Abstract
Particle pollution from urban and industrialized regions in Rio de Janeiro (RJ), Brazil was analyzed for toxic and pro-inflammatory (cytokines: IL-6, IL-8, IL-10) responses in human bronchial epithelial cells. Trace elements contribution was studied. Airborne particulate matter was collected at: three industrial sites Ind-1 (PM10) and Ind-2a and 2b (PM2.5); Centro urban area (PM10) and two rural sites (PM2.5, PM10). PM10 acetone extracts were toxic and did not elicit cytokine release; aqueous extracts were less toxic and stimulated the release of IL-6 and IL-8. PM2.5 aqueous extracts from Ind-2 decreased the release of IL-6 and IL-8. Zinc concentration was higher at the industrial and rural reference sites (Ref-1-2) although metals were not associated to cytokines changes. These results demonstrate that PM from RJ can either increase or decrease cytokine secretion in vitro while being site specific and time dependent.
Keywords: particles, BEAS-2B, industry, cytokines, decrease, metals
Introduction
Atmospheric aerosol or particulate matter (PM) is an essential component of air that can influence climate, visibility, biogeochemical cycling, atmospheric reactivity and affect human health (Griffin, 2013). A worldwide effort to control global PM emissions and protect human health has been recently recognized by respectable institutions. The United States Environmental Protection Agency (USEPA) has established PM2.5 and PM10 National Ambient Air Quality Standards (NAAQS). The primary NAAQS for PM2.5 are 35µg/m3 in a 24hr period plus an annual mean of 12µg/m3 and 150µg/m3 for PM10 (Esworthy, 2013; Particulate matter standards, 1971–2012). The World Health Organization (WHO) established an annual average of 10µg/m3 (PM2.5) and 20µg/m3 (PM10) and a 24hr average of 25µg/m3 (PM2.5) and 50µg/m3 (PM10)(Krzyzanowski and Cohen, 2008). The Brazilian legislation for PM has not been updated since it was initially published in 1990. It established air quality standards for inhalable PM10 but has not yet adopted any PM2.5 standards. The annual PM10 standard for Brazil is 50µg/m3 and daily maximum is 150µg/m3, these are much higher than the recommended WHO levels.
Natural and anthropogenic sources contribute to the total PM global mass emissions and are critical at particular sites. The most abundant natural source of PM is sea salt followed by soil dust resuspended by wind and aerosol particles derived from plant waxes, pollens, spores and other similar materials (Despres et al., 2012). Other natural sources of PM are volcanic ashes. Reports from a 2010 volcanic eruption in Iceland show a 24hr mean concentration of l,230(µ/m3 of PM10 (25 times the established health limit) with a l0min average value over 13,000(µ/m3 (Thorsteinsson et al., 2002). Moreover, Soufriere Hills volcano in the island of Montserrat increased background PM concentrations to a factor of 10 (Moore et al., 2012). Some heavy metals including Cd, Cu, V, F, CI, Zn, and Pb were found enriched in PM aqueous fractions from volcanic aerosol (Smith et al., 2012). Anthropogenic sources contain combustion and industrial processing materials (Bond et al., 2007). The amount of compounds resulting from vehicle tire ware and the combustion of gas and diesel fuel contribute to environmental urban PM. A variety of indoor combustion and human activity also contribute significantly to human exposure. Incinerator burning of waste and land burning are considered additional sources contributing to local as well as global aerosols. All of these sources modulate PM to the extent of causing human respiratory and cardiovascular diseases through inhalation and deposition mechanisms (Chiarelli et al., 2011; Conceição et al, 2001; Ignotti et al., 2010; Lin et al., 1999; Nascimento and Francisco, 2013; Rivero et al., 2005). The mechanisms associated to PM adverse health effects are not well understood, but inflammatory processes and oxidative stress are believed to play a key role (Donaldson and Tran, 2002). Current efforts are focused on the identification of specific constituents of PM from complex mixture, which are linked to human health effects (Griffin, 2013).
Continuous monitoring of PM has been performed in a few regions of Brazil by the Company of Environmental Sanitation Technology (Cetesb) in São Paulo (SP) and Environmental Institute of Rio de Janeiro (RJ) (INEA). SP and RJ established the first air monitoring stations in the 1970s in the metropolitan area of their capitals and are the only ones following PM2.5. Other states: Rio Grande do Sul, Paraná and Minas Gerais (MG) have short-term monitoring stations. Amazon region has been monitored due to biomass burning (Ignotti et al., 2010). The lack of continuous monitoring precludes the establishment of new standards for air quality. Due to the size of the country, the diverse activities of each region and differences in climate, air quality varies widely as well as the chemical composition of the particles. Epidemiological studies performed in SP showed that people are exposed to high concentrations of particles in air (Chiarelli et al., 2011; Conceição et al., 2001; Lin et al., 1999; Nascimento and Francisco, 2013). SP has the biggest motor vehicle fleet of Brazil with approximately 8 million units as compared to 3–5 million in Mexico City, which is highly polluted (Fideicomiso del Distrito Federal, Ciudad de Mexico, 2013; Universia, 2013). Therefore, the contribution of airborne suspended particles is mainly from vehicular emissions. Local PM10 emissions have been shown to impact regional air quality at Santa Catarina (SC), Brazil (Hoinaski et al., 2013).
In RJ, some studies have been performed regarding chemical analyses on PM composition (Gioda et al., 2011a; Godoy et al., 2009; Loyola et al., 2012; Mateus et al., 2013; Paulino et al., 2010; Quiterio et al., 2004a). Various epidemiological studies made in RJ link PM exposure to health conditions such as respiratory diseases (Castro et al., 2009) and increased pediatric visits due to bronchial obstruction (Moura et al., 2009). PM exposure has also been associated to lung cancer mortality (Gouveia et al., 2003; Junger et al., 2005). The mutagenic and genotoxic activity of PM was associated to polycyclic aromatic hydrocarbons (PAH) found in high traffic stations (Miguel et al., 1990; Ramos et al., 2013). Although these epidemiological links to PM have been established, we do not know of any reports published characterizing toxicological responses of PM from RJ. Biological responses studies of PM specific agents using in vitro assays are scarce if not existent. This is the first study to report the effects of PM material from RJ on immunological responses using human lung cells. PM from urban/industrial (Centro, Duque de Caxias, Santa Cruz) and rural (Seropédica) sectors was tested for cytotoxic and proinflammatory capacity using human bronchial epithelial cells in vitro. The contribution of trace elements on these toxicological responses was evaluated by differentially testing with the chelating agent, deferoxamine (DF).
Materials and Methods
Sample Collection and Site Descriptions
PM10 were collected in 2009 and PM2.5 in 2010 by INEA. Standard Brazilian method for inhalable particles sampling, consistent with the USEPA, was used. The collectors were placed at a height of approximately 2m in a large open area. Samples were collected over 24hr every 6 days on fiberglass filters (203 × 254mm, 0.21mm thickness, 0.3 µm diameter, Millipore, USA) using high-volume samplers (AGV-PM10 and AGV-PM2.5, Energética, RJ, Brazil) at an average flow rate of 1.14m3min−1 The average temperature in RJ Metropolitan area ranged from 25 to 26°C for Spring-Summer and from 22 to 23°C for Autumn-Winter. The total rainfall in the studied period was about 1000mm distributed between the hottest months, Spring-Summer, coinciding with the seasonal wet period. The predominant wind direction is from southwest. PM10 samples were collected in June and PM2.5 samples in August-September, during wintertime in Brazil. No rain occurred during or 24hr before samplings.
