Abstract
A new method for the synthesis of 2-aminomethyl functionalized 1,4-benzodiazepin-5-ones is presented. The benzodiazepine core is well-known to interact with biological receptors and many pharmaceutical drugs are derived from this structure. The alkene diamination strategy is employed for the first time for the synthesis of 1,4-benzodiazepinones. In this reaction, copper(2-ethylhexanoate)2 serves as promoter and a range of external amines can be coupled with 2-sulfonamido-N-allyl benzamides to generate the 1,4-benzodiazepinones in good yields.
Keywords: 1, 4-benzodiazepinones; diamination; copper-promoted; alkenes
Benzodiazepines and related compounds are considered privileged structures in medicinal chemistry.1–3 A sampling of 1,4-benzodiazepines and 1,4-benzodiazepinones and their corresponding biological activities are illustrated in Figure 1.4–7 While methods involving coupling of anthranilic acid (2-aminobenzoic acid) derivatives and either amino acids or amines and aldehydes (Ugi reaction) are most frequently used for 1,4-benzodiazepinone synthesis,8,9 an emerging strategy involves coupling of anthranilic acid derivatives with allylic amines followed by transition-metal facilitated ring closure via alkene difunctionalization (Scheme 1). These methods have enabled the synthesis of variously functionalized 1,4-benzodiazepines and 1,4-benzodiazepinones not readily synthesized by the more classical routes.
Figure 1.
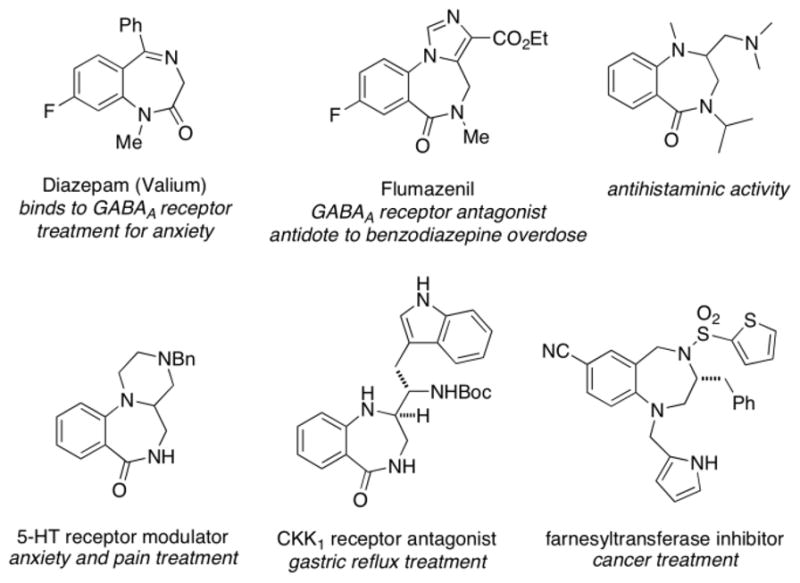
Examples of biologically active 1,4-benzodiazepinones
Scheme 1.
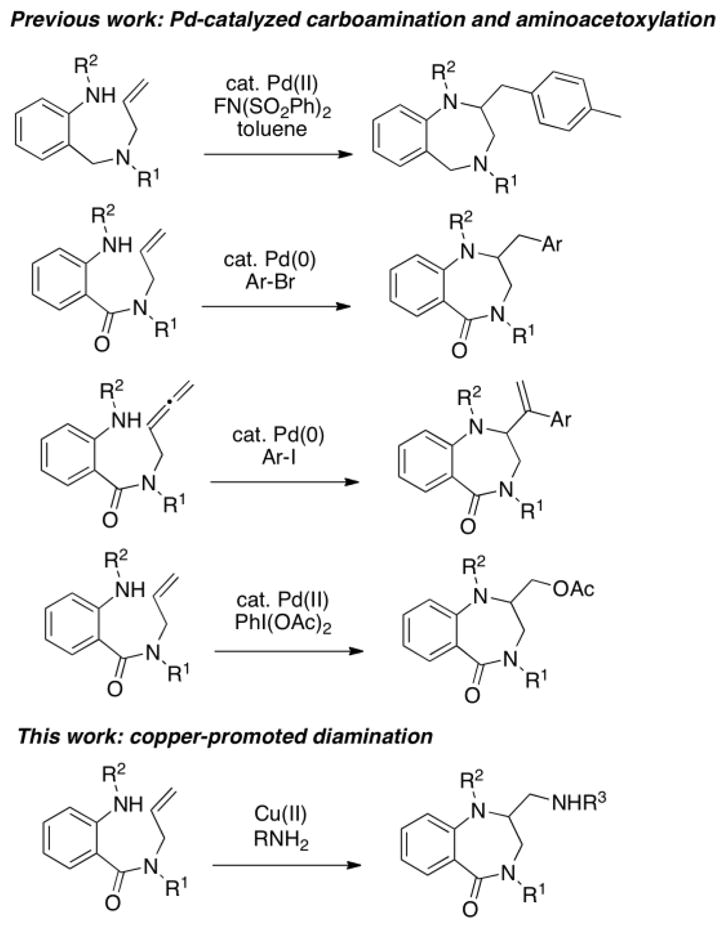
Palladium-catalyzed alkene oxidative amination,10 carboamination11–13 and aminoacetoxylation14 have all been performed to secure the 1,4-benzodiazepinone core while introducing various benzyl, alkenyl and acetoxymethyl functional groups on the benzodiazepine ring (Scheme 1). Despite these advances, no method for benzodiazepine formation via alkene diamination has yet been reported. Aminomethyl-functionalized benzodiazepines have demonstrated interesting biological activity (Fig. 1), thus a versatile method for their synthesis would be a valuable contribution. We report herein a copper-promoted intra/intermolecular alkene diamination for the synthesis of aminomethyl-functionalized 1,4-benzodiazepinones (Scheme 1).
