Abstract
Bathymetric distributions of photosynthetic marine invertebrate species are relatively well studied, however the importance of symbiont zonation (i.e. hosting of distinct algal endosymbiont communities over depth) in determining these depth distributions still remains unclear. Here, we assess the prevalence of symbiont zonation in tropical scleractinian corals by genotyping the Symbiodinium of the 25 most common species over a large depth range (down to 60 m) on a Caribbean reef. Symbiont depth zonation was found to be common on a reef-wide scale (11 out of 25 coral species), and a dominant feature in species with the widest depth distributions. With regards to reproductive strategy, symbiont zonation was more common in broadcasting species, which also exhibited a higher level of polymorphism in the symbiont zonation (i.e. number of different Symbiodinium profiles involved). Species with symbiont zonation exhibited significantly broader depth distributions than those without, highlighting the role of symbiont zonation in shaping the vertical distributions of the coral host. Overall, the results demonstrate that coral reefs can consist of highly structured communities over depth when considering both the coral host and their obligate photosymbionts, which probably has strong implications for the extent of connectivity between shallow and mesophotic habitats.
Keywords: coral reef, depth distribution, symbiont zonation, mesophotic, Symbiodinium
2. Introduction
Tropical scleractinian corals have been documented across a wide bathymetric range (0 to more than 100 m) within the photic zone [1], with coral species exhibiting distinct vertical distributions [2–4]. The strong environmental gradients encountered across this bathymetric range are important drivers in this zonation, particularly factors such as wave action, heterotrophic resource availability and temperature as they can differ greatly at opposite ends of the depth spectrum [4–7]. However, as scleractinian corals depend to a great extent on photosynthesis for their energy requirements due to their obligate symbiosis with dinoflagellates from the genus Symbiodinium [8], the exponential decrease in available irradiance probably plays one of the most important roles in determining the vertical distributions of coral species [4]. Coral species exhibit adaptations along this light gradient to optimize light capture by their endosymbionts, ranging from changes in gross colony morphology [9], skeletal morphology [10], to host pigment composition [11]. In addition, coral populations of the same species at opposite ends of their depth range have been found in association with different Symbiodinium types (e.g. [6,12–14]) that can be physiologically distinct and adapted to different light conditions [15,16]. Such ‘symbiont zonation’ (i.e. a bathymetric shift in associated Symbiodinium) has been observed for a range of different coral species (e.g. [6,12–14]), and probably facilitates distribution of these species across a broad environmental gradient [16]. However, the prevalence of ‘symbiont zonation’ on a reef-wide scale remains unknown, and it is unclear to what extent it underlies the vertical distributions of coral species.
Most assessments of Symbiodinium diversity over depth have been focused either on several species (reviewed in [17]), or community-wide but with low numbers of replicate samples to allow for extensive taxon sampling [18–21]. In addition, most Symbiodinium studies have been carried out over narrow depth spans, often sampling target species over a subsection of their entire depth distribution (but see [6,7,22–24]), limiting the power of detecting symbiont zonation. While depth zonation in associated Symbiodinium has been observed for coral species with both narrow [12,14] and broad [6,7] depth distributions, it remains unclear whether there is a link between the vertical distribution of the coral host and the occurrence of symbiont zonation (e.g. whether symbiont zonation is more common in corals with broader depth distributions). Similarly, endosymbiont zonation has been found in both brooding and broadcasting coral species [17], but it is uncertain whether Symbiodinium zonation is more common in either reproductive mode. Offspring from broadcasting corals generally acquire Symbiodinium from the water column (horizontal symbiont transmission), whereas most brooding corals are thought to acquire Symbiodinium directly from the maternal colony (horizontal symbiont transmission) [25,26]. This difference in endosymbiont acquisition mode could potentially lead to distinct patterns of Symbiodinium diversity over depth [27]. However, assessing links between host traits (i.e. depth distribution and reproductive mode) and patterns of Symbiodinium over depth requires extensive genotyping across both a wide number of species and a broad bathymetric range.
The diversity of Symbiodinium at mesophotic depths remains poorly explored, and as such, the extent of specialization of mesophotic endosymbiont communities remains unclear [27]. Initial studies in the Caribbean identified putative ‘deep-specialist’ Symbiodinium types in the genera Madracis [6], Montastraea [7] and Agaricia [24], pointing towards a certain level of endosymbiont specialization at mesophotic depths. By contrast, preliminary studies on the Great Barrier Reef and in Hawai'i have indicated mesophotic corals associate predominantly with Symbiodinium types commonly found in shallow water [27,28]. Nonetheless, most studies of Symbiodinium diversity have been carried out over relatively shallow depth ranges, and it may well be that specialization only occurs deeper into the mesophotic zone. In terms of coral species distributions [17,29], the upper mesophotic appears to represent a transition zone between the shallow and lower mesophotic [29,30], and a similar pattern may be present for the associated endosymbiont community. However, the extent of specialization of mesophotic Symbiodinium communities and the overlap of endosymbiont communities between shallow and mesophotic depths remains largely untested.
