Abstract
Even before the discovery of hepatitis B virus (HBV), it was known that chimpanzees (Pan troglodytes) are susceptible to human hepatitis viruses. The chimpanzee is the only primate animal model for HBV infections. Much like HBV-infected human patients, chimpanzees can develop acute and chronic HBV infections and consequent hepatitis. Chimpanzees also develop a cellular immune response similar to that observed in humans. For these reasons, the chimpanzee has proven to be an invaluable model for investigations on HBV-driven disease pathogenesis and also the testing of novel antiviral therapies and prophylactic approaches.
The chimpanzee is the only immunocompetent nonhuman host that is fully susceptible to hepatitis B virus (HBV) infection. It has been instrumental in vaccine development and studying virus–host interactions during infection.
Chimpanzees are the most important animal model for HBV research because they are the only immunocompetent nonhuman host fully susceptible to HBV infection. Shortly after the discovery of the Australia antigen and its relation to hepatitis (Blumberg et al. 1968; Prince 1968; Gocke and Kavey 1969), the presence of HBsAg was shown in the sera of chimpanzees (Lichter 1969; Maynard et al. 1971). However, it was not known whether chimpanzees were native hosts of HBV or whether they contracted HBV as a result of transmission from humans. In the early 1970s, it was shown that chimpanzees were fully susceptible to infection with HBV present in human plasma (Maynard et al. 1972; Barker et al. 1973). Much later, HBV strains indigenous to chimpanzees and other nonhuman primates were discovered (Norder et al. 1996; Lanford et al. 1998, 2000; Warren et al. 1999; Grethe et al. 2000; Hu et al. 2000, 2001; MacDonald et al. 2000; Takahashi et al. 2000, 2001; Vartanian et al. 2002; Sall et al. 2005; Dupinay et al. 2013). In 1977, the HBV-associated delta antigen was identified in patients (Rizzetto et al. 1977). Subsequently, studies with the chimpanzee showed that delta antigen is a serological marker of a separate transmissible agent, hepatitis delta virus (HDV) (Rizzetto et al. 1980a,b). Since then, the chimpanzee has also served as an experimental model for HDV natural history and pathogenesis (reviewed in Gerin 2001). In this review, I will focus on the chimpanzee model for HBV infections and its impact on our understanding of HBV pathogenesis and the control of the HBV pandemic.
RECOGNITION OF INFECTIOUS (VIRAL) HEPATITIS IN CHIMPANZEES
A report by Soper and Smith in the late 1930s was most likely the first to provide evidence that a human hepatitis virus could infect nonhuman primates. Hepatitis was apparently transmitted to persons receiving a yellow-fever vaccine, produced in rhesus monkeys (Soper and Smith 1938). In 1961, Hillis documented a small outbreak of hepatitis among chimpanzee handlers at a U.S. Air Force Base, who were in contact with animals arriving from Africa (Hillis 1961). Chimpanzees also became ill from what appeared to be an “unknown” virus at the time. It was suspected that they might have acquired this “human” infection when they were inoculated with human blood, with the intent to protect them from human diseases, shortly after they were captured (Hillis 1961, 1963; Deinhardt et al. 1972). It was recognized that the infectious agent spread between cages, as well as to veterinary personnel, and that the most common transmission was by the fecal–oral route. Although not known at the time, most of these infections were most likely caused by hepatitis A virus (HAV), but infections with HBV might also have occurred. In particular, a chimpanzee developed fulminant hepatitis shortly after arrival at the Delta Regional Primate Research Center (Covington, LA) and two handlers developed severe hepatitis 1–2 months later (Smetana 1965; Smetana and Felsenfeld 1969). Although never confirmed, this was probably one of the earliest observed transmissions of HBV from a nonhuman primate to a human. Similarly, in studies in the 1950s, six chimpanzees developed hepatitis after inoculation with human serum obtained from students at the Willowbrook State School (New York, NY) for mentally disabled children (reviewed in Deinhardt 1976). It was later shown that HBV was endemic among the students at that school. With the identification and characterization of HBV in the 1960s and 1970s (Blumberg 1964; Dane et al. 1970; Millman et al. 1970), it became possible to detect HBV infections with serological tests. In 1969, Hirschman et al. (1969) showed the presence of Australia antigen in chimpanzees. One of the first well-documented cases of transmission of HBV from a chimpanzee to a human was reported in 1970 when a child suffering from liver failure and subjected to cross-circulation with a chimpanzee became positive for HBsAg (Rivers and Keeling 1970). It was later found that the chimpanzee was HBsAg positive for at least 1 year before the cross-circulation event, although the animal did not show signs of any disease (Rivers and Keeling 1970). Nonetheless, it remained obscure for years, whether spontaneous viral hepatitis in chimpanzees was of human or animal origin (Smetana and Felsenfeld 1969; Grethe et al. 2000). Sequence analysis finally showed that HBV is also indigenous to chimpanzees and other nonhuman primates (Vaudin et al. 1988; Grethe et al. 2000). In 1972 and the years following, it was conclusively shown that HBV sera–negative chimpanzees were susceptible to infection with human HBV isolates (Maynard et al. 1972; Barker et al. 1973, 1975a,b; Markenson et al. 1975). These crucial experiments paved the way for future prospective studies of HBV infections, vaccine development, and therapeutic interventions as discussed in the next sections.
ESTABLISHING THE CHIMPANZEE AS AN HBV MODEL SYSTEM
Several studies using limited dilutions of inocula containing different subtypes of human HBV showed an inverse relationship between inoculum size and incubation period (ranging from 2 to 20 wk) (Barker et al. 1975b; Shikata et al. 1977; Tabor et al. 1983b,c). Infectivity titers ranging from 107 to 108 chimpanzee infectious doses (CID)50/ml were measured in patient-derived inocula (Tabor et al. 1983b). Later, when real-time quantitative polymerase chain reaction (PCR) allowed for precise measurements of HBV DNA, it was reported that inocula from chronic HBV carriers, containing as little as three genome equivalents (GE) of HBV DNA, could infect a chimpanzee (Hsia et al. 2006; Komiya et al. 2008). Together with a recent demonstration that one GE of HBV DNA, obtained from a previously infected animal, was sufficient to reproducibly infect chimpanzees (Asabe et al. 2009), these studies showed that HBV does not produce viral DNA-containing particles that are defective. Several studies showed that acute HBV infections can also be induced in chimpanzees following intrahepatic injection of recombinant HBV DNA (Will et al. 1982, 1983, 1985), or after inoculation with HBV produced in tissue culture cells (Acs et al. 1987; Sureau et al. 1988) or in HBV transgenic mice (Guidotti et al. 1999).
Those early studies also showed that acute HBV infection is associated with hepatitis and liver disease, as determined histologically and by elevated serum alanine aminotransferase (sALT) and/or serum aspartate levels (summarized in Tabor et al. 1983b). Interestingly, the severity of disease depended on the inocula (i.e., subtypes), but not the dose of the inoculum (Tabor et al. 1983c). For example, inoculation of three chimpanzees with an HBV strain that caused fulminant hepatitis in human patients also resulted in severe hepatitis in chimpanzees (Ogata et al. 1993). A recent study also showed that low-dose inoculation, with as little as one GE of HBV, resulted in levels of serum and intrahepatic HBV markers, and was cleared with similar kinetics as inoculation with a very high dose of 1010 GE of HBV (Asabe et al. 2009).
Chronic HBV infection, as defined by serum HBsAg positivity for >6 mo, occurred at a similar frequency (5%–10%) in chimpanzees inoculated with different HBV subtypes (Shikata et al. 1980; Tabor et al. 1983b,c), as described for human patients (Hoofnagle 1981). Despite the similarities of the course of HBV infection in human patients and chimpanzees, the severity of hepatitis consistently appeared to be milder in HBV-infected chimpanzees (Barker et al. 1973, 1975a).
Initial efforts to expand HBV infection to other nonhuman primate systems failed. Reports that HBV could also be transmitted to, and serially passaged in, rhesus monkeys could not be confirmed (London et al. 1972). Similarly, a more recent report showed HBV replication in a Barbary macaque (Macaca sylvanus) after transfection of cloned HBV DNA, but serial passage and persistent infection in these animals was not observed (Gheit et al. 2002; Dupinay et al. 2013). In contrast, gibbons (genus Hylobates) at the International Center for Gibbon Studies (Santa Clarita, CA) were positive for markers of ongoing or past HBV infection and phylogenetic analysis of the isolated HBV sequences suggested that the gibbons, most likely, had been infected by transmission of HBV from humans (Lanford et al. 2000). This observation is consistent with two previous studies, providing serological evidence of HBV infection in gibbons inoculated with human HBsAg–positive saliva or semen (Bancroft et al. 1977; Scott et al. 1980). Finally, it was shown that a Macaca fascicularis colony from Mauritius Island was naturally infected with a human HBV isolate (Dupinay et al. 2013). Macaques might become an alternative model to chimpanzees for the study of therapeutic approaches against HBV infections (Bukh et al. 2013).
THE CHIMPANZEE MODEL AS A “SAFETY TEST” TO PREVENT HBV INFECTIONS IN HUMANS
The detailed characterization of the course of HBV infections in chimpanzees, together with its reproducibility, made it possible to use this model to test for the presence of HBV in human serum and blood-derived products. For example, chimpanzee blood was used to show that inactivation of HBV in blood products with heat was only partially successful (Shikata et al. 1978; Hollinger et al. 1984; Purcell et al. 1985; Lelie et al. 1987). Over the next 15 years, many different inactivation strategies were used, including urea/formalin treatment (Tabor et al. 1983a), UV-irradiation alone and in combination with Tween 80 and β-propiolactone (Stephan et al. 1981; Prince et al. 1983a,b), chloroform treatment (Feinstone et al. 1983), exposure to Tween 80 and ether at 4°C (Prince et al. 1984a), glutaraldehyde or ethyl alcohol treatment (Kobayashi et al. 1984), exposure to heat (Hollinger et al. 1984; Kobayashi et al. 1984; Purcell et al. 1985; Lelie et al. 1987), photochemical treatment (Alter et al. 1988; Lin et al. 2005), ion exchange chromatography (Zolton et al. 1985), various disinfectants (Prince et al. 1993), and also antibodies to HBV (Tabor et al. 1980a; Brummelhuis et al. 1983).
These efforts were instrumental in containing the spread of HBV by blood transfusion and blood- or serum-derived products, including clotting factors, immunoglobulins, and HBV vaccines, which were derived from plasma of chronic carriers of HBV (see next section). Nevertheless, it was also recognized that non-A non-B (NANB) hepatitis infection could delay or prevent HBV infection in chimpanzees and lead to ambiguous results in tests for blood-derived products and vaccines (Brotman et al. 1983).
