Abstract
The yeasts Candida and Cryptococcus spp. are important human opportunistic pathogens. Candida spp. rely on skin or mucosal breach to cause bloodstream infection, whereas Cryptococcus spp. exploit depressed cell-mediated immunity characteristic of advanced HIV infection. The treatment for both organisms relies on the administration of rapidly fungicidal agents. In candidaemia, source control is important, with removal of prosthetic material and drainage of collections, as well as hunting for and tailoring therapy to disseminated sites of infection, particularly the eyes and heart. For cryptococcal meningitis, restoration of immune function through antiretroviral therapy (ART) is key, together with careful management of the complications of raised intracranial pressure and relapsed infection, both pre- and post-ART.
Treatments for both candidiasis and cryptococcosis involve the use of fungicidal agents. In addition, source control is critical in the management of candidiasis, and the restoration of immune function is key in cryptococcosis.
C andida species (spp.) form part of the normal flora of the human gastrointestinal and genitourinary tracts. Invasive infection occurs in the context of breach of skin or mucosal integrity, often on a background of prior broad-spectrum antibiotic use and defective host innate or cell-mediated immunity. Additional risk factors for candidaemia include diabetes, prolonged intensive care unit stay, presence of a central venous catheter (CVC), recent abdominal surgery, total parenteral nutrition, corticosteroid treatment, Candida colonization, and extremes of age (Wey et al. 1989; Saiman et al. 2000; Glockner and Cornely 2013). Additional risk factors in neonates include very low birthweight and necrotizing enterocolitis (Farmaki et al. 2007; Filioti et al. 2007).
As the population of susceptible individuals rises, the incidence of invasive candidiasis is increasing (Pfaller and Diekema 2012), with Candida (spp.) now the fourth most common cause of nosocomial bloodstream infection (BSI) in the United States (8%–10% of all nosocomial BSIs) (Wisplinghoff et al. 2004), with similar prevalence reported in Europe (Engel et al. 2007; Vincent et al. 2009). Treatment is with an antifungal agent from the azole, polyene (amphotericin B), or echinocandin drug classes. With widespread use of azoles for prophylaxis and treatment, there has been a shift in epidemiology from Candida albicans to non-albicans species. Recently acquired resistance to echinocandins has also been reported (Alexander et al. 2013). Source control is important, and hematogenous dissemination of Candida can result in seeding and distant foci of infection, requiring specific modification of antifungal therapy.
The following five principles summarize the most important aspects of the management of invasive candidiasis.
PRINCIPLE 1: ADMINISTER AN AGENT LIKELY TO CLEAR Candida RAPIDLY AND EFFECTIVELY
Early, effective, and rapidly fungicidal treatment is essential given that invasive candidiasis has an attributable mortality rate up to 50% (Gudlaugsson et al. 2003; Zaoutis et al. 2005), and mortality is directly correlated with delays in initiation of adequate antifungal therapy (Morrell et al. 2005; Garey et al. 2006).
Local epidemiology should guide empiric management with both the prevalence of different Candida spp. and the rate of fluconazole-resistance being important considerations. For instance, prior azole use in an individual patient is a risk factor for an azole-resistant infection (Bassett et al. 2010).
Because of their efficacy, spectrum, and safety, echinocandins are preferred empirically in hemodynamically unstable and/or neutropenic patients, those with prior azole use, in known non-albicans species, or empirically in centers with high prevalence of non-albicans species (particularly Candida glabrata) or fluconazole resistance. Fluconazole is favored for clinically stable, immunocompetent patients, those with known susceptible isolates, and in centers with a high prevalence of Candida parapsilosis (which has higher MICs to echinocandins) or low azole resistance (Pappas et al. 2009a). To date, there is no evidence to suggest superior efficacy of any particular echinocandin, nor in favor of combination therapy for invasive candidiasis (Table 1).
Table 1.
Recommended therapy for cryptococcal meningoencephalitis in HIV
| Phase | 0–2 wk | 2–10 wk | 10 wk–1 yr |
|---|---|---|---|
| Induction | Amphotericin 0.7–1.0 mg/kg/d + 5FC 100 mg/kg/d Alternative regimens: AmBd + fluconazole 800–1200 mg/d Liposomal AmB 3–4 mg/kg/d; fluconazole 1200 mg/d + 5FC | ||
| Consolidation | Fluconazole 400 mg/d | ||
| Maintenance | Fluconazole 200 mg/d for 6–12 mo or until CD4 >100 and HIV viral load undetectable on ART |
The site of infection, renal and hepatic function, concomitant interacting medicines, specific contraindications, and the adverse reaction profile for individual patients should be taken into consideration.
PRINCIPLE 2: LOOK FOR THE SOURCE AND REMOVE IT WHERE POSSIBLE
Source control is critical in the management of invasive candidiasis. Infected devices should be removed and collections drained wherever possible. Failure to achieve timely source control is associated with mortality in patients with septic shock (Kollef et al. 2012).
The impact of CVC removal on mortality is controversial. Quantitation of cultures taken from the line versus peripherally can help identify catheter-associated candidaemia and prioritization for removal in patients with tunneled lines (Raad et al. 2004).
Candida spp. readily form biofilms on invasive devices (intravenous lines, catheters, prosthetic heart valves, stents, prosthetic joints), contributing to difficulties in clearance of infection because of their higher resistance to both antifungal drugs and host immune responses (Table 2) (Ramage et al. 2005). Activity against biofilm is important particularly when optimal source control is not achievable, and varies among different antifungal agents. In particular, azoles are not active against sessile forms of Candida spp., whereas echinocandins and amphotericin B have both fungicidal activity and good penetration into biofilms on vascular devices (Bassett et al. 2010; Ruhnke et al. 2011).
Table 2.