The wide bathymetric distribution of certain coral species, and the relatively shallow depth to which certain stressors and disturbances are limited, has led to the hypothesis that deep reefs may act as refugia and provide a source of reproduction for their shallow-water counterparts (reviewed in [17]). However, recent molecular ecological studies have demonstrated that corals with broad depth distributions do not necessarily form a single metapopulation [23,30–34], and the ability of a species to act as a source of reproduction should therefore not be based on species distribution alone (i.e. co-occurrence in shallow and deep habitats). The relative difficulties associated with the development of informative genetic markers [35] and, more recently, genotyping-by-sequencing approaches for cnidarians hosting endosymbionts still hamper assessment of host population structure on an ecosystem-wide scale (i.e. across a wide range of species), and genotyping efforts have therefore been restricted to case studies of particular species. However, several studies have found that host populations that harbour distinct Symbiodinium types over depth (e.g. Seriatopora hystrix, Madracis pharensis and Montastraea cavernosa) are genetically divergent [30–33], illustrating that the occurrence of symbiont zonation can be an indication or ‘proxy’ of underlying host genetic structuring. As such, assessment of symbiont zonation across a broad range of species (which can be assessed using a single Symbiodinium marker) may be an important first step towards a better understanding of the potential extent of host population structuring over depth and vertical connectivity of coral communities on a reef-wide scale.
Although we have a reasonable understanding of the vertical distribution of invertebrate species on coral reefs, the role of symbiont zonation underlying these distributions remains poorly understood, particularly on an ecosystem-wide scale. Here, we assess Symbiodinium diversity over a large depth range (2–60 m) and across 25 coral species to determine: (i) the overall prevalence of symbiont depth zonation and (ii) whether the occurrence of symbiont zonation is linked to reproductive mode and/or depth distribution of the host. Symbiodinium identity was established for 16 broadcasting and nine brooding coral species across depth intervals (2, 5, 10, 25, 40, 50 and 60 m) for a total of 1636 coral colonies from the Buoy 0/1 study site in Curaçao, using ITS2/DGGE fingerprinting. Representing one of the largest genotyping efforts carried out on a single reef, this study demonstrates that coral reefs can consist of highly structured communities when taking into account both the coral host and its associated Symbiodinium.
3. Material and methods
3.1. Sample collection
Coral samples were collected at the Buoy 0/1 site situated 500 m west of the CARMABI Institute, Curaçao, Southern Caribbean (12°07′31 N, 68°58′27 W) (figure 1). The study site is characterized by a 50–100 m wide shallow terrace that gently slopes down to 8–12 m, followed by a fore reef slope that shows an abrupt descent down to a 50–60 m deep terrace (figure 1a). Colonies were sampled from 25 coral species at this study site, with the majority of samples being collected during the following sampling periods: July–August 2008, November 2009 and the ‘Catlin Seaview Survey’ expedition in March–April 2013. We also included samples from two previously published datasets of the genus Madracis (n=307; collected between 2004 and 2006) [6,16,32] and Agaricia (n=332; collected between 2004 and 2009) [24] that were obtained at the exact same study site. Care was taken to avoid resampling of colonies in different sampling years. Small coral fragments (approx. 3 cm2) were collected by hammer and chisel at the following depth intervals: 2 m (±1 m), 5 m (±1 m), 10 m (±1 m), 25 m (±2 m), 40 m (±2 m), 50 (±2 m) and 60 m (±2 m) for each species that could be found at that depth. Species identifications were done in situ, and later reconfirmed in the laboratory before processing. Coral tissue samples were stored in ethanol or a salt-saturated 20% DMSO/0.5 M EDTA solution at −20°C. Total DNA was extracted from the tissue using a Qiagen Plant Mini Kit, MoBio Ultra Clean Soil DNA Kit (following the manufacturer's instructions), or a slightly modified method used for black tiger shrimp [23,36].
Figure 1.