PM10
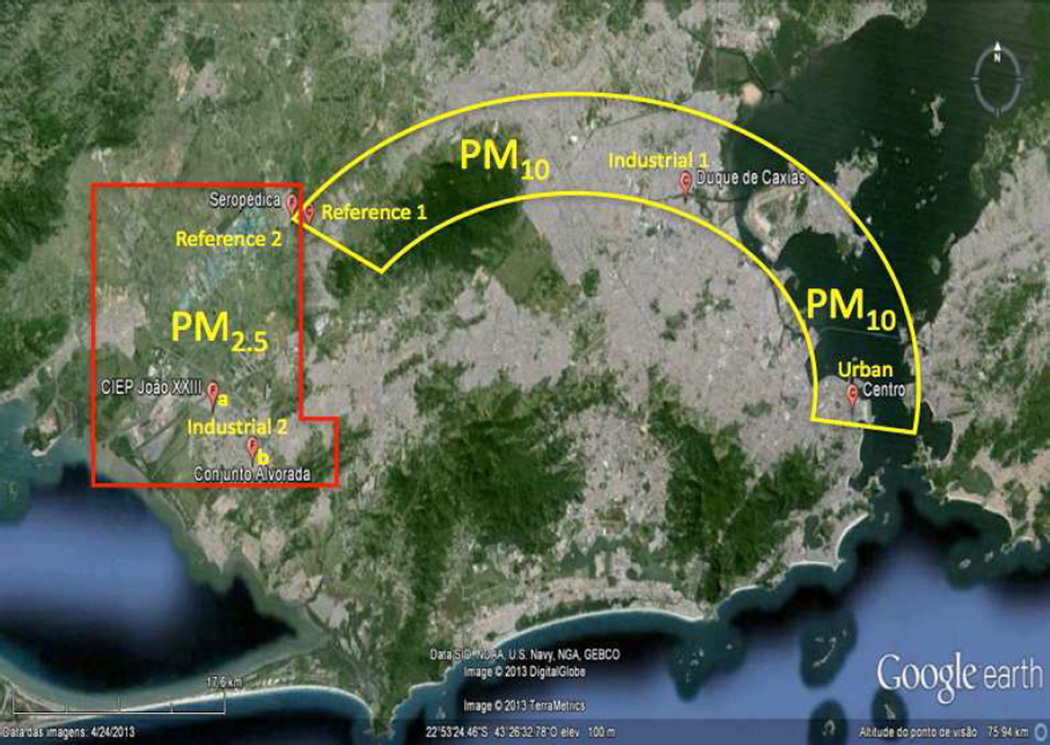
Three sites of different socio-economic background were chosen (Fig. 1). Two sites are located in the metropolitan area of RJ: Centro shown as urban and the industrial area of Duque de Caxias (Ind-1) and the rural reference site in the Seropédica County (Ref-1). Centro is located in the downtown area of RJ and its main influence is heavy vehicular traffic. Duque de Caxias houses about 800 industries including chemical, metallurgical, gas, plastics, furniture, textiles and clothing. In addition one of the largest refineries of the country is located in this area. Seropédica main activity used to be agriculture. The distance between Centro and Duque de Caxias to Seropédica is about 75 and 52km, respectively.
Figure 1.
PM10 and PM2.5 sampling sites in Rio de Janeiro. PM10 stations are contained within the yellow semicircle these are: Duque de Caxias (Industrial-1), Centro (Urban) and Seropédica (Reference/Ref-1). The PM2.5 locations are within the red rectangle: Santa Cruz (CIEP João XXII as Industrial-2a, Conjunto Alvorada as Industrial-2b and Seropédica as Reference/Ref-2. Modified from www.googlemaps.com.
PM2.5
Santa Cruz (Fig. 1) is another important industrial area with several industries such as paint and solvent production, leather manufacture, printing, structural steel manufacture and metallurgy. In 2010, the biggest steel mill in Brazil was settled. Due to its importance to the environment and population around the industrial region, two sampling sites were chosen: a school area (CIEP João XXIII represented as Ind-2a) and a residential area (Conjunto Alvorada marked as Ind-2b). Seropédica was again chosen as rural reference (Ref-2). The distance between the sampling sites (CIEP João XXIII, Conjunto Alvorada and Seropédica) to the industrial area is about 3, 8 and 15km, respectively.
Extraction Procedure
PM10 samples
An aliquot of the filter (¼) was cut and weighted in an analytical balance (Shimadzu, Brazil, ±0.0002g). The filters were subsequently extracted using the following steps: 1) 120ml of n-hexane (Vetec, Brazil) and sonication for 2hr; 2) re-extraction with 120ml of acetone (Vetec, Brazil) and sonication for 2hr; 3) re-extraction with 120ml of ultrapure water (Millipore, USA) and sonication for 2hr. An aliquot of the aqueous extract was analyzed by inductively coupled plasma and optical emission spectrometry (ICP-MS or OES) to determine the metal concentrations. Metals in organic extracts were not analyzed since the ICP flame is extinguished. For cells exposure, organic extracts were dried with a nitrogen stream and aqueous extracts with a Centrivap console (Labconco). The extract mass was determined gravimetrically, subsequently the organic fractions were dissolved in DMSO and the aqueous fractions in water to a concentration of 100mg/ml. Blank filters were analyzed in the same manner. All extracts were stored in individual vials at −20°C until further analyses.
PM2.5 samples
A portion of the filter (¼) was cut and weighed in an analytical balance (Shimadzu, Brazil, ±0.0002g). Extraction was performed in 45.0ml ultrapure water (Millipore, USA), which was sonicated for 2hr. Aliquots of the aqueous extracts were analyzed by ICP OES to study metal composition of the samples. Heating reduced extracts volume and the samples were lyophilized and weighted. Blank filters were analyzed in the same order. All extracts were stored in individual vials at −20°C until further analyses.
Chemical Analysis
Determinations of trace elements (Cd, Cr, Cu, Mn, Ni, Pb, Ti, and V) were performed using an ICP-MS model DRC II (PerkinElmer-Sciex, USA) and major elements (Al, Fe, and Zn) using Optima DV 4300 model (Perkin Elmer, USA), which is able to perform analyses in axial or radial mode of observation. This tool makes it possible to choose the best operational mode to analyze an element in function of background and concentration levels. The analytical solutions were prepared by diluting multi-elemental standards (Titrisol®-Merck, Germany) with concentrations of lOOOmg L−1 , with water and acidified with HNO3 (30–35% w/w) to avoid precipitations. The calibration curves ranged from 5 to 50mg L−1 (ICP OES) and from 50µg L−1 to 100µg L−1 (ICP-MS). External calibration was employed using the Linear Throw Zero statistical model. Duplicates and calibration controls were performed at fifteen samples. A calibration check with external standards was performed to ensure a relative error no more than 10%. The limit of detection (LOD) was calculated based on three times the standard deviation of the blanks. LOD values ranged from 0.11 to 65.8µg L−1 (Gioda et al., 201 la; Mateus et al., 2013).
Cell Culture and Exposures
Human bronchial epithelial cells (BEAS-2B) were obtained from the American Type Culture Collection (ATCC®CRL-9609TM). Cells were cultured according to ATCC protocols in Keratinocyte growth medium (KGM-2, Lonza, Walkersville, MD, USA), maintained at 37°C in humidified atmosphere of 5% CO2 and used at passages 44–59. The cells were seeded at a density of 5 × 104 cells per well onto 96-well plates and incubated for 24hr. PM2.5 and PM10 extracts were diluted in cell media at concentrations ranging from 5 to 250µg/ml and used to expose cells for 24hr. An additional set of cells (n=3 dishes) was also concurrently exposed to PM extracts pre-treated with deferoxamine mesylate (DF, Sigma, Cat No D9533), a metal chelator, at a final concentration of 50µM. Cell supernatants were collected after each exposure and used for cytokine analyses while the adhered cells to the plates were processed to study cell viability.
Cytotoxicity
The Neutral Red bioassay was used to measure cell viability. After a 24hr cell exposure to PM extracts, the treatments were removed and BEAS-2B cells incubated with Neutral Red dye (Sigma, Cat No N2889) at a final concentration of 100µg/ml for 3hr. The dye was then removed; cells were fixed in 1% calcium chloride, 0.5% formaldehyde, rinsed with phosphate buffered saline (PBS) and lysed using 1% acetic acid and 50% ethanol. Cell viability was spectrophotometrically determined at 540nm using an Ultramark microplate reader (Bio Rad, Richmond, CA, USA). Triton-X (25µg/ml) was used as a positive control for cell toxicity. The following negative controls were simultaneously employed in each experiment: media, media with deferoxamine (DF) and water. Cell viability less than 80% was the cut off considered for cytotoxicity.
