Abstract
An ethological approach to attention predicts that organisms orient preferentially to valuable sources of information in the environment. For many gregarious species, orienting to other individuals provides valuable social information but competes with food acquisition, water consumption and predator avoidance. Individual variation in vigilance behaviour in humans spans a continuum from inattentive to pathological levels of interest in others. To assess the comparative biology of this behavioural variation, we probed vigilance rates in free-ranging macaques during water drinking, a behaviour incompatible with the gaze and postural demands of vigilance. Males were significantly more vigilant than females. Moreover, vigilance showed a clear genetic component, with an estimated heritability of 12%. Monkeys carrying a relatively infrequent ‘long’ allele of TPH2, a regulatory gene that influences serotonin production in the brain, were significantly less vigilant compared to monkeys that did not carry the allele. These findings resonate with the hypothesis that the serotonin pathway regulates vigilance in primates and by extension provoke the idea that individual variation in vigilance and its underlying biology may be adaptive rather than pathological.
Keywords: Macaca mulatta, rhesus macaque, serotonin pathway, TPH2, vigilance, social attention, neuroethology, primate
For group-living animals, survival and reproductive success often depend on the appropriate perception of others and adaptive responses to their behaviour. Better information permits better informed action, thus endorsing the hypothesis that the brain has been shaped by natural selection to facilitate acquisition of information about the identity, behavioural states and impending behaviours of others (Allman, 2000; Dunbar, 1998). Vigilance behaviour, visual scanning of the external environment, has been documented in dozens of different species of mammal and bird (Elgar, 1989). Although vigilance can be used to detect predators (Elgar, 1989; Roberts, 1996), it can also be used to gather social information (Chang et al., 2013; Klein, Shepherd, & Platt, 2009). Some gregarious species, like many primates, reduce predation risk by living in groups, but consequently face elevated levels of competition with conspecifics. In these animals, gathering social information may be the primary function of visual monitoring (Chance & Jolly, 1970; Treves, 2000).
Consistent with this idea, recent studies have demonstrated that ringtail lemurs, Lemur catta (Shepherd & Platt, 2008), rhesus macaques, Macaca mulatta (Deaner, Khera, & Platt, 2005) and humans (Hayden et al., 2007) value the opportunity to acquire information about conspecifics. For rhesus macaques, the value of information about the dominance status and reproductive quality of others can substitute for fluid or food rewards (Deaner et al., 2005; Watson, Ghodasra, & Platt, 2009), and for human males the value of information about the attractiveness of females can substitute for money and time, and can motivate work (Hayden et al., 2007). Moreover, wild primates routinely attend to the social interactions of others, and orient visually towards conspecific calls in playback experiments (e.g. Bergman et al., 2003; Cheney & Seyfarth, 1999; Cheney, Seyfarth, & Silk, 1995). These experiments also demonstrate that recent social interactions influence the selectivity of this orienting behaviour, indicating that nonhuman primates remember interactions with opponents and allies and use this information to modulate attention (Cheney et al., 2010; Engh et al., 2006; Wittig et al., 2014; Wittig et al., 2007a; Wittig et al., 2007b).
Consonant with these behavioural studies, specialized populations of neurons in brain areas linked to attention (Klein, Deaner, & Platt, 2008), self-control (Ebitz & Platt, in press) and reward (Klein & Platt, 2013; Watson & Platt, 2012) respond when monkeys have the opportunity to acquire information about others or use this information to guide subsequent visual exploration behaviour (Shepherd et al., 2009). Some of these same structures are also activated in humans when they choose to acquire visual information about others at the expense of monetary rewards (Smith et al., 2010) or effortful labour (Aharon et al., 2001). Together, these observations resonate with the idea that the challenges of social life favoured the evolution of specialized neural circuits mediating the acquisition and utilization of information about other individuals, which evolved from basal circuits mediating information acquisition and utilization in nonsocial contexts (Adams et al., 2012; Chang et al., 2013; Pearson, Watson, & Platt, 2014).
Despite the clear adaptive value of social vigilance, and clear evidence that specific neural circuits have evolved to support this behaviour, individuals often vary substantially in social attention behaviour (Frischen, Bayliss, & Tipper, 2007; Seyfarth & Cheney, 2013; Shepherd, Deaner, & Platt, 2006). The sources and persistence of these differences remain to be understood fully, but some seem to be genetic in origin (Constantino & Todd, 2000; Ebstein et al., 2010; Jamain et al., 2008). In many animals, including mammals, crustaceans and fish, serotonin regulates social behaviour, including aggression and dominance relationships (Edwards & Kravitz, 1997; Higley et al., 1996; Higley et al., 1992). Among primates, in particular, serotonin influences a broad array of social functions (Watson et al., 2009). Indeed, the human psychiatric literature is replete with associations between genetic variation in the serotonin system and behavioural pathology (Caspi et al., 2010; Caspi et al., 2003; Hariri et al., 2005).
Two important proteins in the serotonin system are the serotonin transporter (5-HTT), which removes serotonin from the synaptic space between neurons in the brain, and tryptophan hydroxylase (TH), the enzyme that regulates serotonin production. The genes encoding these two proteins have been repeatedly, although controversially, linked to various psychiatric disorders in humans (Canli et al., 2008; Gao et al., 2012; Hariri et al., 2005; Hariri & Holmes, 2006; Popova & Kulikov, 2010; Waider et al., 2011; Zhou et al., 2005) and influence anxiety-related personality traits among healthy individuals (Gutknecht et al., 2007; Lesch et al., 1996; Reuter, Kuepper, & Hennig, 2007; Sen, Burmeister, & Ghosh, 2004).
One well-studied polymorphism in the gene encoding the serotonin transporter is the 5-HTT length polymorphic region (5-HTTLPR), which consists of a repeating sequence of base pairs. There are two predominant alleles in the human population: the short allele, which has 14 repeat elements, and the long allele, which has 16 (Hariri & Holmes, 2006). The short allele is typically associated with psychiatric disease, anxiety-related traits and activity in the amygdala, a brain region associated with threat detection and vigilance (Hariri et al., 2005; Hariri & Holmes, 2006; Hariri et al., 2002). An orthologous repeat 5-HTTLPR variant exists in rhesus macaques (Canli & Lesch, 2007), and in this manuscript we refer to both human and rhesus polymorphisms as 5-HTTLPR. Rhesus macaques who carry at least one copy of the less common short allele and who are exposed to a stressful period during early development (peer rearing versus maternal rearing), show delayed neural development, impaired serotonergic function, higher aggression and higher stress hormone levels than monkeys who carry two copies of the long 5-HTTLPR allele (Barr et al., 2004; Bennett et al., 2002; Champoux et al., 2002). Moreover, monkeys carrying the short 5-HTTLPR allele are more likely to avoid dominant faces in an attention task, show suppressed risk taking after exposure to dominant faces and have greater physiological arousal in response to dominant faces (Watson et al., 2009). Altered social reactivity in macaques carrying the 5-HTTLPR short allele is consistent with functional imaging studies in humans, in which the short 5-HTTLPR allele is linked to greater amygdala activity when viewing angry faces (Hariri et al., 2005; Hariri et al., 2002).