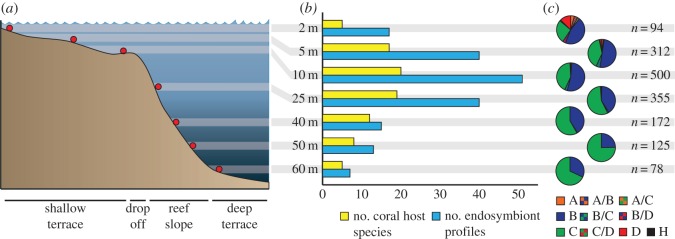
(a) Bathymetric profile at sampling location (Buoy 0/1, Curaçao), (b) number of coral host species and associated Symbiodinium profiles observed across the sampled depth range (2–60 m) and (c) prevalence of clades and clade combinations over depth in the Symbiodinium profiles of sampled specimens (with numbers of sampled colonies indicated).
3.2. Genetic characterization of associated algal endosymbionts (Symbiodinium)
The internal transcribed spacer 2 (ITS2) region of the rDNA for Symbiodinium was amplified for all samples (n=1636) using Symbiodinium-specific primers ([18]) as described in Bongaerts et al. [31]. To identify the dominant Symbiodinium types in each sample, the amplified ITS2 fragments were separated using denaturing gradient gel electrophoresis (DGGE) on a CBScientific System following conditions outlined in Sampayo et al. [14]. Running conditions varied for the Madracis samples (i.e. 30–70% gradient on Bio-Rad DCode system) included from Frade et al. [6], however representative newly acquired Madracis samples were run on both systems to allow for cross-comparison between DGGE profiles. Representative, dominant bands of each characteristic profile were excised (usually from several replicate profiles), eluted overnight in dH2O, re-amplified and purified (using ExoSAP-IT) prior to sequencing. The re-amplified PCR products were sequenced in both the forward and reverse directions (ABI BigDye Terminator chemistry, Australian Genome Research Facility). Chromatograms were analysed using CodonCode aligner with sequences being aligned with MUSCLE and blasted on GenBank (http://www.ncbi.nlm.nih.gov/BLAST). A haplotype network was created using TCS 1.21 [37], treating gaps as a fifth character state. Network analyses were performed separately for Symbiodinium clade B and C because of their high level of divergence. Coral–Symbiodinium associations were compared to previously described associations using the GeoSymbio [38] and SD2-GED [39] databases.
The co-dominant sequences in a given profile may represent either intragenomic variants and/or a mix of distinct Symbiodinium types, however as this inference can sometimes be subjective we named profiles in this study by their co-dominant ITS2 ‘sequences’ (e.g. C3/C3b/C3nN1) rather than symbiont ‘types’ (which can consist of multiple sequences). Known Symbiodinium sequences are indicated by their ITS2 sequence name sensu LaJeunesse (e.g. C3d), novel sequences are identified by a nomenclature that specifies the known sequence to which they are most related, followed by a capital N (indicating novel sequence) and an arbitrary number (e.g. C3b.N1) and incomplete sequences (i.e. shorter length) are identified by a nomenclature that specifies the known sequence to which they are most related (based on their partial sequence) followed by a capital U (indicating full sequence remains ‘unknown’) and an arbitrary number (e.g. C3.U1).
3.3. Mitochondrial DNA sequence analyses of coral colonies
The coral host mitochondrial nad5 non-coding region was amplified using primers F18 (5′-GTCCTTACGTCTTTACACCGAC-3′) and R17 (5′-AAAGACCACTCTAAAGCCCGCT-3′) for randomly picked samples from the shallowest and deepest collection depth of each of the coral species in this study (n=90) to check for potential cryptic diversity across the sampled bathymetric range (as done for Madracis spp. previously by Frade et al. [32]). PCR amplifications were performed using conditions described for the atp6 gene in Bongaerts et al. [24], and products were purified, sequenced, aligned and analysed as specified for the algal endosymbionts (Symbiodinium). Phylogenetic analyses of sequences were performed using maximum likelihood in MEGA 6 [40] under the delayed transition setting and calculation of bootstrap support values based on 1000 replicates. The best-fit model of molecular evolution was selected by hierarchical Akaike information criterion (AIC) using MEGA 6 [40] with a HKY + G model best describing the nad5 data under a log likelihood optimality criterion.
3.4. Statistical analyses
For host species associating with more than one Symbiodinium profile, an (nested) analysis of similarity (ANOSIM) was carried out using a Sorensen resemblance measure in the software package PRIMER v. 6 to test for differences between sampling years (two-way ANOSIM with depth nested within year) and depths (one-way ANOSIM comparing individual depth groups). Data from depths where less than 10 coral colonies were sampled were not included in the ANOSIM. Fisher's exact tests (two-tailed P) were used to investigate the relation between reproductive mode and the occurrence of symbiont zonation, and the relation between reproductive mode and the level of polymorphism observed in symbiont zonation (two to three profiles or more than three profiles) using numbers of species as values. A Mann–Whitney U-test was used to assess differences in Symbiodinium diversity between broadcasting and brooding species. The effect of symbiont zonation and reproductive mode on the depth distribution of the host (determined as the difference between the shallowest and deepest sampling depth) was assessed using two-way ANOVA, with data log(x + 10) transformed to meet the assumptions of normality and homoscedasticity.
