Abstract
When blood-feeding parasites increase seasonally, their deleterious effects may prevent some host species, especially those living in large groups where parasites are numerous, from reproducing later in the summer. Yet the role of parasites in regulating the length of a host's breeding season—and thus the host's opportunity for multiple brooding—has not been systematically investigated. The highly colonial cliff swallow (Petrochelidon pyrrhonota), a temperate-latitude migratory songbird in the western Great Plains, USA, typically has a relatively short (eight to nine week) breeding season, with birds rarely nesting late in the summer. Colonies at which ectoparasitic swallow bugs (Oeciacus vicarius) were experimentally removed by fumigation were over 45 times more likely to have birds undertake a second round of nesting than were colonies exposed to parasites. Late nesting approximately doubled the length of the breeding season, with some birds raising two broods. Over a 27 year period the percentage of birds engaging in late nesting each year increased at a colony site where parasites were removed annually. This trend could not be explained by changes in group size, climate or nesting phenology during the study. The results suggest that ectoparasitism shortens the cliff swallow's breeding season and probably prevents many individuals from multiple brooding. When this constraint is removed, selection may rapidly favour late nesting.
Keywords: cliff swallow, ectoparasites, Petrochelidon pyrrhonota, reproductive phenology, swallow bug, time of breeding
2. Introduction
Blood-feeding ectoparasites often exert strong selective pressure on their hosts and may affect the evolution of life-history traits such as clutch size, immune investment and offspring quality/quantity trade-offs [1–5]. A number of studies have demonstrated negative effects of ectoparasites on nestling growth and survival, especially in colonial hosts where grouping enhances parasite transmission [6–11]. Ectoparasitism may also affect the number of nesting attempts hosts are able to undertake, particularly if infestations increase seasonally to the extent that breeding later—when more parasites are present—is often unsuccessful [12,13]. Ectoparasitism thus could be one of the ecological drivers for the commonly observed seasonal decline in reproductive success for many birds of temperate latitudes [14–18] and might serve to truncate the typical length of the breeding season.
Surprisingly, little is known about how ectoparasites directly affect avian breeding phenology [1]. One study showed that decisions to undertake second broods depended on the extent of parasitism at a site, with birds at lightly infested sites more likely to nest again late in the season [12]. Ectoparasite loads can also impair the productivity of second broods more than that of first broods, with this cost of parasitism increasing over the breeding season [19]. However, because both the decision to nest a second time and the success of late nestings can be affected by a number of environmental factors [16,17,20], determining the role of ectoparasites independent of other effects can be difficult without experimental manipulation of parasite abundance.
In this study, we take advantage of a long-term experiment, in which we annually removed ectoparasitic swallow bugs (Hemiptera: Cimicidae: Oeciacus vicarius) from cliff swallow colonies, to investigate the potential role of ectoparasitism in regulating the length of the breeding season. These deleterious blood-feeding parasites are known to affect many aspects of the cliff swallow's social behaviour and ecology [7,8,21,22]. Bugs increase at swallow colony sites over the summer, and thus the seasonally increasing cost of parasitism might prevent birds from undertaking multiple nesting attempts at infested sites. If swallow bugs represent a seasonal constraint on nesting time in cliff swallows, we predicted that cliff swallows should lengthen the time spent breeding at sites where bugs were removed.
Our measure of breeding-season length was whether cliff swallows initiated late nesting at a colony site. Late nesting was defined as a distinct second round of nesting activity (building nests and laying eggs) at a colony site after nestlings had fledged there, generally leading to a doubling of the length of time birds were present at a site. We investigated how the frequency of late nesting changed over a period of more than 20 years at a parasite-free site and use the results to gain insight into how ectoparasites potentially constrain the length of the cliff swallow's breeding season and the number of breeding attempts it makes.
