Abstract
The exploration of Earth's biodiversity is an exciting and ongoing endeavour. Here, we report a new species of seadragon from Western Australia with substantial morphological and genetic differences to the only two other known species. We describe it as Phyllopteryx dewysea n. sp. Although the leafy seadragon (Phycodurus eques) and the common seadragon (Phyllopteryx taeniolatus) occur along Australia's southern coast, generally among relatively shallow macroalgal reefs, the new species was found more offshore in slightly deeper waters. The holotype was trawled east of the remote Recherche Archipelago in 51 m; additional specimens extend the distribution west to Perth in 72 m. Molecular sequence data show clear divergence from the other seadragons (7.4–13.1% uncorrected divergence in mitochondrial DNA) and support a placement as the sister-species to the common seadragon. Radiographs and micro-computed tomography were used on the holotype of the new species and revealed unique features, in addition to its unusual red coloration. The discovery provides a spectacular example of the surprises still hidden in our oceans, even in relatively shallow waters.
Keywords: biodiversity, Syngnathidae, new species, seadragon
2. Introduction
The fraction of undescribed biodiversity in the ocean is arguably high [1,2], and new fish species are likely to be described, especially from the deeper continental slopes [3]. Here, we describe a remarkable example, a new species of seadragon. Phyllopteryx dewysea n. sp. is only the third known species of seadragon, and the first to be discovered in 150 years, possibly because it lives in slightly deeper waters than its relatives.
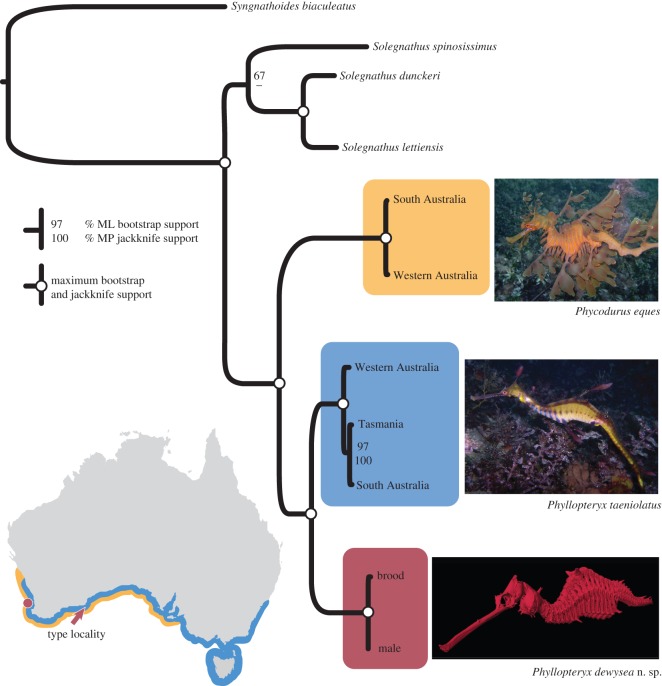
Seadragons (Syngnathidae) are fish of mesmerizing beauty (figure 1), with the leafy seadragon (Phycodurus eques) having more elaborate appendages than the colourful common seadragon (Phyllopteryx taeniolatus). Their ornamentation helps camouflage them among seagrasses and kelp in the shallow coastal waters of southern Australia [4]. The range of the common seadragon spans the entire southern coast from Western Australia to New South Wales and Tasmania, whereas the leafy seadragon has a more restricted distribution from Western Australia to South Australia (map in figure 1). The two known species of seadragons fascinate aquarium visitors around the world, draw many SCUBA divers into the water and are charismatic symbols for conservation of marine species [5,6].
Figure 1.
Distribution and phylogenetic relationships of the three seadragon species. The range of the leafy seadragon is shown in gold, the common seadragon in blue and the type locality of the new seadragon Phyllopteryx dewysea n. sp. is indicated by the red arrow. The red circle represents the collection sites of the three paratypes. The maximum-likelihood tree is based on the partitioned dataset (4416 bp). Numbers next to the nodes indicate bootstrap (top) and jackknife (bottom) values; full support is indicated by white circles.
On a biodiversity survey (part of the Marine Futures project 2007; http://www.marinefutures.fnas.uwa.edu.au/), a male seadragon carrying a brood of offspring was trawled off the coast of Western Australia. The specimen was accessioned at the Western Australian Museum (WAM P33223.002) as a common seadragon, and its uniqueness only subsequently recognized following DNA sequencing. Three additional specimens were located in scientific collections. The holotype of the new seadragon shows a bright red coloration and with transverse body-markings similar to leafy seadragons. However, the body shape more closely resembles the common seadragon. Here, we examine the anatomy and evolutionary history of the new seadragon to determine its systematic placement and formally describe it.