Over the past decade, alkene diamination has emerged as a powerful way to access valuable 1,2-diamines.15–24 Such vicinal diamines have demonstrated significant utility as components of bioactive compounds, as well as in chemical synthesis, where they are useful as either ligands or catalysts in organic and organometallic transformations.15,25,26 Our research group has developed copper-promoted27–29 and copper-catalyzed23 alkene diaminations for the synthesis of aminomethyl functionalized indolines, isoindolines, pyrrolidines, γ-lactams, cyclic ureas, cyclic sulfamides, tetrahydroisoquinolines and tetrahydroquinolines. While transition metal catalyzed cyclization reactions to form larger rings are generally more challenging than five-membered ring formation reactions, the successful precedent set by the palladium-catalyzed alkene difunctionalizations (Scheme 1) convinced us that formation of 1,4-benzodiazepinones via copper-facilitated alkene diamination should be feasible. The reduction in transannular strain due to the high prevalence of sp2-hybridized carbons in the diazepinone backbone may favor cyclization compared to formation of seven-membered rings made up of predominantly Csp3-backbone.
The reaction of N-nosyl-2-amino-N′-allylbenzamide 1a in the presence of TsNH2 (3 equiv), Cu(2-ethylhexanoate)2 and Cs2CO3 (1 equiv) was examined under various conditions for the formation of 1,4-benzodiazepinone 2a (Table 1). Using 300 mol% Cu(2-ethylhexanoate)2 in either xylenes at 130 °C or PhCF3 at 120 °C for 48 h, 100% conversion of substrate 1a was observed and 1,4-benzodiazepinone 2a was isolated in 81% and 76% yields, respectively (Table 1, entries 1 and 2). A decrease in either reaction time or Cu(2-ethylhexanoate)2 loading led to decreased conversion (Table 1, entries 3 and 4). 1,2-Dichloroethane at 105 °C were also viable reaction conditions and provided 80% conversion to 2a after 48 h (Table 1, entry 5). When catalytic Cu(2-ethylhexanoate)2 (30 mol%) in the presence of MnO2 (300 mol %) was used, less than 5% conversion to diamine 2a was observed (Table 1, entry 6).
Table 1.
Optimization of the diaminationa
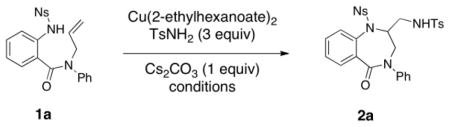
| |||||
|---|---|---|---|---|---|
| Entry | Cu(II) (mol %) | Solvent | Temp (°C) | T (h) | Yield (%) |
| 1 | 300 | xylenes | 130 | 48 | 81b |
| 2 | 300 | PhCF3 | 120 | 48 | 76 b |
| 3 | 300 | PhCF3 | 120 | 24 | 90% conversion |
| 4 | 200 | PhCF3 | 120 | 48 | 64% conversion |
| 5 | 300 | DCE | 105 | 48 | 80% conversion |
| 6 | 30 | xylenes | 130 | 48 | <5% conversion |
Reactions were run in capped glass tubes heated in oil baths.
Isolated yield following chromatography on silica gel. No substrate was present in crude 1H NMR (100% conversion).