Cytokine Measurements
Measurements of IL-6, IL-8 and IL-10 were performed using a Fluorokine Multi-analyte Profiling Kit (catalog number base kit: LUH000; IL-6: LUH206; IL-8: LUH208 and IL-10: LUH217) from R&D Systems, Minneapolis, MN, USA following the manufacturer’s instructions. Lipopolysaccharide (LPS) at 10µg/ml was employed as positive control. Negative controls used for the cytotoxicity experiments remained the same. Cytokine concentrations were determined using the dual laser flow analyzer Luminex 200 (Luminex Corp, Austin, TX, USA). Standard curve for each cytokine was plotted employing a 5-paremeter logistic fit (5-PL). This has been previously reported by our laboratory (Fuentes-Mattei et al., 2010; Rodriguez-Cotto et al., 2013).
Statistical Analyses
Differences between individual groups were evaluated using the unpaired Student’s t Test. The criterion for statistical significance was set at p≤0.05. Statistical analyses were performed using the Graph Pad InStat3 software. Analyses were based on 3 independent experiments per cell response.
Results
PM levels and metal composition
The annual PM10 mean concentrations were 34.2µg/m3 for Ref-1, 46.5µg/m3 for the urban site and 71.1µg/m3 for the Ind-1 (Gioda et al., 2011a). As expected, the lowest concentration for PM10 was obtained at Ref-1, followed by the urban and Ind-1 sites (Gioda et al., 2011a). However, the PM10 annual concentration at all sites exceeded the recommended standards established by WHO (20 µg/m3) which indicates that Brazil should develop a strategic plan to improve its air quality in RJ. In this study, the daily concentration at all these sites also exceeded (Table 1) the WHO standard reference value (50µg/m3). All PM10 aqueous extracts showed Cu and Zn as the most abundant metals in all stations. Although most metals were generally lower at the rural site Al was higher while all other trace metals (Zn, Cu, Cd, Ti and Fe) were higher at the urban and Ind-1 sites.
Table 1.
Trace element concentrations in PM10 aqueous extracts
| Site | Urban | Industrial 1 | Reference 1 |
|---|---|---|---|
| PM (µg/m3) | 84.4 | 63.9 | 86 |
| Metals (µg/ml) | |||
| Al | 0.0980 | 0.1313 | 0.1020 |
| Cd | 0.0042 | 0.0092 | 0.0007 |
| Cr | 0.000 | 0.0039 | 0.000 |
| Cu | 3.6496 | 0.2591 | 0.0808 |
| Fe | 0.1240 | 0.1333 | 0.0282 |
| Ni | 0.0109 | 0.0058 | 0.000 |
| Pb | 0.0069 | 0.0077 | 0.000 |
| Ti | 0.0038 | 0.0038 | 0.0004 |
| V | 0.0165 | 0.0053 | 0.000 |
| Zn | 2.9550 | 2.6361 | 1.5402 |
The annual PM2.5 mean was similar at all sites (12µg/m3 ), a value 20% higher than the WHO standard (10µg/m3) (Mateus et al, 2013). Correspondingly, the daily PM2.5 concentration reported here also exceeded the value recommended by WHO (25µg/m3) (Table 2). Similar to what was found in PM10 aqueous extracts, Zn was also the most abundant element in PM2.5. For CIEP João XXIII and Conjunto Alvorada (Ind-2a and b respectively) two samples were analyzed. A difference in metal concentrations was observed between the samples at these two sites (Table 2). The metal concentrations at Ind-2a1 (August 2010) were consistently higher than in September (Ind-2a2). This is also true for Ind-2b1 (August) and Ind-2b2 (September). Industrial site Ind-1 consistently exhibited higher concentrations of heavy metals compared to the Ind-2 (Table 1,Table 2).
Table 2.
Trace element concentrations in PM2.5 aqueous extracts
| Site | Industrial 2 | Reference 2 | |||
|---|---|---|---|---|---|
| Sample IDa | 2a1 | 2a2 | 2b1 | 2b2 | |
| PM (µg/m3) | 43.4 | 29.4 | 42.8 | 21.1 | 27.8 |
| Metals (µg/ml) | |||||
| Al | 0.02 | <0.06 | 0.01 | <0.06 | <0.06 |
| Cr | <0.004 | 0.001 | <0.004 | <0.004 | 0.001 |
| Cu | 0.033 | 0.021 | 0.028 | 0.035 | 0.043 |
| Fe | 0.178 | 0.071 | 0.224 | 0.042 | 0.071 |
| Mn | 0.030 | 0.017 | 0.045 | 0.012 | 0.019 |
| Ni | 0.011 | <0.01 | 0.015 | 0.001 | <0.01 |
| Ti | 0.006 | 0.005 | 0.007 | 0.002 | 0.003 |
| V | 0.065 | 0.016 | 0.093 | 0.017 | 0.014 |
| Zn | 3.289 | 1.700 | 3.598 | 0.339 | 0.713 |
Industrial 2a1 and 2b1 were collected during August; 2a2 and 2b2 during September
PM10 Effects on Cytotoxicity and Cytokines Secretion
Organic Extracts
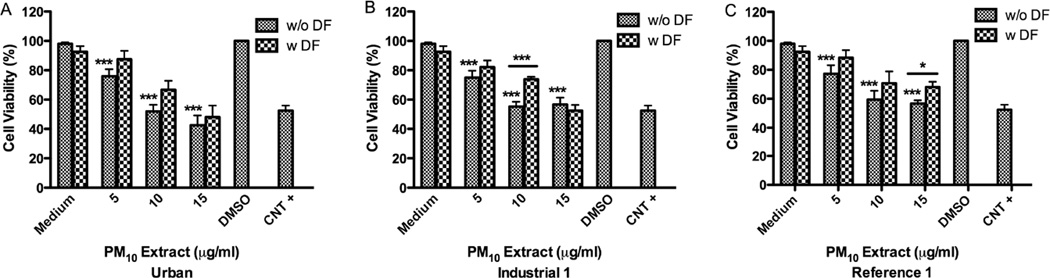
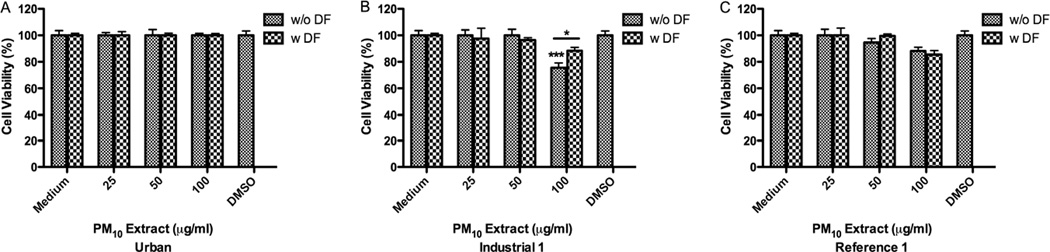
All the PM10 acetone extracts exhibited a toxic effect on BEAS-2B at a concentration of 10µg/ml (Fig. 2). Pre-treatment with DF substantially prevented cytoxicity of both Ind-1 and Ref-1 acetone extracts at 10µg/ml and 15µg/ml (Fig. 2B–C) respectively. Using non-toxic concentration (5µg/ml) these extracts did not increase the release of IL-6, IL-8 or IL-10. Urban and Ref-1 hexane extracts were not toxic at 100µg/ml. However, Ind-1 hexane extract was toxic at 100µg/ml and pre-treatment with DF significantly increased cell viability from 76% to 88% (Fig. 3). Exposure to these extracts did not increase cytokine secretion (IL-6, IL-8 and IL-10) at any of the concentrations tested.
Figure 2.