3. Material and methods
3.1. Study area
We have studied cliff swallows since 1982 in the western Great Plains, USA, centred near the Cedar Point Biological Station (41°13′ N, 101°39′ W) in Keith County, southwestern Nebraska, along the North and South Platte rivers and including portions of Deuel, Garden, Lincoln and Morrill counties [8]. Cliff swallows construct gourd-shaped mud nests, often in dense, synchronously breeding colonies. In our study area, the birds nest mostly on the sides of bridges, in box-shaped road culverts, or underneath overhangs on the sides of cliffs [23]. Colony size varies widely; in our study area it ranges from 2 to 6000 nests (mean±s.e., 404±13, n=2318 colonies), with some birds nesting solitarily. The typical phenology (in the absence of late nesting) is for cliff swallows to first arrive in southwestern Nebraska in mid to late April, for most birds to have initiated egg laying by early June, and for nestlings to have mostly fledged by mid-July. Cliff swallows spend the winter in northeastern Argentina, Uruguay and southwestern Brazil [24], although the wintering range of our specific population is unknown.
Swallow bugs negatively affect nestling cliff swallow development and survival, lowering nestling body mass, inducing anaemia, killing nestlings prior to fledging and increasing nutritional stress and wing asymmetry among birds that fledge [7,8,13,25,26]. Experiments in colonies of different sizes have shown that the effects of bugs are most severe in the largest colonies, where bugs are also the most numerous [7,8], although these colony-size effects have diminished in recent years (C. R. Brown 2014, unpublished data).
3.2. Fumigation and study colonies
Swallow bugs were removed from colonies by lightly misting the outside of all cliff swallow nests and adjacent nesting substrate with a dilute solution of the insecticide Dibrom [7,8]. This chemical works largely as a contact pesticide, although we use the term fumigation to describe parasite removal. Nests were sprayed at 7 to 14 day intervals throughout the nesting season, typically beginning early in the season after birds had initially settled at sites and continuing until nests were no longer active. Dibrom is highly effective against swallow bugs [9], and even a single spraying can greatly reduce the number of bugs at a colony site for the entire season (C. R. Brown 2013, personal observation). Other studies of avian ectoparasites have also used this insecticide [25,27]. Dibrom has little effect on the other common haematophagous ectoparasite of cliff swallows in western Nebraska, the bird flea (Siphontaptera: Ceratophyllidae: Ceratophyllus celsus), so by fumigating, we removed only swallow bugs [8].
Fumigation of colonies began in 1984 and continued at some sites through to 2014. Some sites were fumigated only in certain years, depending on the research question that required parasite removal. Two colony sites, Whitetail and Junkyard, were fumigated each year of the study, beginning in 1984 and 1998, respectively. The fumigated sites were all concrete road culverts, and they did not differ in physical attributes from other culvert sites occupied by cliff swallows in the study area. Non-fumigated colony sites were those with no fumigation of any part and were situated on culverts, bridges and buildings [23]. Only sites monitored by us throughout the summer and visited often enough to know if late nesting occurred were included in these analyses. Colonies active from 1982 to 2014 were included.
Throughout this paper, a colony site refers to a physical structure at a particular locale where birds nested, whereas a colony refers to a collection of individuals at a given site [23]. At any one time, a site could contain only one colony, as the colony was a functional definition consisting of all birds occupying a given site at a particular time. Colonies occurring at a given colony site in different years were considered independent units of analysis, because colony size at a site and the birds resident there often varied widely from year to year. However, to control for possible non-independence between years brought about by site heterogeneity, we controlled for colony site by including it as a random effect in mixed models.
3.3. Measuring late nesting, colony size and initiation date
We assessed whether late nesting occurred at a colony site each year by observing the birds' presence at the site after the early round of nesting had been completed (known by fledging of young from most nests). When the birds' presence at nests at a site after about 15 July suggested they might be nesting, we checked nests for eggs using a dental mirror and flashlight inserted through a nest's tubular mud entrance. For many colonies, we ruled out any late nesting (without checking nests) whenever all birds had vacated the site by 25 July (by which time most cliff swallows had migrated from the study area). Our definition of late nesting as a temporally distinct second round of breeding at a site meant that all colonies included in our analyses had to have been ones that were active during the early round of nesting in May and June (the typical time when cliff swallows nest in the study area) and thus were available to have a late round of nesting. Late nesting typically occurred in nests that had also been occupied in the early round. The birds in the late round at a site had been there during the early round [28] and thus engaged in true double-brooding. There was generally about a 45 to 50 day difference between the mean egg-laying date for the early round at a site and the mean egg-laying date for the late round.
