNA viruses are inhibited by the RNAi pathway.
- 54.Deddouche S, Matt N, Budd A, Mueller S, Kemp C, Galiana-Arnoux D, Dostert C, Antoniewski C, Hoffmann JA, Imler JL. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in Drosophila. Nat Immunol. 2008;9:1425–1432. doi: 10.1038/ni.1664. [DOI] [PubMed] [Google Scholar]
- 55.Paradkar PN, Trinidad L, Voysey R, Duchemin JB, Walker PJ. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc Natl Acad Sci U S A. 2012;109:18915–18920. doi: 10.1073/pnas.1205231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paradkar PN, Duchemin JB, Voysey R, Walker PJ. Dicer-2-dependent activation of Culex Vago occurs via the TRAF-Rel2 signaling pathway. PLoS Negl Trop Dis. 2014;8:e2823. doi: 10.1371/journal.pntd.0002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aliyari R, Wu Q, Li HW, Wang XH, Li F, Green LD, Han CS, Li WX, Ding SW. Mechanism of induction and suppression of antiviral immunity directed by virus-derived small RNAs in Drosophila. Cell Host Microbe. 2008;4:387–397. doi: 10.1016/j.chom.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Q, Luo Y, Lu R, Lau N, Lai EC, Li WX, Ding SW. Virus discovery by deep sequencing and assembly of virus-derived small silencing RNAs. Proc Natl Acad Sci U S A. 2010;107:1606–1611. doi: 10.1073/pnas.0911353107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Myles KM, Morazzani EM, Adelman ZN. Origins of alphavirus-derived small RNAs in mosquitoes. RNA Biol. 2009;6:387–391. doi: 10.4161/rna.6.4.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siu RW, Fragkoudis R, Simmonds P, Donald CL, Chase-Topping ME, Barry G, Attarzadeh-Yazdi G, Rodriguez-Andres J, Nash AA, Merits A, et al. Antiviral RNA interference responses induced by Semliki Forest virus infection of mosquito cells: characterization, origin, and frequency-dependent functions of virus-derived small interfering RNAs. J Virol. 2011;85:2907–2917. doi: 10.1128/JVI.02052-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nandety RS, Fofanov VY, Koshinsky H, Stenger DC, Falk BW. Small RNA populations for two unrelated viruses exhibit different biases in strand polarity and proximity to terminal sequences in the insect host Homalodisca vitripennis. Virology. 2013;442:12–19. doi: 10.1016/j.virol.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 62. Sabin LR, Zheng Q, Thekkat P, Yang J, Hannon GJ, Gregory BD, Tudor M, Cherry S. Dicer-2 processes diverse viral RNA species. PLoS One. 2013;8:e55458. doi: 10.1371/journal.pone.0055458. •The authors analyze the vsiRNA profiles generated during infection of Drosophila cells with divergent ssRNA and dsDNA viruses to identify potential dsRNA signatures produced by each virus that are recognized and processed by Dicer-2.
- 63.Han YH, Luo YJ, Wu Q, Jovel J, Wang XH, Aliyari R, Han C, Li WX, Ding SW. RNA-based immunity terminates viral infection in adult Drosophila in the absence of viral suppression of RNA interference: characterization of viral small interfering RNA populations in wild-type and mutant flies. J Virol. 2011;85:13153–13163. doi: 10.1128/JVI.05518-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brackney DE, Beane JE, Ebel GD. RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification. PLoS Pathog. 2009;5:e1000502. doi: 10.1371/journal.ppat.1000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schnettler E, Ratinier M, Watson M, Shaw AE, McFarlane M, Varela M, Elliott RM, Palmarini M, Kohl A. RNA interference targets arbovirus replication in Culicoides cells. J Virol. 2013;87:2441–2454. doi: 10.1128/JVI.02848-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goic B, Vodovar N, Mondotte JA, Monot C, Frangeul L, Blanc H, Gausson V, Vera-Otarola J, Cristofari G, Saleh MC. RNA-mediated interference and reverse transcription control the persistence of RNA viruses in the insect model Drosophila. Nat Immunol. 2013;14:396–403. doi: 10.1038/ni.2542. ••The authors provide insight into the poorly-understood area of viral persistence.They show that shortly after infection, endogenous reverse transcriptases convert viral RNA into DNA forms that are transcribed to produce dsRNAs that are processed into vsiRNAs. These vsiRNAs contribute to the suppression of virus replication and the establishment of a non-pathogenic, persistent infection.
