Abstract
Bacterial and viral CpG oligonculeotides are unmethylated cytosine-phosphate-guanosine dinucleotide sequences and trigger an innate immune response through activation of the toll-like receptor 9 (TLR9). We have developed synthetic photocaged CpGs via site-specific incorporation of nitropiperonyloxymethyl (NPOM)-caged thymidine residues. These oligonucleotides enable the optical control of TLR9 function and thereby provide light-activation of an immune response. We provide a proof-of-concept model by applying a reporter assay in live cells and by quantification of endogenous production of interleukin 6.
Keywords: oligonucleotide synthesis, light-activation, photocaged nucleotides, immune response, toll-like receptors
The innate immune response is activated by pathogen-associated molecular patterns, such as CpG oligonucleotides.1 CpGs are unmethylated cytosine-phosphate-guanosine dinucleotide sequences. The CG dinucleotide sequence is rare in mammalian genomes; however, it is common in bacterial DNA and viral DNA.2,3 For this reason, the immune system has evolved to recognize the CpG motif in order to trigger a proper response to a bacterial or viral infection.
CpG oligonucleotides were first discovered by Krieg et al. in 1995 when it was demonstrated that bacterial DNA activates an immune response in B cells.4 The mechanism of CpG activation was elucidated five years later by Hemmi et al.5 First, CpG oligomers are internalized through an endosomal pathway (Figure 1). Within the endosome, the CpG DNA site-specifically binds to the Toll-like receptor 9 (TLR9).6 TLR9 undergoes a conformational change once activated by the CpG oligomer.7,8 This stimulates a cascade that upregulates transcription factors such as NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells) and AP1 (activator protein 1), resulting in the expression of pro-inflammatory genes. As a result, a strong immune response is created that leads to the activation of cytotoxic T cells and the production of antibodies in B cells and plasma cells.9
Figure 1.
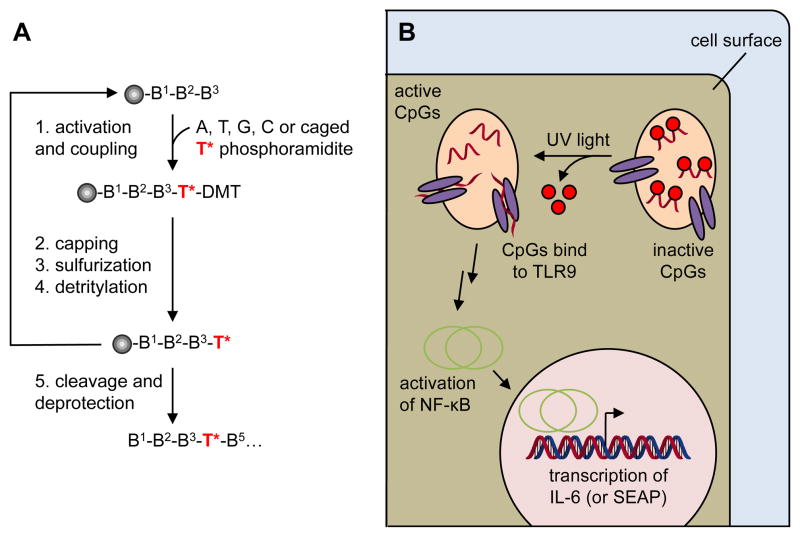
Synthesis and photoactivation of CpGs for optical immune response stimulation. A) Photocaged CpGs are synthesized by standard solid-phase oligonucleotide synthesis via site-specific incorporation of a caged thymidine phosphoramidite. B) Photocaged CpGs delivered into cells will not bind to the TLR9 receptor and thus not activate an immune response. After UV irradiation, the caging groups are removed and the CpGs are able to bind to the TLR9 receptor, undergo a conformational change, which activates NF-κB, leading to the transcription of cytokines and the SEAP reporter gene.