THE CHIMPANZEE MODEL IN HBV VACCINE AND DRUG DEVELOPMENT
It was observed early on that chimpanzees previously exposed to HBV were protected from reinfection with high doses of HBV (Wilson and Logan 1975). Cross-challenging studies indicated that animals developing hepatitis following inoculation of HBsAg from one subtype were protected from challenge with another subtype (Murphy et al. 1974; Maynard et al. 1975; Gerety et al. 1979). Moreover, HBsAg immunization could trigger long-lasting cellular, as well as humoral responses (Ibrahim et al. 1974; Trépo et al. 1975). Together with the observation that immune function was directly related to infection outcome (Wilson and Logan 1975), these studies provided the rationale for vaccine development. Chimpanzee studies were instrumental as they allowed for both vaccine efficacy and safety testing. Thus, with the exception of certain polio vaccine studies in the 1950s (Sabin 1955a,b), the characterization of HBV infection and vaccine development probably reflects the first large-scale use of the chimpanzee model in biomedical research (McAuliffe et al. 1980; Prince et al. 1985).
The first-generation HBV vaccine was developed in the 1970s and was based on purification of HBsAg from the plasma of healthy HBV carrier patients (reviewed in McAuliffe et al. 1980). Evaluation of vaccine safety and efficacy mostly relied on studies in chimpanzees (Hilleman et al. 1975; Purcell and Gerin 1975; Buynak et al. 1976; Gerety et al. 1979; and reviewed in McAuliffe et al. 1980; Tabor et al. 1982; Prince et al. 1985), and these efforts greatly contributed to the licensing and use of the first plasma-derived HBV vaccine in the United States (Immunization Practices Advisory Committee 1982). Despite the success of the first-generation HBV vaccine, there remained a certain risk of contamination of the vaccine with trace amounts of infectious HBV and/or other blood-borne diseases. Thus, alternative vaccines were developed and tested in chimpanzees. These included synthetic peptides (Itoh et al. 1986; Neurath et al. 1986; Emini et al. 1989), antibodies targeting HBV (Stephan et al. 1984; Hong et al. 2004; Kim et al. 2008a,b), HBV proteins produced in cell culture (Tabor et al. 1981), live recombinant viruses expressing HBV proteins (Moss et al. 1984; Lubeck et al. 1989), DNA immunization (Davis et al. 1996; Prince et al. 1997), the use of other viral proteins as immunogens (Prince et al. 1984b; Iwarson et al. 1985; Murray et al. 1987), and recombinant HBsAg produced in yeast. The latter was licensed in 1986 and rapidly replaced the plasma-derived vaccine. In recent years, modified recombinant vaccines were also tested in the chimpanzee model, further emphasizing the importance of this model in HBV vaccine development (Wahl et al. 1989; Fujisawa et al. 1990; Kuroda et al. 1991; Davis et al. 1996; Ogata et al. 1997, 1999; Page et al. 2001; Payette et al. 2006; Kamili et al. 2009; Kamili 2010).
Although the current recombinant vaccines are extremely efficient in preventing new infection, they do not provide any benefit to the millions of people that are chronic carriers and at greatly enhanced risk for developing liver cirrhosis and hepatocellular carcinoma (Prince 2001). Currently available therapeutic treatments for chronic HBV infection, while reducing the viral load, are very rarely curative (Kwon and Lok 2011; Sonneveld and Janssen 2011). Thus, different therapeutic vaccination strategies are under development with the hope of achieving viral clearance by boosting the immune response to HBV (reviewed in Liu et al. 2014). A series of therapeutic vaccination studies has been performed, over the years, in chimpanzees. These included administration of the interferon inducer poly(I/C) (Purcell et al. 1976), DNA-vaccine/boost protocols (Pancholi et al. 2001; Shata et al. 2006), treatments with therapeutic antibodies (Eren et al. 2000, 2006; Dagan and Eren 2003), genetic immunization using a recombinant retroviral vector-expressing HBV core protein (Sällberg et al. 1998), and, recently, immune modulation through Toll-like receptor (TLR)-7 activation (Lanford et al. 2013). Although some of these experiments resulted in prolonged reduction of viral markers and improved immune functions, none were curative, indicating that alternative strategies involving a combination with antiviral treatments may be required to cure chronic HBV infections (Bertoletti and Gehring 2013).
STUDYING HBV PATHOGENESIS IN THE CHIMPANZEE MODEL
The ability to obtain serial liver biopsy samples from HBV-infected chimpanzees allows for investigations of viral and host parameters during the course of acute and chronic HBV infections. For example, studies in the 1980s described the detection of HBV DNA replicative intermediates, as well as covalently closed circular DNA (cccDNA) in infected livers (Shouval et al. 1980; Monjardino et al. 1982; Ruiz-Opazo et al. 1982a,b). Similarly, viral transcripts were identified and characterized to determine their 5′ and 3′ ends (Cattaneo et al. 1983, 1984). Other studies investigated immunopathogenesis in the infected liver using histological and ultrastructural methods (Karasawa et al. 1985a, 1985b; Schaff et al. 1992; Falcon et al. 1993). Besides serological time course studies of viral antigens and their corresponding antibodies, most of these studies were descriptive in nature (Barker et al. 1975b; Takahashi et al. 1979; Tabor et al. 1980b; Klinkert et al. 1986). However, with the recognition that chimpanzee (Patr) class I major histocompatibility complex (MHC) molecules and human leukocyte antigen (HLA) supertypes in humans share overlapping peptide-binding specificities, it became possible to investigate, in detail, the T-cell response to HBV in chimpanzees (Bertoni et al. 1998; McKinney et al. 2000; Sidney et al. 2006, 2007). Real-time quantitative PCR, specific for HBV DNA and cellular genes, as well as DNA microarray analyses, enabled design and execution of comprehensive longitudinal studies on host–virus interactions using chimpanzee sera and tissues from liver biopsies. These advances also permitted investigations on HBV DNA integration in the liver of chronically infected chimpanzees (Mason et al. 2009).
Absence of an Innate Response by HBV-Infected Cells
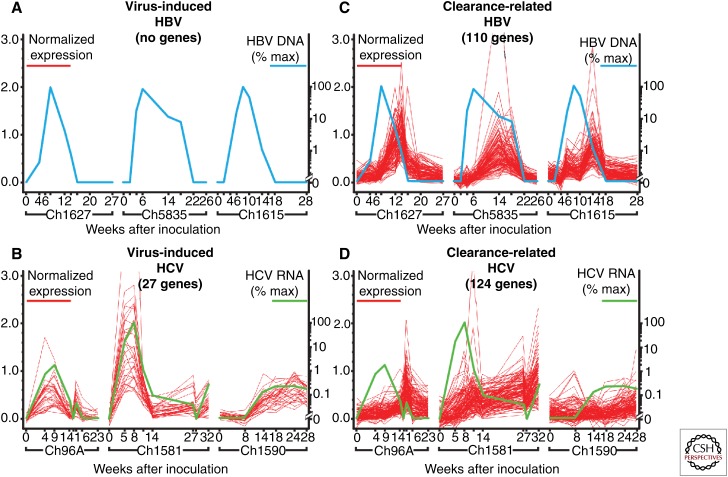
Expression of interferons, a hallmark of many viral infections, activates a cellular innate immune response characterized by transcriptional induction of a large number of genes called interferon-induced genes (ISGs). ISGs, in turn, encode proteins that exert a variety of intracellular antiviral functions to limit virus production and spread (Samuel 1991; Wieland and Chisari 2005). Longitudinal, intrahepatic expression profiling in chimpanzees showed that many ISGs are strongly induced during the spread of hepatitis C virus (HCV) infection, suggesting that HCV is highly visible to the innate immune system (Fig. 1B) (Bigger et al. 2001; Su et al. 2002; Wieland and Chisari 2005; Chisari et al. 2010). Surprisingly, however, intrahepatic gene expression profiling in acutely HBV-infected chimpanzees revealed that HBV does not induce detectable ISGs or other cellular gene expression as it spreads throughout the liver (Fig. 1A) (Wieland et al. 2004a, 2014; Wieland and Chisari 2005). This observation indicated that, unlike HCV, HBV acts like a “stealth virus,” which is invisible to the innate immune system (Wieland et al. 2004a; Wieland and Chisari 2005). The reason might lie in the mechanism of HBV replication. cccDNA is localized in the cell nucleus, whereas most known innate sensor proteins are cytoplasmic, the viral transcripts are capped and polyadenylated, resembling the structure of normal cellular transcripts, and the replicating genome is sequestered within viral capsids in the cytoplasm of infected cells (Summers 1988; Seeger and Mason 2000; Ganem and Prince 2004). Thus, the typical widespread expansion of HBV in the liver may reflect its ability to evade detection mechanisms that would result in interferon production, to which the virus can be exquisitely sensitive, as it has been shown in HBV transgenic mice (Guidotti et al. 1996; Wieland et al. 2000).
Figure 1.
Liver gene expression profile during HBV and HCV infection (Wieland et al. 2004a). (A) Genes correlated with viremia in all acutely HBV-infected chimpanzees. No genes correlated positively or negatively with the level of intrahepatic HBV DNA during the time course of acute HBV infection in all three animals. (B) Intrahepatic gene expression correlated with viremia in three HCV-infected chimpanzees (Su et al. 2002). (C) Liver gene expression profile associated with viral clearance in all three acutely HBV-infected chimpanzees, and (D) that associated with clearance in HCV-infected chimpanzees (Su et al. 2002). Gene identities in HBV- and HCV-infected animals are described in Wieland et al. (2004a) and Su et al. (2002), respectively. Blue and green lines show the intrahepatic HBV DNA or serum HCV RNA as a percentage (% max) of the corresponding peak levels, respectively. For optimal visualization, gene expression levels (red lines) are shown after normalization to the 10th and 90th percentiles for each gene. Values on the x axis represent weeks after inoculation with HBV. (From Wieland et al. 2004a and Su et al. 2002; adapted, with permission, from the National Academy of Sciences © 2004 and 2002, respectively; and from Chisari et al. 2010; with permission from Elsevier Masson SAS © 2010.)
Mechanisms of HBV Clearance
Many studies have shown that the peripheral blood CD4+ and CD8+ T-cell response to HBV is vigorous and multispecific in patients with acute hepatitis who ultimately clear the virus, whereas these are relatively weak in persistently infected patients with chronic hepatitis (Ferrari et al. 1990; Bertoletti et al. 1991; Penna et al. 1991; Rehermann et al. 1995; Tsui et al. 1995). Furthermore, HBV-specific T cells are detectable in the livers of chronically infected patients, where they probably contribute to disease pathogenesis but, for some reason, are unable to control or cure the infection (Ferrari et al. 1987; Bertoletti et al. 1997; Maini et al. 2000; Rehermann 2000). Interestingly, a study examining a relationship between the number of intrahepatic HBV-specific CD8 T cells, extent of liver disease, and levels of HBV replication in chronically infected patients indicated that inhibition of virus replication could occur independently of liver damage. This study also showed that the functionality of HBV-specific CD8 T cells was more important to the control of HBV replication than their absolute number (Maini et al. 2000). Indeed, in the HBV transgenic mouse model, adoptively transferred HBsAg-specific CTL clones not only kill engaged HBV-positive hepatocytes, but also secrete interferon (IFN)-γ, which noncytopathically inhibits HBV gene expression and replication in the rest of the hepatocytes (Moriyama et al. 1990; Ando et al. 1994; Guidotti et al. 1994, 1996). Furthermore, in the HBV transgenic mouse model, HBV replication is inhibited by any stimulus that induces IFN-γ and/or IFN-α/β in the liver (Guidotti et al. 1996; Franco et al. 1997; Cavanaugh et al. 1998; Kakimi et al. 2000; McClary et al. 2000a, 2000b; Pasquetto et al. 2000; Wieland et al. 2000; Isogawa et al. 2005b). This raises the possibility that HBV infections can be controlled by several arms of the immune response and perhaps explains why HBV infections are almost always self-limiting in immunologically competent adults.
