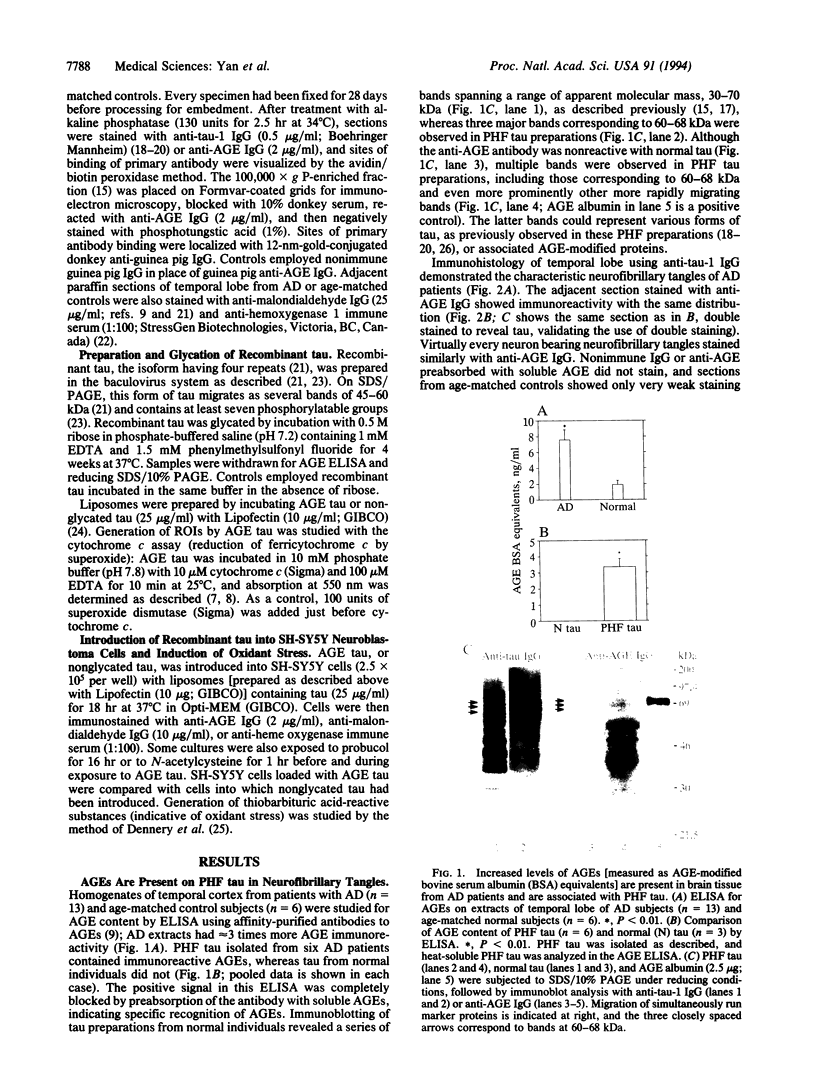
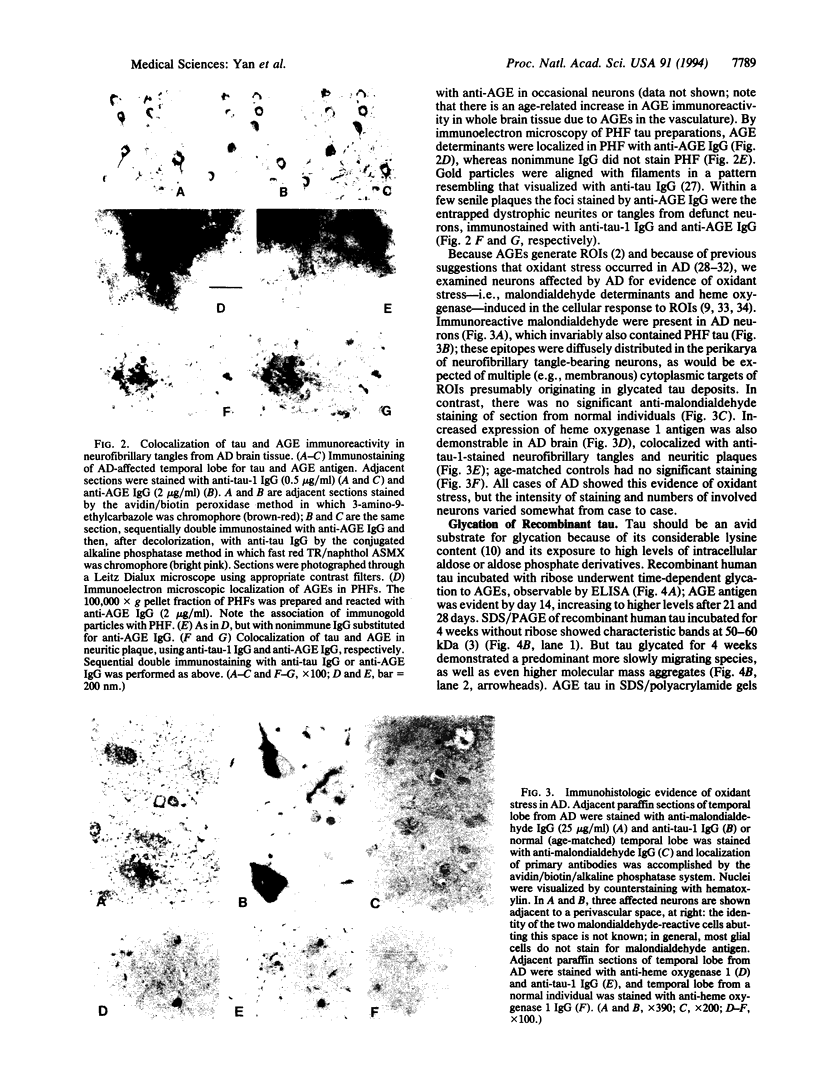
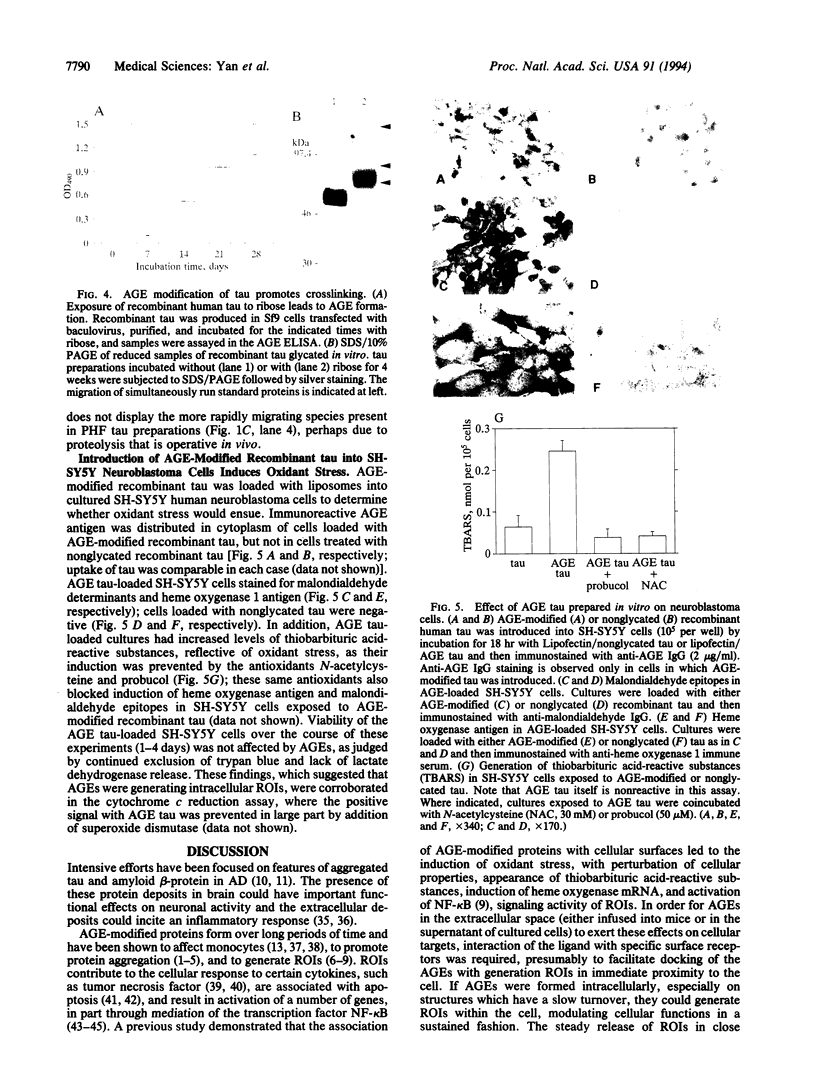
Abstract
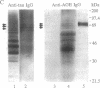
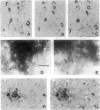
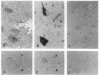
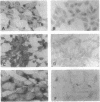
The stability of proteins that constitute the neurofibrillary tangles and senile plaques of Alzheimer disease suggests that they would be ideal substrates for nonenzymatic glycation, a process that occurs over long times, even at normal levels of glucose, ultimately resulting in the formation of advanced glycation end products (AGEs). AGE-modified proteins aggregate, and they generate reactive oxygen intermediates. Using monospecific antibody to AGEs, we have colocalized these AGEs with paired helical filament tau in neurofibrillary tangles in sporadic Alzheimer disease. Such neurons also exhibited evidence of oxidant stress: induction of malondialdehyde epitopes and heme oxygenase 1 antigen. AGE-recombinant tau generated reactive oxygen intermediates and, when introduced into the cytoplasm of SH-SY5Y neuroblastoma cells, induced oxidant stress. We propose that in Alzheimer disease, AGEs in paired helical filament tau can induce oxidant stress, thereby promoting neuronal dysfunction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Hirano T., Taga T., Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990 Aug;4(11):2860–2867. [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Balla G., Jacob H. S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J. W., Vercellotti G. M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992 Sep 5;267(25):18148–18153. [PubMed] [Google Scholar]
- Baynes J. W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991 Apr;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Bucala R., Makita Z., Koschinsky T., Cerami A., Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6434–6438. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennery P. A., Kramer C. M., Alpert S. E. Effect of fatty acid profiles on the susceptibility of cultured rabbit tracheal epithelial cells to hyperoxic injury. Am J Respir Cell Mol Biol. 1990 Aug;3(2):137–144. doi: 10.1165/ajrcmb/3.2.137. [DOI] [PubMed] [Google Scholar]