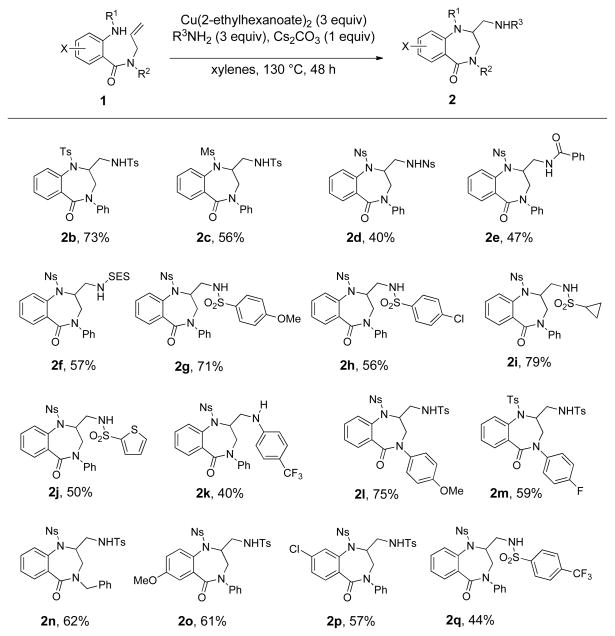
The scope of the diamination reaction was next explored using the reaction conditions in Table 1, entry 1 unless otherwise noted (Scheme 2). As illustrated in Scheme 2, various 2-sulfonamido-N-allyl benzamide derivatives 1 underwent copper-promoted cyclization and coupling with various external amine sources such as tosamide, benzamide, 2-trimethylsilylethylsulfonamide (SESNH2), cyclopropylsulfonamide, 4-trifluoromethylaniline and various arylsulfonamides. Isolated yields of 2-aminomethyl functionalized 1,4-benzodiazapin-5-ones 2 ranged from 40–79%. Lower loading (2 equiv) of both benzamide and 4-trifluoromethylaniline to provide 2e and 2k respectively resulted in higher conversion, possibly due to higher levels of coordination of these amines to the copper(2-ethylhexanoate)2, which might result in reduced reactivity. Interestingly, the nosyl-functionalized substrate that generates 2a (Table 1, entry 1) was more reactive than the tosyl-functionalized substrate that generates 2b (Scheme 2). This is the reverse of the reactivity pattern previously observed in related copper(2-ethylhexanoate)2 promoted alkene aminooxygenation reactions that provide 5-membered pyrrolidine products.30 This may indicate that the formation of a less reversible or more stable N-[Cu] complex31 of 1 could involve deprotonation of the sulfonamide (more facile with N-nosyl than N-tosyl) which could be more kinetically significant in the more challenging seven-membered ring formation.
Scheme 2.
Scope of the benzodiazepinone-forming alkene diamination
An amide whose cyclization/diamination would have resulted in a regioisomeric 1,4-benzodiazepine-5-one did not react (Figure 2). A substrate bearing a 1,1-disubstituted alkene and a substrate lacking an amide alkyl group (in other words, free amide NH) also did not react (Figure 2). Finally, a substrate bearing an internal, styrenyl alkene, did not react (Figure 2). The lack of reactivity with the styrenyl alkene supports a more concerted alkene addition mechanism rather than one based on N-radical formation and addition to the alkene as the N-radical would be expected to be more reactive with the styrenyl alkene than with a terminal alkene.
Figure 2.
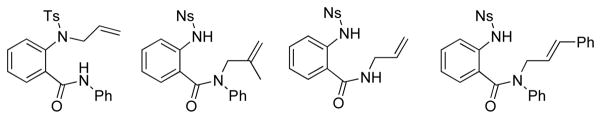
Unreactive substrates
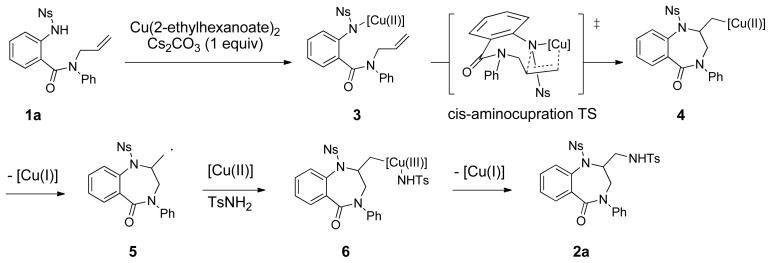
A potential mechanism for the diamination reaction is illustrated in Scheme 3. Following coordination of [Cu(II)] to the sulfonamide of substrate 1a, cis-aminocupration31 through a nine-membered ring transition state provides the unstable organocopper(II) intermediate 4. Homolysis of the C-[Cu(II)] bond results in organic radical 5 and [Cu(I)]. Addition of radical 5 to [Cu(II)] and concomitant coordination of TsNH2 provides an organocopper(III) intermediate 6.32 Carbon-nitrogen bond formation via reductive elimination then generates the 1,4-benzodiazepin-5-one product 2a.