3. Material and methods
3.1. Sampling
Several diagnostic features of the skeleton allowed us to identify additional specimens of the new species that had been catalogued in scientific collections as common seadragons, even when they had lost colour and were unsuitable for genetic analysis. As the holotype was trawled from an unusual depth, we requested and examined specimens from depths of more than 50 m. Most museum specimens, however, do not hold explicit depth records, therefore we also asked for information on individuals collected on vessels, as those are more likely to have been collected offshore. A total of 15 trawled specimens from New South Wales, Tasmania, South Australia and Western Australia (see the electronic supplementary material for details) were located. Of these, two specimens (CSIRO Australian National Fish Collection; ANFC C2269 and C2270) collected near Perth, Western Australia, were trawled from a depth of 72 m and are here designated as paratypes of the new species based on their anatomical similarity to the holotype. The other trawled specimens were common seadragons, unfortunately mostly without depth records, which makes it difficult to infer a depth range for this species. The deepest record for a common seadragon was from 27 to 33 m (WAM P.20854; see the electronic supplementary material for details). We visually inspected 158 lots of common seadragons held at the Western Australian Museum, mostly from the southwestern coast of Australia, and found an additional paratype from Perth without a depth record as it was beachwashed. No specimens of the new species are housed at the South Australian Museum (Ralph Foster 2014, personal communication), which holds 60 lots of common seadragons mainly from South Australia and Victoria.
3.2. Molecular sampling
A piece of the tail of the holotype (preserved in ethanol) was used for DNA extraction as the dermal appendages were missing in this species; the specimen was a male carrying its brood, and we also obtained DNA sequences for one of its brood of embryos in order to obtain the mitochondrial haplotype of the mother. A DNA extraction protocol for formalin-preserved tissues [7] was unsuccessful for two of the paratypes (ANFC C2269 and C2270) and not attempted for the third paratype (WAM P660) because of its advanced age (collected 1919). For comparison with the other two seadragon species, we included genetic samples of wild-caught common seadragons and leafy seadragons from across their respective ranges (table 1). A small tissue clip was taken from the ventral dermal appendages of live animals that were subsequently released. This non-lethal sampling was considered less destructive than fin-clipping as the dermal appendages do not seem to have a locomotory function and are often damaged in otherwise healthy seadragons. We included three specimens of the common seadragon from Western Australia (Shelley Cove, Dunsborough, 17 December 2005), South Australia (Encounter Bay, 8 April 2006) and Tasmania (Blackmans Bay, Hobart, 16 December 2012). For the leafy seadragon, we included two specimens, one from South Australia (Encounter Bay, 3 February 2006), the other from Western Australia (Bremer Bay, 11 December 2005). See the ethics statement for permit details.
Table 1.
Collection details and GenBank accession numbers. WA, Western Australia; SA, South Australia; TAS, Tasmania.
Outgroup samples included three members of Solegnathinae, a group which was identified as the sister-group to the seadragons [8–10]. We included sequences from three species of spiny pipehorses, namely Solegnathus spinosissimus (Auckland Museum Marine Collections MA133942, New Zealand, Southland, Milford Sound, total length (TL) 1900 mm, May 2006), Solegnathus dunckeri (from GenBank, table 1), and Solegnathus lettiensis(ANFC H6340-09/GT192, Western Australia, northwest of Rottnest Island, RV Naturaliste, 100 m depth, 7 April 2006, supplied as Solegnathus guentheri). We also included Syngnathoides biaculeatus (Syngnathoidinae; gift from the Birch Aquarium at Scripps, 28 May 2013), which was shown to be closely related to seadragons and Solegnathinae [8–10].
3.3. DNA extraction and PCR amplification
DNA was isolated with the Qiagen DNeasy blood and tissue kit (Qiagen Sciences, Germantown, MD). Seven marker regions (four mitochondrial, three nuclear) were amplified in a reaction volume of 12.5 μl with the following primer pairs:
— 12S: L1091 and H1478 [11], modified following reference [9], amplified 346 bp of the 12S ribosomal RNA gene, using the thermal profile of 95°C for 15 min, 35 cycles of 95°C for 40 s, 50°C for 40 s, 68°C for 50 s, and a final extension at 68°C for 5 min.
— 16S: L2510 and H3058 [12], which amplify 522 bp of the 16S ribosomal RNA gene, with the same thermal profile as 12S.
— ND4: L11424-ND4 [13] and H12293-Leu [14], amplified an 838 bp fragment of the NADH dehydrogenase subunit four, including transfer RNA histidine and serine, with a thermal profile of 94°C for 9 min, 34 cycles of 94°C for 45 s, 52°C for 45 s, 72°C for 60 s, and a final extension at 72°C for 6 min.
— Control region: L15995 L-PRO [15] and H693.5 12S5r.5 [16], amplified a 1036 bp fragment of the mitochondrial control region, including fragments of the 12S ribosomal RNA gene. The fragment has a repetitive region in the middle of the fragment that impaired the sequencing reaction. To obtain the full-length fragment, we developed an additional primer set for sequencing the flanking regions of the mitochondrial control region (CR-FlanksF 5′-CCCCCACCCCCTTTAAAGAC-3′ and CR-FlanksR 5′-CGAGTCGTATGTGTCCCACC-3′). All fragments were amplified via a thermal profile of 94°C for 3 min, 40 cycles of 94°C for 60 s, 50°C for 30 s, 72°C for 60 s, and a final extension at 72°C for 5 min.
— S7: S7RPEX1F and S7RPEX2R [17] amplified up to 674 bp of the intron 1 and exon 2 of the nuclear S7 ribosomal protein gene with the thermal profile specified in reference [18].
— Aldolase: we used forward and reverse primers as specified in reference [19], amplifying a 382 bp long fragment of a nuclear aldolase-like gene containing both coding and non-coding regions with a thermal profile as outlined in reference [19].