- Ewing J. F., Maines M. D. Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain: heme oxygenase 2 is not a heat shock protein. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5364–5368. doi: 10.1073/pnas.88.12.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Cairns N. J., Crowther R. A. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992 Jan;8(1):159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- Goedert M., Wischik C. M., Crowther R. A., Walker J. E., Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Kondo J. Polypeptide composition of paired helical filaments. Ann Med. 1989;21(2):121–125. doi: 10.3109/07853898909149198. [DOI] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Kirstein M., Brett J., Radoff S., Ogawa S., Stern D., Vlassara H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9010–9014. doi: 10.1073/pnas.87.22.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knops J., Kosik K. S., Lee G., Pardee J. D., Cohen-Gould L., McConlogue L. Overexpression of tau in a nonneuronal cell induces long cellular processes. J Cell Biol. 1991 Aug;114(4):725–733. doi: 10.1083/jcb.114.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S. Alzheimer's disease: a cell biological perspective. Science. 1992 May 8;256(5058):780–783. doi: 10.1126/science.1589757. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Binder L. I., Yen S. H. Alzheimer disease proteins (A68) share epitopes with tau but show distinct biochemical properties. J Neurosci Res. 1990 Mar;25(3):420–430. doi: 10.1002/jnr.490250320. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Liu W. K., Yen S. H. Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res. 1992 Dec 4;597(2):209–219. doi: 10.1016/0006-8993(92)91476-u. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin M. F., DaVolio J., Garcia R. Cationic liposome-mediated incorporation of prostatic acid phosphatase protein into human prostate carcinoma cells. Biochem Biophys Res Commun. 1993 Apr 30;192(2):413–419. doi: 10.1006/bbrc.1993.1431. [DOI] [PubMed] [Google Scholar]