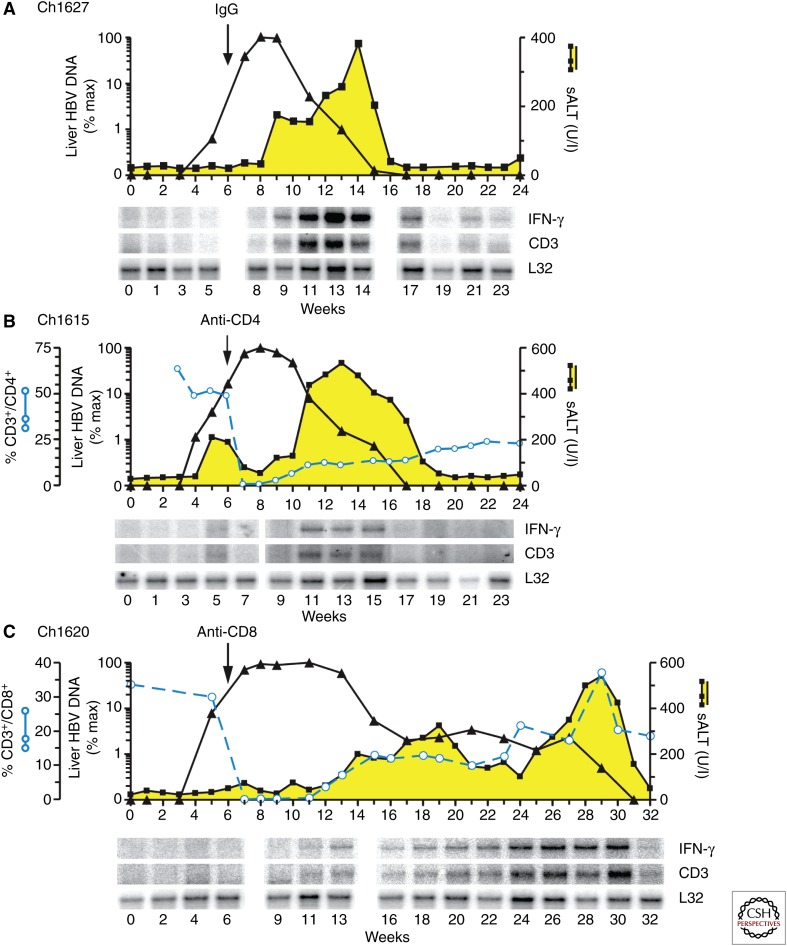
The chimpanzee model made it possible to characterize, in more detail, the cells and mechanisms responsible for viral clearance and liver disease during acute HBV infections. For example, monoclonal antibody-mediated T-cell depletion studies showed that CD4+ T-cell depletion at the peak of infection did not significantly affect the kinetics of HBV clearance and liver disease during acute HBV infection (Fig. 2A,B) (Thimme et al. 2003). In contrast, depletion of CD8+ T cells at the peak of infection profoundly altered the duration and outcome of an acute HBV infection (Fig. 2C) (Thimme et al. 2003). In the absence of CD8+ T cells (wk 8–11), infection remained at peak levels and increases in sALT activity were delayed until wk 13, when CD8+ T cells started to reappear. Furthermore, the time span from the first phase of clearance (wk 15) to eventual termination of the infection was markedly prolonged (Fig. 2C). Importantly, during wk 29, the CD8+ cell numbers returned to baseline levels, which coincided with a surge in sALT activity and termination of the infection. Interestingly, the initial phase of viral clearance in all three animals was associated with intrahepatic CD3 and IFN-γ expression but low sALT activity, suggesting that reduction of HBV levels in this phase might be mostly driven by noncytopathic mechanisms similar to the observations in an earlier study in chimpanzees (Guidotti et al. 1999).
Figure 2.
Course of acute HBV infection in chimpanzees after experimental inoculation with HBV in the presence or absence of CD4+ and CD8+ cells (Thimme et al. 2003). All animals were inoculated with 108 genome equivalents (GE) of HBV intravenously in wk 0 and a monoclonal antibody (see below) in wk 6. (A) Chimpanzee (Ch.) 1627 was injected with irrelevant control antibody (IgG). (B) Chimpanzee 1615 was injected with a CD4-specific monoclonal antibody. (C) Chimpanzee 1620 was injected with a CD8-specific monoclonal antibody. Intrahepatic HBV DNA (black triangles) is expressed as a percentage (% max) of the corresponding peak HBV DNA levels in the liver of each animal. sALT activity (black squares and yellow area) is expressed in units per liter. The number of CD3+ CD4+ or CD3+ CD8+ T cells is expressed as a percentage of the total number of CD3+ T cells in the peripheral blood. Vertical arrows indicate the time of antibody treatment. Total RNA isolated from liver biopsy samples was analyzed for the expression of CD3, IFN-γ, and L32 by a ribonuclease (RNase) protection assay (Thimme et al. 2003). The L32 signals reflect the amount of RNA used in the assay. (From Thimme et al. 2003; adapted, with permission, from the Journal of Virology, American Society for Microbiology © 2003.)
Intrahepatic gene expression profiling of these and other acutely HBV-infected animals confirmed that the early phase of clearance of HBV (Fig. 1C) (Wieland et al. 2004a) and, parenthetically, also for HCV (Fig. 1D) (Su et al. 2002), was temporally associated with the appearance of CD3, CD8, and IFN-γ mRNA. It also showed that other T-cell-derived and IFN-γ-stimulated genes were induced in this phase, suggesting that some of them might contribute to noncytopathic inhibition of HBV replication (Wieland et al. 2004a; Wieland and Chisari 2005). Indeed, although HBV replicative intermediates decreased as much as 50-fold from peak levels during this time, there was little or no attendant liver disease, despite the fact that virtually 100% of the hepatocytes were infected (Guidotti et al. 1999; Thimme et al. 2003; Wieland et al. 2004b), suggesting that noncytopathic mechanisms were active during this early phase of viral clearance. Interestingly, HBV cccDNA also decreased eightfold during the same time period, suggesting that cccDNA could, at least partially, be eliminated from hepatocytes by a noncytolytic mechanism (Guidotti et al. 1999; Wieland et al. 2004b). Thus, the chimpanzee model showed that the mechanism of CD8-dependent cytopathic and noncytopathic clearance of HBV, which was first discovered in the HBV transgenic mouse model (Guidotti et al. 1996), might be operative in the context of a natural HBV infection (Guidotti et al. 1999; Thimme et al. 2003; Wieland et al. 2004b; Murray et al. 2005).
Mechanisms of HBV Persistence
Neonatal tolerance to HBV is believed to be responsible for viral persistence following mother-to-infant transmission (Chisari and Ferrari 1995). Tolerance might be induced by the HBV precore protein (HBeAg), which has the capacity to cross the placenta, and, in HBV transgenic mice, induce neonatal tolerance (Milich et al. 1990). On the other hand, the basis for the blunted immune response, which is characteristic for adult onset chronic HBV infections, is not well understood (reviewed in Guidotti and Chisari 2006; Rehermann 2013). Potential contributing factors to HBV persistence in adults include viral escape mutants with altered B- and T-cell epitopes that can evade the immune response (reviewed in Wieland and Chisari 2005; Guidotti and Chisari 2006), specific inhibition of the adaptive immune response by viral proteins (Brunetto et al. 1991; Hu et al. 1999; Reignat et al. 2002; Chen et al. 2004; Ganem and Prince 2004), and the size of the inoculum as described below.
The Size of the Viral Inoculum Contributes to the Outcome of HBV Infections
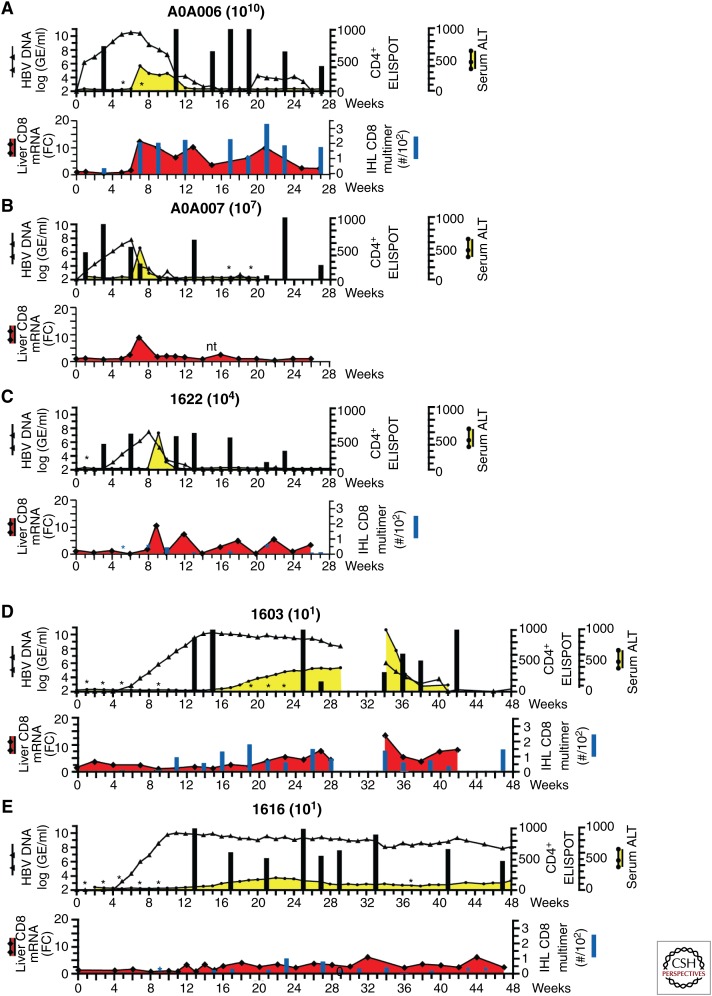
HBV infections in chimpanzees using a wide dose range of an inoculum showed that the size of the inoculum can influence the outcome of HBV infections (Asabe et al. 2009). Animals inoculated with 1010, 107, and 104 GE of HBV developed acute HBV infection and cleared the virus within 8–30 wk after its first detection (Fig. 3A–C). The incubation time was inversely correlated with the inoculum dose, but clearance followed the same pattern as previously observed in several other animals that had been inoculated with 108 GE (Fig. 2A,B) (Guidotti et al. 1999; Thimme et al. 2003). In contrast, two animals inoculated with 10 GE became chronically infected (Fig. 3D,E), with one of them (like many chronically infected humans) ultimately clearing the virus in the context of an acute disease flare-up 42 wk postinfection (p.i.), whereas the other remained infected for 55 wk p.i. when the study was terminated. This suggested that a viral dose >104 GE favors clearance (Fig. 3A–C), whereas doses between 104 and 1 GE favor persistent infections (Fig. 3D,E). Acute infections were associated with early CD4+ T-cell priming, either before or at the onset of detectable viral spread, which coincided with a sharply synchronized influx of HBV-specific CD8+ T cells into the liver and a corresponding increase in sALT activity and histological evidence of acute viral hepatitis. Interestingly, animals that developed chronic or prolonged infection showed the first detectable peripheral CD4+ T-cell response after the virus had infected almost all hepatocytes in the liver (Fig. 3) (Asabe et al. 2009). This correlated with an uncoordinated influx of HBV-specific CD8+ T cells into the liver and a correspondingly asynchronous increase in sALT activity (Asabe et al. 2009).