Several phosphorothioate backbone CpGs are currently in clinical trials either alone or as a vaccine adjuvant due to their ability to increase the generation of antibodies and killer T cells.9 Upon activation, a large number of cytokines are secreted including interleukin (IL)-6, IL-10, and IL-12.10,11,12
The ability to conditionally control an immune response using light as an external trigger has the potential to help elucidate how patterns of TLR activation enable the immune system to identify infection. The use of photocaged small molecules, proteins, and oligonucleotides has enabled precise optical control of many biological processes.13–16 Based on our experience of applying photocaged oligonucleotides to the optical regulation of cellular processes,17–22 we chose to synthesis a photocaged class B CpG oligonucleotide (Figure 1).2 This CpG sequence contains several thymidine residues as potential target sites for the incorporation of nucleobase-caging groups. With our previous success in photocaging phosphorothioate DNA antisense agents that inhibit DNA:RNA interaction23 and photoregulating DNA:protein interactions with caged DNA,24 we hypothesized that it may be possible to regulate an immune response by synthetically installing caged thymidine phosphoramidites within a CpG sequence. The caging groups would inhibit the CpG oligonucleotide from binding to TLR9, and thus suppress the immune response cascade (Figure 1). UV irradiation would remove the caging groups and restore the binding ability of the CpG, activating the TLR9 and subsequently initiate an immune response.
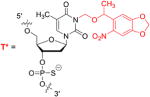
NPOM caged thymidine phosphoramidites were synthesized as previously reported25 and were site-specifically incorporated into the phosphorothioate CpG oligonucleotide ODN2006 (Table 1). The caged thymidine residues were either equally distributed throughout the oligonucleotide (CpG-4A) or concentrated at the two termini (CpG-4B). Equal spacing of the caged thymidine nucleotide would ensure that the caged CpG oligomer would most likely not bind to TLR9; on the other hand, caged thymidine nucleobases on each termini may permit low binding affinity to TLR9, and thus would lead to the faster activation after irradiation. As a positive control, a non-caged CpG oligomer (CpG) and a scrambled negative control (CNTRL) were also synthesized. The negative control oligomer should not be recognized by TLR9 and therefore, no immune response should be elicited.
Table 1.
Sequence of caged CpG oligonucleotides. All sequences are composed of phosphorothioate linkages. The NPOM-caged thymidine residues are indicated by a red T*.
| DNA | Sequence (5′ – 3′) |
|---|---|
| CNTRL | TCCAGAACAAAGGAAACG |
| CpG | TCGTCGTTTTGTCGTTTTGTCGTT |
| CpG-4A | TCGT*CGTTTTGT*CGT*TTTGT*CGTT |
| CpG-4B | T*CGT*CGTTTTGTCGTTTTGT*CGT*T |
| |
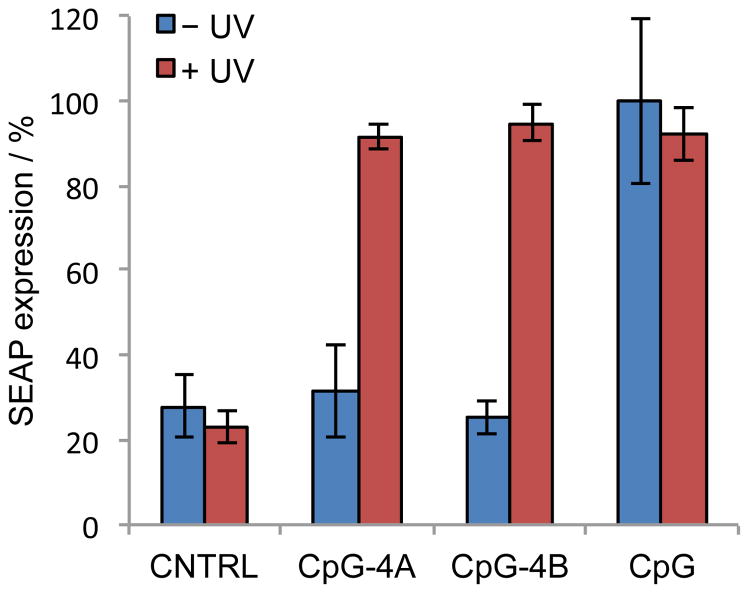
In an initial assay HEK293 cells that were stably transfected with a TLR9 gene were co-transfected with a pNiFty-SEAP (secreted alkaline phosphatase) reporter (Invitrogen) and the CpG oligonucleotides.2 After transfection, the cells were either kept in the dark or briefly irradiated with UV light (365 nm, 5 min). The cells were incubated for 48 h and a media aliquot was removed and assayed for SEAP expression using the Phospha-Light Assay Kit (Applied Biosystems). For normalization purposes, SEAP expression induced by the positive control, non-caged CpG was set to 100%. As expected, treatment with the scrambled control oligonucleotide CNTRL resulted in low luminescence indicating that this