Antifungal classes, agents, spectrum, and dosing in the treatment of invasive candidiasis
| Drug class | ||||||||
|---|---|---|---|---|---|---|---|---|
| Amphotericin B | Echinocandins | Azoles | ||||||
| Drug | AmB deoxycholate | Liposomal AmB | Lipid complex AmB | Anidulafungin | Caspofungin | Micafungin | Fluconazole | Voriconazole |
| Common susceptibility of Candida spp. | ||||||||
| C. albicans | S | S | S to R | S | ||||
| C. glabrata | S | Sa | S-DD to R | S to R | ||||
| C. krusei | S | S | R | S | ||||
| C. lusitaniae | S to R | S | S | S | ||||
| C. parapsilosis | S | S to R | S | S | ||||
| C. tropicalis | S | S | S | S | ||||
| Available formulations | ||||||||
| IV | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Oral | ✓ | ✓ | ||||||
| Dosing for treatment of candidaemia | ||||||||
| Loading dose (first 24 h) in adults | – | – | – | 200 mg | 70 mg | – | 12 mg/kg | 6 mg/kg (12 hourly) |
| Maintenance dose in adults | 0.5–1 mg/kg | 3 mg/kg | 5 mg/kg | 100 mg | 50 mg (70 mg if >85 kg) | 100 mg | 6 mg/kg | 3 mg/kg (12 hourly) |
| Dose adjustment in renal impairment | Contraindicated | None | None | None | None | None | Reduce dose | Avoid IV formulation |
| Dose adjustment in moderate hepatic impairment | Unknown | Unknown | Unknown | None | Dose reduce | None | Reduce maintenance dose | Halve maintenance dose |
| Dose adjustment in severe hepatic impairment | Unknown | Unknown | Unknown | None | Unknown | Unknown | Unknown | Unknown |
AmB, amphotericin B; S, susceptible; S-DD, susceptible dose dependent; R, resistant.
aReports of echinocandin resistance emerging in C. glabrata (Alexander et al. 2013).
PRINCIPLE 3: MODIFY EMPIRIC THERAPY ACCORDING TO SPECIES DETERMINATION AND SUSCEPTIBILITY TESTING
For stable patients with fluconazole-susceptible strains, de-escalation to fluconazole should be considered. Patients who have improved clinically and who have cleared Candida from the bloodstream might be suitable for step-down oral therapy to complete the course. Available oral options include fluconazole, itraconazole, voriconazole, and posaconazole. Fluconazole is preferred for susceptible species, whereas voriconazole may be indicated as step-down therapy for Candida krusei or voriconazole-susceptible C. glabrata (Pappas et al. 2009a).
PRINCIPLE 4: CONFIRM CLEARANCE OF Candida
Treatment efficacy should be assessed by documentation of blood culture sterilization, and treatment continued for 14 d after clearance of Candida, providing there is no evidence of disseminated infection (Pappas et al. 2009a).
Other modifiable factors driving persistence of infection should also be addressed. If clinically feasible, immunosuppressive and antibacterial therapy should be reduced/deescalated or stopped (Labelle et al. 2008) and glycemic control in diabetics optimized.
PRINCIPLE 5: CHECK FOR DISSEMINATED INFECTION AND TREAT ACCORDINGLY
Although invasive candidiasis is commonly limited to the bloodstream, organ involvement following hematogenous dissemination is an important manifestation of the disease and may involve the kidneys, myocardium, liver, spleen, bone, central nervous system (CNS), or eyes (Schwesinger et al. 2005; Thorn et al. 2010). Persistent candidaemia is an important risk factor for disseminated infection (Meister et al. 1977; Donhuijsen et al. 1991; Zaoutis et al. 2004; Masood and Sallah 2005). Symptoms and signs are determined by the type and extent of organ involvement.
Fundoscopy is warranted in all patients to exclude endophthalmitis. Endocarditis should be excluded in diagnosis of cases of persistent candidaemia, known valvular pathology, or any other symptom or sign suggestive of endocardial involvement (Pappas et al. 2009a).
EYE
The reported incidence of Candida endophthalmitis or chorioretinitis in patients with candidaemia ranges between 5% and 78% (Raoult 2011), with most recent studies reporting an incidence in the lower end of this range (Donahue et al. 1994; Benjamin et al. 2003; Rodríguez-Adrían et al. 2003). Antifungal therapy of Candida chorioretinitis is similar to candidaemia, but treatment should be continued for at least 4–6 wk until the resolution of all symptoms and signs (Pappas et al. 2009a).
Therapeutic options in Candida endophthalmitis include amphotericin B, fluconazole, and voriconazole. Owing to excellent penetration into the eye, the addition of flucytosine is suggested by some sources. Failures have been reported with echinocandin therapy, attributable to low tissue penetration (Breit et al. 2005; Gauthier et al. 2005; Sarria et al. 2005). Case reports suggest that patients with decreased visual acuity may benefit from early vitrectomy combined with intravitreal instillation of amphotericin B (Essman et al. 1997; Martínez-Vázquez et al. 1998; Khan et al. 2007).
RENAL TRACT
Removal or exchange of foreign bodies in the renal tract (catheters and stents) is an important aspect of managing invasive candidiasis with a urinary focus (Pappas et al. 2009a). If urine cultures persistently grow Candida spp., an ultrasound should be performed to exclude possible renal parenchyma infection. Fluconazole and amphotericin B are effective options for infections in the renal tract (Pappas et al. 2009a) with addition of flucytosine, which is renally excreted, sometimes recommended in complicated infections or infections caused by non-albicans species (Ruhnke et al. 2011). Neither echinocandins nor voriconazole reach adequate levels in the urine, making them less suitable for treatment of infections involving the lower urinary tract (Deresinski and Stevens 2003; Johnson and Kauffman 2003; Chandrasekar and Sobel 2006; Vazquez and Sobel 2006). Conventional amphotericin may be preferred to lipid formulations because of greater penetration into the renal tract (Pappas et al. 2009a), but its nephrotoxicity can be a problem.
BONE
Bone infection occurs via either hematogenous seeding or exogenous inoculation (e.g., during surgery). Debridement and removal of any prostheses, in addition to systemic antifungal treatment for 6–12 mo, is recommended. If prosthetic material is retained, an agent with good biofilm penetration may be preferable (Ruhnke et al. 2011).