4. Results
4.1. Symbiodinium diversity and host–symbiont associations
Symbiodinium community profiles were assessed for 1636 coral colonies originating from a single reef, of which 375 originated from mesophotic depths (40–60 m). In total, 93 Symbiodinium DGGE profiles were distinguished across the 25 studied coral species (figure 1b), based on a total of 120 diagnostic bands. A large amount of profiles only consisted of a single dominant band (41%), however others consisted of either multiple bands from the same Symbiodinium clade (24%) or multiple bands belonging to different Symbiodinium clades (35%) (figure 2). These diagnostic bands represented 22 known Symbiodinium sequences and 39 novel Symbiodinium sequences (electronic supplementary material, figure S1) (GenBank accession nos. KP178779–KP178809, KF551185–KF551192), with 22 bands yielding incomplete sequences (after multiple sequencing attempts). Most obtained Symbiodinium sequences belonged to either clade C (61%) or clade B (32%), with some belonging to the A, D and H clades which were found exclusively in shallow habitats (less than 3% each) (figure 1c).
Figure 2.
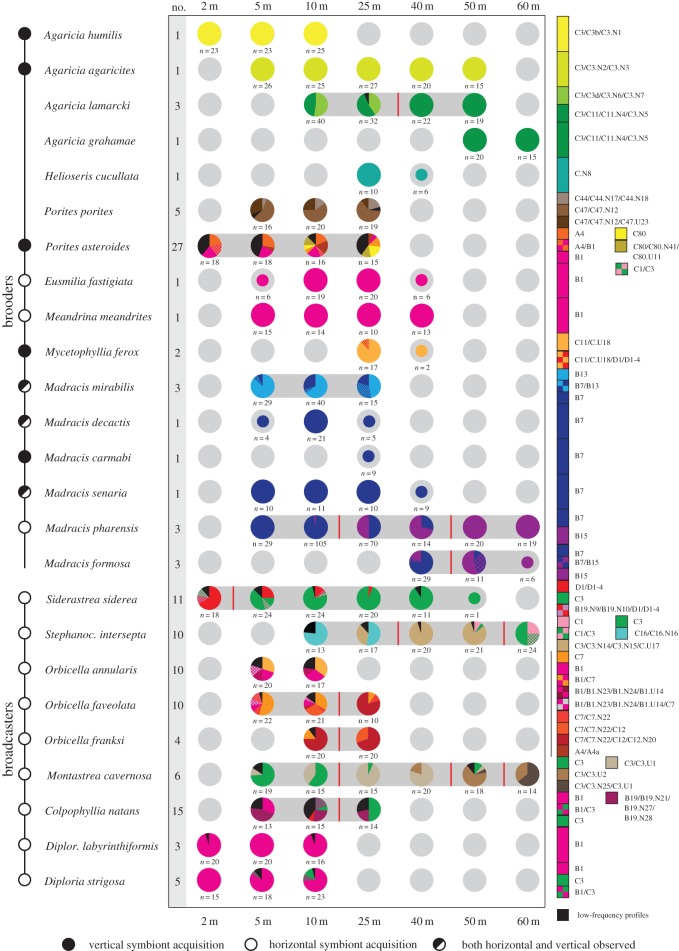
Relative abundances of Symbiodinium ITS2 profiles over depth (2–60 m) for 25 scleractinian species on the Buoy 0/1 study site in Curaçao. Pie graphs joined by grey shading indicate significant changes in Symbiodinium community over depth (ANOSIM), and red lines indicate ‘break points’ where the Symbiodinium community is significantly different at either side of the line. Smaller pie graphs indicate sampling depths where less than 10 colonies were found/sampled (not included in ANOSIM). Coloured circles in front of the species names indicate whether endosymbionts are reportedly acquired vertically, horizontally, facultatively or whether acquisition mode remains unknown (based on [26,34] and P.R.F. & P.B. 2004, personal observations for Madracis). Numbers next to species indicate Symbiodinium profile diversity (i.e. number of observed profiles).