DNA oligomer did not induce an immune response and thus did not trigger SEAP expression (Figure 2). Importantly, no change in SEAP activity was detected in the presence or absence of UV irradiation. Moreover, when kept in the dark, cells treated with the caged CpG-4A showed low levels (30%) of SEAP activity. However, after UV activation and oligonucleotide decaging, an increase in SEAP expression was detected, to the same level as the non-caged control CpG (Figure 2). This indicated that the caged CpG-4A, with the caging groups equally distributed throughout the oligonucleotide, is a viable caging strategy to photoregulate an immune response with excellent off → on switching behavior. Notably, the caged CpG-4B was also inactive when kept in the dark. UV irradiation cleaved the caging groups, and fully restored the activity of CpG-4B to the level of the non-caged CpG control. Therefore, based on this set of experiments, the caging groups can be either concentrated at the two termini or they are spread equally throughout the CpG oligomer in order to inactivate TLR9 activation before light exposure.
Figure 2.
Photochemical activation of CpG function. HEK 293-TLR9 cells were co-transfected with pNiFty-SEAP and the CpG oligonucleotides using X-tremeGENE transfection reagent. The cells were either kept in the dark or irradiated with UV light (365 nm, 5 min). The cells were incubated for 48 h and an aliquot of media was removed and assayed for SEAP expression using the Phospha-Light Assay Kit (Applied Biosystems). Error bars represent standard deviations from three individual experiments.
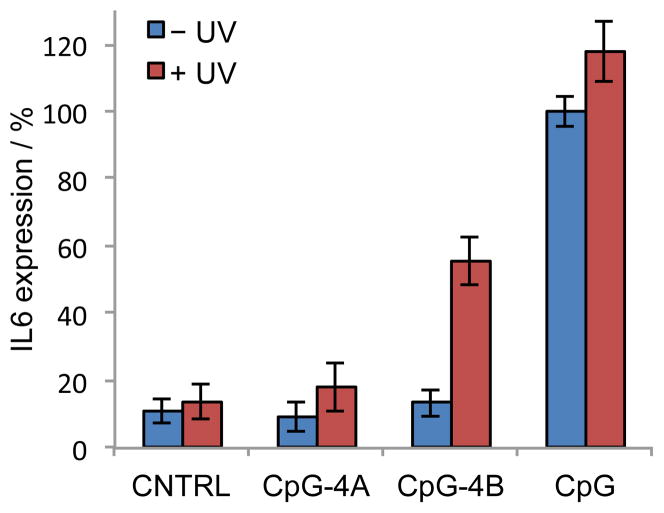
To test the effect of photoactivation of caged CpG oligonucleotides on an endogenous process, instead of a reporter gene, we chose to study the production of IL-6. IL-6 is an immediate downstream effector of TLR9 activation, and therefore a viable target gene. A Human Burkitt’s lymphoma cell line, Namalwa, was treated with the scrambled control, the non-caged CpG, or the caged CpGs. After a 4 h treatment, the supernatant was removed and the cells were resuspended in PBS and either kept in the dark or irradiated with UV light (5 min, 365 nm). Normal growth media was added to the cells and they were incubated for an additional 18 h for cytokine expression. An aliquot of the cell culture media was analyzed by an ELISA assay (Qiagen) for the production of IL-6. For normalization purposes, IL-6 expression induced by the non-caged CpG without UV irradiation (CpG–UV) was set to 100%. In the presence of the scrambled control, less than 10% IL-6 were detected (Figure 3). In agreement with the previous reporter assay (Figure 2), exposure to UV light did not have an effect on IL-6 production as little difference was observed with the CpG control oligonucleotides (CNTRL and CpG). After treatment with caged CpG-4A in the absence of UV irradiation, only a basal level of IL-6 expression was detected–comparable to the scrambled, negative control. However, very little activation of TLR9 was observed after UV irradiation. This is in contrast to the previous reporter assay (Figure 2). Upon treatment with the caged CpG-4B, similar levels of IL-6 expression were observed when these cells were kept in the dark. However, after UV-induced caging group removal, TLR9 was activated and a 5-fold increase of IL-6 expression was observed. However, the same levels as in case of the positive control CpG were not observed. Overall, the photochemical activation of an immune response by caged CpGs can be observed in the upregulation of IL-6 expression.