ENDOCARDIUM
Candida endocarditis is a rare but serious complication (Baddley et al. 2008) and should be excluded by repeated negative blood cultures and a prompt transesophageal echocardiogram wherever suspected (Mylonakis and Calderwood 2001; Pierrotti and Baddour 2002; Tleyjeh et al. 2007). Therapy includes surgical removal of the infected tissue in combination with prolonged antifungal therapy (Steinbach et al. 2005; Salamon et al. 2007; Baddley et al. 2008). Most published guidelines suggest amphotericin B combined with flucytosine for at least 6 wk following valve surgery (Ellis et al. 2001; Pierrotti and Baddour 2002). However, echinocandin therapy is gaining interest because of its superior in vitro activity against biofilms and is recommended as first-line therapy in the more recently published British endocarditis guidelines (Gould et al. 2012). Higher doses may be necessary than those licensed for treatment of candidaemia. Fluconazole is appropriate consolidation therapy for patients with native valve endocarditis, infections caused by C. parapsilosis, and as long-term suppressive therapy in which removal of the prosthetic valve is not possible (Gould et al. 2012).
CNS
Combination of amphotericin B and flucytosine has been advocated for CNS infections, owing to the rapid fungicidal activity of the former, the excellent CNS penetration of the latter and their documented synergistic activity and the clinical efficacy in Candida and cryptococcal infections (Vermes et al. 2000). Alternative options include fluconazole, alone or in combination with flucytosine, particularly for consolidation therapy, and voriconazole, which shows good levels in the CNS (Lutsar et al. 2003) and promising data in patients with CNS aspergillosis (Schwartz et al. 2005). Echinocandin monotherapy is not recommended as penetration into the CNS may be insufficient (Raoult 2011). Antifungal therapy for CNS candidiasis is recommended for at least 4 wk after the resolution of all signs and symptoms of the infection. Removal of any prosthetic material (e.g., drains or shunts) associated with the infection is indicated. Brain abscesses may require neurosurgical drainage (Pappas et al. 2009a).
CRYPTOCOCCOSIS
Cryptococcosis is an infection caused by Cryptococcus neoformans and Cryptococcus gattii. The organism is inhaled into the lungs, where infection may be asymptomatic and resolve spontaneously or present clinically as pneumonia with subsequent hematogenous dissemination to other organs.
Disseminated infection is more common in immunocompromised hosts, usually owing to either HIV infection (Jarvis and Harrison 2007) or iatrogenic immunosuppression following transplantation (Snydman et al. 2008), and may occur following years of latent infection (Garcia-Hermoso et al. 1999; Saha et al. 2007). The central nervous system is the most common site of disease after the lungs, with meningoencephalitis manifesting as headache, meningism, and altered mental status, complicated by seizures and raised intracranial pressure. Focal lesions (cryptococcomas in lung and brain) are more common in C. gattii disease in the immunocompetent host (Mitchell et al. 1995).
Globally, the most common manifestation of cryptococcosis is as cryptococcal meningoencephalitis (CM) in patients with advanced HIV infection and CD4 counts <100 cells/μL. Our antifungal armamentarium against cryptococcosis consists of three drugs: amphotericin B deoxycholate, AmBd (and its liposomal derivatives), flucytosine (5FC), and fluconazole. Despite having one of the most extensively evidence-based treatments among invasive mycoses, acute (10-wk) mortality in CM in HIV remains unacceptably high, ranging from 10%–50% in published cohort and clinical trials, depending on the clinical setting (Perfect et al. 2010).
The management of cryptococcosis is based around seven principles.
Risk Stratification
Patients should be assessed for evidence of dissemination and markers of disease severity at presentation. Patients with mild to moderate pneumonia can be treated with fluconazole 400 mg/d, whereas patients with severe pneumonia (ARDS) or evidence of extrapulmonary dissemination need intravenous AmBd with flucytosine (dosed as for CNS disease; Table 1) for 2 wk followed by fluconazole for 6–12 mo (Perfect et al. 2010).
For HIV-associated CM, established adverse prognostic markers are high baseline fungal burden (using either quantitative cerebrospinal fluid [CSF] cultures or cryptococcal antigen titer) and altered mental status (Glasgow coma scale [GCS] <15 and/or seizures) at presentation (Bicanic et al. 2009). These patients may require longer duration of treatment to sterilize the CSF and more aggressive management of ICP, and are more likely to develop CM-immune reconstitution inflammatory syndrome (IRIS) following initiation of ART (Bicanic et al. 2009a; Boulware et al. 2010; Jarvis et al. 2012).
Clear the Fungus Rapidly
CM treatment can be divided into three phases: induction, consolidation, and maintenance (Table 1) (Perfect et al. 2010). The rate of clearance of cryptococci from the CSF has been shown to be independently associated with both 2- and 10-wk mortality (Bicanic et al. 2009c). The use of the most rapidly fungicidal combinations in the induction phase of treatment (at least 2 wk, and longer in the immunocompetent host and/or C gattii infection) is thus recommended for any patient with CNS disease. The combination of AmBd (0.7–1.0 mg/kg/d) and 5FC (25 mg/kg four times a day) sterilizes the CSF most rapidly (van der Horst et al. 1997; Brouwer et al. 2004; Day et al. 2013), and showed long-term survival benefit in a recent phase III trial (Day et al. 2013). The use of adjunctive interferon (IFN)-γ with AmBd and 5FC leads to even faster rates of clearance and can be considered in refractory cases (Perfect et al. 2010; Jarvis et al. 2012). In organ transplant recipients on concomitant nephrotoxic drugs, liposomal AmB (3–4 mg/kg/d) should be substituted for the deoxycholate preparation (Perfect et al. 2010). Where 5FC is not available, fluconazole 800–1200 mg/d can be combined with AmBd (Pappas et al. 2009; Loyse et al. 2012; Day et al. 2013). Where AmBd cannot be safely administered for 14 d, alternatives include shorter (5–7 d) courses (Muzoora et al. 2012) or the oral combination of fluconazole 1200 mg/d plus 5FC (Nussbaum et al. 2010), currently undergoing phase III randomized controlled trials in Africa (see www.controlled-trials.com/ISRCTN45035509).