Several of the observed Symbiodinium ITS2 sequences in this study have been observed previously for the same species in other Caribbean locations, such as B7 and B13 in Madracis mirabilis, B7 in Madracis pharensis, D1a in Colpophyllia natans, C3 in Diploria strigosa and C12 in Orbicella faveolata [38,39]. However, for many species we observed sequences (in both shallow and deep water) that have not been previously identified in association with those species, ranging from closely related variants (e.g. C16.N16 in Stephanocoenia intersepta) to distinct clades (e.g. D1a in Mycetophyllia ferox) (figure 2). Coral species with a brooding reproductive mode harboured significantly lower numbers of Symbiodinium profiles than species with a broadcasting reproductive mode (Mann–Whitney U-test, U=13, Z=−3.4, p<0.001). An exception was Porites astreoides that hosted a large number of Symbiodinium profiles, containing many novel ITS2 sequences. Broadcast-spawning species harboured higher symbiont diversity and many ITS2 sequence variants were identified that were quite divergent from types previously described for those species (e.g. C7 and C16 in Siderastrea siderea) (figure 2). Additionally, broadcasting species frequently harboured symbionts from at least two different clades, whereas this was uncommon in brooding species.
4.2. Coral host identity
We sequenced the nad5 region for a subset of individuals (n=90) as an initial verification to assess whether shallow and deep populations of collected coral species indeed represent the same ‘species’ (i.e. are not genetically divergent for a conserved DNA region). Lengths of the sequenced nad5 region varied between 621 and 911 bp depending on whether the coral species belonged to, respectively, the robust or complex phylogenetic group, and sequences were aligned separately based on these groupings (GenBank accession nos. KP178822–KP178861, KP178862–KP178900). Most species (15 out of 19) consisted of a single haplotype per species (with no difference between shallow and deep individuals), and the three species consisting of multiple haplotypes (C. natans, S. intersepta and Agaricia lamarcki) showed no clear partitioning between shallow and deep individuals (i.e. haplotypes were shared) (electronic supplementary material, figure S2). The nad5 marker was too conserved to distinguish between Faviidae (e.g. Colpophyllia and Diploria) and members of the Orbicella species complex (O. annularis, O. faveolata and O. franksi). Divergence between Madracis species was assessed in a previous study 32 that reported genetic divergence between shallow and deep populations of M. pharensis at this sampling location (based on the nad5 marker and two nuclear markers).
4.3. Prevalence of symbiont zonation
Depth distributions of individual coral species varied, with absence of Symbiodinium data for a species at a particular sampling depth meaning that the species was not observed at that depth during the more than 75 collection dives (conducted to collect all the specimens) on the study site (figure 2). Eleven species with varying depth distributions (Δ depth = 10–55 m) exhibited significant differences in Symbiodinium community between sampling depths (i.e. symbiont zonation) (figure 2; electronic supplementary material, table S1). In four cases this consisted of a shift between only two and three Symbiodinium profiles (A. lamarcki, M. mirabilis, M. pharensis and M. formosa), and in the other cases consisted of a more complex shift (i.e. involving more than three Symbiodinium profiles). By contrast, eight species associated with a single discernable Symbiodinium profile across their entire depth distribution (Δ depth = 0–45 m) and five species associated with several different Symbiodinium profiles but with no significant differences between depth groups (figure 2). One species (Madracis carmabi) was only found at one sampling depth, but hosted a singled Symbiodinium profile. No significant differences were found between sample groups collected during different fieldwork periods (two-way ANOSIM with depth nested within year) (electronic supplementary material, table S1).
Four out of the five species with the largest depth distributions (Δ depth = 40–55 m; M. pharensis, S. siderea, S. intersepta and M. cavernosa) exhibited significant symbiont zonation (with Agaricia agaricites being the exception), whereas only one of the nine species (O. franksi) with the smallest depth distributions (Δ depth ≤ 15 m) exhibited symbiont zonation (figure 2). With regards to reproductive strategy, only five out of 16 brooding coral species exhibited symbiont zonation, while for broadcast-spawning species more than half did (six out of nine), however there was no significant association (Fisher's exact test, p>0.05) between reproductive strategy and the occurrence of Symbiodinium zonation. Symbiont zonation in all broadcasting coral species was highly polymorphic (i.e. involved more than three Symbiodinium profiles), whereas in all brooding species the zonation was ‘simple’ (i.e. involved two to three Symbiodinium profiles), with the exception of P. astreoides, and this association was significant (Fisher's exact test, p<0.05). Species that exhibited symbiont zonation had significantly larger depth distributions (two-way ANOVA, F1,21=10.0, p<0.005), whereas there was no significant effect of reproductive mode or interaction between reproductive mode and symbiont zonation (figure 3) (electronic supplementary material, table S2).
Figure 3.