Figure 3.
Photoactivation of IL-6 expression through caged CpGs. Namalwa cells were treated with the scrambled control, CNTRL, or non-caged, CpG, and caged CpGs, CpG-4A and CpG-4B. The cells were irradiated (5 min, 365 nm) and incubated for 16 h. An aliquot of the cell culture media was used in an IL-6 ELISA assay (Qiagen). For normalization purposes, IL-6 production by CpG–UV was set to 100% IL-6 expression. Error bars represent standard deviations from three individual experiments.
In summary, we have successfully incorporated NPOM-caged thymidine phosphoramidites into two synthetic CpG oligonucleotides with two different caging strategies: 1) equally distributed caged thymidine residues throughout the oligomer and 2) caging groups concentrated at the 3′ and 5′ termini. In the initial reporter assay, no difference was observed in the activity profile of the two caged CpGs, with each caged CpG being inactive before optical activation and inducing a 4-fold increase in NF-κB-driven gene expression upon irradiation with UV light. However, when measuring the endogenous expression of the pro-inflammatory IL-6, the caged CpG-4B containing nucleobase caging groups clustered close to the 3′ and 5′ termini, a much more pronounced photoactivation was observed, compared to the evenly caged CpG-4A. Since our group has reported several examples where the presence of caging groups prevents DNA hybridization21,22,26,27 and DNA:protein interactions,24 it is conceivable that these caged nucleotides prevent the binding of the CpG oligonucleotide to TLR9. The central 15-nucleotide section of CpG-4B is not interrupted by caging groups and the oligonucleotide may intermittently interact with the receptor. However, since TLR9 activation is highly sequence specific,7 the toll-like receptor is not activated until the caging groups are removed, allowing full binding to TLR9 and activating the immune response cascade.
The ability to conditionally induce an immune response with an external trigger may have therapeutic potential in a variety of diseases including HIV,28 hepatitis B,29 allergens,30 bioterror agents like anthrax,11 and even cancer.12 Optical regulation of the innate immune response could be a versatile tool to study signaling cascades involved in immune responses and spatio-temporally stage activation of different TLRs in conjunction with light-activated small molecule triggers,31 in order to decipher these complex processes.
Acknowledgments
This research was supported in part by the National Institutes of Health (GM079114). We would like to thank Dr. Gregg Dean (Colorado State University) for the HEK 293-TLR9 cell line.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klinman DM. Nat Rev Immunol. 2004;4:249. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 2.Bauer S, Kirschning CJ, Häcker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Proc Natl Acad Sci U S A. 2001;98:9237. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad-Nejad P, Häcker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Eur J Immunol. 2002;32:1958. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 4.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. Nature. 1995;374:546. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 5.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. Nature. 2000;408:740. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 6.Hanagata N. Int J Nanomedicine. 2012;7:2181. doi: 10.2147/IJN.S30197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kindrachuk J, Potter JE, Brownlie R, Ficzycz AD, Griebel PJ, Mookherjee N, Mutwiri GK, Babiuk LA, Napper S. J Biol Chem. 2007;282:13944. doi: 10.1074/jbc.M608089200. [DOI] [PubMed] [Google Scholar]