Following induction treatment, 8-wk consolidation therapy with fluconazole 400 mg/d is recommended, followed by maintenance treatment with 200 mg/d for 6–12 mo or, for HIV-infected patients, until immune restoration with CD4 count >100 cells/μL with an undetectable viral load on antiretroviral therapy (ART) (Perfect et al. 2010).
Minimize Drug Toxicity
Patients should have regular monitoring of complete blood count, electrolytes, and renal function. Amphotericin B deoxycholate is nephrotoxic, resulting in rising creatinine and potassium and magnesium wasting within days of starting treatment (Gallis et al. 1990). This predictable adverse event can be minimized by using preemptive daily fluid loading with 1 L normal saline (Branch 1988; Mayer et al. 2002) and potassium and magnesium replacement (Girmenia et al. 2005), increasing hydration, and omitting doses if the creatinine rises greater than twofold from baseline (see WHO cryptococcosis guidelines, www.who.int/hiv/pub/cryptococcal_disease2011/en) or switching to liposomal AmB preparations for the remainder of treatment (Perfect et al. 2010). 5FC levels should be monitored, where available, and its dose should be adjusted in renal impairment as drug accumulation increases the risk of bone marrow toxicity (Perfect et al. 2010). AmBd-induced anemia is a significant and less well-recognized adverse effect, resulting in a drop in hemoglobin from baseline of 2–3 g/dL (Joly et al. 1996; Sharkey et al. 1996; Leenders et al. 1998; Bicanic et al. 2008), which may necessitate transfusion. Adverse effects are reversible on stopping treatment (Bicanic et al. 2008). Maintenance dose fluconazole is well tolerated with few drug interactions.
Manage Raised Intracranial Pressure
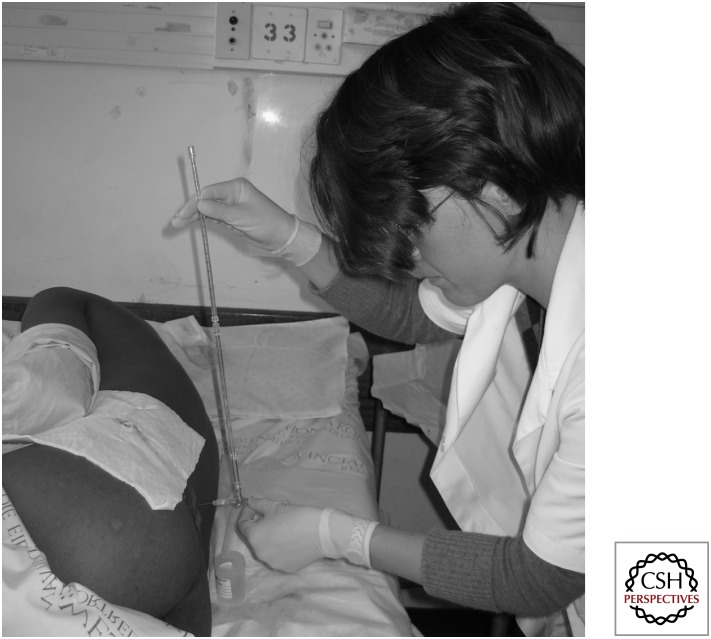
Raised ICP (defined as CSF opening pressure >20 cm H2O) is extremely common in CM, occurring in 60%–80% of patients in the context of high fungal burden (Graybill et al. 2000; Bicanic et al. 2009b) causing CSF outflow obstruction and a communicating hydrocephalus, and is associated with mortality (Graybill et al. 2000; de Vedia et al. 2013). Associated morbidities of visual and hearing loss are sometimes profound and irreversible (Rex et al. 1993; Graybill et al. 2000). All patients should have a baseline lumbar puncture with CSF opening pressure (OP) measurement (Fig. 1) (Perfect et al. 2010). Daily therapeutic lumbar punctures, up to a maximum of 20–30 mL CSF, for those with persistently raised OP >25 cm H2O, provide immediate symptom relief as well as possibly abrogating the adverse impact of raised ICP on survival (Jarvis et al. 2014). Those with refractory high CSF OP and progressive visual or other neurological impairment should be considered for a temporary lumbar drain (Perfect et al. 2010).
Figure 1.
Bedside measurement of CSF opening pressure at lumbar puncture using a manometer.
Restore Host Immunity while Managing Excessive Inflammation
Patients with cryptococcosis are usually immunocompromised, either iatrogenically in organ transplantation, or through CD4 T-cell loss in HIV infection. Production of proinflammatory cytokines tumor necrosis factor α and interferon-γ via CD4 T-cell macrophage interactions is important for fungal clearance and survival (Jarvis et al. 2013b). Adjunctive IFN-γ augments the clearance of infection by AmBd and 5FC (Jarvis et al. 2012). Restoration of host immunity, either by cautiously lowering doses of immunosuppressive agents in solid organ transplant recipients (Snydman et al. 2008) or by commencing ART in HIV-infected patients, is needed to completely clear the cryptococcal infection.
The timing of ART commencement should be individually tailored following induction therapy of CM. Exact timing (between 2–6 wk from start of induction CM treatment, as per the WHO guidelines, www.who.int/hiv/pub/cryptococcal_disease2011/en) depends on the fungal burden at presentation and induction antifungal regimens used, resolution of symptoms and presence of concomitant opportunistic infections, in order to balance the risks of too little versus excessive immune responses in the form of IRIS. A recently completed phase III trial showed excess mortality whether ART is commenced very early at 1–2 wk, median 9 d from start of AmBd-based therapy, compared to 5 wk (Boulware et al. 2013).
An ongoing phase III trial is examining the role of adjunctive steroids in acute CM (see www.controlled-trials.com/ISRCTN59144167). Steroids are currently not recommended to treat acute CM or raised ICP, but are recommended in cases of CM-IRIS refractory to antifungal therapy and management of raised ICP (prednisolone 0.5–1.0 mg/kg/d, tapered over 2–6 wk) (Perfect et al. 2010).