Figure 3.
Peripheral CD4+ T-cell responses against HBV core protein and intrahepatic CD8+ T-cell responses in chimpanzees infected with a dose range of HBV as described in Asabe et al. (2009). 1010 (A), 107 (B), 104 (C), and 101 (D,E) genome equivalents (GE). The upper panel in each part represents the serum HBV DNA as a black line and sALT as a yellow shaded area and the results of peripheral CD4+ T-cell ELISPOT assays are overlaid as black bars. Cryopreserved peripheral blood mononucleated cells (PBMCs) were thawed and stimulated in vitro with HBV core protein and the numbers of IFN-γ-producing cells were determined by ELISPOT assay as described in Asabe et al. (2009). The data are shown as number of spots at each time point minus the number of spots before inoculation per million PBMCs. The lower panel shows the total number of intrahepatic HBV-specific CD8+ T cells per 102 total CD8+ T cells as filled blue bars (right axis) and fold induction (FC) of intrahepatic CD8 messenger RNA (mRNA) compared with two preinoculation time points as a shaded red area (left axis). Intrahepatic lymphocytes were expanded antigen-nonspecifically in vitro and tested with all the corresponding Patr/peptide multimer complexes as described in Asabe et al. (2009). *Tested and negative. nt, not tested. (From Asabe et al. (2009); reproduced/amended, with permission, from the American Society for Microbiology © 2009; and from Chisari et al. (2010); with permission, from Elsevier Masson SAS © 2010.)
Early Priming of the CD4 T-Cell Response Is Required for Viral Clearance
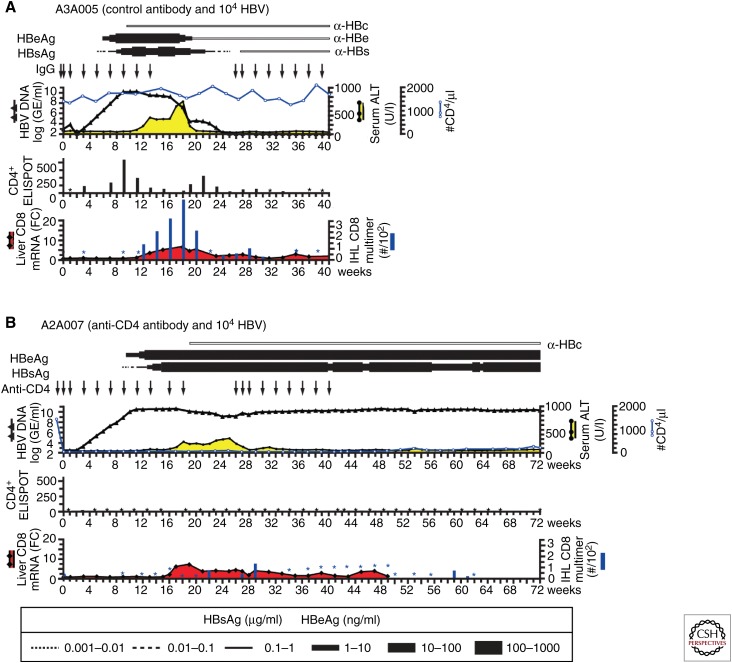
The above results suggested that an early CD4+ T-cell response to HBV infection is required to induce an effective CD8+ T-cell response, which is able to clear the infection. Indeed, inoculation of an animal that was depleted of CD4+ cells before inoculation with a virus dose of 104 GE (Fig. 4A,B) resulted in persistent HBV infection (Asabe et al. 2009). Importantly, as shown in Figure 2B, CD4+ T-cell depletion, using the same antibody at the peak of HBV infection, had no impact on the outcome of infection (Thimme et al. 2003). Together, these results suggest that the timing of CD4+ T-cell priming relative to the kinetics of viral spread is important in determining the magnitude and quality of the subsequent CD8+ T-cell response to HBV and, therefore, the outcome of HBV infection. It is conceivable that CD4+ T-cell priming, which occurred before detectable viremia in some animals infected with a high dose of HBV, could have been triggered by subviral particles that are present at a large molar excess relative to infectious virions in the inoculum (Fig. 3B,C) (Asabe et al. 2009). In contrast, the reduced amount of subviral particles present in low-dose inocula might not be sufficient to induce an early CD4+ T-cell response. As a consequence, CD8+ T-cell priming might occur in the liver, resulting in T-cell inactivation, tolerance, or apoptosis (Bertolino et al. 2002; Crispe 2003; Isogawa et al. 2005a).
Figure 4.
Course of HBV infections, peripheral CD4+ T-cell responses against HBV core protein, and intrahepatic CD8+ T-cell responses in chimpanzees without (A), or with (B) CD4 immunodepletion as described in Asabe et al. (2009). Serum HBV DNA levels are shown as a black line and sALT as a yellow shaded area. Horizontal bars represent serum HBe and HBs antigen levels and the open horizontal bars represent the presence of anti-HBc, -HBe, and -HBs antibodies. The amount of each protein is reflected by the thickness of each bar as indicated in the legend. The numbers of CD4+ T cells per μL of whole blood were shown as closed squares (top panel, right axis). Arrows on the top panels represent injections of control antibody (A), or anti-CD4 antibody (B). Peripheral CD4+ T-cell IFN-γ ELISPOT assays against HBV core protein (middle panel) and detection of intrahepatic HBV-specific CD8+ T cells (bottom panel, left axis) were as described in Figure 3, except that freshly prepared cells were used instead of using cryopreserved cells. Fold induction (FC) of intrahepatic CD8 mRNA compared with two preinoculation time points is shown as a shaded red area (bottom panel, right axis). *Tested and negative. (From Asabe et al. 2009; reproduced/amended, with permission, from the American Society for Microbiology © 2009; and from Chisari et al. (2010); with permission, from Elsevier Masson SAS © 2010.)
CONCLUDING REMARKS
Given the restricted host range of HBV, the chimpanzee model system has been, and still is, critical for the study of HBV infections. Even before HBV was characterized at the molecular level, chimpanzee infection experiments have been crucial in recognizing that hepatitis could be attributed to diverse viral agents transmitted by different routes of infection. With the molecular characterization of HBV, use of the chimpanzee model immediately led to greatly improved safety of blood products used by human patients. Most importantly, the chimpanzee model system enabled the quick development and testing of a very effective prophylactic vaccine, which is still in use today. Besides the immediate impact of those studies on public health, by preventing spread of HBV infection, the chimpanzee model also provided unique opportunities to perform longitudinal studies of the virus–host interactions during acute HBV infection. Those studies revealed that HBV acts like a stealth virus early in infection, remaining undetected and spreading until the onset of the adaptive immune response several weeks later. However, HBV can be controlled when properly activated HBV-specific CD8+ T cells enter the liver, recognize antigen, kill infected cells, and secrete IFN-γ, which triggers a broad-based cascade that amplifies the inflammatory process and also has noncytopathic antiviral activity against HBV. But, absence of proper CD4+ T-cell priming early in infection, as observed in low-dose infections, induces functionally impaired CD8+ T-cell responses, resulting in the establishment of persistent infections. Efforts to restore an effective CD8+ T-cell response in the chimpanzee have not yet been successful. Funding constraints, National Institutes of Health (NIH) restrictions on the use of the chimpanzee model, and the high costs involved with this animal model will increasingly limit its use for biomedical research and antiviral drug testing. Whether alternative animal models for HBV infections, such as the woodchuck, can replace the chimpanzee model remains to be seen.
ACKNOWLEDGMENTS
I thank all of my colleagues who made valuable contributions to the work cited in this review, especially Francis V. Chisari (The Scripps Research Institute, La Jolla, CA), Robert Purcell (NIAID, National Institutes of Health, Bethesda, MD), Luca Guidotti (The Scripps Research Institute, La Jolla, CA), Robert Thimme (University Hospital Freiburg, Freiburg, Germany), David Milich (Vaccine Research Institute of San Diego, San Diego, CA), Antonio Bertolleti (Singapore Institute for Clinical Sciences, Singapore), Carlo Ferrari (Laboratory of Viral Immunopathology, Parma, Italy), and Barbara Rehermann (NIDDK, National Institutes of Health, Bethesda, MD). Thank you to Francis V. Chisari and Urtzi Garaigorta for helpful discussions and critical reading of the manuscript. S.F.W. is supported by National Institutes of Health (NIH) Grants AI20001, CA40489, and CA54560 to Francis V. Chisari. This is manuscript number 26097 of The Scripps Research Institute.