- 67.Loy JD, Mogler MA, Loy DS, Janke B, Kamrud K, Scura ED, Harris DL, Bartholomay LC. dsRNA provides sequence-dependent protection against infectious myonecrosis virus in Litopenaeus vannamei. J Gen Virol. 2012;93:880–888. doi: 10.1099/vir.0.038653-0. [DOI] [PubMed] [Google Scholar]
- 68.Bartholomay LC, Loy DS, Dustin Loy J, Harris DL. Nucleic-acid based antivirals: augmenting RNA interference to 'vaccinate' Litopenaeus vannamei. J Invertebr Pathol. 2012;110:261–266. doi: 10.1016/j.jip.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Chao JA, Lee JH, Chapados BR, Debler EW, Schneemann A, Williamson JR. Dual modes of RNA-silencing suppression by Flock House virus protein B2. Nat Struct Mol Biol. 2005;12:952–957. doi: 10.1038/nsmb1005. [DOI] [PubMed] [Google Scholar]
- 70.Qi N, Zhang L, Qiu Y, Wang Z, Si J, Liu Y, Xiang X, Xie J, Qin CF, Zhou X, et al. Targeting of dicer-2 and RNA by a viral RNA silencing suppressor in Drosophila cells. J Virol. 2012;86:5763–5773. doi: 10.1128/JVI.07229-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sullivan CS, Ganem D. A virus-encoded inhibitor that blocks RNA interference in mammalian cells. J Virol. 2005;79:7371–7379. doi: 10.1128/JVI.79.12.7371-7379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh G, Popli S, Hari Y, Malhotra P, Mukherjee S, Bhatnagar RK. Suppression of RNA silencing by Flock house virus B2 protein is mediated through its interaction with the PAZ domain of Dicer. FASEB J. 2009;23:1845–1857. doi: 10.1096/fj.08-125120. [DOI] [PubMed] [Google Scholar]
- 73.Berry B, Deddouche S, Kirschner D, Imler JL, Antoniewski C. Viral suppressors of RNA silencing hinder exogenous and endogenous small RNA pathways in Drosophila. PLoS One. 2009;4:e5866. doi: 10.1371/journal.pone.0005866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Neal ST, Samuel GH, Adelman ZN, Myles KM. Mosquito-borne viruses and suppressors of invertebrate antiviral RNA silencing. Viruses. 2014;6:4314–4331. doi: 10.3390/v6114314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 76.Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC. A slicer-mediated mechanism for repeat-associated siRNA 5' end formation in Drosophila. Science. 2007;315:1587–1590. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- 77.Scott JC, Brackney DE, Campbell CL, Bondu-Hawkins V, Hjelle B, Ebel GD, Olson KE, Blair CD. Comparison of dengue virus type 2-specific small RNAs from RNA interference-competent and -incompetent mosquito cells. PLoS Negl Trop Dis. 2010;4:e848. doi: 10.1371/journal.pntd.0000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Morazzani EM, Wiley MR, Murreddu MG, Adelman ZN, Myles KM. Production of virus-derived ping-pong-dependent piRNA-like small RNAs in the mosquito soma. PLoS Pathog. 2012;8:e1002470. doi: 10.1371/journal.ppat.1002470. ••This was the first report of vpiRNAs with ping-pong signatures in somatic tissues of Aedes mosquitoes, implicating a somatic piRNA pathway in the control of arbovirus infection.