— Tmo-4c4: Tmo-4c4F and Tmo-4c4R [20] amplified a 559 bp long coding fragment of the nuclear Tmo-4c4 gene with the thermal profile specified in reference [18].
PCR products were purified with Exo-SAP-IT (USB Affymetrix) and sequencing was completed with the corresponding PCR primers (Eurofins MWG Operon, Inc.). Overlapping fragments were edited and then merged into consensus sequences in Geneious v. 6.0.3 (Biomatters Ltd.). All sequences were deposited on GenBank (table 1).
Sequences for each marker were aligned on the MAFFT v. 7 server [21,22]. Most markers aligned without gaps, but gaps were inserted into the control region fragment resulting in an aligned length of 1084 bp, and S7-like protein aligned to a length of 685 bp. Alignments were deposited on TreeBASE (S15971).
3.4. Distance-based analysis
Uncorrected and model-corrected genetic distances were calculated in PAUP* v. 4b10 [23]. The appropriate model selected by jModelTest v. 2.1.4 [24] employing the Akaike information criterion was GTR + G for the mitochondrial dataset (control region, 16S, 12S, ND4) with a gamma parameter of 0.283. For S7 and aldolase, the chosen model was HKY, and K80 + I for Tmo-4c4 with a proportion of invariant sites of 0.723.
3.5. Phylogenetic analysis
To assess the phylogenetic affinities of the seadragons, we conducted maximum-parsimony (MP) and maximum-likelihood (ML) analyses. To check for data congruence, we analysed each nuclear marker individually, while we treated the mitochondrial markers as a single locus (2790 bp), and finally concatenated the datasets (4416 bp) in Mesquite v. 2.75 [25].
Maximum-parsimony analyses were conducted in PAUP*, with unordered and equally weighted characters, heuristic searches with 1000 random addition-sequence replicates and tree bisection and reconnection (TBR) branch swapping and with zero-length branches collapsed. To assess clade confidence, parsimony jackknifing was conducted with 1000 jackknife replicates, each with 100 random addition replicates, 37% character deletion, heuristic search option and TBR branch swapping.
Maximum-likelihood analyses were conducted in raxmlGUI v. 0.93 [26,27] employing the GTR + G model for each of the partitions. The dataset was partitioned for the different loci, for introns and exons of aldolase and S7, and additionally for the codon positions for the protein-coding regions of Tmo-4c4 and the exons of aldolase and S7, and the protein-coding regions and tRNA segments of ND4 (partitions given in the electronic supplementary material, file S2). One thousand replicate ML inferences were performed and clade confidence was assessed with 1000 bootstrap pseudo-replicates employing the thorough bootstrapping algorithm (option ‘-b’). The analyses were rooted with Syngnathoides biaculeatus, with the three Solegnathus species unconstrained. Sequence matrices and resulting trees were deposited on TreeBASE (S15971).
3.6. Morphology and skeletal structure
Morphometric measurements were taken of the holotype and three paratypes, and compared with scaled photographs from field observations of leafy (n=25) and common seadragons (n=20; for details, see electronic supplementary material, file S1). The descriptive morphology follows [4], and proportional measurements were taken as in reference [28].
Micro-computed tomography (μCT) of the holotype was conducted at the Cartilage Tissue Engineering laboratory at the University of California San Diego. The specimen was imaged in a Skyscan 1076 (Kontich, Belgium), wrapped in ethanol-soaked gauze, and positioned in a sealed LDPE container. Imaging was conducted at 36 μm isotropic voxel size, applying an electrical potential of 100 kVp and a current of 100 μA, with a 1.0 mm aluminium filter. A beam-hardening correction algorithm was applied during image reconstruction. The reconstructed file size was 1000×1000×5482 pixels (xyz).
DICOM stacks were processed and segmented in Amira v. 5.4 (Visage Imaging, Inc.), and visualized in Maya 2014 service pack 4 (Autodesk, Inc.). X-ray radiographs of all seadragon species were obtained at Scripps Institution of Oceanography.
4. Results
4.1. Genetic distance
The new species showed clear genetic divergences in mitochondrial and nuclear sequences of a comparable magnitude to those between the established species of seadragons, both in uncorrected and model-corrected distances. As an example, the mitochondrial markers of the new species differed by 7.4% to Phyllopteryx taeniolatus and by 13.1% to Phycodurus eques, which is similar to the 11.6% distance between the two known species (uncorrected pairwise distances). Model-corrected distances (GTR + G) of the mitochondrial data showed 9.9% divergence between the new species and Phyllopteryx taeniolatus, and 22.3% to Phycodurus eques, similar to the two known species that diverged by 18.9%. Comparisons for the three nuclear loci showed a similar pattern (electronic supplementary material, tables S2–S4). The mitochondrial haplotype of the male was 6 bp different from its brood, which represented the mother's mitochondrial haplotype. The father and his offspring were genetically identical for the three nuclear markers.
4.2. Phylogenetic placement
All analyses retrieved the seadragons as a well-supported clade with respect to Syngnathoides biaculeatus and Solegnathinae (figure 1; for individual gene trees see electronic supplementary material, figure S3). Among seadragons, the new species was supported as the sister-species to the common seadragon, and the leafy seadragon as the sister to this clade (figure 1; electronic supplementary material, figure S3a,c,d). The nuclear S7 was incongruent with the other markers, supporting the leafy seadragon as the sister to the new seadragon (electronic supplementary material, figure S3b). MP and ML analyses gave similar topologies within the seadragons.