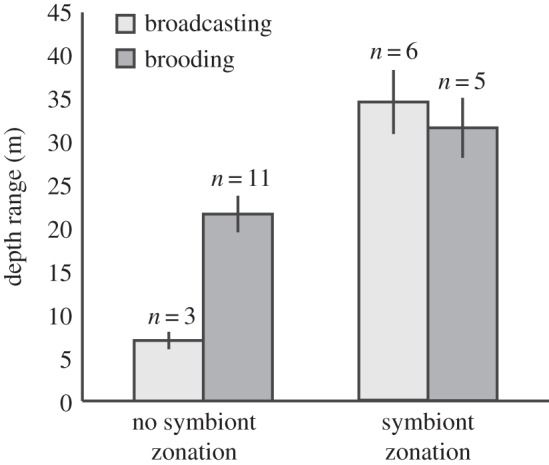
Mean depth distribution ranges for broadcasting and brooding species with and without symbiont zonation. Error bars indicate s.e.m.
4.4. Mesophotic Symbiodinium diversity
Compared with shallow depths (5–25 m), the diversity of scleractinian corals and associated Symbiodinium diversity (both in terms of absolute numbers and numbers of profiles per species) at mesophotic depths (40–60 m) was substantially lower (figure 1b). Depth-generalist species that occurred down to 40–50 m depth mostly harboured the same Symbiodinium profile as in shallower water (10–25 m), however depth-generalist species that extend down to 60 m depth (i.e. S. intersepta, M. cavernosa and M. pharensis) all exhibited distinct Symbiodinium profiles at their deeper extremes (figure 2). Nonetheless, the common symbiont profiles associated with S. intersepta at 60 m represent Symbiodinium types (C1 and C3) that commonly occur on shallow reefs (but not in this species at the study location). Depth-‘breakpoints’ were assessed by evaluating pairwise differences between depths for each species (electronic supplementary material, table S1), with ‘breakpoints’ indicating where Symbiodinium communities from all depths above and below are significantly different from each other (e.g. for A. lamarcki the ‘breakpoint’ is located between 25 and 40 m, as the Symbiodium community at 10 m=25 m≠40 m=50 m). The depth at which these ‘breakpoints’ occurred varied between species, however they were most frequently observed between 10 and 25 m (n=6), with only one breakpoint observed at shallower depths (i.e. between 2 and 5 m). The remainder occurred at the intersection of the mesophotic zone (between 25 and 40 m; n=2) or within the mesophotic zone (between 40 and 50 m or 50 and 60 m; n=5).
5. Discussion
Depth zonation in associated Symbiodinium has been demonstrated for individual scleractinian coral species and genera, with populations hosting distinct endosymbionts at different ends of the depth spectrum (e.g. [12,13,16,41,42]). Here, we demonstrate through extensive genotyping that such symbiont zonation is in fact common among scleractinian corals, with nearly half of the studied species showing significant changes in their Symbiodinium community over depth (figure 2). In addition, we found that species exhibiting symbiont zonation have significantly wider depth distributions, which is particularly apparent in broadcasting species (figure 3). We conclude that endosymbiont zonation plays an important role in determining the bathymetric distribution of Caribbean corals, with its overall prevalence leading to highly structured coral host–endosymbiont communities over depth.
Overall symbiont diversity was found to be high, corroborating previous studies in other Caribbean regions [18,21], with only a quarter of the obtained sequences representing known types that have been observed in other parts of the Caribbean. It also corroborates the dominance of clade B and C in this region, with the occurrence of other clades (A, D and H) being restricted almost exclusively to shallow water (10 m or less) [18,21] (figure 1c). The lower Symbiodinium diversity at mesophotic depths (40–60 m) is in line with previous observations of decreasing diversity over smaller depth ranges in the Caribbean [18,21]. Many new host–Symbiodinium associations were observed, with most coral species harbouring profiles containing Symbiodinium sequences (ranging from closely related variants to distinct clades) that have not been previously identified in association with those species (figure 2). Observing novel host–Symbiodinium associations is common when surveying new geographical regions [43], mesophotic depths [27] or when increasing sample sizes [14], and demonstrates the importance of expanded surveying to broaden our understanding of specificity and diversity in the coral–algal symbiosis. The many novel associations (and ITS2 sequence types) observed here highlight the extensive diversity underlying the geographical and ecological distribution of Caribbean corals.