The TPH2 enzyme is another important regulator of serotonergic function and there exist several polymorphisms in the human TPH2 gene (Gao et al., 2012; Popova & Kulikov, 2010). Unlike 5-HTTLPR, there is no single TPH2 polymorphism that has been particularly well studied or characterized, although several are linked to psychiatric disease, altered amygdala activity and anxiety-related personality traits in a manner similar to 5-HTTLPR (Popova & Kulikov, 2010). In the current study we focus on a rhesus-specific polymorphism, referred to here as the TPH2 insertion polymorphism (TPH2IP), in which 159 additional base pairs are inserted into the untranslated region at the terminal end of the gene (Chen et al., 2006). Alleles associated with TPH2IP are either long (i.e. with insertion) or short (without insertion); the short allele is more common (80% frequency in captive rhesus macaques; Chen et al., 2006).
The precise influence of these polymorphisms on serotonin in vivo remains mysterious. In vitro assays suggest the short 5-HTTLPR and TPH2IP alleles decrease expression of 5-HTT and TPH2, respectively (Bennett et al., 2002; Chen et al., 2006; Greenberg et al., 1999; Heils et al., 1996), which should theoretically increase the amount of circulating 5-HT in both cases. This increase in 5-HT may eventually be downregulated or stabilized via compensatory mechanisms, such as negative feedback loops mediated by the 5-HT1a receptor (Christian et al., 2013). In support of this hypothesis, a study in mice found that a TPH2 polymorphism altered 5-HT synthesis rate, affected anxiety-related behaviours and desensitized 5-HT1a receptors, but did not alter brain serotonin concentration (Berger et al., 2012). Similarly, there is no association between 5-HTTLPR genotype and the level of 5HTT density as measured by positron emission tomography in adult humans (PET) (Kobiella et al., 2011; Shioe et al., 2003; Willeit et al., 2001), although amygdala volume and 5-HT1a receptor binding both vary with this genotype (Kobiella, 2011, page 102; David, 2005, page 144). Genetic variation in both TPH2 and 5-HTT may shape the brain during development (Berger et al., 2012; Kobiella et al., 2011), consistent with observations that psychiatric disease emerges from the interaction of childhood and serotonin genotype (reviewed in Caspi et al., 2010; Hariri & Holmes, 2006).
Although precisely how genetic variation in the serotonergic system influences neural circuit function remains unclear, this variation is capable of shaping behavioural phenotypes. For example, both 5-HTTLPR and TPH2IP influence affiliative behaviours in free-ranging rhesus macaques (Brent et al., 2013). Specifically, rhesus macaques carrying the less frequent serotonin gene variants (short for 5-HTTLPR, long for TPH2IP) tend to have fewer friends and allies, as indexed by position in the grooming network, due to either poor social skill or lack of interest in others. Notably, both male and female monkeys on the periphery of the social network tend to have lower reproductive fitness than those in the centre (Brent et al., 2013), consistent with evidence in female baboons linking stronger social bonds with greater fitness (Seyfarth, Silk, & Cheney, 2012; Silk, Alberts, & Altmann, 2003; Silk et al., 2009; Silk et al., 2010). These findings suggest that individual differences in social attention and other social functions in both rhesus macaques and humans arise, in part, from differences in the way the serotonin system influences the neural circuits that mediate social vigilance behaviour.
Such continuous variation in behavioural traits within a population may reflect a spectrum of alternative behavioural strategies, any or all of which may prove successful within a particular social environment. Genetic variation in the serotonin system in nonhuman primates may influence how individuals cope with intragroup competition. According to this model, phenotypic heterogeneity reflects variations in fitness offered by each strategy depending on the dynamics of the local social environment (Dobson & Brent, 2013). Testing this idea systematically will require determining how genes shape behaviour, fitness, and underlying neural circuit structure and function across primates, including humans.
As a step towards that goal, here we investigate whether variations in the serotonergic polymorphisms 5-HTTLPR and TPH2IP shape vigilance in free-ranging rhesus macaques. We focus on the trade-off between vigilance and drinking behaviours, which provides a standardized context to assess individual variation in social information gathering. We first characterize interindividual differences in vigilance and relate these to sex, age and dominance rank. Next, we explore whether the serotonin-related genetic variations 5-HTTLPR and TPH2IP influence vigilance rates, independent of sex, age and rank. Finally, we explore these gene-specific variations in the context of a more comprehensive statistical model containing information on relatedness based on pedigree, which allows us to differentiate the specific contribution of these candidate genes and to estimate the heritability of vigilance behaviour.
METHODS
Study Population
Rhesus macaques are Old World monkeys that live in social groups containing multiple adult males and females (Melnick, Pearl, & Richard, 1984). Males usually leave their natal groups at the onset of puberty, and subsequently change groups approximately every 4 years throughout their lives (Datta, 1988; Melnick et al., 1984). Females remain in the group in which they were born for life (Pusey & Packer, 1987). Social groups are organized hierarchically. Adult males usually dominate all females. Between males, dominance is established by physical combat and is temporally dynamic (Thierry, 2000). Females form stable linear dominance hierarchies (Chikazawa et al., 1979), with daughters securing the rank immediately below their mothers prior to puberty. The female hierarchy is further structured into matrilines, which are made up of females that are closely related and close in dominance rank. Rhesus societies are characterized by low levels of social tolerance and high levels of aggression (Balasubramaniam et al., 2012; Sueur et al., 2011; Thierry, Iwaniuk, & Pellis, 2000) in captivity (Hinde & Rowell, 1962; Southwick, 1967), in the wild (Southwick, Beg, & Siddiqi, 1965) and on Cayo Santiago, Puerto Rico (Altmann, 1962).
Subjects were 122 rhesus macaques (N = 74 females, N = 48 males), residing on the island of Cayo Santiago. This colony was established in 1938 with monkeys from a single founding population consisting of 409 individuals from India (Rawlins & Kessler, 1986). At the time of this study, there were approximately 1400 individuals on the 15 ha island. There were between six and nine social groups during our study period. All animals are individually identified by a three-character name, which is tattooed on the chest and inner thigh, and by a unique combination of notches in the skin of their ears, both of which are administered at 1 year of age by the trained staff at the field station. No predators are present on the island and monkeys are habituated to the daily presence of human investigators, colony managers and census-takers. Monkeys are provisioned once daily with commercially supplied monkey chow (8773, Teklad NIB Primate Diet Modified). The monkeys also browse freely on the island’s vegetation. Water is supplied ad libitum from rain-water collection troughs. Monkeys also drink water from rainwater puddles that form on the island.
This study was approved by the Institutional Animal Care and Use Committee of the University of Puerto Rico (protocol number A6850108).
Pedigree Data and Genetic Parentage Assignment
We obtained pedigree data from the Caribbean Primate Research Center (CPRC) long-term database. This database contains maternal assignments based on census information (i.e. based on behaviours of putative mothers, such as lactation) for all animals from the founding population onwards, as well as maternity and paternity based on analysis of 29 microsatellite markers for most animals born since 1990 (~2886 monkeys). Maternity and paternity based on genetic data was known for 117 (95.9%) of the animals in our study. We used census information to determine maternal identity when genetic maternity (N = 4) was unknown. There is little evidence for high rates of inbreeding on Cayo Santiago, with virtually no difference in blood polymorphism or mitochondrial haplotype diversity between this population and wild Indian rhesus macaques (Blomquist, 2009). Genetic diversity on Cayo Santiago is similar to that in wild rhesus populations (Blomquist, 2008; Duggleby et al., 1986).