Effect of deferoxamine (DF) on BEAS-2B cytotoxicity to PM10 acetone extracts from: Urban (A), Industrial-1 (B) and Reference-1 (C) sites. Triton X (+CNT) 25µg/ml was used as a positive control and dimethylsulfoxide (DMSO) 0.1% as carrier. DF was used at 50µM. A value of 80%) was considered cytotoxic. Bars represent mean % cell viability ± SEM, ***p<0.001, *p<0.05 compared to DMSO or to adjacent treatment (n=3).
Figure 3.
Effect of deferoxamine (DF) on BEAS-2B cytotoxicity to PM10 hexane extracts from: Urban (A), Industrial-1 (B) and Reference-1 (C) sites. Triton X (+CNT) 25µg/ml was used as a positive control and dimethysulfoxide (DMSO) 0.1% as carrier. DF was used at 50µM. A value of 80% was considered cytotoxic. Bars represent mean % cell viability ± SEM, ***p<0.0019 *p<0.05 compared to DMSO or to adjacent treatment (n=3).
Aqueous Extracts
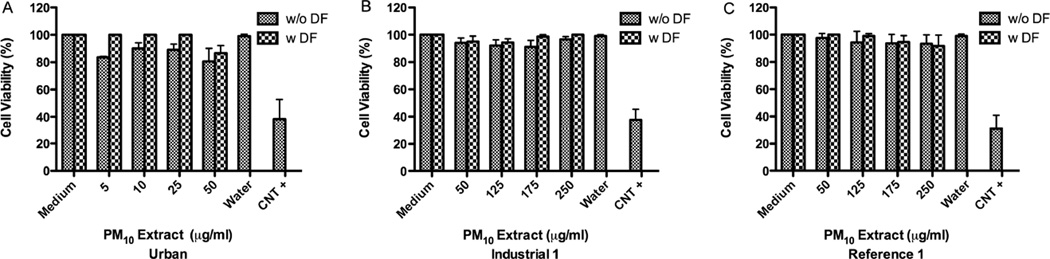
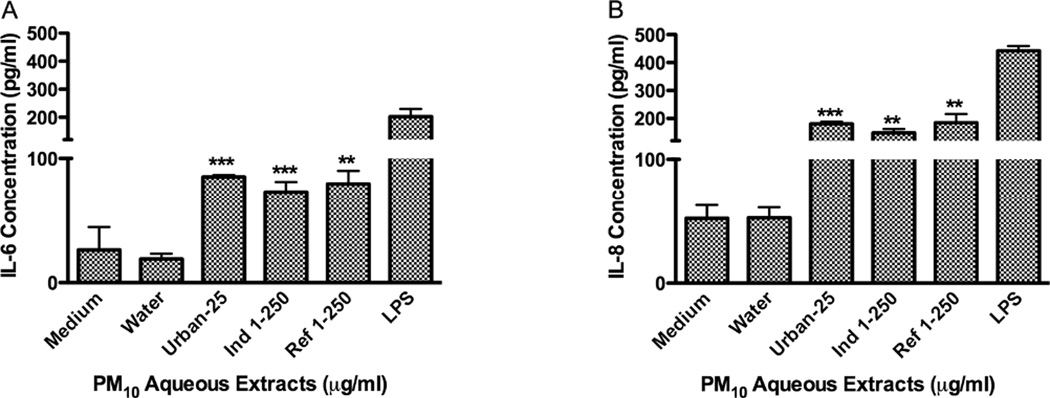
The urban aqueous extract was not toxic to BEAS-2B at 25µg/ml; however, Ind-1 and Ref-1 were not toxic even at a concentration of 250µg/ml (Fig. 4). Aqueous extracts were less toxic than organic extracts and they significantly increased the release of IL-6 and IL-8 from BEAS-2B after 24hr (Fig. 5). The urban aqueous extract increased the release of these cytokines at a concentration as low as 25µg/ml while Ind-1 and Ref-1 required a higher concentration of 250µg/ml. Evidence was not found for the release of IL-10 at the time point tested of 24hr.
Figure 4.
Effect of deferoxamine (DF) on BEAS-2B cytotoxicity to PM10 aqueous extracts from: Urban (A), Industrial-1 (B) and (C) Reference-1 sites. Triton X (+CNT) 25µg/ml was used as a positive control. DF was used at 50µM. A value of 80% was considered cytotoxic. Bars represent mean % cell viability ± SEM (n=3).
Figure 5.
Effect of PM10 aqueous extracts from Urban, Industrial-1 and Reference-1 on IL-6 (A) and IL-8 (B) secretions in BEAS-2B. Lipopolysaccharide (LPS, 10µg/ml) was used as positive control. Bars represent cytokine mean concentration ± SEM, ***p<0.001, **p<0.01, compared to water (n=3).
PM2.5 Effects on Cytotoxicity and Cytokines Secretion
Aqueous Extracts
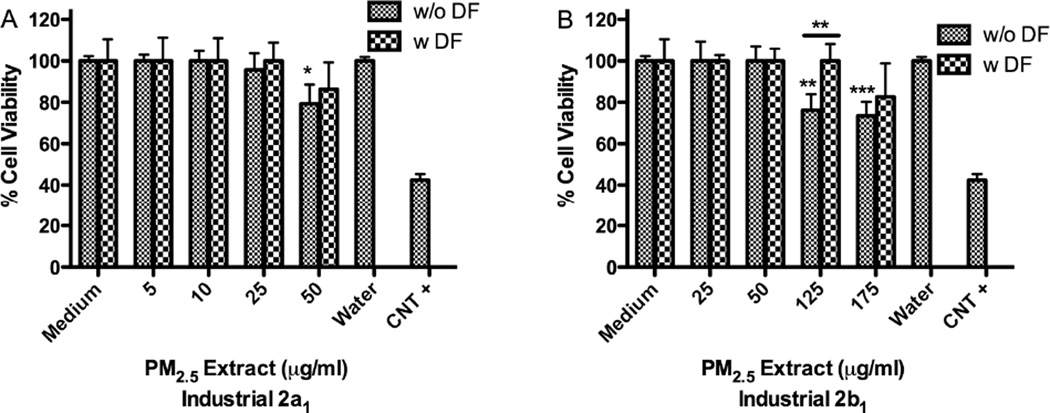
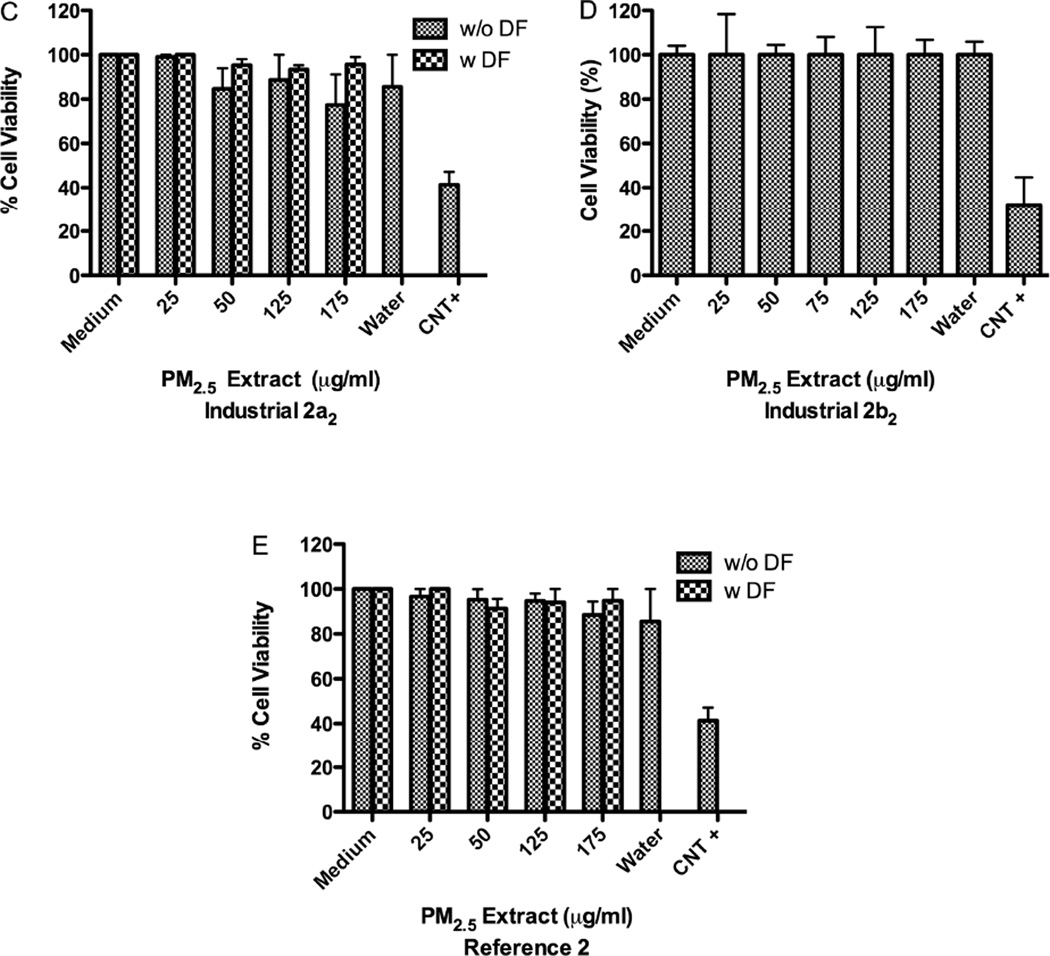
Extracts from samples collected at Ind-2a1 and 2b1 stations at 50µg/ml and 125µg/ml, respectively were more toxic than Ind-2a2-2b2 extracts both at 175µg/ml (Fig. 6). Ind-2a1 extract was toxic at 50µg/ml (Fig. 6A) while a similar extract from that same station (Ind-2a2) taken on a different month was not toxic until 175µg/ml (Fig. 6C). Aqueous extract from Ref-2 was not toxic to BEAS-2B even at a concentration of 175µg/ml (Fig. 6E). The concentration of metals in the air at Ind-2a and 2b stations was higher during the first collection period than during the second. There are days when the airborne particulate matter collected at the Ind-2 site exhibited the same toxicity profile as that collected at the Ref-2 site. However, we also found that the material collected in August was more toxic than that collected in September (Fig. 6A–B).