Symbiont zonation was observed in four out of five coral species with the widest depth distributions, indicating that a change in Symbiodinium over depth is a common trait associated with ‘depth-generalist’ coral species. The association with different endosymbionts over depth probably enables these species to thrive under the different environmental conditions encountered along their broad depth distribution ranges [16], through adaptation to particular light [15], temperature [44,45] or potentially other environmental conditions such as light spectrum or nutrient availability [16]. Nonetheless, the fact that Agaricia agaricites associated with a single Symbiodinium profile along its entire bathymetric distribution (5–50 m) (figure 2) demonstrates that symbiont zonation is not an obligate strategy for depth-generalist species [24]. A similar lack of symbiont zonation has been observed for the Indo-Pacific coral species Pachyseris speciosa, which hosted predominantly a single Symbiodinium profile despite its broad depth distribution in northwest Australia (10–60 m) [22]. While the observed patterns point towards symbiont zonation being more common in species with large depth distributions, symbiont zonation was also observed over very small depth ranges (e.g. in Orbicella franski: Δ depth = 15 m), which is not entirely surprising as certain environmental gradients (e.g. incident irradiance, ultraviolet light and wave action) are most pronounced across shallow depth ranges. Given the inability of ITS2-DGGE to identify Symbiodinium at background levels [46] and the fact that only dominant bands are used for profile assignment, assessment of changes in associated Symbiodinium diversity over depth remains conservative as it ignores subtle changes in relative Symbiodinium abundances. As such, it could be that the frequency of symbiont zonation remains underestimated, however this would not affect the conclusion that Symbiodinium zonation is common on a reef-wide scale.
Clear differences were observed between reproductive strategies in the diversity of associated Symbiodinium, with broadcasters always associating with multiple Symbiodinium profiles (sometimes representing different clades) and with patterns of symbiont zonation always involving at least four (but often more) different Symbiodinium profiles. By contrast, brooders often associated with a single Symbiodinium profile, and when exhibiting symbiont zonation this usually only involved two to three distinct profiles representing a single clade (with P. astreoides as exception). At first, these differences seem to be associated with the different symbiont acquisition modes (respectively, horizontal and vertical) commonly associated with broadcasting and brooding species [25]. Indeed, the broadcasting species studied here reportedly acquire their Symbiodinium horizontally [34,47] (figure 2), and the higher Symbiodinium diversity and prevalence of symbiont zonation may be facilitated by the ‘open nature’ of the coral–algal symbiosis representative of this acquisition mode [48–50]. The many novel associations that were observed for these species may be a further consequence of this potential ‘flexibility’, and highlights the potential role this acquisition mode has played in the ecological and geographical range extensions of these coral species. By contrast, many horizontally transmitting species in the Indo-Pacific appear to associate with relatively few Symbiodinium types [20,51,52], which in part may be a consequence of the overall lower Symbiodinium diversity in this region compared with the Caribbean [19].
Brooding species appear to exhibit a variety of Symbiodinium acquisition strategies, including vertical (e.g. Agaricia humilis), horizontal (e.g. Eusmilia fastigiata) and facultative symbiont transmission (e.g. Madracis mirabilis) [47] (figure 2). In addition, a recent study reports that Symbiodinium can sometimes be acquired through both horizontal and vertical symbiont acquisition (e.g. Stylophora pistillata [50]). Thus, while the transmission mode remains unclear for some of the brooding species, the observed pattern is that of low diversity on a localized scale, in line with the relatively ‘closed nature’ of a vertical symbiont acquisition mode. In a vertical symbiont acquisition mode, different Symbiodinium associations are thought to arise through (co-)evolutionary processes and/or (relatively rare) host–symbiont recombination events [24,51] that can become fixed through ecological selection or geographical isolation. Such processes are probably responsible for the novel associations observed for these brooding species, and more broadly for geographical differences in Symbiodinium diversity of brooding species, rather than merely reflecting symbiotic ‘flexibility’. Similarly, on the Great Barrier Reef, where initially diverse associations where described for several vertically transmitting pocilloporid species (e.g. [14,52]), it later became apparent that these ‘species’ in fact comprise multiple lineages/species with specific endosymbiont associations (e.g. [31,53]). Nonetheless, while Symbiodinium diversity associated with individual brooding species is often low on a localized scale, the overall diversity can in fact be high due to biogeographic differences, with higher levels of diversity compared with horizontally transmitting species [54]. The brooding species Porites astreoides represents an exception with its extremely high local Symbiodinium diversity and indicates that Symbiodinium acquisition in this brooding species may not be limited to only maternal acquisition [55], and potentially represents a more ‘open’ symbiosis.