Annual colony sizes were based on first nesting attempts (the early round) and defined as the number of active nests (ones with at least one egg laid) each year. Colony sizes were determined by direct counts of all active nests, known from viewing nest contents, or by estimation based on nest counts of portions of a colony site or from the number of birds present [8].
At Whitetail only, the number of nests active in the late round of nesting was determined each year by either regularly checking all nests in late July and August or estimating the number of active nests from the number of previously banded adults mist-netted at the site after 15 July. The number of late nests was not available for some years. We did not attempt to estimate the number of nests in the late round at any of the other colony sites, where we simply scored the presence or absence of late nesting.
We determined a given colony's initiation date, by recording when the first cliff swallows were observed at the site that year and remained daily thereafter [29]. We monitored colony sites for arriving birds throughout the nesting season, and if initiation date could not be determined exactly or estimated to the nearest 3 days by virtue of our visit schedule, that colony was not included in initiation-date analyses.
As a measure of the relative earliness of each nesting season across the study area independent of a given site's phenology, we used the annual date on which the first juvenile cliff swallow was captured in a mist net (at any colony) in the course of our extensive mark-recapture programme [30,31] conducted each year of the study (except 1982, 1983 and 2014) at 20–35 colonies annually. We netted virtually daily throughout the season, and thus were likely to detect when the first juveniles fledged at any site.
3.4. Measuring body mass
As a measure of the quality and condition of individuals initially settling in different colonies, we used the body mass of cliff swallows captured in mist nets at both fumigated and non-fumigated colony sites throughout the study area. We restricted the analysis only to birds captured relatively early in the season when a given colony was engaged in nest-building and egg laying. We thus standardized our body masses temporally with respect to nesting stage, rather than date per se [8], given the wide variance in when colonies initiate within a nesting season [29]. Birds were captured during the course of extensive mark-recapture work [30,31], primarily in mist nets, and weighed to the nearest 0.5 g using a Pesola scale. Sex of cliff swallows was determined using presence/absence of a brood patch or cloacal protuberance [8]. From 1984 to 2013, we had body-mass measurements for 13 287 birds in fumigated colonies and 29 254 birds in non-fumigated colonies. Masses were averaged for any individual caught more than once per year during the designated nesting stage. Each individual was used only once—the year it was first captured—in these analyses, and the same individuals were thus not represented in multiple years.
3.5. Palmer drought severity index
As an overall measure of annual climatic conditions during the study (and thus influences on the cliff swallow's aerial insect food), we used the modified Palmer drought severity index (PDSI) for Nebraska's Climate Division 7 (southwest Nebraska) available from the National Oceanic and Atmospheric Administration (NOAA) at www.ncdc.noaa.gov/cag/time-series/. Division 7 encompasses the centre of our study area, and the regional metrics should be broadly representative of climatic conditions the birds experienced each season. The PDSI is a measure of drought intensity used by NOAA, and it integrates both local temperature and rainfall data into a single index useful in describing soil moisture and extent of runoff [32,33]. Lower values of the PDSI indicate more severe drought. Because cliff swallow colonies are active primarily from May through to July, and thus are most likely to be affected by the climatic environment at that time, we used three-month averages for May–July as determined by NOAA.
3.6. Statistical analyses
Logistic mixed models were constructed in SAS [34] using Proc GLIMMIX. This allowed specification of colony site as a random effect, with the categorical-dependent variable being whether a colony site exhibited late nesting. Fixed effects were fumigation status (yes/no, categorical), year, colony size, colony initiation date, date of first juvenile capture and PDSI. Multiple regression of the extent of late nesting each year at Whitetail was performed using Proc GLM in SAS. Body mass of birds in fumigated and non-fumigated colonies was compared with Proc MIXED in SAS, with colony site as a random effect and with fixed effects being whether a site was fumigated, colony size, sex and year.