4.3. Morphology
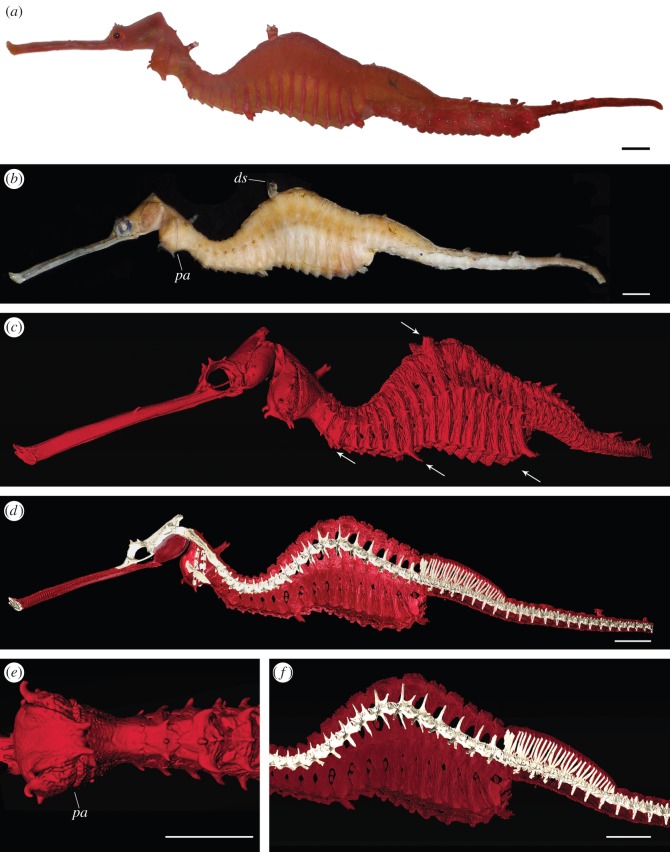
The new species has morphological features that clearly distinguish it from the other two seadragons (table 2; see also electronic supplementary material, file S1). In life, the holotype is easily distinguished by its red colour (figure 2a), and pink vertical bars that extend halfway up the body to the lateral trunk ridges (figure 2b). The more uniform coloration differentiates it from the common seadragon, which has numerous colourful spots and blotches (figure 1). These spots are always distinct, even in preserved specimens (see electronic supplementary material, figure S1) allowing differentiation of the two species even when the pigmentation is faded. The patterning also differs from the tan-yellow leafy seadragon, which has vertical bars that extend all the way up the body and are white with purple rims (figure 1). The X-ray radiographs and μCT showed that the new species has 18 trunk vertebrae with the corresponding outer bony plates (see electronic supplementary material, videos S1 and S2). Leafy seadragons have variable ring counts between 17 and 18. All common seadragons examined had only 17 trunk rings. The body shape of the new seadragon is reminiscent of the common seadragon, and different from the wave-like body of the leafy seadragon (figure 3). Morphometric measurements, however, did not reveal any perfectly diagnostic characters (table 3). The new species also has an enlarged pectoral area compared to the other species (pa in figure 2b,e). Even in large-bodied common seadragons (electronic supplementary material, figure S1d), the area is not as inflated as in the new species. In contrast to the backwards-facing pair of dorsal spines in common and leafy seadragons, the new seadragon has a forward-pointing pair (ds in figure 2b).
Table 2.
Comparison of diagnostic traits among the three seadragon species.
| Phycodurus eques (n=25) | Phyllopteryx taeniolatus (n=20) | Phyllopteryx dewysea n. sp. (n=4) | |
|---|---|---|---|
| trunk rings | 17–18 | 17 | 18 |
| head spine | present | present | absent |
| third ventral spine on trunk ring | 16 | 16 or absent | 17 |
| dorsal spine on trunk ring 11 orients | backward (figure 3a) | backward (figure 3b) | forward (figure 2b, figure 3c) |
| coloration | yellow/pink and white bars | yellow spots and blue ridges | red/pink bars |
| dermal appendages | complex, multilobate | simple, spatulate | unknown |
| lateral trunk and lateral tail ridge | continuous | discontinuous | discontinuous |
Figure 2.
Holotype of the new seadragon Phyllopteryx dewysea n. sp. (a) On-deck shortly after being trawled; (b) preserved, with tip of tail and eggs removed for DNA extraction; pa pectoral area; ds facing dorsal spine; (c–f) three-dimensional scan generated by μCT; (c) outer bony plates, arrows pointing to different enlarged spines; (d) left half of the plates removed to reveal parts of the skull, pectoral girdle and spine (in white); (e) ventral view of the enlarged pectoral area; (f) detail of the trunk region. Scale bars, 1 cm.
Figure 3.