Figure 6.
Effect of deferoxamine (DF) on BEAS-2B cytotoxicity to PM2.5 aqueous extracts from: (A) Industrial-2a1 and (B) Ind-2b1 (August), (C) Ind-2a2 and (D) Ind-2b2 (September) and (E) Reference-2. Triton X (+CNT) 25µg/ml was used as positive control. DF was used at 50µM. A value of 80% was considered cytotoxic. Bars represent mean % cell viability ± SEM, ***p<0.001, **p<0.01, *p<0.05 compared with medium (n=3).
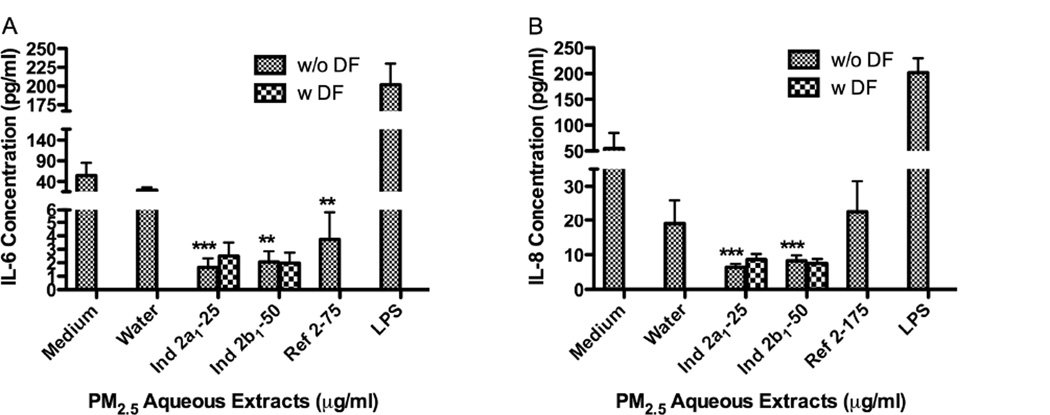
Three of the four sample extracts tested from the Ind-2 sites decreased the release of IL-6 (Ind-2a1 at 25µg/ml, Ind-2b1 at 50µg/ml and Ref-2 at 175µg/ml) (Fig. 7A). Aqueous extracts from Ind-2a and b reduced IL-6 as much as 26 folds from that of controls while extracts from Ref-2 reduced it by 15 folds. IL-8 released was also decreased 9 folds in extracts from both Ind-2a,2b (Fig. 7B). To our surprise none of the extracts from PM2 5 increased the release of IL-6 or IL-8 as did the PM10 aqueous extracts. None of the extracts increased or decreased the expression of IL-10 at the time point tested. Two samples (Ind-2a2 and Ind-2b2) were not tested for cytokine secretion.
Figure 7.
Effect of PM2.5 aqueous extracts on BEAS-2B cytokines secretion, IL-6 (A) and IL-8 (B). Lipopolysaccharide (LPS, 10µg/ml) was used as positive control. Bars represent mean cytokine concentration ± SEM, ***p<0.001, **p<0.01, compared to water, n=3.
Discussion
Increases in airborne PM levels are commonly observed from May to September in RJ (Gioda et al., 2011a). This is due to reduction in wind speed during winter, associated to low relative humidity and seasonal rainfall. One PM2.5 sample period coincided with the opening of a large steel plant in the area of Santa Cruz Ind-2a1. During the first months the company was under operational trials and undesirable emissions were released. The differences in metal concentrations in PM2.5 for CIEP João XXIII (Ind-2a1,2a2) compared to that of Conjunto Alvorada (Ind-2b1,2b2) can be attributed to higher levels in Cu, Fe, V, Mn, Ni, and Zn in August vs. September. The toxicity of the extracts can also be attributed to the differences in collection periods, August greater than September. This is evidenced by the wide difference in metal concentration between Ind-2a1 vs. Ind-2a2. The metal load in the aqueous extract can explain most of the toxicity found in Ind-2b1 and Ind-2a2; however, this is not the case for the extracts obtained in station Ind-2a1. The toxicity at this station is caused by additional factors other than metal alone. The results of this study reveal that Zn plays a key role in RJ environment and could be associated to toxicity and immune responses. This is consistent in part with findings of studies in downtown and suburban areas characterized by industrial and vehicular emissions, including natural input. Enrichment factors found in the downtown area for Zn, Cu, Pb and Cd were in the range of 21–3237ng/m3, indicating an important contribution of anthropogenic sources. In the suburban area, Zn levels were unusually high (596.8–5475.4ng/m3) and may be attributed to the proximity of a company that produces lubricants (Paulino et al., 2010; Quiterio et al., 2004b). High concentrations of Zn and Al in PM2.5 (Mateus et al., 2013) have been attributed to various industrial sources. Furthermore, it has been reported that metal content at these industrial sites were twice as high as that of a rural site (Gioda et al., 2011a).