- Liu W. K., Dickson D. W., Yen S. H. Heterogeneity of tau proteins in Alzheimer's disease. Evidence for increased expression of an isoform and preferential distribution of a phosphorylated isoform in neurites. Am J Pathol. 1993 Feb;142(2):387–394. [PMC free article] [PubMed] [Google Scholar]
- Liu W. K., Ksiezak-Reding H., Yen S. H. Abnormal tau proteins from Alzheimer's disease brains. Purification and amino acid analysis. J Biol Chem. 1991 Nov 15;266(32):21723–21727. [PubMed] [Google Scholar]
- McGeer P. L., Akiyama H., Itagaki S., McGeer E. G. Immune system response in Alzheimer's disease. Can J Neurol Sci. 1989 Nov;16(4 Suppl):516–527. doi: 10.1017/s0317167100029863. [DOI] [PubMed] [Google Scholar]
- Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J. 1989 Oct 15;263(2):539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaetova-Ladinska E. B., Harrington C. R., Roth M., Wischik C. M. Biochemical and anatomical redistribution of tau protein in Alzheimer's disease. Am J Pathol. 1993 Aug;143(2):565–578. [PMC free article] [PubMed] [Google Scholar]
- Mullarkey C. J., Edelstein D., Brownlee M. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990 Dec 31;173(3):932–939. doi: 10.1016/s0006-291x(05)80875-7. [DOI] [PubMed] [Google Scholar]
- Pappolla M. A., Omar R. A., Kim K. S., Robakis N. K. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer's disease. Am J Pathol. 1992 Mar;140(3):621–628. [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Williamson J. R., Brownlee M. Glucose and diabetic vascular disease. FASEB J. 1992 Aug;6(11):2905–2914. doi: 10.1096/fasebj.6.11.1644256. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Sugioka K., Nakano M. O2- generation and lipid peroxidation during the oxidation of a glycated polypeptide, glycated polylysine, in the presence of iron-ADP. Biochim Biophys Acta. 1990 Mar 12;1043(1):27–33. doi: 10.1016/0005-2760(90)90106-8. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Tsuchiya S. Superoxide production from nonenzymatically glycated protein. FEBS Lett. 1988 Aug 29;236(2):406–410. doi: 10.1016/0014-5793(88)80066-8. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Yan S. D., Brett J., Mora R., Nowygrod R., Stern D. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products. J Clin Invest. 1993 May;91(5):2155–2168. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–21602. [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Carney J. M., Starke-Reed P. E., Oliver C. N., Stadtman E. R., Floyd R. A., Markesbery W. R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Taneda S., Richey P. L., Miyata S., Yan S. D., Stern D., Sayre L. M., Monnier V. M., Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville M. J., Percy M. E., Bergeron C., Yoong L. K., Grima E. A., McLachlan D. R. Localization and quantitation of 68 kDa neurofilament and superoxide dismutase-1 mRNA in Alzheimer brains. Brain Res Mol Brain Res. 1991 Jan;9(1-2):1–8. doi: 10.1016/0169-328x(91)90123-f. [DOI] [PubMed] [Google Scholar]
- Subbarao K. V., Richardson J. S., Ang L. C. Autopsy samples of Alzheimer's cortex show increased peroxidation in vitro. J Neurochem. 1990 Jul;55(1):342–345. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- Verbeek M. M., Otte-Höller I., Westphal J. R., Wesseling P., Ruiter D. J., de Waal R. M. Accumulation of intercellular adhesion molecule-1 in senile plaques in brain tissue of patients with Alzheimer's disease. Am J Pathol. 1994 Jan;144(1):104–116. [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Manogue K. R., Dinarello C. A., Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988 Jun 10;240(4858):1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- Yan S. D., Schmidt A. M., Anderson G. M., Zhang J., Brett J., Zou Y. S., Pinsky D., Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994 Apr 1;269(13):9889–9897. [PubMed] [Google Scholar]
- Zemlan F. P., Thienhaus O. J., Bosmann H. B. Superoxide dismutase activity in Alzheimer's disease: possible mechanism for paired helical filament formation. Brain Res. 1989 Jan 2;476(1):160–162. doi: 10.1016/0006-8993(89)91550-3. [DOI] [PubMed] [Google Scholar]