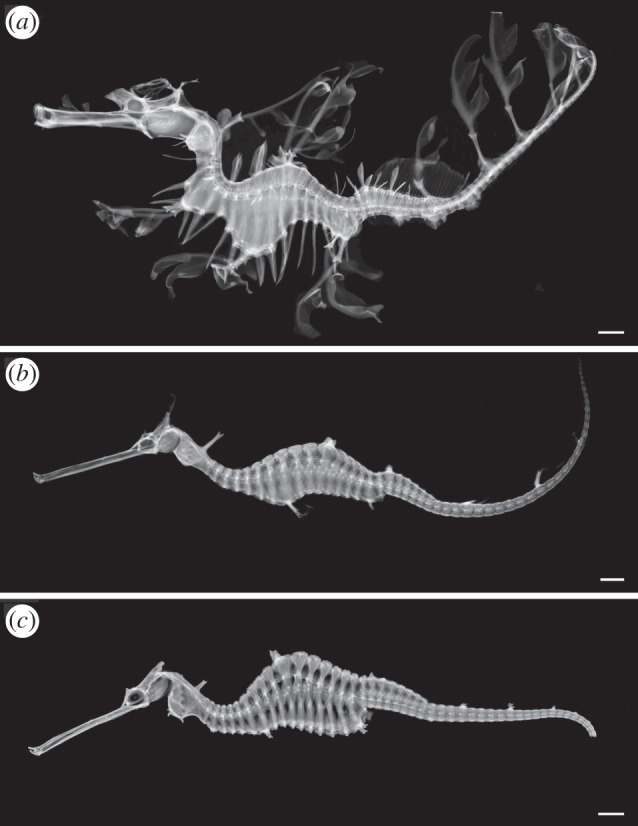
Comparison of the skeleton of the three species of seadragons. X-ray radiographs of (a) the leafy seadragon Phycodurus eques, SIO 04-28; (b) the common seadragon Phyllopteryx taeniolatus, SIO 84-300; (c) Phyllopteryx dewysea n. sp., holotype WAM P33223.002. Scale bars, 1 cm.
Table 3.
Morphometric measurements for the three seadragon species. Values for Phyllopteryx dewysea are given separately for the holotype and as ranges for the three paratypes. Proportions are given as % of the total length (TL), head, or snout length. The tail length of the holotype was measured from the photograph (figure 1a) as the tail is incomplete in the preserved specimen.
| Phycodurus eques (n=25) | Phyllopteryx taeniolatus (n=20) | Phyllopteryx dewysea n. sp. (holotype | three paratypes) | |
|---|---|---|---|
| total length (TL, mm) | 218–401 | 238–427 | 240 | 221–259 |
| trunk length (mm) | 67–114 | 70–135 | 67 | 61–79 |
| tail length (mm) | 114–221 | 98–234 | 111 | 92–123 |
| proportions (%) | |||
| trunk length : TL | 25–42 | 24–34 | 28 | 28–31 |
| tail length : TL | 45–69 | 42–58 | 46 | 41–48 |
| head length : TL | 18–33 | 17–29 | 26 | 22–31 |
| snout length : head length | 53–77 | 61–72 | 68 | 65–74 |
| snout depth : snout length | 11–20 | 7–13 | 7 | 7–10 |
| orbital diameter : head length | 10–13 | 8–13 | 12 | 10–13 |
| postorbital length : head length | 23–36 | 11–28 | 20 | 18–24 |
| neck depth : TL | 4–11 | 3–7 | 5 | 3–4 |
| trunk depth 8th segment : TL | 8–18 | 4–14 | 8 | 7–9 |
| trunk depth 11th segment : TL | 8–17 | 6–13 | 11 | 9–12 |
| trunk depth preanal segment : TL | 6–12 | 4–9 | 9 | 6–8 |
4.4. Systematics
Phyllopteryx dewysean. sp. (figure 1, figure 2a–f, figure 3c, tables 2 and 3, electronic supplementary material figure S2a,b and videos S1–S2).
4.4.1. Holotype
The holotype repository is the Western Australian Museum, Perth: WAM P33223.002, as Phyllopteryx taeniolatus, 240 mm TL, male with brood attached; eggs removed for DNA extraction, tail clipped. The species has been registered with Zoobank under the following LSID: BEF4C635-D5DD-4F70-99B6-88157AA88C7C.
4.4.2. Type locality
Australia, Western Australia, Recherche Archipelago, east of Middle Island, trawled at 51 m, Marine Futures Survey 2007, station MF-MI-007, 10 October 2007, 34°01.589′0 S, 123°21.55′0 E to 34°01.30′ S, 123°21.42′ E.
4.4.3. Paratypes
Three paratypes are designated based on morphological similarity to the holotype (table 3 and electronic supplementary material, file S1). CSIRO Marine and Atmospheric Research, ANFC C2269 and C2270, as Phyllopteryx lucasi (=taeniolatus), two specimens, 236 and 259 mm TL, sex undetermined; trawled west of Garden Island, Western Australia, 32° S, 115° E (coordinates approximated), 72 m, May 1956.
WAM P660, as Phyllopteryx foliatus,one specimen, 221 mm TL, sex undetermined; beachwashed onto Cottesloe Beach, Western Australia, 31°59′ S, 115°45′ E, August 1919.
4.4.4. Etymology
Named for Mary ‘Dewy’ Lowe, for her love of the sea and her support of seadragon conservation and research, without which this new species would not have been discovered.