4. Results
Of 1183 non-fumigated colonies, 19 (1.6%) had late nesting activity, compared with 49 (75.4%) of 65 fumigated colonies. Using 636 colonies and controlling for colony site as a random effect, only fumigation status of a colony (yes/no) was a significant predictor of whether late nesting occurred (F1,548=5.24, p=0.022). Colony size (F1,548=0.03, p=0.86), colony initiation date (F1,548=0.41, p=0.52), date of first juvenile capture (F1,548=0.10, p=0.75), year (F1,594=0.36, p=0.55) and annual PDSI (F1,594=0.26, p=0.61) were not significant predictors of the presence or absence of late nesting. When the analysis was re-run with an additional 612 colonies (that did not have known initiation dates or were from 3 years without first juvenile capture dates), the results were similar (for fumigation status, F1,1131=5.15, p=0.023; p≥0.55 for all others). Colony size, colony initiation date, first juvenile capture date, year and PDSI also had no significant effect on whether colonies exhibited late nesting when fumigated and non-fumigated colonies were analysed separately (p≥0.59 for each).
For Whitetail, the percentage of first-round nests that were re-occupied for late nesting increased significantly over time (figure 1), varying from 2.1% in 1986 to a high of 16.7% in 2007. Only year was a significant predictor of the percentage of nests re-occupied for late nesting (F1,15=31.6, p<0.001, β=0.49), with Whitetail colony initiation date (F1,15=0.08, p=0.79), annual first juvenile capture date in the study area (F1,15=0.0, p=0.99), Whitetail colony size (F1,15=0.46, p=0.51) and annual PDSI (F1,15=0.94, p=0.35) not explaining any significant variation. Results were similar when colony initiation date and first juvenile capture date were removed from the model.
Figure 1.
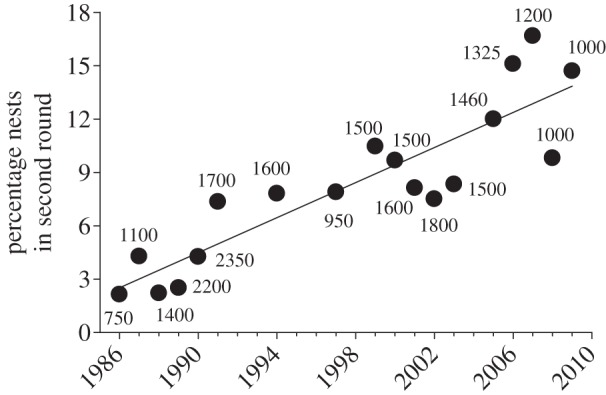
Percentage of cliff swallow nests in the first round of nesting that were active in the second round at a fumigated colony (Whitetail) each year that late nesting occurred. Percentage increased significantly with year (rs=0.89, p<0.0001, n=18 years). Line indicates best-fit least-squares regression. Numbers by dots indicate colony size (approximate number of nests in first round).
When controlling for colony site as a random effect, body mass of cliff swallows soon after initial settlement varied significantly with sex (F1,∼42 000=1621, p<0.0001) and year (F27,∼42 000=76.9, p<0.0001) but did not vary with a site's fumigation status (F1,∼42 000=2.36, p=0.12) or with colony size (F1,∼42 000=0.86, p=0.35). Similar results were obtained when each year was analysed separately: in only 2 years (2006, 1997) was fumigation status a significant predictor of body mass (β=−1.26 and −1.99, p=0.02 and 0.002, respectively), and in the other 25 years fumigation status was not significant (p≥0.11 on all). For Whitetail, mean body mass of birds at settlement each year did not change significantly over time (rs=−0.05, p=0.82, n=26 years).