5-HTTLPR and TPH2IP Genetics
DNA sample collection and serotonin-related polymorphism assessment were performed as described previously (Brent et al., 2013). We assessed polymorphisms using PCRs with previously described primers. The 5-HTTLPR polymorphism of the serotonin transporter gene (SLC6A4) and the TPH2 polymorphism (159 bp insertion in the 3′ UTR) are both characterized by long and short promoter regions (Hariri & Holmes, 2006) (Chen et al., 2006). We grouped individuals that carried at least one copy of the minor allele for each locus (short allele for 5-HTTLPR, long allele for TPH2), as is the standard for studies with few individuals homozygous for the minor allele (see Table 1) (Canli et al., 2008). We note, however, that modelling the genotypes additively, based on the number of minor alleles carried, yielded statistically identical results to those reported in the current paper. We refer to these alleles as ‘short’ or ‘long’ for the remainder of the paper.
Table 1.
Number of rhesus macaques of each genotype for 5-HTTLPR and TPH2IP
| Sex | 5-HTTLPR |
TPH2IP |
||||
|---|---|---|---|---|---|---|
| LL | LS | SS | LL | LS | SS | |
| Female | 36 (0.49) | 27 (0.37) | 11 (0.15) | 5 (0.07) | 29 (0.39) | 40 (0.54) |
| Male | 26 (0.54) | 16 (0.33) | 6 (0.13) | 2 (0.04) | 26 (0.54) | 20 (0.42) |
| Total | 62 (0.51) | 43 (0.35) | 17 (0.14) | 7 (0.06) | 55 (0.45) | 60 (0.49) |
Note that the short (‘S’) allele is the less frequent allele for 5-HTTLPR, but the long (‘L’) allele is the less frequent allele for TPH2IP. Relative frequencies for each sex are reported in parentheses. For purposes of analysis, SS animals and heterozygotes were grouped together for 5-HTTLPR, and LL animals and heterozygotes were grouped together for TPH2IP.
Behavioural Data Collection
Data on vigilance behaviour was collected during three video recording sessions during January 2012, June 2012 and January 2014. Monkeys drinking from troughs or puddles were recorded on video. During the first session, we gathered video data opportunistically upon successful identification of the subject; during the second session, we specifically targeted and videorecorded monkeys homozygous for the short allele of 5-HTTLPR because of their scarcity in the first data collection session and their small sample size (<10%; see Brent et al., 2013). In the third session, were collected a second set of videos for monkeys already present in the data set in order to conduct repeated measures analysis. In total, 279 videos of 122 monkeys were collected, with a mean length of 84.5 s per video. Observations shorter than 30 s were discarded. In the final analysis, there were 75 females and 48 males in our data set, ranging in age from 4 to 28 years. All videos were recorded from about 10 m away from the focal monkey in order to avoid disturbing ongoing behaviour (Hirsch, 2002).
During videorecording, facial characteristics, ear notches and chest tattoos were used to identify subjects. Variables such as age and sex of the subject were derived from previously collected census data. Dominance rank of subjects was determined using observational data collected during focal animal samples and ad libitum, which were collected as part of a long-term behavioural research project. We determined dominance rank from the direction and outcome of submissive interactions between dyads. Behaviour used to assess dominance rank was collected over 9 months within a calendar year. Dominance data was only included when vigilance data were collected during the same year of dominance assessment.
Video Coding
All video data were coded using Tinbergen Alpha software (Adams, 2014) and coded data were preprocessed using MatLab (MathWorks, Natick, MA, U.S.A.). The coder was blind to genotype and other attributes of the subjects. Each video was inspected and coded according to three general types of behaviour: ‘drinking’, ‘vigilance’ and ‘other’ (Fig. 1). Drinking behaviour was recorded as beginning from the time the monkey’s lips touched the water source until the lips left the water. Vigilance behaviour was recorded as a pause in eating, drinking or locomotor activity, accompanied by visual orienting. The posture during vigilance (sitting or standing) was also coded. The ‘other’ behaviour code included irrelevant behaviours such as monkey–monkey interactions (e.g. grooming) or the focal monkey travelling from one drinking spot to another.
Figure 1.
Costs and benefits of vigilance behaviour in free-ranging rhesus macaques. Top: drinking necessitates a head-down posture incompatible with vigilance behaviour. Bottom: animals periodically interrupt drinking to monitor their surroundings. This male, 29H, was vigilant during 47.6% of the filmed drinking bout.
Because the visual targets of vigilance could not be identified by the investigators, we established criteria in order to differentiate between vigilance and nonvigilant resting states. We wanted to ensure that behaviour coded as vigilance in our study was restricted to behaviours in which the monkey appeared to be actively gathering information about the environment, rather than passively resting while staring off into the distance. To do this, we encoded all head swivels, characterized by a turn of the head at least 45 degrees from the current position. We then calculated the duration of all putative ‘looking bouts’, defined as the time intervals between a head swivel and the previous and subsequent states (drinking, other, or next swivel or posture change). The mean looking bout length was 3.7 s, and any looking bout longer than one standard deviation above the mean (SD = 2.7 s) was considered to not meet the criteria for vigilance behaviour. Only looking bouts that met the criteria were included in the analysis. For each movie, vigilance was calculated as the summed duration of all looking bouts that met the criteria, divided by the length of the movie.
For each video, we additionally coded the number of other monkeys in proximity (defined as within 10 m of the focal monkey), whether the focal monkey was eating during the video bout, whether female focal monkeys had infants in direct contact (clinging or standing directly beneath) and whether drinking occurred at a puddle or trough.
Analysis
We used linear mixed models to test whether demographic and genetic variables were associated with vigilance (Pinheiro et al., 2014). We used rate of vigilance behaviour as our dependent measure, monkey identity as a random effect and age, sex, number of other monkeys in proximity and TPH2 and 5-HTTLPR allele status as fixed effects. A Q–Q plot of the residuals indicated that our dependent variable was normally distributed, and residual plots showed linearity and absence of heteroscedasticity, indicating that linear models were appropriate. To explore whether dominance rank influenced vigilance, we coded rank for each individual for whom we had rank information (N = 78) into one of three ordinal rank categories (‘high,’ ‘medium’ and ‘low’) consisting of approximately the same number of individuals. Because we had rank information for only a subset of individuals, we ran models on the subset data in addition to our other analyses, with identical factors and the additional fixed effect of rank. We note that the outcomes of the genetic influences on vigilance were identical across models inclusive and exclusive of rank information. Because of power limitations, we were not able to inspect interactions between TPH2 allele status, rank and sex (N = 1 low-ranking male with long TPH2IP).