4.4.5. Diagnosis
Eighteen trunk segments. Head crest without appendage. Pectoral area enlarged (pa in figure 2b,e). Dorsally enlarged spine on trunk ring 11 pointed forward (ds in figure 2b). Paired enlarged ventral spines on rings 8 and 17 (figure 3c). Body deepest on ring 12, behind dorsal spine. Spines on lateral trunk ridge not continuous with lateral tail ridges (figure 2c).
4.4.6. Brief description of holotype
Preserved colour light brown. Live colour ruby red, with pink vertical bars on each trunk segment and light markings on the snout (figure 2a). Enlarged spines dorsally on neck and trunk ring 11; ventrally on rings 3, 8, 17 (arrows in figure 2c; see also videos S1–S2 included in the electronic supplementary material). Presence and shape of dermal appendages on enlarged spines unknown. Lateral trunk ridges not confluent with lateral but with inferior tail ridges. Trunk dorsally forming pronounced arch, highest point ring 12, behind enlarged dorsal spines (figure 3c). Fin rays: pectoral 22; dorsal unknown; anal 4.
4.4.7. Distribution and bathymetry
The type locality (arrow in map of figure 1) at 50+ m shows a complex habitat of a mixed reef and sandy habitat [29,30]. Two paratypes were collected from the continental shelf near Perth (72 m, circle in figure 1); the habitat at this site is unknown. The fourth paratype, collected at Cottesloe, Perth, does not have depth data, as it was washed up on the beach.
4.4.8. Remarks
The placement of this species within Phyllopteryx was informed by the phylogeny of seadragons (figure 1). The new species lies as the sister group to Phyl. taeniolatus and this does not allow for its inclusion within the other seadragon genus, Phycodurus. We made the decision to place it within Phyllopteryx rather than erect a new genus, as this provides information about the closer relationship of Phyl. dewysea with Phyl. taeniolatus than with Phyc. eques.
An expanded description of the type series of Phyl. dewysea, of Phyl. taeniolatus and Phyc. eques, including detailed comparisons, can be found in the electronic supplementary material, file S1.
5. Discussion
The discovery of a new species of seadragon represents an important finding. It is genetically distinct from the two known seadragons, with supporting evidence from morphology and habitat. Although the two previously known seadragon species were described in the nineteenth century, Phyl. dewysea remained unrecognized for almost a century, after being first collected in 1919. This seadragon may occasionally be washed ashore, and be subjected to trawling, but may not have been recognized (as was the case with the samples located for this study) owing to a superficial similarity to common seadragons. Nevertheless, it seems surprising that this species escaped recognition until now given the public interest in seadragons. A possible explanation is that it may occur in depths beyond recreational SCUBA range: three of our specimens of Phyl. dewysea came from relatively deep water (51 and 72 m) a few kilometres offshore. The bathymetric range of common and leafy seadragons is insufficiently known, but they seem to be mostly restricted to inshore areas [4,31]. Clearly, two sampling localities in offshore waters do not make Phyl. dewysea an exclusive deeper water species, but the coloration of the holotype may be explained by a deeper habitat: although the red colour is conspicuous when out of the water (figure 2a), red light is rapidly absorbed with depth and so being red may effectively render the seadragon cryptic [32].
Basic biological information on habitat preferences and distribution are needed to assess the status of Phyl. dewysea in the wild, and underpin any future management plans. The few records of the new seadragon from southwestern Australia that we currently have cannot give a satisfactory picture of the distributional range. Furthermore, the records off the coast of Perth are almost 60 years old and it is unclear if Phyl. dewysea still occurs in this region. The urban development surrounding the Perth region has been significant in the past century [33], including changes in water quality and vegetation [34,35] that may have also affected the offshore habitat of Phyl. dewysea.
Only the retention of the unrecognized Phyl. dewyseaspecimens in museum collections allowed for its discovery as a new species. This highlights the significance of natural history collections as a treasury of new species [36], even decades after their collection [37]. Another invaluable component of biodiversity discovery consists of exploratory expeditions such as the Marine Futures project that trawled the holotype and mapped its habitat [29]: the on-board documentation provided the only available photograph of a fresh Phyl. dewysea, a currently unique representation of its natural coloration (figure 2a).
Southwestern Australia hosts a large number of marine fish [38], and is considered a hotspot for future discovery [3]. Phyl. dewysea is an illustration of the surprises that are waiting in the ocean, but many open questions remain regarding its range, habitat and biology. The other two seadragons are considered Near Threatened by the IUCN [6,39], and their conservation is of public interest [5,40]. The new seadragon is no less charismatic and deserves to become a flagship species for the discovery of biodiversity.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Marine Futures project for collecting and photographing the holotype. Glenn Moore and Sue Morrison (WAM) are thanked for supplying the deck photo, the holotype and one of the paratypes. Al Graham (ANFC) kindly provided two paratypes and tissue samples. Severine Hannam (Auckland War Memorial Museum) and Leslee Matsushige (Birch Aquarium, UCSD) provided tissues for DNA analysis. We thank Phil Hastings (Scripps Institution of Oceanography, UCSD) for taking the radiographs and supplying specimens, Esther Cory who conducted the μCT scan, and Rachel Berquist for assistance. Kayla Krantz is thanked for laboratory assistance. Dave Catania (California Academy of Sciences), Mark McGrouther (Australian Museum), Ralph Foster (South Australian Museum) are thanked for photographs of common seadragons. We are grateful to Mary Lowe and the Lowe Family Foundation for financial support.