5. Discussion
The extent of ectoparasitism by swallow bugs was clearly associated with the length of the breeding season in cliff swallows of western Nebraska. Colonies with parasite removal via fumigation were over 45 times more likely to have a round of late nesting (and thus a nesting season approximately doubled in length) than were colonies with typical numbers of swallow bugs. Late nesting was unrelated to colony size, phenology of the nesting season or climatic conditions in a given year. That late nesting in Nebraska cliff swallows can be successful and confers potentially higher lifetime fitness than raising only an early single brood [28] suggests that the regular presence of ectoparasites in colonies under natural conditions shortens the birds' nesting season and prevents some individuals from attempting multiple broods.
Our experimental parasite removal establishes the absence of swallow bugs as a causal factor in the initiation of late nesting in cliff swallows. We were able to rule out other possibilities that might have contributed to the observed results. For example, local resources (e.g. the birds' food) could change over time [20], or earlier breeding times in cliff swallows, brought about by responses to increasing drought severity [29], might allow these birds more time in the summer to complete late nesting. However, in either case, we should have seen an effect of year and/or PDSI on the incidence of late nesting at both fumigated and non-fumigated sites, which we did not. Late nesting also seemed unrelated to local phenology (i.e. colony initiation date).
Could the increased late nesting at fumigated sites reflect non-random sorting of high-quality individuals into those colonies? If so, those individuals might have been more capable of nesting late by virtue of their superior condition. The body masses of cliff swallows soon after settlement in colonies do not support this scenario: individual quality or condition, at least as measured by body mass [8], did not differ between fumigated and non-fumigated sites. In addition, colony size itself had no effect on the incidence of late nesting, suggesting that all individuals regardless of their colony-size propensity [35] were equally likely or unlikely to engage in late nesting. Furthermore, because fumigation of a site each year did not begin until after individuals had settled, it is unlikely that individuals had the opportunity to choose their colony (and thus assort) based on whether a site was being fumigated that year. Even at a perennially fumigated site (Whitetail), there was no evidence that the quality of the residents there (as measured by body mass) changed over time as more birds engaged in late nesting.
Cliff swallows' responding to ectoparasites by avoiding late nesting is perhaps not surprising, given the highly deleterious effects of swallow bugs and the other ways cliff swallows adjust their behaviour to bug infestations [7,8,21,25]. Many nests in our study area are so infested with bugs by the time nestlings fledge that neither the adults nor the juveniles ever return to the nests (C. Brown 1984–2014, personal observation), and at these sites the lack of late nesting is not surprising. By contrast, at fumigated sites, parents often lead the juveniles back to the nest to sleep at night for several days after fledging, and the adults themselves (even those who do not attempt late nesting) sit in the empty nests for long periods after the juveniles have permanently vacated the site [36]. In Oklahoma, severe ectoparasite infestations caused entire colonies to abandon nesting sites [13].
While the much greater frequency of late nesting at fumigated colonies compared with non-fumigated ones was striking, perhaps even more surprising was the increase in the extent of late nesting over time at one site (figure 1). This trend could not be explained by changes in breeding phenology, climate or social environment (i.e. colony size). Because late nesting is often perpetrated largely by previous nesters who thus augment their annual reproductive success, the increase over time suggests potential selection for individuals that double-brood. This is supported by brood-size and survival analyses of breeding adults and offspring from early and late nests, which indicate that late nesters have at least equivalent (and perhaps greater) lifetime fitness as those birds that rear only an early brood [28]. However, despite the potential advantages to rearing a second brood, only a relatively small percentage of birds nested late even at fumigated sites such as Whitetail. Other costs of late nesting (e.g. declining food availability, delayed start of autumn migration) independent of ectoparasitism may prevent some individuals from double-brooding, particularly for those who are not among the earliest birds to arrive in the spring [28].