To determine whether TPH2 or 5-HTTLPR allele status specifically contributed to variation in vigilance behaviour, we additionally estimated additive genetic variances for vigilance alongside genetic and demographic effects using generalized linear mixed models fitted in a Bayesian framework using Markov chain Monte Carlo (MCMC) methods in the R package MCMCglmm (Hadfield, 2010). This type of model is commonly known as the ‘animal model’ in evolutionary biology and animal breeding literatures (Wilson et al., 2010; Blomquist & Brent, 2014) and is used here to disambiguate polymorphism-specific influences from more general relatedness influences on phenotype. We ran a full model using our complete data set that included sex, age, number of monkeys in proximity and allele status as fixed effects, and individual and relatedness as random effects. We also ran an identical model, with the addition of rank as a fixed effect, on the subset of data for which we had dominance rank information.
RESULTS
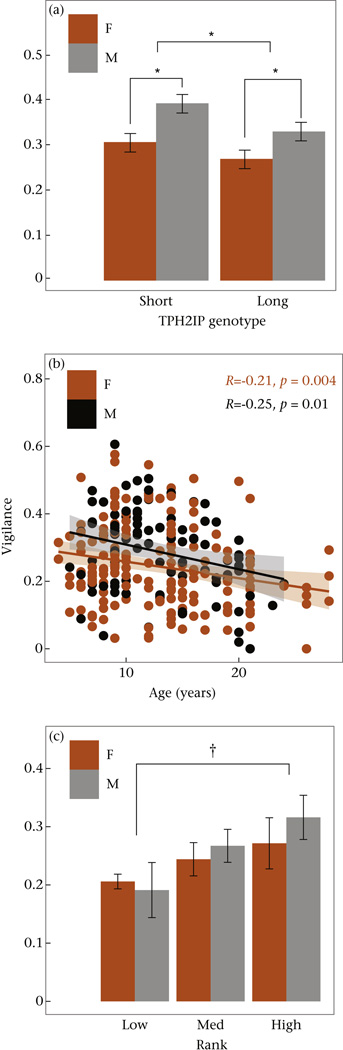
We scored 279 movies from 122 individuals (N = 74 females, N = 48 males) for vigilance while drinking. Movies had a mean ± SD duration of 83.36 ± 55.35 s. Vigilance, the amount time spent looking or scanning, varied from 0 to 80.2% of videos, with a mean ± SD of 25.9 ± 12.7%. We found that age, sex and TPH2IP allele status were significant predictors of differences in vigilance rates (ANOVA: age: F1,152 = 10.68, P = 0.0013; sex: F1,118 = 5.17, P = 0.025; TPH2IP: F1,118 = 8.30, P = 0.005; Fig. 2). The number of monkeys in proximity and 5-HTTLPR allele status were not significant predictors of vigilance rates (both P >0.56). When we included all two-way interaction terms, only the main effects of age, sex and TPH2IP allele status were significant predictors of vigilance (ANOVA: age: F1,130 = 10.38, P = 0.0013; sex: F1,114 = 4.99, P = 0.024; TPH2IP: F1,114 = 8.18, P = 0.005), and no interaction terms were significant (all P >0.11). These findings indicate that monkeys with a copy of the less common TPH2IP long allele were significantly less vigilant than monkeys that did not carry a copy of the long allele. Additionally, these results show that 5-HTTLPR allele status did not influence vigilance, that monkeys showed significantly less vigilance as they aged and that males were more vigilant than females.
Figure 2.
Effects of (a) serotonin-related genotype (short vs long allele of the TPH2 polymorphism), (b) age/sex and (c) dominance rank on vigilance behaviour in free-ranging rhesus macaques. In (a) and (c), values are means ± SD. In (b), the shaded area represents the 95% confidence interval. For presentation purposes, a single outlier (14-year-old male with 80% vigilance) has been removed. †P = 0.014 (Tukey post hoc comparison between low- and high-ranking animals); *P <0.03.
We initially avoided analysing the sexes separately because it would degrade our sample size, a particularly important consideration when running models including relatedness as a covariate. However, to establish whether these effects were the same for each sex, we also examined the contribution of these variables to vigilance in each sex separately. We found that TPH2IP allele status was a significant predictor of differences in vigilance rates in males (ANOVA: F1,45 = 4.94, P = 0.03) and approached significance in females (ANOVA: F1,100 = 3.58, P = 0.06), with TPH2IP long carriers showing less vigilance. Age was also significantly related to vigilance in both males (ANOVA: F1,50 = 5.12, P = 0.028) and females (ANOVA: F1,100 = 5.86, P = 0.017), although the number of animals in proximity and 5-HTTLPR allele status were not (all P >0.2).
We next explored whether TPH2IP allele status specifically influenced vigilance behaviour, or whether this allele status merely served as a nonspecific marker of relatedness. We ran a MCMCglmm containing the relatedness matrix for the entire population and found that TPH2IP status, as well as sex and age, had nonzero contributions to vigilance behaviour (see Table 2). Monkeys spent a mean amount of 33% of the time engaged in vigilance. Relative to females, males spent an additional 4.2% of time being vigilant. Monkeys carrying a copy of the long (minor) TPH2IP allele spent 5.2% less time being vigilant compared to monkeys homozygous for the more common TPH2IP short allele. Vigilance decreased with age, going down 0.5% with each additional year. Thus, variation in TPH2IP, as well as sex and age, significantly influenced vigilance independent of overall genetic relatedness between individuals. Heritability of vigilance was estimated at 12.2%.
Table 2.
Estimates of factors influencing vigilance in free-ranging rhesus macaques based on output of Markov chain Monte Carlo general linear model
| Posterior mean | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|
| Intercept* | 0.33 | 0.28 | 0.38 |
| Sex (male)* | 0.042 | 0.009 | 0.078 |
| TPH2IP genotype (long)* | −0.052 | −0.084 | −0.022 |
| 5-HTTLPR genotype (short) | −0.004 | −0.035 | 0.035 |
| Age* | −0.0049 | −0.0080 | −0.0019 |
| Group size | −0.000070 | −0.0046 | 0.0032 |
Values indicate that the mean rate of vigilance during the movies was 33%. Males had an additional 4.2% increase in vigilance versus females, and long TPH2IP carriers had a 5.2% decrease in vigilance compared with short TPH2IP subjects. Age was inversely related to vigilance, with a decrease of 0.049% vigilance for each additional year.
An asterisk indicates those factors with 95% confidence intervals (CI) that did not overlap zero. Categorical factors in parentheses indicate the level used for comparison to baseline.
Because we only had dominance rank information for a subsample of monkeys, we ran separate models to explore the effect of social status on vigilance behaviour. In this model, TPH2IP allele status remained significantly related to vigilance (ANVOA: F1,70 = 5.08, P = 0.025). Rank showed a tendency towards significance (F1,70 = 2.65, P = 0.078). We performed post hoc tests of multiple comparisons on the effect of rank on vigilance, and found that high-status animals were significantly more vigilant than low-status animals (Tukey multiple comparisons of means: Z = 2.811, P = 0.013). All other comparisons between ranks were not significant (P >0.15). We next constructed a MCMCglmm on this subset of data. Although the 95% CI for the fixed effect of sex in this model contained zero, dominance rank as well as age, TPH2IP status and relatedness had nonzero contributions to vigilance. These data suggest that higher-ranking animals display greater vigilance than lower-ranking animals.