Organic extracts from PM10 were highly toxic to BEAS-2B at 10µg/ml, since the toxic effect was observed for all stations including the reference site. Most PM research performed in Brazil relates to coarse PM10 while little emphasis has been given to PM2.5, perhaps due to the lack on PM2.5 regulations. PM10 chemical composition has been previously reported at Ref-1 site and includes sea salt, NH4NO3, (NH4)2SO4, water-soluble organic carbon and soil as the predominant component (Gioda et al., 2011a). Cytokine induction in Ref-1 can be associated with the organic fraction of PM. The PM10 aqueous extract at the urban site increased IL-6 and IL-8 at much lower concentration than extracts from both the Ind-1 and Ref-1 sites. Urban PM has a strong component of fossil fuel due to concentration of engine emissions and burning in the city as compared to the other sites. It has been well established that, PM10, PM2.5 and diesel exhaust particles (DEP) can induce the secretion of inflammatory cytokines and chemokines in epithelial cells and macrophages (Alfaro-Moreno et al., 2007; Becker et al., 2005; Fuentes-Mattei et al., 2010; Gioda et al., 2011b; Hiraiwa and van Eeden, 2013;Ortiz-Martínez et al., 2010; Rodriguez-Cotto et al., 2013; Schwarze et al., 2013). In addition, cytokine induction can be mainly attributed to the effects of heavy metals in PM2.5 (Rodriguez-Cotto et al., 2013) and residual oil fly ash (Carter et al., 1997). However, here we report a decrease of both IL-6 and IL-8 by Industrial-2 extracts and demonstrate that heavy metals are not responsible for it since the effect was not reverted by DF pretreatment. Moreover, inhalation studies at high doses of urban PM2.5 from the city of SP, showed impairment of lung function by increasing elastic and viscoelastic mechanistic properties (Riva et al., 2011). PM from SP and distributed in PAH fractions was shown to cause mutagenicity and DNA adduct formation in Salmonella strains (Umbuzeiro et al., 2008). Finally, other studies have linked ambient PM2.5 and PM10 from SP to significant cardiopulmonary alterations, blood parameters in vivo and increased blood pressure (Chiarelli et al., 2011; Rivero et al., 2005).
Mean PM10 concentrations as high as 206µg/m3 have been recorded at MG (247 miles Northwest of RJ). This was attributed to the re-suspension of outdoor dust and living conditions (Deschamps et al., 2013) which showed toxic and pro-inflammatory responses (IL-8 release) of high concentrations of indoor dust particles: 250–500µg/ml and 100–500µg/ml, respectively. In fact, higher asthma symptoms (wheezing) were reported in 28.5% of school students (Camargos et al., 1999). Concentrations above 10µg/m3 in PM10 were associated with respiratory emergency room visits in children and adolescents younger than 13 years of age; for cardiovascular diseases, the effect was acute and mainly for the 45 to 64 age group (Braga-Ferreira et al., 2007).
Few reports exist on IL-6 or IL-8 inhibition due to PM exposure (Alfaro-Moreno et al., 2007; Alfaro-Moreno et al., 2009; Fuentes-Mattei et al., 2010; Gioda et al., 2011b; Schwarze et al., 2013). It has been reported that exposure to PM could induce (Becker et al., 2005) or inhibit (Veranth et al., 2006) IL-8 cellular secretion. IL-10 is a pleiotropic immunosuppressive or immunostimulatory cytokine, having an effect in different cell types, suppressing the release of pro-inflammatory cytokines such as IL-6 and IL-8 (Barnes et al., 1998). However, IL-10 was not increased or decreased in this study and by that it should not be related to the observed reduction. These findings indicate that PM effects on cytokine secretion can vary depending on the nature and specific constituents in PM. Furthermore, PM2.5 aqueous extracts from RJ clearly exhibit an inverse effect on IL-6 and IL-8 immune response in BEAS-2B. All PM10 extracts increased the secretion of these cytokines while all PM2.5 extracts decreased them. Both discrete industrial sectors the Ind-1 (PM10) and the Ind-2 (PM2.5) site are separated from each other. However, the Ref-1 (PM10) and Ref-2 (PM2.5) were located in the general rural area but downwind from Ind-2. In addition to site difference and particle size distribution we also need to consider time of the collection. The PM10 samples were collected in 2009 while PM2.5 was collected in 2010. This could explain the differences seen between samples providing constituent differences in PM compositions. These results suggest that the regulation of IL-6 and IL-8 expression varies between PM10 complex mixture compared to PM2.5. The control of IL-6 and IL-8 expression is related to different factors (Martin et al., 1997). IL-6 expression is related to at least three signal-transduction pathways: protein kinase C, cAMP/protein kinase A, and calcium ionophore (Alfaro-Moreno et al., 2009; Martin et al., 1997). IL-8 is up regulated by the phosphorylation of Erk 1/2. The phosphorylation of p38 reduces the phosphorylation of Erk 1/2 and this leads to a down-regulation of IL-8 which is also related to the inhibition of EGFR (Alfaro-Moreno et al., 2009). The cause and reasons associated with IL-6 and IL-8 reduced secretion by PM2.5 at the Ind-2 site is unknown and requires simultaneous mechanistic evaluation. However, here we demonstrate that decrease of both cytokines cannot be attributed to metals alone since the basal levels of these cytokines were not restored with DF pretreatment (Fig. 7). Therefore, we propose that polar organic constituents found in the Ind-2 PM2.5 extract triggers this phenomenon. Other researchers have reported similar findings with high polar organic DEP-extracts (IL-8 inhibition) in BEAS-2B cells, despite stimulating IL-6 (Schwarze et al., 2013). We have also reported similar findings for polar extracts of ambient PM (Fuentes-Mattei et al., 2010). The mechanisms associated with the decrease of cytokines by PM constituents in human lung cells are unclear at this time. These findings set the bases for investigating specific constituents in PM responsible for cytokine decrease.
Highlights.
-
✓
Rio de Janeiro Industrial 2 PM2.5 extracts decreased IL-6 and IL-8 secretion.
-
✓
Rio de Janeiro Industrial 1 PM10 extracts increased IL-6 and IL-8 secretion.
-
✓
PM10 acetone extracts from Rio de Janeiro were very toxic to lung cells BEAS-2B.
-
✓
Heavy metals were higher at the Industrial site (Ind) 1 compared to the Ind 2.
-
✓
Heavy metals were not responsible for the decrease in IL-6 and IL-8 levels.