This study demonstrates perhaps the strongest effect yet of a blood-sucking ectoparasite on the length of its host's breeding season and on the host's ability to undertake multiple broods. Our results suggest that parasitism may be an overlooked factor contributing to the wide variability both among and within species in propensity to double-brood in a given year [37–43]. In the case of cliff swallows, their extreme degree of coloniality probably exacerbates the shortened nesting season imposed by parasites because the birds are exposed to such large numbers of swallow bugs by virtue of their large group sizes. This could be one reason why the less social but ecologically similar barn swallow often produces multiple broods in a season in North America [44,45], while the more colonial cliff swallow does not [24]. In geographical areas where swallow bugs are less numerous, multiple brooding should be more common, which appears to be the case [46]. Our experimental results suggest that cliff swallows have a strong degree of plasticity in breeding phenology and that some individuals have the capacity to respond quickly to an absence of parasites.
Acknowledgements
We thank numerous research assistants, especially Laurie Doss and Jerri Hoskyn, who helped fumigate nests in the early years of the study. The R. Clary, D. Dunwoody, D. Knight and L. Soper families, and the Union Pacific Railroad provided access to land. Amy Moore and Catherine Page managed databases. The School of Biological Sciences at the University of Nebraska-Lincoln allowed us to use the Cedar Point Biological Station. Amy Moore, Valerie O'Brien and Erin Roche provided useful discussion.
Ethics statement
This work was approved by the University of Tulsa's Institutional Animal Care and Use Committee under protocol TU-0020 and was conducted in accordance with the laws of the United States of America.
Data accessibility
Original data deposited in Dryad, .
Author contributions
C.R.B. designed study, did fieldwork, analysed data and wrote the manuscript. M.B.B. did fieldwork.
Funding statement
We thank the National Science Foundation (most recently, DEB-1019423), the National Institutes of Health (AI057569), the National Geographic Society, the Erna and Victor Hasselblad Foundation, Princeton University, Yale University and the University of Tulsa for financial support.
Competing interests
The authors have no competing interests.
References
- 1.Møller AP, Allander K, Dufva R. 1990. Fitness effects of parasites on passerine birds: a review. In Population biology of passerine birds (eds J Blondel, A Gosler, JD Lebreton, R McCleery), pp. 269–280. Berlin, Germany: Springer. [Google Scholar]
- 2.Møller AP, Merino S, Brown CR, Robertson RJ. 2001. Immune defense and host sociality: a comparative study of swallows and martins. Am. Nat. 158, 136–145. (doi:10.1086/321308) [DOI] [PubMed] [Google Scholar]
- 3.Loye JE, Zuk M. (eds). 1991. Bird-parasite interactions: ecology, evolution and behaviour. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Richner H, Heeb P. 1995. Are clutch and brood size patterns in birds shaped by ectoparasites? Oikos 73, 435–441. (doi:10.2307/3545973) [Google Scholar]
- 5.Clayton DH, Moore J. 1997. Host-parasite evolution: general principles and avian models. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Duffy DC. 1983. The ecology of tick parasitism on densely nesting Peruvian seabirds. Ecology 64, 110–119. (doi:10.2307/1937334) [Google Scholar]
- 7.Brown CR, Brown MB. 1986. Ectoparasitism as a cost of coloniality in cliff swallows (Hirundo pyrrhonota). Ecology 67, 1206–1218. (doi:10.2307/1938676) [Google Scholar]