DISCUSSION
We found that vigilance in rhesus macaques is genetically heritable and that it is influenced by variation in TPH2, a gene coding for the enzyme that regulates serotonin synthesis in the brain. Specifically, monkeys carrying the less frequent, long allele at TPH2IP showed lower levels of visual monitoring during drinking than monkeys that had two copies of the short allele.
We cautiously interpret this finding as preliminary evidence for reduced interest in or motivation to observe others in monkeys that carry a copy of the long allele at TPH2IP. Consistent with this interpretation, rhesus macaques on Cayo Santiago that carry copies of the minor allele in both the TPH2IP and 5-HTTLPR polymorphism (i.e. long and short alleles, respectively) live on the fringes of the social network (Brent et al., 2013). A previous study found that captive rhesus macaques carrying the short allele of 5-HTTLPR spend less time viewing the eyes and faces of other monkeys (Watson et al., 2009). Unexpectedly, we found no influence of 5-HTTLPR on vigilance behaviour in our study, suggesting that variation in TPH2IP and 5-HTTLPR exert their effects in subtly different ways or that our power to detect effects of 5-HTTLPR was limited. As reviewed above, because of the heterogeneous and complex nature of the serotonergic system, the exact influence of these polymorphisms on serotonin function in vivo remains unknown.
We note that the monkeys on Cayo Santiago have no natural predators, although agonistic interactions with conspecifics are relatively common (Boelkins & Wilson, 1972). Moreover, previous studies in both captive and wild populations suggest that vigilance in many species of primates primarily functions to observe conspecifics rather than to detect predators (reviewed in Treves, 2000; see also Seyfarth & Cheney, 2013). For example, vigilance occurs even among captive primates, for whom predators are absent (Keverne et al., 1978; Maestripieri, 1993), and a study that identified the targets of monitoring in two species of primates found that most vigilance was directed towards other members of the group (Treves, 1999). Larger groups offer greater protection from predation, both because of a dilution of risk for individual animals and because a greater number of individuals facilitates the detection of predator danger (Elgar, 1989; Roberts, 1996). Consequently, in most mammals and birds, vigilance decreases with increasing group size (Elgar, 1989; Roberts, 1996). In contrast, a meta-analysis of studies of vigilance in 10 different species of primates found little evidence for decreases in vigilance with increasing group size, suggesting a modulation or absence of the predator detection function of vigilance in primates (Treves, 2000). In our study, we found no relationship between the number of monkeys present in the immediate vicinity (10 m radius) and the level of vigilance displayed. We note that this is not incompatible with a social monitoring function for vigilance, since visual social information is typically available on Cayo Santiago even when no monkeys are within our defined range of proximity. Inter- and intragroup conflict and group movement can be visually assessed from afar and provide useful information even if the activity does not immediately or directly influence the subject. Consistent with this idea, wild Japanese macaques, Macaca fuscata, monitor group members more often when they are spread over a large area in order to prevent separation from the group (Suzuki & Sugiura, 2011).
Although visual monitoring does not vary with group size in primates, it is often sensitive to social context. For example, female mountain gorillas, Gorilla gorilla beringei, spend more time monitoring individuals with whom they have had aggressive interactions (Watts, 1998). Both male and female chimpanzees, Pan troglodytes schweinfurthii, display heightened vigilance in the presence of a less-associated group member (Kutsukake, 2006). Male baboons, Papio cynocephalus ursinus, show greater monitoring in open environments with greater opportunity to view conspecifics and fewer threats of predation, consistent with a social strategy rather than a survival strategy (Cowlishaw, 1998).
Although vigilance in our test population seems likely to be driven by social factors, it is possible that identical neurobiological circuits mediate the detection of threats from both predators and conspecifics. Myriad regions in the rhesus brain, including those associated with reward (Klein & Platt, 2013), learning (Watson & Platt, 2012) and visual orienting (Klein et al., 2008) behaviours, contain cells that respond to both potential social threats (dominant faces) and reproductive information (female perinea). Even brain areas and circuits classically thought to mediate threat vigilance, such as the amygdala (Chang & Platt, 2014; Hoffman et al., 2007) and autonomic systems linked to norepinephrine (Ebitz, Pearson, & Platt, 2014) respond to social stimuli. Although the neurobiological representation of predator avoidance remains unknown, these findings militate against the existence of separate neural systems for classic predator detection and social vigilance. Instead, we speculate that a wild population of rhesus macaques exposed to predators may express similar or even greater variation in vigilance-related phenotypes in comparison to our findings from Cayo Santiago. Notably, the competing threats of predators and conspecifics are thought to be responsible for the absence of correlation between group size and vigilance in primates: as group size increases, the threat of predation decreases, but the threat of aggression increases, effectively cancelling each other out (Treves, 1999, 2000).
In the current study, the detection of imminent social threats is but one of the functions of vigilance. Disparate types of visual information can have equal value, as shown by laboratory studies of rhesus macaques in which male monkeys forgo juice rewards in order to display images of dominant faces as well as the perinea of females (Adams, 2014; Deaner et al., 2005) (although we note that the valuation of dominant faces is sensitive to 5-HTTLPR genotype, with reduced value of these stimuli in short allele carriers; Watson et al., 2009). Gathering non-threat-related visual social information, although perhaps less urgent, may be as important as detecting threats. Knowledge about group movement (Stueckle & Zinner, 2008; Suzuki & Sugiura, 2011), the location and behaviour of offspring (Cheney & Seyfarth, 1980), dominance relationships between other individuals (Bergman et al., 2003; Cheney et al., 1995) and reproductive states of potential mates (Dubuc et al., 2009; Higham et al., 2011) and competitors (Watson et al., 2012) may all influence selection for the most adaptive behaviour in a given context. This information could be relevant immediately, as in the case of a male assessing an approaching female during mating season. In fact, red colobus monkeys, Procolobus badius, and redtail monkeys, Cercopithecus ascanius, increase vigilance during the mating season (Treves, 1999), and talapoin monkeys, Miopithecus talapoin, increase visual monitoring when placed in groups with potentially reproductive females (Keverne et al., 1978). Primates also have an impressive memory capacity, as evidenced by playback studies showing that monkeys remember recent interactions with conspecifics when deciding how to respond to a particular vocalization (Cheney et al., 2010; Engh et al., 2006; Wittig et al., 2014; Wittig et al., 2007a; Wittig et al., 2007b). Knowledge of third-party rank relationships may be used when recruiting allies during agonistic encounters (Silk, 1999). Thus, even if social information is not immediately relevant, it can be used later to inform action.
There is no doubt that extended phenotypes in primates, including social attention, are complicated phenomena, and that the neural circuits involved are multifold and complex. It is certain that TPH2IP accounts for only a limited portion of the variation in social attention, and many other genes and pathways likely shape the underlying processes. In fact, there is some scepticism within the scientific community regarding whether variation within a single gene is sufficient to influence phenotypic variability. Although multiple genes often contribute to variation in phenotype (Shao, 2008, page 145), single genes can and do influence complex behaviours (Hoover, 2011, page 109). For example, there are several monogenic disorders that are associated with altered social behaviour in autism (Ghosh, 2013, page 146).