- 79.Zambon RA, Vakharia VN, Wu LP. RNAi is an antiviral immune response against a dsRNA virus in Drosophila melanogaster. Cell Microbiol. 2006;8:880–889. doi: 10.1111/j.1462-5822.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 80.Chotkowski HL, Ciota AT, Jia Y, Puig-Basagoiti F, Kramer LD, Shi PY, Glaser RL. West Nile virus infection of Drosophila melanogaster induces a protective RNAi response. Virology. 2008;377:197–206. doi: 10.1016/j.virol.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schnettler E, Donald CL, Human S, Watson M, Siu RW, McFarlane M, Fazakerley JK, Kohl A, Fragkoudis R. Knockdown of piRNA pathway proteins results in enhanced Semliki Forest virus production in mosquito cells. J Gen Virol. 2013;94:1680–1689. doi: 10.1099/vir.0.053850-0. •The authors provide the first direct evidence for the involvement of Piwi clade Ago proteins in antiviral defense in Aedes mosquito cells.
- 82.Obbard DJ, Jiggins FM, Halligan DL, Little TJ. Natural selection drives extremely rapid evolution in antiviral RNAi genes. Curr Biol. 2006;16:580–585. doi: 10.1016/j.cub.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 83.Bernhardt SA, Simmons MP, Olson KE, Beaty BJ, Blair CD, Black WC. Rapid intraspecific evolution of miRNA and siRNA genes in the mosquito Aedes aegypti. PLoS One. 2012;7:e44198. doi: 10.1371/journal.pone.0044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adelman ZN, Anderson MA, Liu M, Zhang L, Myles KM. Sindbis virus induces the production of a novel class of endogenous siRNAs in Aedes aegypti mosquitoes. Insect Mol Biol. 2012;21:357–368. doi: 10.1111/j.1365-2583.2012.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang Y, Mi Z, Zhuang L, Ma M, An X, Liu W, Cao W, Tong Y. Presence of entomobirnaviruses in Chinese mosquitoes in the absence of Dengue virus co-infection. J Gen Virol. 2013;94:663–667. doi: 10.1099/vir.0.048231-0. [DOI] [PubMed] [Google Scholar]
- 86.Hess AM, Prasad AN, Ptitsyn A, Ebel GD, Olson KE, Barbacioru C, Monighetti C, Campbell CL. Small RNA profiling of Dengue virus-mosquito interactions implicates the PIWI RNA pathway in anti-viral defense. BMC Microbiol. 2011;11:45. doi: 10.1186/1471-2180-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brackney DE, Scott JC, Sagawa F, Woodward JE, Miller NA, Schilkey FD, Mudge J, Wilusz J, Olson KE, Blair CD, et al. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl Trop Dis. 2010;4:e856. doi: 10.1371/journal.pntd.0000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vodovar N, Bronkhorst AW, van Cleef KW, Miesen P, Blanc H, van Rij RP, Saleh MC. Arbovirus-derived piRNAs exhibit a ping-pong signature in mosquito cells. PLoS One. 2012;7:e30861. doi: 10.1371/journal.pone.0030861. •One of the first reports identifying vpiRNAs with clear ping-pong signatures in arbovirus-infected mosquito cells.
- 89.Leger P, Lara E, Jagla B, Sismeiro O, Mansuroglu Z, Coppee JY, Bonnefoy E, Bouloy M. Dicer-2- and Piwi-mediated RNA interference in Rift Valley fever virus-infected mosquito cells. J Virol. 2013;87:1631–1648. doi: 10.1128/JVI.02795-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Mierlo JT, van Cleef KW, van Rij RP. Defense and counterdefense in the RNAi-based antiviral immune system in insects. Methods Mol Biol. 2011;721:3–22. doi: 10.1007/978-1-61779-037-9_1. [DOI] [PubMed] [Google Scholar]