Ethics statement
Non-lethal clipping of the dermal appendages with subsequent release of the seadragons was authorized by the following authorities: Department of Primary Industries and Regions South Australia (PIRSA), exemption number 9901842 to the Fisheries Act 1982, §59; Department of Conservation & Land Management, Western Australia, licence SF 5126; Department of Primary Industries and Water Wild Fisheries Branch, Tasmania, permit 12112 issued under §14 of the Living Marine Resources Management Act 1995. Sampling followed an animal use protocol (S12285) approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, San Diego and was approved by the Department of Primary Industries, Parks, Water & Environment (DPIPWE) Animal Ethics Committee (AEC) of the Tasmanian Government (AEC project no. 11/2012-13). Tissues were transferred with IACUC approval (tissue transfer permit T07215).
Data accessibility
DNA sequences: GenBank accessions KM201544–KM201604. Phylogenetic data: TreeBASE accession number S15971. http://purl.org/phylo/treebase/phylows/study/TB2:S15971.
Author contributions
J.S. carried out the molecular and morphological data generation and analysis, and drafted the manuscript; N.G.W. and G.W.R. collected field data, coordinated the study and drafted the manuscript. All authors gave final approval for publication.
Funding statement
This research was supported by the Lowe Family Foundation.
Competing interests
We have no competing interests.
References
- 1.Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. 2011. How many species are there on Earth and in the ocean? PLoS Biol. 9, 1001127 (doi:10.1371/journal.pbio.1001127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appeltans W, et al. 2012. The magnitude of global marine species diversity. Curr. Biol. 22, 2189–2202. (doi:10.1016/j.cub.2012.09.036) [DOI] [PubMed] [Google Scholar]
- 3.Eschmeyer WN, Fricke R, Fong JD, Polack DA. 2010. Marine fish diversity: history of knowledge and discovery (Pisces). Zootaxa 2525, 19–50. [Google Scholar]
- 4.Dawson CE. 1985. Indo-Pacific pipefishes (Red Sea to the Americas). Ocean Springs, Mississippi: Gulf Coast Research Laboratory. [Google Scholar]
- 5.Martin-Smith KM, Vincent ACJ. 2006. Exploitation and trade of Australian seahorses, pipehorses, sea dragons and pipefishes (family Syngnathidae). Oryx 40, 141–151. (doi:10.1017/S003060530600010X) [Google Scholar]
- 6.Connolly R. 2006. Phycodurus eques. The IUCN Red List of Threatened Species. Version 2014.3. http://www.iucnredlist.org.
- 7.Campos PF, Gilbert TMP. 2012. DNA extraction from formalin-fixed material. In Ancient DNA: methods and protocols (eds B Shapiro, M Hofreiter), pp. 81–85. New York, NY: Humana Press, Springer Science+Business Media. [DOI] [PubMed] [Google Scholar]
- 8.Wilson AB, Orr JW. 2011. The evolutionary origins of Syngnathidae: pipefishes and seahorses. J. Fish Biol. 78, 1603–1623. (doi:10.1111/j.1095-8649.2011.02988.x) [DOI] [PubMed] [Google Scholar]
- 9.Wilson AB. 2001. Male pregnancy in seahorses and pipefishes (family Syngnathidae): rapid diversification of paternal brood pouch morphology inferred from a molecular phylogeny. J. Hered. 92, 159–166. (doi:10.1093/jhered/92.2.159) [DOI] [PubMed] [Google Scholar]
- 10.Wilson NG, Rouse GW. 2010. Convergent camouflage and the non-monophyly of ‘seadragons’ (Syngnathidae: Teleostei): suggestions for a revised taxonomy of syngnathids. Zool. Scr. 39, 551–558. (doi:10.1111/j.1463-6409.2010.00449.x) [Google Scholar]
- 11.Kocher TD, Thomas WK, Meyer A, Edwards SV, Paabo S, Villablanca FX, Wilson AC. 1989. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc. Natl Acad. Sci. USA 86, 6196–6200. (doi:10.1073/pnas.86.16.6196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palumbi S, Martin A, Romano S, McMillan W, Stice L, Grabowski G. 1991. The simple fool's guide to PCR. Honolulu, HI: Special Publications Department Zoology, University of Hawaii. [Google Scholar]
- 13.Miya M, Nishida M. 1999. Organization of the mitochondrial genome of a deep-sea fish, Gonostoma gracile (Teleostei: Stomiiformes): first example of transfer RNA gene rearrangements in bony fishes. Mar. Biotechnol. 1, 416–426. (doi:10.1007/PL00011798) [DOI] [PubMed] [Google Scholar]
- 14.Inoue JG, Miya M, Tsukamoto K, Nishida M. 2003. Evolution of the deep-sea gulper eel mitochondrial genomes: large-scale gene rearrangements originated within the eels. Mol. Biol. Evol. 20, 1917–1924. (doi:10.1093/molbev/msg206) [DOI] [PubMed] [Google Scholar]
- 15.Meyer A, Morrissey JM, Schartl M. 1994. Recurrent origin of a sexually selected trait in Xiphophorus fishes inferred from a molecular phylogeny. Nature 368, 539–542. (doi:10.1038/368539a0) [DOI] [PubMed] [Google Scholar]