A critical step in the candidate gene approach is appropriate selection and a priori knowledge about a particular gene, allowing formation of meaningful hypotheses (Tabor, Risch, & Myers, 2002). A large body of literature, spanning the fields of neurobiology, animal behaviour and psychiatry, implicate the serotonin system in social behaviour and attention. Within this literature there is high consistency in the effects of genetic variation in the serotonin system across species. Not only do humans and rhesus macaques show behavioural differences that correlate similarly with 5-HTTLPR genotype (reviewed in Canli & Lesch 2007), but mice, which lack this genetic variation, show similar behavioural alterations when the serotonin transporter gene is experimentally mutated (Murphy et al., 2001). It is also worth noting the prevalent clinical use of serotonin reuptake inhibitors to treat psychiatric disease (Stokes, 1998), which provides unequivocal evidence that relatively small molecular perturbations can induce dramatic behavioural changes. Because TPH2IP and 5-HTTLPR are both known to induce expression changes in proteins crucial for serotonergic function, it is plausible that these polymorphisms exert measurable effects on the organism’s behaviour.
In addition to the impact of serotonin on attention, we also found that male rhesus macaques are more vigilant than females. Prior studies in primates have found inconsistent patterns of sex differences in vigilance, which often vary in specific contexts like mating season (Treves, 1999) or during grooming bouts (Cowlishaw, 1998). The sex differences found in our study could well be tied to sex-related incentives for social information gathering in rhesus macaques: female ranks depend on lineage and are relatively stable across time, whereas male ranks are determined by contests and coalition building (Datta, 1988; Melnick et al., 1984). Male dominance rank is thus less stable and depends more strongly on the current social environment. Heightened male vigilance may also reflect a greater tendency for males to assess female reproductive status.
Although variation in serotonin-related genotypes predispose humans towards psychiatric disease (Caspi et al., 2010; Hariri & Holmes, 2006), the persistence of minor alleles within free-ranging and freely breeding populations of humans and rhesus macaques suggest an adaptive function to this variation (Dobson & Brent, 2013). Genetic variants at TPH2 and elsewhere interact with one another as well as with the environment (Canli et al., 2008; Caspi et al., 2010; Hariri & Holmes, 2006), resulting in a heterogeneous population representing a wide distribution of vigilance phenotypes. Variation in vigilance phenotypes may reflect distinct behavioural strategies for success in a dynamic social context (Dobson & Brent, 2013), even though the tails of the phenotypic distribution may be maladaptive in both rhesus macaques and humans. These findings suggest that pathological vigilance in contemporary human populations arises, in part, from variation in a genetically regulated adaptive trait shared with rhesus macaques and possibly other primates (Waider et al., 2011). Together, these observations endorse a comparative, evolutionary approach to psychiatric disease.
Highlights.
In rhesus macaques, individual variation in vigilance has a genetic basis (heritability ~12%).
Males are more vigilant than females, but vigilance declines with age.
High-status monkeys tend to be more vigilant than low-status monkeys.
Gene polymorphism in TPH2 influences vigilance and is linked to social isolation.
Less common ‘long’ allele carriers (high TPH2) are less vigilant than ‘short’ allele homozygotes.
Acknowledgments
We thank the Caribbean Primate Research Center for logistical and technical support for the work reported here. This research was supported by R01 MH096875, R01 MH089484, and the Duke Institute for Brain Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams GK. Tinbergen Alpha. Release 1. Geneva, Switzerland: Zenodo; 2014. http://zenodo.org/record/13009#.VNj3Ji7w-7A. [Google Scholar]
- Adams GK, Watson KK, Pearson J, Platt ML. Neuroethology of decisionmaking. Current Opinion in Neurobiology. 2012;22:982–989. doi: 10.1016/j.conb.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Allman JM. Evolving brains. New York, NY: W.H. Freeman; 2000. [Google Scholar]
- Altmann SA. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Annals of the New York Academy of Sciences. 1962;102:338–435. doi: 10.1111/j.1749-6632.1962.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam KN, Dittmar K, Berman CM, Butovskaya M, Cooper MA, Majolo B, et al. Hierarchical steepness, counter-aggression, and macaque social style scale. American Journal of Primatology. 2012;74:915–925. doi: 10.1002/ajp.22044. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing condition and rh5-HTTLPR interact to influence limbic– hypothalamic–pituitary–adrenal axis response to stress in infant macaques. Biological Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bennett A, Lesch K, Heils A, Long J, Lorenz J, Shoaf S, et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Molecular Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Berger SM, Weber T, Perreau-Lenz S, Vogt MA, Gartside SE, Maser-Gluth C, et al. A functional Tph2 C1473G polymorphism causes an anxiety phenotype via compensatory changes in the serotonergic system. Neuropsychopharmacology. 2012;37:1986–1998. doi: 10.1038/npp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM. Hierarchical classification by rank and kinship in baboons. Science. 2003;302:1234–1236. doi: 10.1126/science.1087513. [DOI] [PubMed] [Google Scholar]
- Blomquist G. Genetic variation for life history and morphology in the Cayo Santiago female rhesus macaques (Macaca mulatta) American Journal of Physical Anthropology. 2008;135(Suppl. 46):70. [Google Scholar]
- Blomquist GE. Fitness-related patterns of genetic variation in rhesus macaques. Genetica. 2009;135:209–219. doi: 10.1007/s10709-008-9270-x. [DOI] [PubMed] [Google Scholar]
- Boelkins RC, Wilson AP. Intergroup social dynamics of the Cayo Santiago rhesus (Macaca mulatta) with special reference to changes in group membership by males. Primates. 1972;13:125–139. [Google Scholar]
- Brent LJ, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, et al. Genetic origins of social networks in rhesus macaques. Scientific Reports. 2013;3:1042. doi: 10.1038/srep01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Congdon E, Todd Constable R, Lesch KP. Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on neural correlates of affective processing. Biological Psychology. 2008;79:118–125. doi: 10.1016/j.biopsycho.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch K-P. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Chance MRA, Jolly CJ. Social groups of monkeys, apes and men. London U.K.: Cape; 1970. [Google Scholar]
- Chang SW, Brent LJ, Adams GK, Klein JT, Pearson JM, Watson KK, et al. Neuroethology of primate social behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10387–10394. doi: 10.1073/pnas.1301213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Platt ML. Amygdala: eyes wide open. Current Biology. 2014;24:R1000–R1002. doi: 10.1016/j.cub.2014.08.044. [DOI] [PubMed] [Google Scholar]
- Chen G, Novak M, Hakim S, Xie Z, Miller G. Tryptophan hydroxylase-2 gene polymorphisms in rhesus monkeys: association with hypothalamic– pituitary–adrenal axis function and in vitro gene expression. Molecular Psychiatry. 2006;11:914–928. doi: 10.1038/sj.mp.4001870. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Moscovice LR, Heesen M, Mundry R, Seyfarth RM. Contingent cooperation between wild female baboons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9562–9566. doi: 10.1073/pnas.1001862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. Vocal recognition in free-ranging vervet monkeys. Animal Behaviour. 1980;28:362–367. [Google Scholar]