- 8.Brown CR, Brown MB. 1996. Coloniality in the cliff swallow: the effect of group size on social behavior. Chicago, IL: University of Chicago Press. [Google Scholar]
- 9.Brown CR, Brown MB. 2004. Empirical measurement of parasite transmission between groups in a colonial bird. Ecology 85, 1619–1626. (doi:10.1890/03-0206) [Google Scholar]
- 10.Hoogland JL. 1995. The black-tailed prairie dog: social life of a burrowing mammal. Chicago, IL: University of Chicago Press. [Google Scholar]
- 11.Rifkin JL, Nunn CL, Garamszegi LZ. 2012. Do animals living in larger groups experience greater parasitism? A meta-analysis. Am. Nat. 180, 70–82. (doi:10.1086/666081) [DOI] [PubMed] [Google Scholar]
- 12.Møller AP. 1990. Effects of parasitism by a haematophagous mite on reproduction in the barn swallow. Ecology 71, 2345–2357. (doi:10.2307/1938645) [Google Scholar]
- 13.Loye JE, Carroll SP. 1991. Nest ectoparasite abundance and cliff swallow colony site selection, nestling development, and departure time. In Bird-parasite interactions: ecology, evolution and behavior (eds JE Loye, M Zuk), pp. 222–242. Oxford, UK: Oxford University Press. [Google Scholar]
- 14.Klomp H. 1970. The determinants of clutch size in birds: a review. Ardea 58, 1–125 [Google Scholar]
- 15.Perrins CM. 1970. The timing of birds' breeding seasons. Ibis 112, 242–255. (doi:10.1111/j.1474-919X.1970.tb00096.x) [Google Scholar]
- 16.Price T, Kirkpatrick M, Arnold SJ. 1988. Directional selection and the evolution of breeding date in birds. Science 240, 798–799. (doi:10.1126/science.3363360) [DOI] [PubMed] [Google Scholar]
- 17.Rowe L, Ludwig D, Schluter D. 1994. Time, condition, and the seasonal decline of avian clutch size. Am. Nat. 143, 698–722. (doi:10.1086/285627) [Google Scholar]
- 18.Verhulst S, Nilsson JA. 2008. The timing of birds' breeding seasons: a review of experiments that manipulated timing of breeding. Phil. Trans. R. Soc. B 363, 399–410. (doi:10.1098/rstb.2007.2146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lope F, González G, Pérez JJ, Møller AP. 1993. Increased detrimental effects of ectoparasites on their bird hosts during adverse environmental conditions. Oecologia 95, 234–240. (doi:10.1007/BF00323495) [DOI] [PubMed] [Google Scholar]
- 20.Husby A, Kruuk LEB, Visser ME. 2009. Decline in the frequency and benefits of multiple brooding in great tits as a consequence of a changing environment. Proc. R. Soc. B 276, 1845–1854. (doi:10.1098/rspb.2008.1937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown CR, Brown MB. 1992. Ectoparasitism as a cause of natal dispersal in cliff swallows. Ecology 73, 1718–1723. (doi:10.2307/1940023) [Google Scholar]
- 22.Brown CR, Brown MB. 1999. Fitness components associated with laying date in the cliff swallow. Condor 101, 230–245. (doi:10.2307/1369986) [Google Scholar]
- 23.Brown CR, Brown MB, Roche EA. 2013. Spatial and temporal unpredictability of colony size in cliff swallows across 30 years. Ecol. Monogr. 83, 511–530. (doi:10.1890/12-2001.1) [Google Scholar]
- 24.Brown CR, Brown MB. 1995. Cliff swallow (Hirundo pyrrhonota). In The birds of North America (eds A Poole, F Gill), no. 149 Philadelphia, PA: Academy of Natural Sciences. [Google Scholar]
- 25.Chapman BR, George JE. 1991. The effects of ectoparasites on cliff swallow growth and survival. In Bird-parasite interactions: ecology, evolution and behavior (eds JE Loye, M Zuk), pp. 69–92. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Brown CR, Brown MB. 2002. Ectoparasites cause increased bilateral asymmetry of naturally selected traits in a colonial bird. J. Evol. Biol. 15, 1067–1075. (doi:10.1046/j.1420-9101.2002.00474.x) [Google Scholar]