Acknowledgements
Supported by National Institute of General Medical Sciences of the National Institutes of Health (NIH, R25GM061838). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Thanks to FAPERJ and CNPq for financial support and CAPES (Luiz Drude, NCT-TMCOCEAN Project coordinator), which provided Vinicius L. Mateus scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfaro-Moreno E, Nawrot TS, Nemmar A, Nemery B. Particulate matter in the environment: pulmonary and cardiovascular effects. Curr. Opin. Pulmon. Med. 2007;13:98–106. doi: 10.1097/MCP.0b013e328013f47e. http://dx.doi.org/10.1097/MCP.0b013e328013f47e. PMid:17255799. [DOI] [PubMed] [Google Scholar]
- Alfaro-Moreno E, Torres V, Miranda J, Martínez L, García-Cuellar C, Nawrot TS, Vanaudenaerde B, Hoet P, Ramírez-López P, Rosas I, Nemery B, Osornio-Vargas AR. Induction of IL-6 and inhibition of IL-8 secretion in the human airway cell line Calu-3 by urban particulate matter collected with a modified method of PM sampling. Environmental Research. 2009;109:528–535. doi: 10.1016/j.envres.2009.02.010. http://dx.doi.org/10.1016/j.envres.2009.02.010. PMid:19304283. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacological reviews. 1998;50(4):515–596. [PubMed] [Google Scholar]
- Becker S, Mundandhara S, Devlin RB, Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies. Toxicol. Appl. Pharmacol. 2005;207:S269–S275. doi: 10.1016/j.taap.2005.01.023. http://dx.doi.org/10.1016/j.taap.2005.01.023 PMid:15993911. [DOI] [PubMed] [Google Scholar]
- Bond TC, Bhardwaj E, Dong R, Jogani R, Jung S, Roden C, Streets DG, Tratmann NM. Historical emissions of black and organic carbon aerosol from energy-related combustion 1850-2000. Global Biogeochemical Cycles. 2007;21:GB2018. http://dx.doi.org/10.1029/2006GB002840. [Google Scholar]
- Braga-Ferreira AL, Amador Pereira LA, Procópio M, Afonso de André P, do Nascimento Saldiva PH. Association between air pollution and respiratory and cardiovascular diseases in Itabira, Minas Gerais State, Brazil. Mineração e Efeitos Adversos na Saúde Mineração e Efeitos Adversos na SaúdeS571. Cad. Saúde Pública. 2007;23(4):S570–S578. doi: 10.1590/s0102-311x2007001600017. [DOI] [PubMed] [Google Scholar]
- Camargos Paulo AM, Castro RM, Feldman JS. Prevalencia de síntomas relacionados con el asma en escolares de Campos Gerais (MG) Brasil Rev. Panam Salud Publica. 1999;6(1) doi: 10.1590/s1020-49891999000600002. http://dx.doi.org/10.1590/S102049891999000600002. [DOI] [PubMed] [Google Scholar]
- Carter JD, Ghio A, Samet J, Devlin RB. Cytokine production by human airway epithelial cells after exposure to an air pollution particle is metal dependent. Toxicol. Appl. Pharmacol. Vol. 1997;146:180–188. doi: 10.1006/taap.1997.8254. [DOI] [PubMed] [Google Scholar]
- Castro HA, Cunha MF, Mendonça GAS, Junger WL, Cruz JC, Leon AP. Efeitos da poluição do ar na função respiratória de escolares, Rio de Janeiro, RJ. Revista Saúde Pública. 2009;43:26–34. doi: 10.1590/s0034-89102009000100004. http://dx.doi.org/10.1590/S0034-89102009000100004. PMid:19169573. [DOI] [PubMed] [Google Scholar]
- Chiarelli P, Pereira L, Saldiva P, Filho C, Garcia M, Braga A, Martins L. The association between air pollution and blood pressure in traffic controllers in SantoAndré, São Paulo, Brazil. Environmental Research. 2011;111:650–655. doi: 10.1016/j.envres.2011.04.007. http://dx.doi.org/10.1016/j.envres.2011.04.007. PMid:21570068. [DOI] [PubMed] [Google Scholar]
- Conceição G, Miraglia S, Kishi H, Saldiva P, Singer J. Air Pollution and Child Mortality: A Time-Series Study in São Paulo, Brazil. Environmental Health Perspectives. 2001;109(3):347–350. doi: 10.1289/ehp.109-1240551. http://dx.doi.org/10.1289/ehp.01109s3347. http://dx.doi.org/10.2307/3434781. PMid:11427383. PMCid:PMC1240551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps E, Weidler P, Friedrich F, Weiss C, Diabaté S. Characterization of indoor dust from Brazil and evaluation of the cytotoxicity in A549 lung cells. Environ Geochem Health. 2013 doi: 10.1007/s10653-013-9560-9. [DOI] [PubMed] [Google Scholar]
- Despres VR, Huffman JA, Burrows SM, Hoose C, Safatov AS, Buryak G, Fröhlich-Nowoiski J, Elbert W, Andreae MO, Pöschi U, Jaenicke R. Primary biological aerosol particles in the atmosphere: a review. Tellus Series B - Chemical and Physical Meteorolog. 2012;64:15598. http://dx.doi.org/10.3402/tellusb.v64i0.15598. [Google Scholar]
- Donaldson K, Tran C. Inflammation caused by particles and fibers. InhalToxicol. 2002;14:p5–p27. doi: 10.1080/089583701753338613. http://dx.doi.org/10.1080/089583701753338613. PMid:12122558. [DOI] [PubMed] [Google Scholar]
- Esworthy R. Air quality: EPA’s changesto the particulate matter (PM) standard..CRS Report for Congress. Congressional Research Service. 2013:43. PMid:23977205 PMCid:PMC3747154. [Google Scholar]
- Fideicomiso para el mejoramiento de las vias de comunicacion del Distrito Federal, Ciudad de Mexico. Retrieved on November 2013. http://www.fimevic.df.gob.mx/problemas/1diagnostico.htm. [Google Scholar]
- Fuentes-Mattei E, Rivera E, Gioda A, Sánchez-Rivera D, Roman-Velazquez FR, Jiménez-Velez BD. Use of human bronchial epithelial cells (BEAS-2B) to study immunological markers resulting from exposure to PM2.5 organic extract from Puerto Rico. Toxicol. Appl. Pharm. 2010;243:381–389. doi: 10.1016/j.taap.2009.12.009. http://dx.doi.org/10.1016/j.taap.2009.12.009. PMid:20026096. PMCid:PMC2925116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioda A, Amaral B, Monteiro I, Saint’Pierre T. Chemical composition, sources, solubility, and transport of aerosol trace elements in a tropical region. J. Environ. Monit. 2011a;13:21–34. doi: 10.1039/c1em10240k. http://dx.doi.org/10.1039/c1em10240k. PMid:21677995. [DOI] [PubMed] [Google Scholar]
- Gioda A, Fuentes-Mattei E, Jimenez-Velez B. Evaluation of cytokine expression in BEAS cells exposed to fine particulate matter (PM2.5) from specialized indoor environments. J. Environ. Health Res. 2011b;21:106–119. doi: 10.1080/09603123.2010.515668. http://dx.doi.org/10.1080/09603123.2010.515668 PMid:21424968. PMCid:PMC3785544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy MLDP, Godoy JM, Roldão LA, Soluri D, Donagemma RA. Atmos. Environ. 2009;43:2366. [Google Scholar]
- Gouveia N, Mendonça GAS, Leon AP, et al. ”Poluição do ar e efeitos na saúdenas populações de duas grandes metrópoles brasileiras”. Epidemiologia e Serviços de Saúde. 2003;12:29–240. [Google Scholar]
- Griffin RJ. The Sources and Impacts of Tropospheric Particulate Matter. Nature Education Knowledge. 2013;4(5):1. http://www.nature.com/scitable/knowledge/library/the-sources-and-impacts-of-tropospheric-particulate-102760478. [Google Scholar]
- Hiraiwa K, van Eeden SF. Review Article: Contribution of Lung Macrophages to the Inflammatory Responses Induced by Exposure to Air Pollutants. Mediators of Inflammation. 2013 doi: 10.1155/2013/619523. http://dx.doi.org/10.1155/2013/619523. PMid:24058272. PMCid:PMC3766602. [DOI] [PMC free article] [PubMed]
- Hoinaski L, Franco D, Stuetz R, Sivret E, Melo Lisboa H. Investigation of PM10 sources in Santa Catarina, Brazil through graphical interpretation analysis combined with receptor modeling. Environmental Technology. 2013 doi: 10.1080/21622515.2013.772659. http://dx.doi.org/10.1097/MCP.0b013e328013f47e. PMid:17255799. [DOI] [PubMed]
- Ignotti E, Valente J, Longo K, Freitas S, Hacon S, Netto P. Impact on human health of particulate matter emitted from burnings in the Brazilian Amazon region. Rev. Saúde Pública. 2010 doi: 10.1590/s0034-89102010000100013. http://dx.doi.org/10.1590/S0034-89102010000100013. [DOI] [PubMed]