- Cheney DL, Seyfarth RM. Recognition of other individuals’ social relationships by female baboons. Animal Behaviour. 1999;58:67–75. doi: 10.1006/anbe.1999.1131. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM, Silk JB. The responses of female baboons (Papio cynocephalus ursinus) to anomalous social interactions: evidence for causal reasoning? Journal of Comparative Psychology. 1995;109:134. doi: 10.1037/0735-7036.109.2.134. [DOI] [PubMed] [Google Scholar]
- Christian BT, Wooten DW, Hillmer AT, Tudorascu DL, Converse AK, Moore CF, et al. Serotonin Transporter genotype affects serotonin 5-HT1A binding in primates. Journal of Neuroscience. 2013;33:2512–2516. doi: 10.1523/JNEUROSCI.4182-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Genetic structure of reciprocal social behavior. American Journal of Psychiatry. 2000;157:2043–2045. doi: 10.1176/appi.ajp.157.12.2043. [DOI] [PubMed] [Google Scholar]
- Cory JS, Myers JH. The ecology and evolution of insect baculoviruses. Annual Review of Ecology, Evolution, and Systematics. 2003:239–272. [Google Scholar]
- Cowlishaw G. The role of vigilance in the survival and reproductive strategies of desert baboons. Behaviour. 1998:431–452. [Google Scholar]
- Datta S. The acquisition of dominance among free-ranging rhesus monkey siblings. Animal Behaviour. 1988;36:754–772. [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Current Biology. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Dobson SD, Brent LJ. On the evolution of the serotonin transporter linked polymorphic region (5-HTTLPR) in primates. Frontiers in Human Neuroscience. 2013;7:588. doi: 10.3389/fnhum.2013.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Brent LJ, Accamando AK, Gerald MS, MacLarnon A, Semple S, Heistermann M, Engelhardt A. Sexual skin color contains information about the timing of the fertile phase in free-ranging Macaca mulatta. International Journal of Primatology. 2009;30:777–789. [Google Scholar]
- Duggleby CR, Haseley PA, Rawlins RG, Kessler MJ. An overview of blood group genetic studies on the Cayo Santiago rhesus monkeys. In: Kessler MJ, Rawlins RG, editors. The Cayo Santiago macaques. Albany, NY: SUNY Press; 1986. pp. 269–282. [Google Scholar]
- Dunbar RI. The social brain hypothesis. Brain. 1998;9:178–190. [Google Scholar]
- Ebitz RB, Pearson JM, Platt ML. Pupil size and social vigilance in rhesus macaques. Frontiers in Neuroscience. 2014;8:100. doi: 10.3389/fnins.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitz RB, Platt ML. Neuronal activity in primate dorsal anterior cingulate cortex signals task conflict and predicts adjustments in pupil-linked arousal. Neuron. doi: 10.1016/j.neuron.2014.12.053. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Chew SH, Zhong S, Knafo A. Genetics of human social behavior. Neuron. 2010;65:831–844. doi: 10.1016/j.neuron.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Kravitz EA. Serotonin, social status and aggression. Current Opinion in Neurobiology. 1997;7:812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- Elgar MA. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biological Reviews. 1989;64:13–33. doi: 10.1111/j.1469-185x.1989.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Engh AL, Hoffmeier RR, Cheney DL, Seyfarth RM. Who, me? Can baboons infer the target of vocalizations? Animal Behaviour. 2006;71:381–387. [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychological Bulletin. 2007;133:694. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Pan Z, Jiao Z, Li F, Zhao G, Wei Q, et al. TPH2 gene polymorphisms and major depression: a meta-analysis. PLoS One. 2012;7:e36721. doi: 10.1371/journal.pone.0036721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. American Journal of Medical Genetics. 1999;88:83–87. [PubMed] [Google Scholar]
- Gutknecht L, Jacob C, Strobel A, Kriegebaum C, Müller J, Zeng Y, et al. Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. International Journal of Neuropsychopharmacology. 2007;10:309–320. doi: 10.1017/S1461145706007437. [DOI] [PubMed] [Google Scholar]
- Hadfield JD. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. Journal of Statistical Software. 2010;33:1–22. [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Parikh PC, Deaner RO, Platt ML. Economic principles motivating social attention in humans. Proceedings of the Royal Society B: Biological Sciences. 2007;274:1751–1756. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Higham JP, Hughes KD, Brent LJ, Dubuc C, Engelhardt A, Heistermann M, et al. Familiarity affects the assessment of female facial signals of fertility by free-ranging male rhesus macaques. Proceedings of the Royal Society B: Biological Sciences. 2011;278:3452–3458. doi: 10.1098/rspb.2011.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley J, King S, Jr., Hasert M, Champoux M, Suomi S, Linnoila M. Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology. 1996;14:67–76. doi: 10.1016/S0893-133X(96)80060-1. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman P, Taub D, Higley S, Suomi SJ, Linnoila M, et al. Cerebrospinal fluid monoamine and adrenal correlates of aggression in freeranging rhesus monkeys. Archives of General Psychiatry. 1992;49:436–441. doi: 10.1001/archpsyc.1992.01820060016002. [DOI] [PubMed] [Google Scholar]
- Hinde R, Rowell T. Communication by postures and facial expressions in the rhesus monkey (Macaca mulatta) Proceedings of the Zoological Society of London. 1962;138:1–21. [Google Scholar]
- Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facialexpression and gaze-selective responses in the monkey amygdala. Current Biology. 2007;17:766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Hoover K, Grove M, Gardner M, Hughes DP, McNeil J, Slavicek J. A gene for an extended phenotype. Science. 2011;333:1401. doi: 10.1126/science.1209199. [DOI] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne E, Leonard R, Scruton D, Young S. Visual monitoring in social groups of talapoin monkeys (Miopithecus talapoin) Animal Behaviour. 1978;26:933–944. [Google Scholar]
- Klein JT, Deaner RO, Platt ML. Neural correlates of social target value in macaque parietal cortex. Current Biology. 2008;18:419–424. doi: 10.1016/j.cub.2008.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JT, Platt ML. Social information signaling by neurons in primate striatum. Current Biology. 2013;23:691–696. doi: 10.1016/j.cub.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JT, Shepherd SV, Platt ML. Social attention and the brain. Current Biology. 2009;19:R958–R962. doi: 10.1016/j.cub.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiella A, Reimold M, Ulshöfer D, Ikonomidou V, Vollmert C, Vollstädt-Klein S, et al. How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: the roles of in vivo serotonin transporter expression and amygdala structure. Translational Psychiatry. 2011;1:e37. doi: 10.1038/tp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake N. The context and quality of social relationships affect vigilance behaviour in wild chimpanzees. Ethology. 2006;112:581–591. [Google Scholar]
- Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Vigilance costs of allogrooming in macaque mothers. American Naturalist. 1993;141:744–753. doi: 10.1086/285503. [DOI] [PubMed] [Google Scholar]
- Melnick DJ, Pearl MC, Richard A. Male migration and inbreeding avoidance in wild rhesus monkeys. American Journal of Primatology. 1984;7:229–243. doi: 10.1002/ajp.1350070303. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Li Q, Engel S, Wichems C, Andrews A, Lesch K-P, et al. Genetic perspectives on the serotonin transporter. Brain Research Bulletin. 2001;56:487–494. doi: 10.1016/s0361-9230(01)00622-0. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Watson KK, Platt ML. Decision making: the neuroethological turn. Neuron. 2014;82:950–965. doi: 10.1016/j.neuron.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D. R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-117. 2014 http://CRAN.R-project.org/package=nlme.