- 16.Hrbek T, Farias IP. 2008. The complete mitochondrial genome of the pirarucu (Arapaima gigas, Arapaimidae, Osteoglossiformes). Genet. Mol. Biol. 31, 293–302. (doi:10.1590/S1415-47572008000200024) [Google Scholar]
- 17.Chow S, Hazama K. 1998. Universal PCR primers for S7 ribosomal protein gene introns in fish. Mol. Ecol. 7, 1255–1256. (doi:10.1046/j.1365-294x.1998.00406.x) [PubMed] [Google Scholar]
- 18.Teske PR, Beheregaray LB. 2009. Evolution of seahorses' upright posture was linked to Oligocene expansion of seagrass habitats. Biol. Lett. 5, 521–523. (doi:10.1098/rsbl.2009.0152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teske PR, Cherry MI, Matthee CA. 2004. The evolutionary history of seahorses (Syngnathidae: Hippocampus): molecular data suggest a West Pacific origin and two invasions of the Atlantic Ocean. Mol. Phylogenet. Evol. 30, 273–286. (doi:10.1016/S1055-7903(03)00214-8) [DOI] [PubMed] [Google Scholar]
- 20.Streelman JT, Karl SA. 1997. Reconstructing labroid evolution with single-copy nuclear DNA. Proc. R. Soc. Lond. B 264, 1011–1020. (doi:10.1098/rspb.1997.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katoh K. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066. (doi:10.1093/nar/gkf436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. (doi:10.1093/molbev/mst010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swofford D. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Sunderland, Massachusetts: Sinauer Associates. [Google Scholar]
- 24.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 (doi:10.1038/nmeth.2109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maddison W, Maddison D. Mesquite: a modular system for evolutionary analysis. 2011 Version 2.75. http://mesquiteproject.org .
- 26.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. (doi:10.1093/bioinformatics/btl446) [DOI] [PubMed] [Google Scholar]
- 27.Silvestro D, Michalak I. 2011. raxmlGUI: a graphical front-end for RAxML. Org. Divers. Evol. 12, 335–337. (doi:10.1007/s13127-011-0056-0) [Google Scholar]
- 28.Lourie SA. 2003. A new pygmy seahorse, Hippocampus denise (Teleostei: Syngnathidae) from the Indo-Pacific. Zool. Stud. 42, 284–291. [Google Scholar]
- 29.Radford B, Van Niel KP, Holmes K. WA Marine Futures: benthic modelling and mapping final report. 2008 University of Western Australia. http://static.weboffice.uwa.edu.au/files/marine_futures/0_Overview%20Information/MF_Spatial%20Report%20final.pdf .
- 30.Kendrick GA, et al. Characterising the fish habitats of the Recherche Archipelago: FRDC 2001/060 Final Report. 2005. Fisheries Research and Development Corporation and University of Western Australia. [Google Scholar]
- 31.Kuiter RH. 1993. Coastal fishes of south-eastern Australia. Honolulu, HI: University of Hawaii Press. [Google Scholar]
- 32.Johnsen S. 2002. Cryptic and conspicuous coloration in the pelagic environment. Proc. R. Soc. Lond. B 269, 243–256. (doi:10.1098/rspb.2001.1855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearce RJ. 1991. Management of the marine environment in Western Australia: an ecosystem approach. Mar. Pollut. Bull. 23, 567–572. (doi:10.1016/0025-326X(91)90735-B) [Google Scholar]
- 34.Walker DI, McComb AJ. 1992. Seagrass degradation in Australian coastal waters. Mar. Pollut. Bull. 25, 191–195. (doi:10.1016/0025-326X(92)90224-T) [Google Scholar]
- 35.Cambridge ML, Chiffings AW, Brittan C, Moore L, McComb AJ. 1986. The loss of seagrass in Cockburn Sound, Western Australia. II. Possible causes of seagrass decline. Aquat. Bot. 24, 269–285. (doi:10.1016/0304-3770(86)90062-8) [Google Scholar]
- 36.Bebber DP, et al. 2010. Herbaria are a major frontier for species discovery. Proc. Natl Acad. Sci. USA 107, 22169–22171. (doi:10.1073/pnas.1011841108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontaine B, Perrard A, Bouchet P. 2012. 21 years of shelf life between discovery and description of new species. Curr. Biol. 22, 943 (doi:10.1016/j.cub.2012.10.029) [DOI] [PubMed] [Google Scholar]
- 38.Hutchins JB. 2001. Biodiversity of shallow reef fish assemblages in Western Australia using a rapid censusing technique. Rec. West. Aust. Museum 20, 247–270. [Google Scholar]
- 39.Connolly R. Phyllopteryx taeniolatus. 2006 The IUCN Red List of Threatened Species. Version 2014.3. http://www.iucnredlist.org .
- 40.Vincent ACJ, Foster SJ, Koldewey HJ. 2011. Conservation and management of seahorses and other Syngnathidae. J. Fish Biol. 78, 1681–1724. (doi:10.1111/j.1095-8649.2011.03003.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: GenBank accessions KM201544–KM201604. Phylogenetic data: TreeBASE accession number S15971. http://purl.org/phylo/treebase/phylows/study/TB2:S15971.