Prevent CM Unmasked by ART Using CRAG Screening and Preemptive Treatment
The simple and inexpensive cryptococcal polysaccharide antigen (CRAG) test has excellent sensitivity and specificity for detection of CRAG in serum, plasma, and CSF (Jarvis et al. 2011b). Cryptococcal antigenemia, indicating early dissemination from the lung, precedes symptomatic meningitis by weeks to months (French et al. 2002), with reported prevalence in cohorts of HIV-infected patients with CD4 <100 cells/μL ranging between 2% and 21% (Meya et al. 2010; Jarvis et al. 2011a). In many countries in sub-Saharan Africa with high prevalence of HIV and cryptococcal coinfection where patients start ART at very low CD4 counts, an increasing proportion of CM now occurs as unmasking disease following commencement of ART (Jarvis et al. 2010). Reflex CRAG screening of all ART-naïve patients with CD4 <100 cells/μL with lumbar puncture (LP) to rule out meningitis and preemptive fluconazole therapy at 800 mg/d before ART start is likely to be a cost-effective intervention (Meya et al. 2010; Jarvis et al. 2013a), and is being piloted in centers in South Africa and Uganda.
Address Complications Contributing to Persistent or Relapsed Infection
Patients may either be slow to respond to initial treatment, or relapse following an initial response to therapy, either before or after starting ART. Factors causing slow response to induction therapy of CM include use of drugs that are slow to sterilize the CSF, such as fluconazole-based regimens or poor adherence to consolidation or maintenance therapy, and raised ICP, which can develop on therapy and persist even beyond the point of CSF sterilization (Bicanic et al. 2009b).
For slow clearance despite appropriately dosed AmB-based induction regimens, prolongation of the induction course or adjuvant IFN-γ may be considered (Perfect et al. 2010). Raised ICP usually responds to repeated large volume therapeutic LPs so long as tolerated by the patient, or temporary lumbar drains (Perfect et al. 2010). VP shunting is rarely indicated.
In immunocompetent patients with C. gattii infections, cryptococcomas in lung or brain may respond poorly to antifungal therapy alone, even when administered for the recommended 6 wk. Large cryptococcomas may require steroids and/or debulking surgery for associated mass effect (Perfect et al. 2010).
The development of antifungal drug resistance is rare in the context of AmB-based induction regimens, but has been described following use of fluconazole monotherapy for induction (Bicanic et al. 2006). Minimum inhibitory concentration (MIC) testing of the initial C. neoformans strain is not recommended. For a patient suffering a relapse during fluconazole therapy, paired initial and relapse isolate fluconazole MIC testing is recommended. An MIC rise >2 dilutions from baseline, or absolute values >16 μg/mL suggest development of resistance to fluconazole (Perfect et al. 2010), and patients should be retreated with an AmBd-based induction regimen followed by maintenance on weekly AmBd 1 mg/kg (Powderly et al. 1992). Relapses following ART with negative cultures of CSF should be managed as IRIS following exclusion of alternative diagnoses, with continuation of ART and antifungal therapy, management of raised ICP and consideration of steroids in refractory cases.
Footnotes
Editors: Arturo Casadevall, Aaron P. Mitchell, Judith Berman, Kyung J. Kwon-Chung, John R. Perfect, and Joseph Heitman
Additional Perspectives on Human Fungal Pathogens available at www.perspectivesinmedicine.org
REFERENCES
- Alexander B, Johnson M, Pfeiffer C, Jiménez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56: 1724–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddley JW, Benjamin DK Jr, Patel M, Miró J, Athan E, Barsic B, Bouza E, Clara L, Elliott T, Kanafani Z, et al. 2008. Candida infective endocarditis. Eur J Clin Microbiol Infect Dis 27: 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett M, Mikulska M, Viscoli C. 2010. Bench-to-bedside review: Therapeutic management of invasive candidiasis in the intensive care unit. Crit Care 14: 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin DJ, Poole C, Steinbach W, Rowen J, Walsh T. 2003. Neonatal candidemia and end-organ damage: A critical appraisal of the literature using meta-analytic techniques. Pediatrics 112: 634–640. [DOI] [PubMed] [Google Scholar]
- Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. 2006. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: The role of fluconazole resistance and immune reconstitution. Clin Infect Dis 43: 1069–1070. [DOI] [PubMed] [Google Scholar]
- Bicanic T, Wood R, Meintjes G, Rebe K, Brouwer AE, Loyse A, Bekker LG, Jaffar S, Harrison T. 2008. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: A randomized trial. Clin Infect Dis 47: 123–130. [DOI] [PubMed] [Google Scholar]
- Bicanic T, Meintjes G, Rebe K, Williams A, Loyse A, Wood R, HAYES M, Jaffar S, Harrison TS. 2009a. Immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis: A prospective study. J Acquir Immune Defic Syndr 51: 130–134. [DOI] [PubMed] [Google Scholar]
- Bicanic T, Brouwer AE, Meintjes G, Rebe K, Limmathurotsakul D, Chierakul W, Teparrakkul P, Loyse A, White NJ, Wood R. 2009b. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS 23: 701–706. [DOI] [PubMed] [Google Scholar]
- Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, Rebe K, Loyse A, Jarvis J, Bekker L. 2009c. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: Analysis of a combined cohort of 262 patients. Clin Infect Dis 49: 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware DR, Meya DB, Bergemann TL, Wiesner DL, Rhein J, Musubire A, Lee SJ, Kambugu A, Janoff EN, Bohjanen PR. 2010. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: A prospective cohort study. PLoS Med 7: e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, Taseera K, Nabeta HW, Schutz C, Williams DA, et al. 2014. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. New Engl J Med 370: 2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch RA. 1988. Prevention of amphotericin B–induced renal impairment: A review on the use of sodium supplementation. Arch Intern Med 148: 2389. [PubMed] [Google Scholar]