Footnotes
Editors: Christoph Seeger and Stephen Locarnini
Additional Perspectives on Hepatitis B and Delta Viruses available at www.perspectivesinmedicine.org
REFERENCES
- Acs G, Sells MA, Purcell RH, Price P, Engle R, Shapiro M, Popper H. 1987. Hepatitis B virus produced by transfected Hep G2 cells causes hepatitis in chimpanzees. Proc Natl Acad Sci 84: 4641–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter HJ, Creagan RP, Morel PA, Wiesehahn GP, Dorman BP, Corash L, Smith GC, Popper H, Eichberg JW. 1988. Photochemical decontamination of blood components containing hepatitis B and non-A, non-B virus. Lancet 2: 1446–1450. [DOI] [PubMed] [Google Scholar]
- Ando K, Guidotti LG, Wirth S, Ishikawa T, Missale G, Moriyama T, Schreiber RD, Schlicht HJ, Huang SN, Chisari FV. 1994. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J Immunol 152: 3245–3253. [PubMed] [Google Scholar]
- Asabe S, Wieland SF, Chattopadhyay PK, Roederer M, Engle RE, Purcell RH, Chisari FV. 2009. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol 83: 9652–9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft WH, Snitbhan R, Scott RM, Tingpalapong M, Watson WT, Tanticharoenyos P, Karwacki JJ, Srimarut S. 1977. Transmission of hepatitis B virus to gibbons by exposure to human saliva containing hepatitis B surface antigen. J Infect Dis 135: 79–85. [DOI] [PubMed] [Google Scholar]
- Barker LF, Chisari FV, McGrath PP, Dalgard DW, Kirschstein RL, Almeida JD, Edington TS, Sharp DG, Peterson MR. 1973. Transmission of type B viral hepatitis to chimpanzees. J Infect Dis 127: 648–662. [DOI] [PubMed] [Google Scholar]
- Barker LF, Maynard JE, Purcell RH, Hoofnagle JH, Berquist KR, London WT. 1975a. Viral hepatitis, type B, in experimental animals. Am J Med Sci 270: 189–195. [DOI] [PubMed] [Google Scholar]
- Barker LF, Maynard JE, Purcell RH, Hoofnagle JH, Berquist KR, London WT, Gerety RJ, Krushak DH. 1975b. Hepatitis B virus infection in chimpanzees: Titration of subtypes. J Infect Dis 132: 451–458. [DOI] [PubMed] [Google Scholar]
- Bertoletti A, Gehring AJ. 2013. Immune therapeutic strategies in chronic hepatitis B virus infection: Virus or inflammation control? PLoS Pathog 9: e1003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A, Ferrari C, Fiaccadori F, Penna A, Margolskee R, Schlicht HJ, Fowler P, Guilhot S, Chisari FV. 1991. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc Natl Acad Sci 88: 10445–10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A, D’Elios MM, Boni C, De Carli M, Zignego AL, Durazzo M, Missale G, Penna A, Fiaccadori F, Del Prete G, et al. 1997. Different cytokine profiles of intraphepatic T cells in chronic hepatitis B and hepatitis C virus infections. Gastroenterology 112: 193–199. [DOI] [PubMed] [Google Scholar]
- Bertolino P, McCaughan GW, Bowen DG. 2002. Role of primary intrahepatic T-cell activation in the “liver tolerance effect.” Immunol Cell Biol 80: 84–92. [DOI] [PubMed] [Google Scholar]
- Bertoni R, Sette A, Sidney J, Guidotti LG, Shapiro M, Purcell R, Chisari FV. 1998. Human class I supertypes and CTL repertoires extend to chimpanzees. J Immunol 161: 4447–4455. [PubMed] [Google Scholar]
- Bigger CB, Brasky KM, Lanford RE. 2001. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol 75: 7059–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg BS. 1964. Polymorphisms of the serum proteins and the development of iso-precipitins in transfused patients. Bull NY Acad Med 40: 377–386. [PMC free article] [PubMed] [Google Scholar]
- Blumberg BS, Sutnick AI, London WT. 1968. Hepatitis and leukemia: Their relation to Australia antigen. Bull NY Acad Med 44: 1566–1586. [PMC free article] [PubMed] [Google Scholar]
- Brotman B, Prince AM, Huima T, Richardson L, van den Ende MC, Pfeifer U. 1983. Interference between non-A, non-B and hepatitis B virus infection in chimpanzees. J Med Virol 11: 191–205. [DOI] [PubMed] [Google Scholar]
- Brummelhuis HG, Over J, Duivis-Vorst CC, Wilson-de Stürler LA, Ates G, Hoek PJ, Reerink-Brongers EE. 1983. Contributions to the optimal use of human blood: IX. Elimination of hepatitis B transmission by (potentially) infectious plasma derivatives. Vox Sang 45: 205–216. [DOI] [PubMed] [Google Scholar]
- Brunetto MR, Giarin MM, Oliveri F, Chiaberge E, Baldi M, Alfarano A, Serra A, Saracco G, Verme G, Will H, et al. 1991. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc Natl Acad Sci 88: 4186–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J, Lanford RE, Purcell RH. 2013. Persistent human hepatitis B virus infection in cynomolgus monkeys: A novel animal model in the search for a cure? Hepatology 58: 1533–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buynak EB, Roehm RR, Tytell AA, Bertland AU, Lampson GP, Hilleman MR. 1976. Development and chimpanzee testing of a vaccine against human hepatitis B. Proc Soc Exp Biol Med 151: 694–700. [DOI] [PubMed] [Google Scholar]
- Cattaneo R, Will H, Hernandez N, Schaller H. 1983. Signals regulating hepatitis B surface antigen transcription. Nature 305: 336–338. [DOI] [PubMed] [Google Scholar]
- Cattaneo R, Will H, Schaller H. 1984. Hepatitis B virus transcription in the infected liver. EMBO J 3: 2191–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh VJ, Guidotti LG, Chisari FV. 1998. Inhibition of hepatitis B virus replication during adenovirus and cytomegalovirus infections in transgenic mice. J Virol 72: 2630–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MT, Billaud JN, Sällberg M, Guidotti LG, Chisari FV, Jones J, Hughes J, Milich DR. 2004. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc Natl Acad Sci 101: 14913–14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari FV, Ferrari C. 1995. Hepatitis B virus immunopathogenesis. Annu Rev Immunol 13: 29–60. [DOI] [PubMed] [Google Scholar]
- Chisari FV, Isogawa M, Wieland SF. 2010. Pathogenesis of hepatitis B virus infection. Pathol Biol 58: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe IN. 2003. Hepatic T cells and liver tolerance. Nat Rev Immunol 3: 51–62. [DOI] [PubMed] [Google Scholar]
- Dagan S, Eren R. 2003. Therapeutic antibodies against viral hepatitis. Curr Opin Mol Ther 5: 148–155. [PubMed] [Google Scholar]
- Dane DS, Cameron CH, Briggs M. 1970. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet 1: 695–698. [DOI] [PubMed] [Google Scholar]
- Davis HL, McCluskie MJ, Gerin JL, Purcell RH. 1996. DNA vaccine for hepatitis B: Evidence for immunogenicity in chimpanzees and comparison with other vaccines. Proc Natl Acad Sci 93: 7213–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt F. 1976. Hepatitis in primates. Adv Virus Res 20: 113–157. [DOI] [PubMed] [Google Scholar]
- Deinhardt F, Wolfe L, Junge U, Holmes AW. 1972. Viral hepatitis in non-human primates. Can Med Assoc J 106: 468–472. [PMC free article] [PubMed] [Google Scholar]
- Dupinay T, Gheit T, Roques P, Cova L, Chevallier-Queyron P, Tasahsu S-I, Le Grand R, Simon F, Cordier G, Wakrim L, et al. 2013. Discovery of naturally occurring transmissible chronic hepatitis B virus infection among Macaca fascicularis from Mauritius Island. Hepatology 58: 1610–1620. [DOI] [PubMed] [Google Scholar]
- Emini EA, Larson V, Eichberg J, Conard P, Garsky VM, Lee DR, Ellis RW, Miller WJ, Anderson CA, Gerety RJ. 1989. Protective effect of a synthetic peptide comprising the complete preS2 region of the hepatitis B virus surface protein. J Med Virol 28: 7–12. [DOI] [PubMed] [Google Scholar]
- Eren R, Ilan E, Nussbaum O, Lubin I, Terkieltaub D, Arazi Y, Ben-Moshe O, Kitchinzky A, Berr S, Gopher J, et al. 2000. Preclinical evaluation of two human anti-hepatitis B virus (HBV) monoclonal antibodies in the HBV-trimera mouse model and in HBV chronic carrier chimpanzees. Hepatology 32: 588–596. [DOI] [PubMed] [Google Scholar]
- Eren R, Landstein D, Terkieltaub D, Nussbaum O, Zauberman A, Ben-Porath J, Gopher J, Buchnick R, Kovjazin R, Rosenthal-Galili Z, et al. 2006. Preclinical evaluation of two neutralizing human monoclonal antibodies against hepatitis C virus (HCV): A potential treatment to prevent HCV reinfection in liver transplant patients. J Virol 80: 2654–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon V, Baranosky N, Castro FO, Montero C, González M, Hayes O, Ancheta O, Gra B, Mandado S. 1993. Ultrastructural and immunocytochemical characteristics of hepatocytes from hepatitis B virus infected chimpanzees. Tissue Cell 25: 865–873. [DOI] [PubMed] [Google Scholar]
- Feinstone SM, Mihalik KB, Kamimura T, Alter HJ, London WT, Purcell RH. 1983. Inactivation of hepatitis B virus and non-A, non-B hepatitis by chloroform. Infect Immun 41: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C, Penna A, Giuberti T, Tong MJ, Ribera E, Fiaccadori F, Chisari FV. 1987. Intrahepatic, nucleocapsid antigen-specific T cells in chronic active hepatitis B. J Immunol 139: 2050–2058. [PubMed] [Google Scholar]
- Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, Giuberti T, Cavalli A, Petit MA, Fiaccadori F. 1990. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol 145: 3442–3449. [PubMed] [Google Scholar]
- Franco A, Guidotti LG, Hobbs MV, Pasquetto V, Chisari FV. 1997. Pathogenetic effector function of CD4-positive T helper 1 cells in hepatitis B virus transgenic mice. J Immunol 159: 2001–2008. [PubMed] [Google Scholar]
- Fujisawa Y, Kuroda S, van Eerd PM, Schellekens H, Kakinuma A. 1990. Protective efficacy of a novel hepatitis B vaccine consisting of M (pre-S2 + S) protein particles (a third generation vaccine). Vaccine 8: 192–198. [DOI] [PubMed] [Google Scholar]
- Ganem D, Prince AM. 2004. Hepatitis B virus infection—Natural history and clinical consequences. N Engl J Med 350: 1118–1129. [DOI] [PubMed] [Google Scholar]
- Gerety RJ, Tabor E, Purcell RH, Tyeryar FJ. 1979. Summary of an international workshop on hepatitis B vaccines. N Engl J Med 140: 642–648. [DOI] [PubMed] [Google Scholar]
- Gerin JL. 2001. Animal models of hepatitis delta virus infection and disease. ILAR J 42: 103–106. [DOI] [PubMed] [Google Scholar]
- Gheit T, Sekkat S, Cova L, Chevallier M, Petit M-A, Hantz O, Lesénéchal M, Benslimane A, Trépo C, Chemin I. 2002. Experimental transfection of Macaca sylvanus with cloned human hepatitis B virus. J Gen Virol 83: 1645–1649. [DOI] [PubMed] [Google Scholar]