- Junger WL, Leon AP, Mendonça GAS. Associação entre mortalidade diária por câncer de pulmão e poluição do ar no município do Rio de Janeiro: um estudo ecológico de séries temporais. Revista Brasileira de Cancerologia. 2005;51:111–115. [Google Scholar]
- Krzyzanowski M, Cohen A. Update of WHO air quality guidelines. Air Qual. Atmos. Health. 2008;1:7–13. http://dx.doi.org/10.1007/s11869-008-0008-9. [Google Scholar]
- Lin C, Martins M, Farhat S, Pope C, 3rd, Conceição G, Anastácio V, Hatanaka M, Andrade W, Hamaue W, Böhm G, Saldiva P. Air pollution and respiratory illness of children in São Paulo, Brazil. Paediatric and Perinatal Epidemiology. 1999;13(4):475–488. doi: 10.1046/j.1365-3016.1999.00210.x. http://dx.doi.org/10.1046/j.1365-3016.1999.00210.x. PMid:10563367. [DOI] [PubMed] [Google Scholar]
- Loyola J, Arbilla G, Quiterio SL, Escaleira V, Minho AS. Trace Metals in the Urban Aerosols of Rio de Janeiro City. Journal of the Brazilian Chemical Society. 2012;23:628–638. http://dx.doi.org/10.1590/S0103-50532012000400007. [Google Scholar]
- Martin LD, Rochelle LG, Fischer BM, Krunkosky TM, Adler KB. Airway epithelium as an effector of inflammation: molecular regulation of secondary mediators. Eur. Respir. J. 1997;10:2139–2146. doi: 10.1183/09031936.97.10092139. http://dx.doi.org/10.1183/09031936.97.10092139. PMid:9311517. [DOI] [PubMed] [Google Scholar]
- Mateus V, Monteiro I, Rocha R, Saint’Pierre T, Gioda A. Study of the chemical composition of particulate matter from Rio de Janeiro metropolitan region, Brazil, by inductively coupled plasma-mass spectrometry and optical emission spectrometry. Spectrochimica Acta Part B. 2013;86:131–136. http://dx.doi.org/10.1016/j.sab.2013.03.003. [Google Scholar]
- Miguel A, Daisy J, Sousa J. Comparative study of the mutagenic and genotoxic activity associated with inhalable particulate matter in Rio de Janeiro air. Environmental and Molecular Mutagenesis. 1990;15(1):36–43. doi: 10.1002/em.2850150106. http://dx.doi.org/10.1002/em.2850150106. PMid:2137085. [DOI] [PubMed] [Google Scholar]
- Moore KR, Duffell H, Nicholl A, Searl A. Montserrat. Geological Society, London, Memoirs. 2002;21:557–566. [Google Scholar]
- Moura M, Junger WL, Mendonça GAES, de Leon AP. Air quality and emergency pediatric care for symptoms of bronchial obstruction categorized by age bracket in Rio de Janeiro, Brazil. Caderno Saúde Pública. 2009;25(3):635–644. doi: 10.1590/s0102-311x2009000300018. http://dx.doi.org/10.1590/S0102-311×2009000300018. PMid:19300852. [DOI] [PubMed] [Google Scholar]
- Nascimento L, Francisco J. Particulate matter and hospital admission due to arterial hypertension in a medium-sized Brazilian city. Cad. Saúde Pública. Rio de Janeiro. 2013;29(8):1565–1571. doi: 10.1590/0102-311x00127612. http://dx.doi.org/10.1590/0102-311×00127612. [DOI] [PubMed] [Google Scholar]
- Ortiz-Martínez M, Rivera-Ramirez E, Mendez-Torres L, Jiménez-Vélez BD. Theophanides M, Theophanides T, editors. Role of chemical and biological constituents of PM10 from Saharan dust in the exacerbation of asthma in Puerto Rico. In: Biodiversity Science for Humanity. Athens Institute for Educations & Research. 2010:101–118. [Google Scholar]
- Particulate matter (PM) standards, Table of historical PM NAAQS, History of the National Ambient Air Quality Standards for particulate matter during the period 1971–2012. http://www.epa.gov/ttn/naaqs/standards/pm/s_pm_history.html#2.
- Paulino SA, Quiterio SL, Escaleira V, Arbilla G. Evolution of Particulate Matter and Associated Metal Levels in the Urban Area of Rio de Janeiro. Brazil. Bulletin of Environmental Contamination and Toxicology. 2010;84(3):315–318. doi: 10.1007/s00128-009-9931-1. http://dx.doi.org/10.1007/s00128-009-9931-1. PMid:20041227. [DOI] [PubMed] [Google Scholar]
- Quiterio SL, Escaleira V, Sousa CRS, Maia LFPG, Arbilla G. Metals in Airborne Particulate Matter in Downtown Rio de Janeiro. Brazil. Bulletin of Environmental Contamination and Toxicology. 2004b;72(5):916–922. doi: 10.1007/s00128-004-0331-2. http://dx.doi.org/10.1007/s00128-004-0331-2. PMid:15266686. [DOI] [PubMed] [Google Scholar]
- Quiterio S, Sousa da Silva C, Arbilla G, Escaleira V. Metals in airborne particulate matter in the industrial district of Santa Cruz, Rio de Janeiro, in an annual period. Atmospheric Environment. 2004a;38:321–331. http://dx.doi.org/10.1016/j.atmosenv.2003.09.017. [Google Scholar]
- Ramos de Rainho C, Machado Corrêa S, Mazzei JL, Fortes Aiub CA, Felzenszwalb I. Genotoxicity of Polycyclic Aromatic Hydrocarbons and Nitro-Derived in Respirable Airborne Particulate Matter Collected from Urban Areas of Rio de Janeiro (Brazil) BioMed Research International. 2013;2013:9. doi: 10.1155/2013/765352. Article ID 765352, http://dx.doi.org/10.1155/2013/765352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D, Magalhães C, Lopes A, Lanças T, Mauad T, Malm O, Valença S, Saldiva P, Faffe D, Zin W. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhalation Toxicology. 2011;23(5):257–267. doi: 10.3109/08958378.2011.566290. http://dx.doi.org/10.3109/08958378.2011.566290. PMid:21506876. [DOI] [PubMed] [Google Scholar]
- Rivero D, Soares S, Filho G, Saiki M, Godleski J, Antonangelo L, Dolhnikoff M, Saldiva P. Acute cardiopulmonary alterations induced by fine particulate matter of São Paulo, Brazil. Toxicological Sciences. 2005;85:898–905. doi: 10.1093/toxsci/kfi137. http://dx.doi.org/10.1093/toxsci/kfi137. PMid:15746007. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cotto R, Ortiz-Martínez M, Rivera Rámirez E, Mendez L, Dávila JC, Jiménez-Vélez BD. African dust storms reaching Puerto Rican coast stimulate the secretion of IL-6 and IL-8 and cause cytotoxicity to human bronchial epithelial cells (BEAS-2B) Health, Special Issue: PM2.5 Effects on Health. 2013;5(10B):14–28. doi: 10.4236/health.2013.510A2003. http://dox.doi.org/10.4236/health.2013.510A2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze PE, Totlandsdal AI, Låg M, Refsnes M, Holme JA, Øvrevik J. Inflammation-Related Effects of Diesel Engine Exhaust Particles: Studies on Lung Cells In Vitro. BioMed Research International.Volume. 2013;2013:13. doi: 10.1155/2013/685142. http://dx.doi.org/10.1155/2013/685142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith David B, Robert A, Zielinski WI, Rose, Huebert BJ. Water-soluble material on aerosols collected within volcanic eruption clouds. Journal of Geophysical Research: Oceans. 2012;87(C7):1978–2012. [Google Scholar]
- Thorsteinsson T, Jóhannsson T, Stohl A, Kristiansen NI. High levels of particulate matter in Iceland due to direct ash emissions by the Eyjafjallajökull eruption and resuspension of deposited ash. Journal of Geophysical Research: Solid Earth. 2002;117(B9):1978–2012. [Google Scholar]
- Umbuzeiro G, Franco A, Martins M, Kummrow F, Carvalho L, Schmeiser H, Leykauf J, Stiborova M, Claxton L. Mutagenicity and DNA adduct formation of PAH, nitro-PAH, and oxy-PAH fractions of atmospheric particulate matter from S˜ao Paulo. Brazil. Mutation Research. 2008;652:72–80. doi: 10.1016/j.mrgentox.2007.12.007. http://dx.doi.org/10.1016/j.mrgentox.2007.12.007. PMid:18294902. [DOI] [PubMed] [Google Scholar]
- Universia. Demasiados vehículos circulan en el DF. 2013 http://noticias.universia.net.mx/actualidad/noticia/2013/06/21/1032169/demasiados-vehiculos-circulan-df.html\.
- Veranth JM, Moss TA, Chow JC, Labban R, Nichols WK, Walton JC, et al. Correlation of in vitro cytokine responses with the chemical composition of soil-derived particulate matter. Environ. Health Perspect. 2006;114:341–349. doi: 10.1289/ehp.8360. http://dx.doi.org/10.1289/ehp.8360. PMid:16507455. PMCid:PMC1392226. [DOI] [PMC free article] [PubMed] [Google Scholar]