- Breit S, Hariprasad S, Mieler W, Shah G, Mills M, Grand M. 2005. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am J Ophthalmol 139: 135–140. [DOI] [PubMed] [Google Scholar]
- Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ, Harrison TS. 2004. Combination antifungal therapies for HIV-associated cryptococcal meningitis: A randomised trial. Lancet 363: 1764–1767. [DOI] [PubMed] [Google Scholar]
- Chandrasekar P, Sobel J. 2006. Micafungin: A new echinocandin. Clin Infect Dis 42: 1171–1178. [DOI] [PubMed] [Google Scholar]
- Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, Phu NH, Nghia HD, Phong ND, Thai CQ. 2013. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 368: 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deresinski S, Stevens D. 2003. Caspofungin. Clin Infect Dis 36: 1445–1457. [DOI] [PubMed] [Google Scholar]
- De Vedia L, Arechavala A, Calderón M, Maiolo E, Rodríguez A, Lista N, Di Virgilio E, Cisneros J, Prieto R. 2013. Relevance of intracranial hypertension control in the management of Cryptococcus neoformans meningitis related to AIDS. Infection 41: 1073–01077. [DOI] [PubMed] [Google Scholar]
- Donahue SP, Greven CM, Zuravleff JJ, Eller AW, Nguyen MH, Peacock JE Jr, Wagener MW, Yu VL. 1994. Intraocular candidiasis in patients with candidemia. Clinical implications derived from a prospective multicenter study. Ophthalmology 101: 1302–1309. [DOI] [PubMed] [Google Scholar]
- Donhuijsen K, Pfaffenbach B, Samandari S, Leder L. 1991. Autopsy results of deep mycoses in hematologic neoplasms 1053 patients. Mycoses 34: 25–27. [PubMed] [Google Scholar]
- Ellis ME, Al-Abdely H, Sandridge A, Greer W, Ventura W. 2001. Fungal endocarditis: Evidence in the world literature 1965–1995. Clin Infect Dis 32: 50–62. [DOI] [PubMed] [Google Scholar]
- Engel C, Brunkhorst F, Bone H, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, et al. 2007. Epidemiology of sepsis in Germany: Results from a national prospective multicenter study. Intensive Care Med 33: 606–618. [DOI] [PubMed] [Google Scholar]
- Essman TF, Flynn HW Jr, Smiddy WE, Brod RD, Murray TG, Davis JL, Rubsamen PE. 1997. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers 28: 185–194. [PubMed] [Google Scholar]
- Farmaki E, Evdoridou J, Pouliou T, Bibashi E, Panagopoulou P, Filioti J, Benos A, Sofianou D, Kremenopoulos G, Roilides E. 2007. Fungal colonization in the neonatal intensive care unit: Risk factors drug susceptibility, and association with invasive fungal infections. Am J Perinatol 24: 127–135. [DOI] [PubMed] [Google Scholar]
- Filioti J, Spiroglou K, Panteliadis CP, Roilides E. 2007. Invasive candidiasis in pediatric intensive care patients: Epidemiology, risk factors, management, and outcome. Intensive Care Med 33: 1272–1283. [DOI] [PubMed] [Google Scholar]
- French N, Gray K, Watera C, Nakiyingi J, Lugada E, Moore M, Lalloo D, Whitworth JA, Gilks CF. 2002. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 16: 1031–1038. [DOI] [PubMed] [Google Scholar]
- Gallis HA, Drew RH, Pickard WW. 1990. Amphotericin B: 30 years of clinical experience. Rev Infect Dis 12: 308–329. [DOI] [PubMed] [Google Scholar]
- Garcia-Hermoso D, Janbon G, Dromer F. 1999. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol 37: 3204–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin Infect Dis 43: 25–31. [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Nork TM, Prince R, Andes D. 2005. Subtherapeutic ocular penetration of caspofungin and associated treatment failure in Candida albicans endophthalmitis. Clin Infect Dis 41: e27–e28. [DOI] [PubMed] [Google Scholar]
- Girmenia C, Cimino G, Di Cristofano F, Micozzi A, Gentile G, Martino P. 2005. Effects of hydration with salt repletion on renal toxicity of conventional amphotericin B empirical therapy: A prospective study in patients with hematological malignancies. Support Care Cancer 13: 987–992. [DOI] [PubMed] [Google Scholar]
- Glöckner A, Cornely OA. 2013. Practical considerations on current guidelines for the management of invasive candidiasis. Mycoses 56: 11–20. [DOI] [PubMed] [Google Scholar]
- Gould F, Denning D, Elliott T, Foweraker J, Perry JD, Prendergast BD, Sandoe JA, Spry MJ, Watkin RW, Working Party of the British Society for Antimicrobial Chemotherapy. 2012. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: A report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 67: 269–289. [DOI] [PubMed] [Google Scholar]
- Graybill JR, Sobel J, Saag M, Van Der Horst C, Powderly W, Cloud G, Riser L, Hamill R, Dismukes WE. 2000. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. Clin Infect Dis 30: 47–54. [DOI] [PubMed] [Google Scholar]
- Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 37: 1172–1177. [DOI] [PubMed] [Google Scholar]
- Jarvis JN, Harrison TS. 2007. HIV-associated cryptococcal meningitis. AIDS 21: 2119–2129. [DOI] [PubMed] [Google Scholar]
- Jarvis JN, Meintjes G, Harrison TS. 2010. Outcomes of cryptococcal meningitis in antiretroviral naive and experienced patients in South Africa. J Infect 60: 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis JN, Harrison TS, Govender N, Lawn SD, Longley N, Bicanic T, Maartens G, Venter F, Bekker L, Wood R, et al. 2011a. Routine cryptococcal antigen screening for HIV-infected patients with low CD4+ T-lymphocyte counts-time to implement in South Africa? S Afr Med J 101: 232–234. [DOI] [PubMed] [Google Scholar]