- 27.Moss WW, Camin JH. 1970. Nest parasitism, productivity, and clutch size in purple martins. Science 168, 1000–1003. (doi:10.1126/science.168.3934.1000) [DOI] [PubMed] [Google Scholar]
- 28.Brown CR, Roche EA, O'Brien VA. 2015. Costs and benefits of late nesting in cliff swallows. Oecologia 177, 413–421. (doi:10.1007/s00442-014-3095-3) [DOI] [PubMed] [Google Scholar]
- 29.Brown CR, Brown MB. 2014. Breeding time in a migratory songbird is predicted by drought severity and group size. Ecology 95, 2736–2744. (doi:10.1890/14-0425.1) [Google Scholar]
- 30.Brown CR, Brown MB. 2004. Group size and ectoparasitism affect daily survival probability in a colonial bird. Behav. Ecol. Sociobiol. 56, 498–511. (doi:10.1007/s00265-004-0813-6) [Google Scholar]
- 31.Roche EA, Brown CR, Brown MB, Lear KM. 2013. Recapture heterogeneity in cliff swallows: increased exposure to mist nets leads to net avoidance. PLoS ONE 8, 58092 (doi:10.1371/journal.pone.0058092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer WC. 1965. Meteorological drought, Research Paper 45. Washington, DC: United States Department of Commerce. [Google Scholar]
- 33.Heddinghaus TR, Sabol P. 1991. A review of the Palmer Drought Severity Index and where do we go from her? In Proceedings of the 7th Conference on Applied Climatology, pp. 242–246. Boston, MA: American Meteorological Society. [Google Scholar]
- 34.SAS Institute. 2004. SAS/STAT user's guide, version 9.1. Cary, NC: SAS Institute. [Google Scholar]
- 35.Brown CR, Brown MB. 2000. Heritable basis for choice of group size in a colonial bird. Proc. Natl Acad. Sci. USA 97, 14825–14830. (doi:10.1073/pnas.97.26.14825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown CR. 1998. Swallow summer. Lincoln, NE: University of Nebraska Press. [Google Scholar]
- 37.Brown CR. 1978. Double-broodedness in purple martins in Texas. Wilson Bull. 90, 239–247 [Google Scholar]
- 38.Nolan V., Jr 1978. The ecology and behavior of the prairie warbler Dendroica discolor. Washington, DC: Ornithological Monograph 26, American Ornithologists' Union. [Google Scholar]
- 39.Geupel GR, DeSante DF. 1990. Incidence and determinants of double brooding in wrentits. Condor 92, 67–75. (doi:10.2307/1368384) [Google Scholar]
- 40.Mahony NA, Vander Haegen WM, Walker BL, Krannitz PG. 2001. Male incubation and multiple brooding in sagebrush Brewer's sparrows. Wilson Bull. 113, 441–444. (doi:10.1676/0043-5643(2001)113[0441:MIAMBI]2.0.CO;2) [Google Scholar]
- 41.Nagy LR, Holmes RT. 2005. To double-brood or not? Individual variation in the reproductive effort in black-throated blue warblers (Dendroica caerulescens). Auk 122, 902–914. (doi:10.1642/0004-8038(2005)122[0902:TDONIV]2.0.CO;2) [Google Scholar]
- 42.Monroe AP, Hallinger KK, Brasso RL, Cristol DA. 2008. Occurrence and implications of double brooding in a southern population of tree swallows. Condor 110, 382–386. (doi:10.1525/cond.2008.8341) [Google Scholar]
- 43.Jacobs AC, Reader LL, Fair JM. 2013. What determines the rates of double brooding in the western bluebird? Condor 115, 386–393. (doi:10.1525/cond.2013.120085) [Google Scholar]
- 44.Ramstack JM, Murphy MT, Palmer MR. 1998. Comparative reproductive biology of three species of swallows in a common environment. Wilson Bull. 110, 233–243 [Google Scholar]
- 45.Brown CR, Brown MB. 1999. Barn swallow (Hirundo rustica). In The birds of North America (eds A Poole, F Gill), no. 452 Philadelphia, PA: Academy of Natural Sciences. [Google Scholar]
- 46.Weaver HB, Brown CR. 2004. Brood parasitism and egg transfer in cave swallows (Petrochelidon fulva) and cliff swallows (P. pyrrhonota) in south Texas. Auk 121, 1122–1129. (doi:10.1642/0004-8038(2004)121[1122:BPAETI]2.0.CO;2) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data deposited in Dryad, .