Scheme 3.
Proposed diamination mechanism
In summary, the synthesis of 2-aminomethyl functionalized 1,4-benzodiazepin-5-ones 2 has been enabled by copper-promoted alkene diamination. Investigation into the bioactivity of these interesting new compounds is underway and will be reported in due course.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (GM078383) for support of this work, the National Science Foundation chemistry REU program at UB (CHE-1262771) for a summer fellowship to J.P.M. and the Brazilian Ministry of Education for a summer fellowship to M.M.M.F. (CAPES program).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Hadjipavlou-Litina D, Hansch C. Chem Rev. 1994;94:1483–1505. [Google Scholar]
- 2.Costantino L, Barlocco D. Curr Med Chem. 2006;13:65–85. [PubMed] [Google Scholar]
- 3.Melancon BJ, Hopkins CR, Wood MR, Emmitte KA, Niswender CW, Christopoulos A, Conn PJ, Lindsley CW. J Med Chem. 2012;55:1445–1464. doi: 10.1021/jm201139r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cale AD. 4,705,853. US Patent. 1987
- 5.Herrero S, Garcia-Lopez MT, Cenarruzabeitia E, Del Rio J, Herranz R. Tetrahedron. 2003;59:4491–4499. [Google Scholar]
- 6.Wang Y, Brewer JT, Akritopoulou-Zanze I, Djuric SW, Pohlki F, Braje W, Relo A-L. 8,518,933. US Patent. 2011
- 7.Hunt JT, Ding CZ, Batorsky R, Bednarz M, Bhide R, Cho Y, Chong S, Chao S, Gullo-Brown J, Guo P, Kim SH, Lee FYF, Leftheris K, Miller A, Mitt T, Patel M, Penhallow BA, Ricca C, Rose WC, Schmidt R, Slusarchyk WA, Vite G, Manne V. J Med Chem. 2000;43:3587–3595. doi: 10.1021/jm000248z. [DOI] [PubMed] [Google Scholar]
- 8.Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 9.Ellman JA. Acc Chem Res. 1996;29:132–143. [Google Scholar]
- 10.Beccalli EM, Broggini G, Paladino G, Penoni A, Zoni C. J Org Chem. 2004;69:5627–5630. doi: 10.1021/jo0495135. [DOI] [PubMed] [Google Scholar]
- 11.Neukom JD, Aquino AS, Wolfe JP. Org Lett. 2011;13:2196–2199. doi: 10.1021/ol200429a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigamonti M, Prestat G, Broggini G, Poli G. J Organomet Chem. 2014;760:149–155. [Google Scholar]
- 13.Rosewall CF, Sibbald PA, Liskin DV, Michael FE. J Am Chem Soc. 2009;131:9488–9489. doi: 10.1021/ja9031659. [DOI] [PubMed] [Google Scholar]
- 14.Manick AD, Duret G, Tran DN, Berhal F, Prestat G. Org Chem Front. 2014;1:1058–1061. [Google Scholar]
- 15.de Jong S, Nosal DG, Wardrop DJ. Tetrahedron. 2012;68:4067–4105. doi: 10.1016/j.tet.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingalis EL, Sibbald PA, Kaminsky W, Michael FE. J Am Chem Soc. 2013;135:8854–8856. doi: 10.1021/ja4043406. [DOI] [PubMed] [Google Scholar]
- 17.Olson DE, Su JY, Roberts A, Du Bois J. J Am Chem Soc. 2014;136:13506–13509. doi: 10.1021/ja506532h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muniz K, Martinez C. J Org Chem. 2013;78:2168–2174. doi: 10.1021/jo302472w. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y-F, Zhu X, Chiba S. J Am Chem Soc. 2012;134:3679–3682. doi: 10.1021/ja2120629. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Pu W, Xiong T, Li Y, Zhou X, Sun K, Liu Q, Zhang Q. Angew Chem Int Ed. 2013;52:2529–2533. doi: 10.1002/anie.201209142. [DOI] [PubMed] [Google Scholar]
- 21.Zhu M-K, Chen Y-C, Loh T-P. Chem Eur J. 2013;19:5250–5254. doi: 10.1002/chem.201203832. [DOI] [PubMed] [Google Scholar]