- Jarvis JN, Percival A, Bauman S, Pelfrey J, Meintjes G, Williams GN, Longley N, Harrison TS, Kozel TR. 2011b. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin Infect Dis 53: 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis JN, Meintjes G, Rebe K, Williams GN, Bicanic T, Williams A, Schutz C, Bekker L, Wood R, Harrison TS. 2012. Adjunctive interferon- immunotherapy for the treatment of HIV-associated cryptococcal meningitis: A randomized controlled trial. AIDS 26: 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis JN, Harrison TS, Lawn SD, Meintjes G, Wood R, Cleary S. 2013a. Cost effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South Africa. PLoS ONE 8: e69288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis JN, Casazza JP, Stone HH, Meintjes G, Lawn SD, Levitz SM, Harrison TS, Koup RA. 2013b. The phenotype of the Cryptococcus-specific CD4 memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. J Infect Dis 207: 1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, Longley N, et al. 2014. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: Implications for improving outcomes. Clin Infect Dis 58: 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LB, Kauffman CA. 2003. Voriconazole: A new triazole antifungal agent. Clin Infect Dis 36: 630–637. [DOI] [PubMed] [Google Scholar]
- Joly V, Aubry P, Ndayiragide A, Carrière I, Kawa E, Mlika-Cabanne N, Aboulker J, Coulaud J, Larouze B, Yeni P. 1996. Randomized comparison of amphotericin B deoxycholate dissolved in dextrose or intralipid for the treatment of AIDS-associated cryptococcal meningitis. Clin Infect Dis 23: 556–562. [DOI] [PubMed] [Google Scholar]
- Khan FA, Slain D, Khakoo RA. 2007. Candida endophthalmitis: Focus on current and future antifungal treatment options. Pharmacotherapy 27: 1711–1721. [DOI] [PubMed] [Google Scholar]
- Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. 2012. Septic shock attributed to Candida infection: Importance of empiric therapy and source control. Clin Infect Dis 54: 1739–1739. [DOI] [PubMed] [Google Scholar]
- Kullberg BJ, Verweij PE, Akova M, Arendrup MC, Bille J, Calandra T, Cuenca-Estrella M, Herbrecht R, Jacobs F, Kalin M, et al. 2011. European expert opinion on the management of invasive candidiasis in adults. Clin Microbiol Infect 17: 1–12. [DOI] [PubMed] [Google Scholar]
- Labelle AJ, Micek ST, Roubinian N, Kollef MH. 2008. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med 36: 2967–2972. [DOI] [PubMed] [Google Scholar]
- Leenders AC, Daenen S, Jansen RL, Hop WC, Lowenberg B, Wijermans PW, Cornelissen J, Herbrecht R, Lelie HVD, Hoogsteden HC. 1998. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br J Haematol 103: 205–212. [DOI] [PubMed] [Google Scholar]
- Liechty CA, Solberg P, Were W, Ekwaru JP, Ransom RL, Weidle PJ, Downing R, Coutinho A, Mermin J. 2007. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health 12: 929–935. [DOI] [PubMed] [Google Scholar]
- Loyse A, Wilson D, Meintjes G, Jarvis JN, Bicanic T, Bishop L, Rebe K, Williams A, Jaffar S, Bekker L, et al. 2012. Comparison of the early fungicidal activity of high-dose fluconazole, voriconazole, and flucytosine as second-line drugs given in combination with amphotericin B for the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis 54: 121–128. [DOI] [PubMed] [Google Scholar]
- Lutsar I, Roffey S, Troke P. 2003. Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin Infect Dis 37: 728–732. [DOI] [PubMed] [Google Scholar]
- Martínez-Vázquez C, Fernández-Ulloa J, Bordón J, Sopeña B, de la Fuente J, Ocampo A, Rubianes M. 1998. Candida albicans endophthalmitis in brown heroin addicts: Response to early vitrectomy preceded and followed by antifungal therapy. Clin Infect Dis 27: 1130–1133. [DOI] [PubMed] [Google Scholar]
- Masood A, Sallah S. 2005. Chronic disseminated candidiasis in patients with acute leukemia: Emphasis on diagnostic definition and treatment. Leuk Res 29: 493–501. [DOI] [PubMed] [Google Scholar]
- Mayer J, Doubek M, Doubek J, Horký D, Scheer P, Štěpánek M. 2002. Reduced nephrotoxicity of conventional amphotericin B therapy after minimal nephroprotective measures: Animal experiments and clinical study. J Infect Dis 186: 379–388. [DOI] [PubMed] [Google Scholar]
- Meister H, Heymer B, Schäfer H, Haferkamp O. 1977. Role of Candida albicans in granulomatous tissue reactions: I. In vitro degradation of C. albicans and immunospecificity of split products. J Infect Dis 135: 224–234. [DOI] [PubMed] [Google Scholar]
- Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, Kamya MR, Bohjanen PR, Boulware DR. 2010. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4 cell count ≤ 100 cells/μL who start HIV therapy in resource-limited settings. Clin Infect Dis 51: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DH, Sorrell TC, Allworth AM, Heath CH, McGregor AR, Papanaoum K, Richards MJ, Gottlieb T. 1995. Cryptococcal disease of the CNS in immunocompetent hosts: Influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis 20: 611–616. [DOI] [PubMed] [Google Scholar]
- Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of candida bloodstream infection until positive blood cultures are obtained: A potential risk factor for hospital mortality. Antimicrob Agents Chemother 49: 3640–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzoora CK, Kabanda T, Ortu G, Ssentamu J, Hearn P, Mwesigye J, Longley N, Jarvis JN, Jaffar S, Harrison TS. 2012. Short course amphotericin B with high dose fluconazole for HIV-associated cryptococcal meningitis. J Infect 64: 76–81. [DOI] [PubMed] [Google Scholar]
- Mylonakis E, Calderwood SB. 2001. Infective endocarditis in adults. N Engl J Med 345: 1318–1330. [DOI] [PubMed] [Google Scholar]