- 8.Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DC, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, Espevik T, Golenbock DT. Nat Immunol. 2007;8:772. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- 9.Holtick U, Scheulen ME, von Bergwelt-Baildon MS, Weihrauch MR. Expert Opin Investig Drugs. 2011;20:361. doi: 10.1517/13543784.2011.553187. [DOI] [PubMed] [Google Scholar]
- 10.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. Expert Rev Vaccines. 2011;10:499. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rynkiewicz D, Rathkopf M, Sim I, Waytes AT, Hopkins RJ, Giri L, DeMuria D, Ransom J, Quinn J, Nabors GS, Nielsen CJ. Vaccine. 2011;29:6313. doi: 10.1016/j.vaccine.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 12.Karbach J, Neumann A, Atmaca A, Wahle C, Brand K, von Boehmer L, Knuth A, Bender A, Ritter G, Old LJ, Jäger E. Clin Cancer Res. 2011;17:861. doi: 10.1158/1078-0432.CCR-10-1811. [DOI] [PubMed] [Google Scholar]
- 13.Baker AS, Deiters A. ACS chemical biology. 2014;9:1398. doi: 10.1021/cb500176x. [DOI] [PubMed] [Google Scholar]
- 14.Deiters A. Chembiochem : a European journal of chemical biology. 2010;11:47. doi: 10.1002/cbic.200900529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HM, Larson DR, Lawrence DS. ACS chemical biology. 2009;4:409. doi: 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer G, Heckel A. Angew Chem Int Ed Engl. 2006;45:4900. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q, Deiters A. Accounts of chemical research. 2014;47:45. doi: 10.1021/ar400036a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemphill J, Govan J, Uprety R, Tsang M, Deiters A. J Am Chem Soc. 2014;136:7152. doi: 10.1021/ja500327g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Govan JM, Young DD, Lusic H, Liu Q, Lively MO, Deiters A. Nucleic acids research. 2013;41:10518. doi: 10.1093/nar/gkt806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prokup A, Hemphill J, Deiters A. J Am Chem Soc. 2012;134:3810. doi: 10.1021/ja210050s. [DOI] [PubMed] [Google Scholar]
- 21.Govan JM, Uprety R, Hemphill J, Lively MO, Deiters A. ACS chemical biology. 2012;7:1247. doi: 10.1021/cb300161r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govan JM, Lively MO, Deiters A. J Am Chem Soc. 2011;133:13176. doi: 10.1021/ja204980v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young DD, Lusic H, Lively MO, Yoder JA, Deiters A. Chembiochem : a European journal of chemical biology. 2008;9:2937. doi: 10.1002/cbic.200800627. [DOI] [PubMed] [Google Scholar]
- 24.Young DD, Govan JM, Lively MO, Deiters A. Chembiochem : a European journal of chemical biology. 2009;10:1612. doi: 10.1002/cbic.200900090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lusic H, Young DD, Lively MO, Deiters A. Organic letters. 2007;9:1903. doi: 10.1021/ol070455u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young DD, Edwards WF, Lusic H, Lively MO, Deiters A. Chemical Communications. 2008:462. doi: 10.1039/b715152g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young DD, Garner RA, Yoder JA, Deiters A. Chem Commun (Camb) 2009:568. doi: 10.1039/b819375d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper CL, Davis HL, Angel JB, Morris ML, Elfer SM, Seguin I, Krieg AM, Cameron DW. AIDS. 2005;19:1473. doi: 10.1097/01.aids.0000183514.37513.d2. [DOI] [PubMed] [Google Scholar]
- 29.Halperin SA, Ward B, Cooper C, Predy G, Diaz-Mitoma F, Dionne M, Embree J, McGeer A, Zickler P, Moltz KH, Martz R, Meyer I, McNeil S, Langley JM, Martins E, Heyward WL, Martin JT. Vaccine. 2012;30:2556. doi: 10.1016/j.vaccine.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 30.Tighe H, Takabayashi K, Schwartz D, Van Nest G, Tuck S, Eiden JJ, Kagey-Sobotka A, Creticos PS, Lichtenstein LM, Spiegelberg HL, Raz E. J Allergy Clin Immunol. 2000;106:124. doi: 10.1067/mai.2000.107927. [DOI] [PubMed] [Google Scholar]
- 31.Ryu KA, Stutts L, Tom JK, Mancini RJ, Esser-Kahn AP. J Am Chem Soc. 2014;136:10823. doi: 10.1021/ja412314j. [DOI] [PMC free article] [PubMed] [Google Scholar]