- Gocke DJ, Kavey NB. 1969. Hepatitis antigen. Correlation with disease and infectivity of blood-donors. Lancet 1: 1055–1059. [DOI] [PubMed] [Google Scholar]
- Grethe S, Heckel JO, Rietschel W, Hufert FT. 2000. Molecular epidemiology of hepatitis B virus variants in nonhuman primates. J Virol 74: 5377–5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG, Chisari FV. 2006. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol 1: 23–61. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, Chisari FV. 1994. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci 91: 3764–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4: 25–36. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284: 825–829. [DOI] [PubMed] [Google Scholar]
- Hilleman MR, Buynak EB, Roehm RR, Tytell AA, Bertland AU, Lampson GP. 1975. Purified and inactivated human hepatitis B vaccine: Progress report. Am J Med Sci 270: 401–404. [DOI] [PubMed] [Google Scholar]
- Hillis WD. 1961. An outbreak of infectious hepatitis among chimpanzee handlers at a United States Air Force Base. Am J Hyg 73: 316–328. [DOI] [PubMed] [Google Scholar]
- Hillis WD. 1963. Viral hepatitis associated with sub-human primates. Transfusion 3: 445–454. [DOI] [PubMed] [Google Scholar]
- Hirschman RJ, Shulman NR, Barker LF, Smith KO. 1969. Virus-like particles in sera of patients with infectious and serum hepatitis. JAMA 208: 1667–1670. [PubMed] [Google Scholar]
- Hollinger FB, Dolana G, Thomas W, Gyorkey F. 1984. Reduction in risk of hepatitis transmission by heat-treatment of a human factor VIII concentrate. J Infect Dis 150: 250–262. [DOI] [PubMed] [Google Scholar]
- Hong HJ, Ryu CJ, Hur H, Kim S, Oh HK, Oh MS, Park SY. 2004. In vivo neutralization of hepatitis B virus infection by an anti-preS1 humanized antibody in chimpanzees. Virology 318: 134–141. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH. 1981. Serologic markers of hepatitis B virus infection. Annu Rev Med 32: 1–11. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Purcell RH, Farshid M, Lachenbruch PA, Yu M-YW. 2006. Quantification of hepatitis B virus genomes and infectivity in human serum samples. Transfusion 46: 1829–1835. [DOI] [PubMed] [Google Scholar]
- Hu Z, Zhang Z, Doo E, Coux O, Goldberg AL, Liang TJ. 1999. Hepatitis B virus X protein is both a substrate and a potential inhibitor of the proteasome complex. J Virol 73: 7231–7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Margolis HS, Purcell RH, Ebert J, Robertson BH. 2000. Identification of hepatitis B virus indigenous to chimpanzees. Proc Natl Acad Sci 97: 1661–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Javadian A, Gagneux P, Robertson BH. 2001. Paired chimpanzee hepatitis B virus (ChHBV) and mtDNA sequences suggest different ChHBV genetic variants are found in geographically distinct chimpanzee subspecies. Virus Res 79: 103–108. [DOI] [PubMed] [Google Scholar]
- Ibrahim AB, Vyas GN, Prince AM. 1974. Studies on delayed hypersensitivity to hepatitis B antigen in chimpanzees. Clin Exp Immunol 17: 311–318. [PMC free article] [PubMed] [Google Scholar]
- Immunization Practices Advisory Committee. 1982. Inactivated hepatitis B virus vaccine. MMWR 31: 317–328. [PubMed] [Google Scholar]
- Isogawa M, Furuichi Y, Chisari FV. 2005a. Oscillating CD8+ T cell effector functions after antigen recognition in the liver. Immunity 23: 53–63. [DOI] [PubMed] [Google Scholar]
- Isogawa M, Robek MD, Furuichi Y, Chisari FV. 2005b. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol 79: 7269–7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Takai E, Ohnuma H, Kitajima K, Tsuda F, Machida A, Mishiro S, Nakamura T, Miyakawa Y, Mayumi M. 1986. A synthetic peptide vaccine involving the product of the pre-S(2) region of hepatitis B virus DNA: Protective efficacy in chimpanzees. Proc Natl Acad Sci 83: 9174–9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwarson S, Tabor E, Thomas HC, Snoy P, Gerety RJ. 1985. Protection against hepatitis B virus infection by immunization with hepatitis B core antigen. Gastroenterology 88: 763–767. [DOI] [PubMed] [Google Scholar]
- Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med 192: 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamili S. 2010. Infectivity and vaccination efficacy studies in animal models of HBV S and pol gene mutants. Antivir Ther 15: 477–485. [DOI] [PubMed] [Google Scholar]
- Kamili S, Sozzi V, Thompson G, Campbell K, Walker CM, Locarnini S, Krawczynski K. 2009. Efficacy of hepatitis B vaccine against antiviral drug-resistant hepatitis B virus mutants in the chimpanzee model. Hepatology 49: 1483–1491. [DOI] [PubMed] [Google Scholar]
- Karasawa T, Shikata T, Abe K, Kanayama S, Noro M, Oda T. 1985a. HBV-associated ultrastructures in the chimpanzees’ livers with experimental hepatitis B. Acta Pathol Jpn 35: 1333–1342. [DOI] [PubMed] [Google Scholar]
- Karasawa T, Shikata T, Abe K, Kondo R, Noro M, Oda T. 1985b. Ultrastructural studies on liver cell necrosis and lymphocytes in experimental hepatitis B. Acta Pathol Jpn 35: 1359–1374. [DOI] [PubMed] [Google Scholar]
- Kim S-H, Kim S-H, Oh HK, Ryu CJ, Park SY, Hong HJ. 2008a. In vivo hepatitis B virus-neutralizing activity of an anti-HBsAg humanized antibody in chimpanzees. Exp Mol Med 40: 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-H, Shin YW, Hong K-W, Chang K-H, Ryoo K-H, Paik S-H, Kim J-M, Brotman B, Pfahler W, Prince AM. 2008b. Neutralization of hepatitis B virus (HBV) by human monoclonal antibody against HBV surface antigen (HBsAg) in chimpanzees. Antiviral Res 79: 188–191. [DOI] [PubMed] [Google Scholar]
- Klinkert MQ, Theilmann L, Pfaff E, Schaller H. 1986. Pre-S1 antigens and antibodies early in the course of acute hepatitis B virus infection. J Virol 58: 522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Tsuzuki M, Koshimizu K, Toyama H, Yoshihara N, Shikata T, Abe K, Mizuno K, Otomo N, Oda T. 1984. Susceptibility of hepatitis B virus to disinfectants or heat. J Clin Microbiol 20: 214–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y, Katayama K, Yugi H, Mizui M, Matsukura H, Tomoguri T, Miyakawa Y, Tabuchi A, Tanaka J, Yoshizawa H. 2008. Minimum infectious dose of hepatitis B virus in chimpanzees and difference in the dynamics of viremia between genotype A and genotype C. Transfusion 48: 286–294. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fujisawa Y, Iino S, Akahane Y, Suzuki H. 1991. Induction of protection level of anti-pre-S2 antibodies in humans immunized with a novel hepatitis B vaccine consisting of M (pre-S2 + S) protein particles (a third generation vaccine). Vaccine 9: 163–169. [DOI] [PubMed] [Google Scholar]
- Kwon H, Lok AS. 2011. Hepatitis B therapy. Nat Rev Gastroenterol Hepatol 8: 275–284. [DOI] [PubMed] [Google Scholar]
- Lanford RE, Chavez D, Brasky KM, Burns RB3, Rico-Hesse R. 1998. Isolation of a hepadnavirus from the woolly monkey, a New World primate. Proc Natl Acad Sci 95: 5757–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford RE, Chavez D, Rico-Hesse R, Mootnick A. 2000. Hepadnavirus infection in captive gibbons. J Virol 74: 2955–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, et al. 2013. GS-9620, an oral agonist of Toll-like Receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 144: 1508–1517 1517.e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelie PN, Reesink HW, Niessen J, Brotman B, Prince AM. 1987. Inactivation of 1015 chimpanzee-infectious doses of hepatitis B virus during preparation of a heat-inactivated hepatitis B vaccine. J Med Virol 23: 289–295. [DOI] [PubMed] [Google Scholar]
- Lichter EA. 1969. Chimpanzee antibodies to Australia antigen. Nature 224: 810–811. [DOI] [PubMed] [Google Scholar]
- Lin L, Hanson CV, Alter HJ, Jauvin V, Bernard KA, Murthy KK, Metzel P, Corash L. 2005. Inactivation of viruses in platelet concentrates by photochemical treatment with amotosalen and long-wavelength ultraviolet light. Transfusion 45: 580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Kosinska A, Lu M, Roggendorf M. 2014. New therapeutic vaccination strategies for the treatment of chronic hepatitis B. Virol Sin 29: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London WT, Alter HJ, Lander J, Purcell RH. 1972. Serial transmission in rhesus monkeys of an agent related to hepatitis-associated antigen. J Infect Dis 125: 382–389. [DOI] [PubMed] [Google Scholar]
- Lubeck MD, Davis AR, Chengalvala M, Natuk RJ, Morin JE, Molnar-Kimber K, Mason BB, Bhat BM, Mizutani S, Hung PP. 1989. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc Natl Acad Sci 86: 6763–6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald DM, Holmes EC, Lewis JC, Simmonds P. 2000. Detection of hepatitis B virus infection in wild-born chimpanzees (Pan troglodytes verus): Phylogenetic relationships with human and other primate genotypes. J Virol 74: 4253–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A, et al. 2000. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 191: 1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markenson JA, Gerety RJ, Hoofnagle JH, Barker LF. 1975. Effects of cyclophosphamide on hepatitis B virus infection and challenge in chimpanzees. J Infect Dis 131: 79–87. [DOI] [PubMed] [Google Scholar]
- Mason WS, Low HC, Xu C, Aldrich CE, Scougall CA, Grosse A, Clouston A, Chavez D, Litwin S, Peri S, et al. 2009. Detection of clonally expanded hepatocytes in chimpanzees with chronic hepatitis B virus infection. J Virol 83: 8396–8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard JE, Hartwell WV, Berquist KR. 1971. Hepatitis-associated antigen in chimpanzees. J Infect Dis 123: 660–664. [DOI] [PubMed] [Google Scholar]
- Maynard JE, Berquist KR, Krushak DH, Purcell RH. 1972. Experimental infection of chimpanzees with the virus of hepatitis B. Nature 237: 514–515. [DOI] [PubMed] [Google Scholar]
- Maynard JE, Krushak DH, Bradley DW, Berquist KR. 1975. Infectivity studies of hepatitis A and B in non-human primates. Dev Biol Stand 30: 229–235. [PubMed] [Google Scholar]
- McAuliffe VJ, Purcell RH, Gerin JL. 1980. Type B hepatitis: A review of current prospects for a safe and effective vaccine. Rev Infect Dis 2: 470–492. [DOI] [PubMed] [Google Scholar]