- 22.Hong KB, Johnston JN. Org Lett. 2014;16:3804–3807. doi: 10.1021/ol501693j. [DOI] [PubMed] [Google Scholar]
- 23.Turnpenny BW, Chemler SR. Chem Sci. 2014;5:1786–1793. doi: 10.1039/C4SC00237G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, Cornwall RG, Du H, Zhao B, Shi Y. Acc Chem Res. 2014;47:3665–3678. doi: 10.1021/ar500344t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucet D, Le Gall T, Mioskowski C. Angew Chem Int Ed. 1998;37:2580–2627. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht L, Jiang H, Jorgensen KA. Chem Eur J. 2014;20:358–368. doi: 10.1002/chem.201303982. [DOI] [PubMed] [Google Scholar]
- 27.Zabawa TP, Kasi D, Chemler SR. J Am Chem Soc. 2005;127:11250–11251. doi: 10.1021/ja053335v. [DOI] [PubMed] [Google Scholar]
- 28.Zabawa TP, Chemler SR. Org Lett. 2007;9:2035–2038. doi: 10.1021/ol0706713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sequeira FC, Turnpenny BW, Chemler SR. Angew Chem Int Ed. 2010;49:6365–6368. doi: 10.1002/anie.201003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paderes MC, Chemler SR. Org Lett. 2009;11:1915–1918. doi: 10.1021/ol9003492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paderes MC, Belding L, Fanovic B, Dudding T, Keister JB, Chemler SR. Chem Eur J. 2012;18:1711–1726. doi: 10.1002/chem.201101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochi JK, Bemis A, Jenkins CL. J Am Chem Soc. 1968;90:4616–4625. [Google Scholar]
- 33.Representative diamination procedure (Table 1, entry 1): 4-methyl-N-((1-((4-nitrophenyl)sulfonyl)-5-oxo-4-phenyl-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl)methyl)benzenesulfonamide (2a): N-allyl-2-((4-nitrophenyl)sulfonamido)-N-phenylbenzamide (1a) (50 mg, 0.11 mmol, 1 equiv) in a glass tube equipped with a magnetic stir bar was treated with Cs2CO3 (37.1 mg, 0.11 mmol, 1 equiv), p-toluenesulfonamide (58.6 mg, 0.34 mmol, 3 equiv) and copper(2-ethylhexanoate)2 (120 mg, 0.34 mmol, 3 equiv) followed by 0.6 mL (0.2 M) of xylenes. The tube was capped and the reaction mixture was stirred at 130 °C for 48 h. The reaction mixture was allowed to cool to room temperature and then was diluted with CH2Cl2 (10 mL). This mixture was then washed with sat. aq. EDTANa2 (2 x 10 mL) and the two layers were separated. The aqueous layer was extracted with CH2Cl2 (3 x 25 mL). The organic layers were combined, dried with Na2SO4 and concentrated in vacuo to yield the crude product, which was purified by flash chromatography on silica gel (20–80% EtOAc in hexanes gradient) to afford the benzodiazepinone 2a in 81% yield (56 mg, 0.09 mmol) as a white solid. mp: 118–130 °C; 1H NMR (400 MHz, CDCl3) δ 2.48 (s, 3H), 3.02–2.97 (m, 1H), 3.15–3.12 (m, 1H), 3.44 (dd, J = 15.6, 12.4 Hz, 1H), 3.86 (dd, J = 15.6, 4.8 Hz, 1H), 4.72–4.67 (m, 1H), 5.20 (t, J = 6.0 Hz, 1H), 6.88 (d, J = 8.4 Hz, 2H), 7.20–7.17 (m, 2H), 7.28–7.24 (m, 2H), 7.36 (d, J = 8.0 Hz, 2 H), 7.57–7.51 (m, 2 H), 7.74–7.70 (m, 3H), 7.78 (d, J = 8.0 Hz, 2H), 8.09 (d, J = 8.8 Hz, 2H) ppm; 13C NMR (75 MHz, CDCl3) δ 21.6, 44.5, 51.8, 62.2, 123.9, 124.5, 126.8, 127.3, 128.8, 129.0, 130.0, 130.2, 130.8, 130.9, 132.4, 132.5, 135.4, 136.0, 141.0, 143.6, 144.1, 150.3, 166.9 ppm; IR (neat, thin film) ν 3266, 2925, 2360, 1644, 1596, 1531, 1494, 1455, 1402, 1349, 1164, 1091 cm−1; HRMS (ESI) calcd. for C29H27O7N4S2 [M+H]+ 607.1316, found 607.1318
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.