- Nussbaum JC, Jackson A, Namarika D, Phulusa J, Kenala J, Kanyemba C, Jarvis JN, Jaffar S, Hosseinipour MC, Kamwendo D, et al. 2010. Combination flucytosine and high-dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: A randomized trial in Malawi. Clin Infect Dis 50: 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, et al. 2009a. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48: 503–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas PG, Chetchotisakd P, Larsen RA, Manosuthi W, Morris MI, Anekthananon T, Sungkanuparph S, Supparatpinyo K, Nolen TL, Zimmer LO. 2009b. A phase II randomized trial of amphotericin B alone or combined with fluconazole in the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis 48: 1775–1783. [DOI] [PubMed] [Google Scholar]
- Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50: 291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M, Diekema D. 2012. The epidemiology of invasive candidiasis. ASM, Washington, DC. [Google Scholar]
- Pierrotti LC, Baddour LM. 2002. Fungal endocarditis, 1995–2000. Chest 122: 302–310. [DOI] [PubMed] [Google Scholar]
- Powderly WG, Saag MS, Cloud GA, Robinson P, Meyer RD, Jacobson JM, Graybill JR, Sugar AM, Mcauliffe VJ, Follansbee SE. 1992. A controlled trial of fluconazole or amphotericin B to prevent relapse of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome. N Engl J Med 326: 793–798. [DOI] [PubMed] [Google Scholar]
- Raad I, Hanna H, Boktour M, Girgawy E, Danawi H, Mardani M, Kontoyiannis D, Darouiche R, Hachem R, Bodey GP. 2004. Management of central venous catheters in patients with cancer and candidemia. Clin Infect Dis 38: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Ramage G, Saville SP, Thomas DP, López-Ribot JL. 2005. Candida biofilms: An update. Eukaryot Cell 4: 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex JH, Larsen RA, Dismukes WE, Cloud GA, Bennett JE. 1993. Catastrophic visual loss due to Cryptococcus neoformans meningitis. Medicine 72: 207–224. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Adrián LJ, King RT, Tamayo-Derat LG, Miller JW, Garcia CA, Rex JH. 2003. Retinal lesions as clues to disseminated bacterial and candidal infections: Frequency, natural history, and etiology. Medicine (Baltimore) 82: 187–202. [DOI] [PubMed] [Google Scholar]
- Ruhnke M, Rickerts V, Cornely OA, Buchheidt D, Glöckner A, Heinz W, Höhl R, Horré R, Karthaus M, Kujath P, et al. 2011. Diagnosis and therapy of Candida infections: Joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses 54: 279–310. [DOI] [PubMed] [Google Scholar]
- Saha D, Goldman D, Shao X, Casadevall A, Husain S, Limaye A, Lyon M, Somani J, Pursell K, Pruett T. 2007. Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clin Vaccine Immunol 14: 1550–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, Blumberg HM, Patterson JE, Rinaldi M, Edwards JE, et al. 2000. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J 19: 319–324. [DOI] [PubMed] [Google Scholar]
- Salamon SA, Fuursted K, Egeblad H, Petersen E, Ott P. 2007. Candida albicans tricuspid and pulmonic valve endocarditis: Challenge of relapsing risk and role of combined medical treatment and surgery. Scand J Infect Dis 39: 641–644. [DOI] [PubMed] [Google Scholar]
- Sarria JC, Bradley JC, Habash R, Mitchell KT, Kimbrough RC, Vidal AM. 2005. Candida glabrata endophthalmitis treated successfully with caspofungin. Clin Infect Dis 40: e46–e48. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA, Schuler U, Lutsar I, Toke P, Thiel E. 2005. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 106: 2641–2645. [DOI] [PubMed] [Google Scholar]
- Schwesinger G, Junghans D, Schröder G, Bernhardt H, Knoke M. 2005. Candidosis and aspergillosis as autopsy findings from 1994 to 2003. Mycoses 48: 176–180. [DOI] [PubMed] [Google Scholar]
- Sharkey PK, Graybill JR, Johnson ES, Hausrath SG, Pollard RB, Kolokathis A, Mildvan D, Fan-Havard P, Eng RH, Patterson TF. 1996. Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis 22: 308–314. [PubMed] [Google Scholar]
- Snydman DR, Singh N, Dromer F, Perfect JR, Lortholary O. 2008. Cryptococcosis in solid organ transplant recipients: Current state of the science. Clin Infect Dis 47: 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach WJ, Perfect JR, Cabell CH, Fowler VG, Corey GR, Li JS, Zaas AK, Benjamin DK Jr. 2005. A meta-analysis of medical versus surgical therapy for Candida endocarditis. J Infect 51: 230–247. [DOI] [PubMed] [Google Scholar]
- Thorn JL, Gilchrist KB, Sobonya RE, Gaur NK, Lipke PN, Klotz SA. 2010. Postmortem candidaemia: Marker of disseminated disease. J Clin Pathol 63: 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tleyjeh IM, Abdel-Latif A, Rahbi H, Scott CG, Bailey KR, Steckelberg JM, Wilson WR, Baddour LM. 2007. A systematic review of population-based studies of infective endocarditis. Chest 132: 1025–1035. [DOI] [PubMed] [Google Scholar]
- Van Der Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, Sobel JD, Johnson PC, Tuazon CU, Kerkering T, Moskovitz BL. 1997. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med 337: 15–21. [DOI] [PubMed] [Google Scholar]
- Vazquez JA, Sobel JD. 2006. Anidulafungin: A novel echinocandin. Clin Infect Dis 43: 215–222. [DOI] [PubMed] [Google Scholar]
- Vermes A, Guchelaar HJ, Dankert J. 2000. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother 46: 171–179. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302: 2323–2329. [DOI] [PubMed] [Google Scholar]
- Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. 1989. Risk factors for hospital-acquired candidemia. A matched case–control study. Arch Intern Med 149: 2349–2353. [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide. Clin Infect Dis 39: 309–317. [DOI] [PubMed] [Google Scholar]
- Zaoutis TE, Greves HM, Lautenbach E, Bilker WB, Coffin SE. 2004. Risk factors for disseminated candidiasis in children with candidaemia. Paediatr Infect Dis 23: 635–641. [DOI] [PubMed] [Google Scholar]
- Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. 2005. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: A propensity analysis. Clin Infect Dis 41: 1232–1239. [DOI] [PubMed] [Google Scholar]