- McClary H, Koch R, Chisari FV, Guidotti LG. 2000a. Inhibition of hepatitis B virus replication during Schistosoma mansoni infection in transgenic mice. J Exp Med 192: 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClary H, Koch R, Chisari FV, Guidotti LG. 2000b. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol 74: 2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney DM, Erickson AL, Walker CM, Thimme R, Chisari FV, Sidney J, Sette A. 2000. Identification of five different Patr class I molecules that bind HLA supertype peptides and definition of their peptide binding motifs. J Immunol 165: 4414–4422. [DOI] [PubMed] [Google Scholar]
- Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. 1990. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci 87: 6599–6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman I, Loeb LA, Bayer ME, Blumberg BS. 1970. Australia antigen (a hepatitis-associated antigen): Purification and physical properties. J Exp Med 131: 1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monjardino J, Fowler MJ, Montano L, Weller I, Tsiquaye KN, Zuckerman AJ, Jones DM, Thomas HC. 1982. Analysis of hepatitis virus DNA in the liver and serum of HBe antigen positive chimpanzee carriers. J Med Virol 9: 189–199. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Guilhot S, Klopchin K, Moss B, Pinkert CA, Palmiter RD, Brinster RL, Kanagawa O, Chisari FV. 1990. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science 248: 361–364. [DOI] [PubMed] [Google Scholar]
- Moss B, Smith GL, Gerin JL, Purcell RH. 1984. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature 311: 67–69. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Maynard JE, Le Bouvier GL. 1974. Viral subtypes and cross-protection in hepatitis B virus infections of chimpanzees. Intervirology 3: 378–381. [DOI] [PubMed] [Google Scholar]
- Murray K, Bruce SA, Wingfield P, van Eerd P, de Reus A, Schellekens H. 1987. Protective immunisation against hepatitis B with an internal antigen of the virus. J Med Virol 23: 101–107. [DOI] [PubMed] [Google Scholar]
- Murray JM, Wieland SF, Purcell RH, Chisari FV. 2005. Dynamics of hepatitis B virus clearance in chimpanzees. Proc Natl Acad Sci 102: 17780–17785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath AR, Kent SB, Parker K, Prince AM, Strick N, Brotman B, Sproul P. 1986. Antibodies to a synthetic peptide from the preS 120–145 region of the hepatitis B virus envelope are virus neutralizing. Vaccine 4: 35–37. [DOI] [PubMed] [Google Scholar]
- Norder H, Ebert JW, Fields HA, Mushahwar IK, Magnius LO. 1996. Complete sequencing of a gibbon hepatitis B virus genome reveals a unique genotype distantly related to the chimpanzee hepatitis B virus. Virology 218: 214–223. [DOI] [PubMed] [Google Scholar]
- Ogata N, Miller RH, Ishak KG, Purcell RH. 1993. The complete nucleotide sequence of a pre-core mutant of hepatitis B virus implicated in fulminant hepatitis and its biological characterization in chimpanzees. Virology 194: 263–276. [DOI] [PubMed] [Google Scholar]
- Ogata N, Zanetti AR, Yu M, Miller RH, Purcell RH. 1997. Infectivity and pathogenicity in chimpanzees of a surface gene mutant of hepatitis B virus that emerged in a vaccinated infant. J Infect Dis 175: 511–523. [DOI] [PubMed] [Google Scholar]
- Ogata N, Cote PJ, Zanetti AR, Miller RH, Shapiro M, Gerin J, Purcell RH. 1999. Licensed recombinant hepatitis B vaccines protect chimpanzees against infection with the prototype surface gene mutant of hepatitis B virus. Hepatology 30: 779–786. [DOI] [PubMed] [Google Scholar]
- Page M, Jones CD, Bailey C. 2001. A novel, recombinant triple antigen hepatitis B vaccine (Hepacare). Intervirology 44: 88–97. [DOI] [PubMed] [Google Scholar]
- Pancholi P, Lee DH, Liu Q, Tackney C, Taylor P, Perkus M, Andrus L, Brotman B, Prince AM. 2001. DNA prime/canarypox boost-based immunotherapy of chronic hepatitis B virus infection in a chimpanzee. Hepatology 33: 448–454. [DOI] [PubMed] [Google Scholar]
- Pasquetto V, Guidotti LG, Kakimi K, Tsuji M, Chisari FV. 2000. Host–virus interactions during malaria infection in hepatitis B virus transgenic mice. J Exp Med 192: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payette PJ, Ma X, Weeratna RD, McCluskie MJ, Shapiro M, Engle RE, Davis HL, Purcell RH. 2006. Testing of CpG-optimized protein and DNA vaccines against the hepatitis B virus in chimpanzees for immunogenicity and protection from challenge. Intervirology 49: 144–151. [DOI] [PubMed] [Google Scholar]
- Penna A, Chisari FV, Bertoletti A, Missale G, Fowler P, Giuberti T, Fiaccadori F, Ferrari C. 1991. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J Exp Med 174: 1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince AM. 1968. An antigen detected in the blood during the incubation period of serum hepatitis. Proc Natl Acad Sci 60: 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince AM. 2001. Perspectives on prophylactic and therapeutic immunization against hepatitis B and C viruses. Transfus Clin Biol 8: 467–470. [DOI] [PubMed] [Google Scholar]
- Prince AM, Stephan W, Brotman B. 1983a. β-Propiolactone/ultraviolet irradiation: A review of its effectiveness for inactivation of viruses in blood derivatives. Rev Infect Dis 5: 92–107. [DOI] [PubMed] [Google Scholar]
- Prince AM, Stephan W, Kotitschke R, Brotman B. 1983b. Inactivation of hepatitis B and non-A, non-B viruses by combined use of Tween 80, β-propiolactone, and ultraviolet irradiation. Thromb Haemost 50: 534–536. [PubMed] [Google Scholar]
- Prince AM, Horowitz B, Brotman B, Huima T, Richardson L, van den Ende MC. 1984a. Inactivation of hepatitis B and Hutchinson strain non-A, non-B hepatitis viruses by exposure to Tween 80 and ether. Vox Sang 46: 36–43. [DOI] [PubMed] [Google Scholar]
- Prince AM, Vnek J, Brotman B. 1984b. An affordable multideterminant plasma-derived hepatitis B virus vaccine. IARC Sci Publ 63: 355–372. [PubMed] [Google Scholar]
- Prince AM, Brotman B, Purcell RH, Gerin JL. 1985. A final report on safety and immunogenicity of a bivalent aqueous subunit HBV vaccine. J Med Virol 15: 399–419. [DOI] [PubMed] [Google Scholar]
- Prince DL, Prince HN, Thraenhart O, Muchmore E, Bonder E, Pugh J. 1993. Methodological approaches to disinfection of human hepatitis B virus. J Clin Microbiol 31: 3296–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince AM, Whalen R, Brotman B. 1997. Successful nucleic acid based immunization of newborn chimpanzees against hepatitis B virus. Vaccine 15: 916–919. [DOI] [PubMed] [Google Scholar]
- Purcell RH, Gerin JL. 1975. Hepatitis B subunit vaccine: A preliminary report of safety and efficacy tests in chimpanzees. Am J Med Sci 270: 395–399. [PubMed] [Google Scholar]
- Purcell RH, London WT, McAuliffe VJ, Palmer AE, Kaplan PM, Gerin JL, Wagner J, Popper H, Lvovsky E, Wong DC, et al. 1976. Modification of chronic hepatitis-B virus infection in chimpanzees by administration of an interferon inducer. Lancet 2: 757–761. [DOI] [PubMed] [Google Scholar]
- Purcell RH, Gerin JL, Popper H, London WT, Cicmanec J, Eichberg JW, Newman J, Hrinda ME. 1985. Hepatitis B virus, hepatitis non-A, non-B virus and hepatitis delta virus in lyophilized antihemophilic factor: Relative sensitivity to heat. Hepatology 5: 1091–1099. [DOI] [PubMed] [Google Scholar]
- Rehermann B. 2000. Intrahepatic T cells in hepatitis B: Viral control versus liver cell injury. J Exp Med 191: 1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B. 2013. Pathogenesis of chronic viral hepatitis: Differential roles of T cells and NK cells. Nat Med 19: 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M, Moss B, Sette A, Chisari FV. 1995. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med 181: 1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reignat S, Webster GJM, Brown D, Ogg GS, King A, Seneviratne SL, Dusheiko G, Williams R, Maini MK, Bertoletti A. 2002. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med 195: 1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers SL, Keeling M. 1970. A study of the incidence of Australia antigen and antibody in nonhuman primates. Vox Sang 19: 270–272. [PubMed] [Google Scholar]
- Rizzetto M, Canese MG, Aricò S, Crivelli O, Trépo C, Bonino F, Verme G. 1977. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 18: 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M, Canese MG, Gerin JL, London WT, Sly DL, Purcell RH. 1980a. Transmission of the hepatitis B virus-associated delta antigen to chimpanzees. J Infect Dis 141: 590–602. [DOI] [PubMed] [Google Scholar]
- Rizzetto M, Hoyer B, Canese MG, Shih JW, Purcell RH, Gerin JL. 1980b. DDelta agent: Association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci 77: 6124–6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Opazo N, Chakraborty PR, Shafritz DA. 1982a. Characterization of viral genomes in the liver and serum of chimpanzee long-term hepatitis B virus carriers: A possible role for supercoiled HBV-DNA in persistent HBV infection. J Cell Biochem 19: 281–292. [DOI] [PubMed] [Google Scholar]
- Ruiz-Opazo N, Chakraborty PR, Shafritz DA. 1982b. Evidence for supercoiled hepatitis B virus DNA in chimpanzee liver and serum Dane particles: Possible implications in persistent HBV infection. Cell 29: 129–136. [DOI] [PubMed] [Google Scholar]
- Sabin AB. 1955a. Behavior of chimpanzee avirulent poliomyelitis viruses in experimentally infected human volunteers. Am J Med Sci 230: 1–8. [DOI] [PubMed] [Google Scholar]
- Sabin AB. 1955b. Immunization of chimpanzees and human beings with avirulent strains of poliomyelitis virus. Ann NY Acad Sci 61: 1050–1056. [DOI] [PubMed] [Google Scholar]
- Sall AA, Starkman S, Reynes JM, Lay S, Nhim T, Hunt M, Marx N, Simmonds P. 2005. Frequent infection of Hylobates pileatus (pileated gibbon) with species-associated variants of hepatitis B virus in Cambodia. J Gen Virol 86: 333–337. [DOI] [PubMed] [Google Scholar]
- Sällberg M, Hughes J, Javadian A, Ronlov G, Hultgren C, Townsend K, Anderson CG, O’Dea J, Alfonso J, Eason R, et al. 1998. Genetic immunization of chimpanzees chronically infected with the hepatitis B virus, using a recombinant retroviral vector encoding the hepatitis B virus core antigen. Hum Gene Ther 9: 1719–1729. [DOI] [PubMed] [Google Scholar]