- Popova NK, Kulikov AV. Targeting tryptophan hydroxylase 2 in affective disorder. Expert Opinion on Therapeutic Targets. 2010;14:1259–1271. doi: 10.1517/14728222.2010.524208. [DOI] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ. The Cayo Santiago macaques: History, behavior, and biology. Albany, NY: SUNY Press; 1986. [Google Scholar]
- Reuter M, Kuepper Y, Hennig J. Association between a polymorphism in the promoter region of the TPH2 gene and the personality trait of harm avoidance. International Journal of Neuropsychopharmacology. 2007;10:401–404. doi: 10.1017/S1461145706007073. [DOI] [PubMed] [Google Scholar]
- Roberts G. Why individual vigilance declines as group size increases. Animal Behaviour. 1996;51:1077–1086. [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxietyrelated personality traits. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;127:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. Affiliation, empathy, and the origins of theory of mind. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10349–10356. doi: 10.1073/pnas.1301223110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth RM, Silk JB, Cheney DL. Variation in personality and fitness in wild female baboons. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16980–16985. doi: 10.1073/pnas.1210780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd SV, Deaner RO, Platt ML. Social status gates social attention in monkeys. Current Biology. 2006;16:R119–R120. doi: 10.1016/j.cub.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Shepherd SV, Klein JT, Deaner RO, Platt ML. Mirroring of attention by neurons in macaque parietal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9489–9494. doi: 10.1073/pnas.0900419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd SV, Platt ML. Spontaneous social orienting and gaze following in ringtailed lemurs (Lemur catta) Animal Cognition. 2008;11:13–20. doi: 10.1007/s10071-007-0083-6. [DOI] [PubMed] [Google Scholar]
- Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, et al. No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse. 2003;48:184–188. doi: 10.1002/syn.10204. [DOI] [PubMed] [Google Scholar]
- Silk JB. Male bonnet macaques use information about third-party rank relationships to recruit allies. Animal Behaviour. 1999;58:45–51. doi: 10.1006/anbe.1999.1129. [DOI] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, et al. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proceedings of the Royal Society B: Biological Sciences. 2009;276:3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, et al. Strong and consistent social bonds enhance the longevity of female baboons. Current Biology. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Smith DV, Hayden BY, Truong T-K, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. Journal of Neuroscience. 2010;30:2490–2495. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick CH. An experimental study of intragroup agonistic behavior in rhesus monkeys (Macaca mulatta) Behaviour. 1967;28:182–209. doi: 10.1163/156853967x00235. [DOI] [PubMed] [Google Scholar]
- Southwick CH, Beg MA, Siddiqi MR. Rhesus monkeys in north India. In: DeVore I, editor. Primate behavior: Field studies of monkeys and apes. New York, NY: Holt, Rinehart & Winston; 1965. pp. 111–159. [Google Scholar]
- Stokes PE. Ten years of fluoxetine. Depression and Anxiety. 1998;8:1–4. [PubMed] [Google Scholar]
- Stueckle S, Zinner D. To follow or not to follow: decision making and leadership during the morning departure in chacma baboons. Animal Behaviour. 2008;75:1995–2004. [Google Scholar]
- Sueur C, Petit O, De Marco A, Jacobs A, Watanabe K, Thierry B. A comparative network analysis of social style in macaques. Animal Behaviour. 2011;82:845–852. [Google Scholar]
- Suzuki M, Sugiura H. Effects of proximity and activity on visual and auditory monitoring in wild Japanese macaques. American Journal of Primatology. 2011;73:623–631. doi: 10.1002/ajp.20937. [DOI] [PubMed] [Google Scholar]
- Tabor HK, Risch NJ, Myers RM. Candidate-gene approaches for studying complex genetic traits: practical considerations. Nature Reviews Genetics. 2002;3:391–397. doi: 10.1038/nrg796. [DOI] [PubMed] [Google Scholar]
- Thierry B, Iwaniuk AN, Pellis SM. The influence of phylogeny on the social behaviour of macaques (Primates: Cercopithecidae, genus Macaca) Ethology. 2000;106:713–728. [Google Scholar]
- Treves A. Within-group vigilance in red colobus and redtail monkeys. American Journal of Primatology. 1999;48:113–126. doi: 10.1002/(SICI)1098-2345(1999)48:2<113::AID-AJP3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Treves A. Theory and method in studies of vigilance and aggregation. Animal Behaviour. 2000;60:711–722. doi: 10.1006/anbe.2000.1528. [DOI] [PubMed] [Google Scholar]
- Waider J, Araragi N, Gutknecht L, Lesch K-P. Tryptophan hydroxylase-2 (TPH2) in disorders of cognitive control and emotion regulation: a perspective. Psychoneuroendocrinology. 2011;36:393–405. doi: 10.1016/j.psyneuen.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Watson KK, Ghodasra JH, Furlong MA, Platt ML. Visual preferences for sex and status in female rhesus macaques. Animal Cognition. 2012;15:401–407. doi: 10.1007/s10071-011-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Ghodasra JH, Platt ML. Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS One. 2009;4:e4156. doi: 10.1371/journal.pone.0004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Platt ML. Social signals in primate orbitofrontal cortex. Current Biology. 2012;22:2268–2273. doi: 10.1016/j.cub.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DP. A preliminary study of selective visual attention in female mountain gorillas (Gorilla gorilla beringei) Primates. 1998;39:71–78. [Google Scholar]
- Willeit M, Stastny J, Pirker W, Praschak-Rieder N, Neumeister A, Asenbaum S, et al. No evidence for in vivo regulation of midbrain serotonin transporter availability by serotonin transporter promoter gene polymorphism. Biological Psychiatry. 2001;50:8–12. doi: 10.1016/s0006-3223(00)01123-9. [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Reale D, Clements MN, Morrissey MM, Postma E, Walling CA, et al. An ecologist’s guide to the animal model. Journal of Animal Ecology. 2010;79:13–26. doi: 10.1111/j.1365-2656.2009.01639.x. [DOI] [PubMed] [Google Scholar]
- Wittig RM, Crockford C, Langergraber KE, Zuberbühler K. Triadic social interactions operate across time: a field experiment with wild chimpanzees. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20133155. doi: 10.1098/rspb.2013.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig RM, Crockford C, Seyfarth RM, Cheney DL. Vocal alliances in chacma baboons (Papio hamadryas ursinus) Behavioral Ecology and Sociobiology. 2007a;61:899–909. [Google Scholar]
- Wittig RM, Crockford C, Wikberg E, Seyfarth RM, Cheney DL. Kinmediated reconciliation substitutes for direct reconciliation in female baboons. Proceedings of the Royal Society B: Biological Sciences. 2007b;1115;274:1109, 1115. doi: 10.1098/rspb.2006.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Roy A, Lipsky R, Kuchipudi K, Zhu G, Taubman J, et al. Haplotype-based linkage of tryptophan hydroxylase 2 to suicide attempt, major depression, and cerebrospinal fluid 5-hydroxyindoleacetic acid in 4 populations. Archives of General Psychiatry. 2005;62:1109–1118. doi: 10.1001/archpsyc.62.10.1109. [DOI] [PubMed] [Google Scholar]