- Samuel CE. 1991. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology 183: 1–11. [DOI] [PubMed] [Google Scholar]
- Schaff Z, Eder G, Eder C, Lapis K. 1992. Ultrastructure of normal and hepatitis virus infected human and chimpanzee liver: Similarities and differences. Acta Morphol Hung 40: 203–214. [PubMed] [Google Scholar]
- Scott RM, Snitbhan R, Bancroft WH, Alter HJ, Tingpalapong M. 1980. Experimental transmission of hepatitis B virus by semen and saliva. J Infect Dis 142: 67–71. [DOI] [PubMed] [Google Scholar]
- Seeger C, Mason WS. 2000. Hepatitis B virus biology. Microbiol Mol Biol Rev 64: 51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shata MTM, Pfahler W, Brotman B, Lee D-H, Tricoche N, Murthy K, Prince AM. 2006. Attempted therapeutic immunization in a chimpanzee chronic HBV carrier with a high viral load. J Med Primatol 35: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata T, Karasawa T, Abe K, Uzawa T, Suzuki H, Oda T, Imai M, Mayumi M, Moritsugu Y. 1977. Hepatitis B e antigen and infectivity of hepatitis B virus. J Infect Dis 136: 571–576. [DOI] [PubMed] [Google Scholar]
- Shikata T, Karasawa T, Abe K, Takahashi T, Mayumi M, Oda T. 1978. Incomplete inactivation of hepatitis B virus after heat treatment at 60°C for 10 hours. J Infect Dis 138: 242–244. [DOI] [PubMed] [Google Scholar]
- Shikata T, Karasawa T, Abe K. 1980. Two distinct types of hepatitis in experimental hepatitis B virus infection. Am J Pathol 99: 353–368. [PMC free article] [PubMed] [Google Scholar]
- Shouval D, Chakraborty PR, Ruiz-Opazo N, Baum S, Spigland I, Muchmore E, Gerber MA, Thung SN, Popper H, Shafritz DA. 1980. Chronic hepatitis in chimpanzee carriers of hepatitis B virus: Morphologic, immunologic, and viral DNA studies. Proc Natl Acad Sci 77: 6147–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidney J, Asabe S, Peters B, Purton K-A, Chung J, Pencille TJ, Purcell R, Walker CM, Chisari FV, Sette A. 2006. Detailed characterization of the peptide binding specificity of five common Patr class I MHC molecules. Immunogenetics 58: 559–570. [DOI] [PubMed] [Google Scholar]
- Sidney J, Peters B, Moore C, Pencille TJ, Ngo S, Masterman K-A, Asabe S, Pinilla C, Chisari FV, Sette A. 2007. Characterization of the peptide-binding specificity of the chimpanzee class I alleles A 0301 and A 0401 using a combinatorial peptide library. Immunogenetics 59: 745– 751. [DOI] [PubMed] [Google Scholar]
- Smetana HF. 1965. Experimental and spontaneous viral pepatitis in primates. Lab Invest 14: 1366–1374. [PubMed] [Google Scholar]
- Smetana HF, Felsenfeld AD. 1969. Viral hepatitis in subhuman primates and its relationship to human viral hepatitis. Virchows Arch A Pathol Pathol Anat 348: 309–327. [DOI] [PubMed] [Google Scholar]
- Sonneveld MJ, Janssen HLA. 2011. Chronic hepatitis B: Peginterferon or nucleos(t)ide analogues? Liver Int 31: 78–84. [DOI] [PubMed] [Google Scholar]
- Soper FL, Smith HH. 1938. Yellow fever vaccination with cultivated virus and immune and hyperimmune serum. Am J Trop Med s1–s18: 111–134. [Google Scholar]
- Stephan W, Berthold H, Prince AM. 1981. Effect of combined treatment of serum containing hepatitis B virus with β-propiolactone and ultraviolet irradiation. Vox Sang 41: 134–138. [DOI] [PubMed] [Google Scholar]
- Stephan W, Prince AM, Brotman B. 1984. Modulation of hepatitis B infection by intravenous application of an immunoglobulin preparation that contains antibodies to hepatitis B e and core antigens but not to hepatitis B surface antigen. J Virol 51: 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R, Wieland S, Bukh J, Purcell RH, Schultz PG, et al. 2002. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci 99: 15669–15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J. 1988. The replication cycle of hepatitis B viruses. Cancer 61: 1957–1962. [DOI] [PubMed] [Google Scholar]
- Sureau C, Eichberg JW, Hubbard GB, Romet-Lemonne JL, Essex M. 1988. A molecularly cloned hepatitis B virus produced in vitro is infectious in a chimpanzee. J Virol 62: 3064–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor E, Aronson DL, Gerety RJ. 1980a. Removal of hepatitis-B-virus infectivity from factor-IX complex by hepatitis-B immune-globulin. Experiments in chimpanzees. Lancet 2: 68–70. [DOI] [PubMed] [Google Scholar]
- Tabor E, Frosner G, Deinhardt F, Gerety RJ. 1980b. Hepatitis B e antigen and antibody: Detection by radioimmunoassay in chimpanzees during experimental hepatitis B. J Med Virol 6: 91–99. [DOI] [PubMed] [Google Scholar]
- Tabor E, Copeland JA, Mann GF, Howard CR, Skelly J, Snoy P, Zuckerman AJ, Gerety RJ. 1981. Nondetection of infectious hepatitis B virus in a human hepatoma cell line producing hepatitis B surface antigen. Intervirology 15: 82–86. [DOI] [PubMed] [Google Scholar]
- Tabor E, Howard CR, Skelly J, Snoy P, Goudeau A, Zuckerman AJ, Gerety RJ. 1982. Immunogenicity in chimpanzees of experimental hepatitis B vaccines prepared from intact hepatitis B virus, purified polypeptides, or polypeptide micelles. J Med Virol 10: 65–74. [DOI] [PubMed] [Google Scholar]
- Tabor E, Buynak E, Smallwood LA, Snoy P, Hilleman M, Gerety RJ. 1983a. Inactivation of hepatitis B virus by three methods: Treatment with pepsin, urea, or formalin. J Med Virol 11: 1–9. [DOI] [PubMed] [Google Scholar]
- Tabor E, Purcell RH, Gerety RJ. 1983b. Primate animal models and titered inocula for the study of human hepatitis A, hepatitis B, and non-A, non-B hepatitis. J Med Primatol 12: 305–318. [PubMed] [Google Scholar]
- Tabor E, Purcell RH, London WT, Gerety RJ. 1983c. Use of and interpretation of results using inocula of hepatitis B virus with known infectivity titers. J Infect Dis 147: 531–534. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Miyakawa Y, Gotanda T, Mishiro S, Imai M, Mayumi M. 1979. Shift from free “small” hepatitis B e antigen to IgG-bound “large” form in the circulation of human beings and a chimpanzee acutely infected with hepatitis B virus. Gastroenterology 77: 1193–1199. [PubMed] [Google Scholar]
- Takahashi K, Brotman B, Usuda S, Mishiro S, Prince AM. 2000. Full-genome sequence analyses of hepatitis B virus (HBV) strains recovered from chimpanzees infected in the wild: Implications for an origin of HBV. Virology 267: 58–64. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Mishiro S, Prince AM. 2001. Novel hepatitis B virus strain from a chimpanzee of Central Africa (Pan troglodytes troglodytes) with an unusual antigenicity of the core protein. Intervirology 44: 321–326. [DOI] [PubMed] [Google Scholar]
- Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. 2003. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 77: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trépo C, Vnek J, Prince AM. 1975. Delayed hypersensitivity and Arthus reaction to purified hepatitis B surface antigen (HBsAg) in immunized chimpanzees. Clin Immunol Immunopathol 4: 528–537. [DOI] [PubMed] [Google Scholar]
- Tsui LV, Guidotti LG, Ishikawa T, Chisari FV. 1995. Posttranscriptional clearance of hepatitis B virus RNA by cytotoxic T lymphocyte-activated hepatocytes. Proc Natl Acad Sci 92: 12398–12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian J-P, Pineau P, Henry M, Hamilton WD, Muller MN, Wrangham RW, Wain-Hobson S. 2002. Identification of a hepatitis B virus genome in wild chimpanzees (Pan troglodytes schweinfurthi) from East Africa indicates a wide geographical dispersion among equatorial African primates. J Virol 76: 11155–11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudin M, Wolstenholme AJ, Tsiquaye KN, Zuckerman AJ, Harrison TJ. 1988. The complete nucleotide sequence of the genome of a hepatitis B virus isolated from a naturally infected chimpanzee. J Gen Virol 69: 1383–1389. [DOI] [PubMed] [Google Scholar]
- Wahl M, Iwarson S, Snoy P, Gerety RJ. 1989. Failure of hepatitis B immune globulin to protect against exp infection in chimpanzees. J Hepatol 9: 198–203. [DOI] [PubMed] [Google Scholar]
- Warren KS, Heeney JL, Swan RA, Heriyanto, Verschoor EJ. 1999. A new group of hepadnaviruses naturally infecting orangutans (Pongo pygmaeus). J Virol 73: 7860–7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland SF, Chisari FV. 2005. Stealth and cunning: Hepatitis B and hepatitis C viruses. J Virol 79: 9369–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland SF, Guidotti LG, Chisari FV. 2000. Intrahepatic induction of α/β interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J Virol 74: 4165–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Thimme R, Purcell RH, Chisari FV. 2004a. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci 101: 6669–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland SF, Spangenberg HC, Thimme R, Purcell RH, Chisari FV. 2004b. Expansion and contraction of the hepatitis B virus transcriptional template in infected chimpanzees. Proc Natl Acad Sci 101: 2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland SF, Asabe S, Engle RE, Purcell RH, Chisari FV. 2014. Limited hepatitis B virus replication space in the chronically hepatitis C virus-infected liver. J Virol 88: 5184–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will H, Cattaneo R, Koch HG, Darai G, Schaller H, Schellekens H, van Eerd PM, Deinhardt F. 1982. Cloned HBV DNA causes hepatitis in chimpanzees. Nature 299: 740–742. [DOI] [PubMed] [Google Scholar]
- Will H, Darai G, Deinhardt F, Schellekens H, Schaller H. 1983. Hepatitis B after infection of a chimpanzee with cloned HBV DNA. Dev Biol Stand 54: 131–133. [PubMed] [Google Scholar]
- Will H, Cattaneo R, Darai G, Deinhardt F, Schellekens H, Schaller H. 1985. Infectious hepatitis B virus from cloned DNA of known nucleotide sequence. Proc Natl Acad Sci 82: 891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Logan LM. 1975. Hepatitis B core antigen in the immunosuppressed chimpanzee. Dev Biol Stand 30: 240–243. [PubMed] [Google Scholar]
- Zolton RP, Padvelskis JV, Kaplan PM. 1985. Removal of hepatitis B virus infectivity from human γ-globulin prepared by ion-exchange chromatography. Vox Sang 49: 381–389. [DOI] [PubMed] [Google Scholar]