The associated endosymbionts at the lower reaches of this study (50–60 m) appear to represent a specialized community, consisting mostly of Symbiodinium that are not observed in shallow water (except, for example, those associated with A. agaricites) (figure 2), which is in contrast with preliminary observations from the Indo-Pacific region [27,28]. For some species (e.g. Stephanocoenia intersepta and Montastraea cavernosa), the symbiont community was distinct even between different depths within the mesophotic zone, which has been observed previously for M. cavernosa in the Bahamas [7,33]. Interestingly, the deepest S. intersepta populations (60 m) associated at our study site with the generalist types Symbiodinium C1 and/or C3 that are commonly found in shallow water [56]. The presence of these Symbiodinium at mesophotic depths could be interpreted as a lack of a specialized endosymbiont community, however the absence of these types within the shallow S. intersepta populations at our study site points towards (local) specialization of this host–Symbiodinium association to mesophotic conditions (60 m). As such, ecological implications of ‘depth-specialization’ should therefore be assessed by looking at the combination of symbiotic partners and on a localized scale (i.e. by comparing shallow versus deep assemblages at a single location). Alternatively, it may be that these Symbiodinium are physiologically distinct through recent adaptation to different environmental conditions, as adaptive variation has been demonstrated in Symbiodinium C1 previously [57]. At upper mesophotic depths (40 m) of the study site, most of the dominant Symbiodinium profiles can be found also at shallower depths for the same species. This indicates that there is substantial overlap in Symbiodinium associations between the upper reaches of the mesophotic zone and intermediate/shallow depths for depth-generalist coral species, and reinforces the idea of the upper mesophotic as a transition zone between the shallow and lower mesophotic reef [29,30].
Symbiont zonation has been found to be associated with genetic structuring of the coral host in several brooding and a broadcasting species [7,31–34], and as such can be indicative of underlying host genetic differentiation. The patterns of Symbiodinium observed here on a reef-wide scale might therefore be reflective of highly structured coral host populations over depth and genetically distinct populations at lower mesophotic depths. On the other hand, there is considerable overlap in host–Symbiodinium associations observed between shallow (10–25 m) and upper mesopotic depths (40 m), highlighting the potential connectedness of these habitats. Nonetheless, host genetic differentiation across habitats has also been observed between populations hosting the same Symbiodinium type [31,58], and conversely a lack of host differentiation has been observed for species exhibiting symbiont zonation [59]. Our initial screening of host genetic differentiation was aimed at identifying potential cryptic species diversity and did not reveal any gross genetic differences, other than the depth-divergence of Madracis pharensis that was reported in Frade et al. [32]. However, the targeted mitochondrial region (nad5) was too conserved to assess population-level genetic differentiation (and within the robust clade to assess even intrageneric differences), and the presence of host genetic differentiation underlying the Symbiodinium zonation remains to be assessed. The prevalence of Symbiodinium zonation stresses the importance of such future host-focused studies, in particular in assessing reef-wide patterns of vertical connectivity.
In conclusion, this study demonstrates the prevalence of endosymbiont zonation underlying the vertical distributions of Caribbean scleractinian corals and indicates that Symbiodinium zonation appears to play an important role in facilitating the broad depth ranges of depth-generalist species. Future studies should assess whether this pattern is consistent with other geographical regions and whether endosymbiont zonation is also pervasive in other non-scleractinian photosynthetic marine invertebrates. Overall, we clearly need a better understanding of the ecological and evolutionary interactions between coral hosts and their endosymbionts, through high-resolution genetic studies of both symbiotic partners, to further elucidate reef-wide patterns of host–Symbiodinium structuring and the interconnectedness of habitats on coral reefs.
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank Mark Vermeij, Norbert Englebert, Carlos Winterdaal, Judith Bakker and Joshua Boldt for logistical support.
Data accessibility
Sequence data have been deposited in GenBank under the following accession nos. KP178779–KP178809, KP178822–KP178900.
Funding statement
The study was conducted as part of the ‘Catlin Seaview Survey’ funded by Catlin Group Limited, with support from the Australian Research Council (ARC) Centre of Excellence for Coral Reef Studies and the Global Change Institute.
References
- 1.Kahng SE, Garcia-Sais JR, Spalding HL, Brokovich E, Wagner D, Weil E, Hinderstein L, Toonen RJ. 2010. Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29, 255–275. (doi:10.1007/s00338-010-0593-6) [Google Scholar]
- 2.Goreau TF, Wells JW. 1967. The shallow-water Scleractinia of Jamaica: revised list of species and their vertical distribution range. Bull. Mar. Sci. 17, 442–453. [Google Scholar]
- 3.Sheppard C. 1982. Coral populations on reef slopes and their major controls. Mar. Ecol. Prog. Ser. 7, 83–115. (doi:10.3354/meps007083) [Google Scholar]
- 4.Veron JEN, Stafford-Smith M. 2000. Corals of the world. Townsville, Australia: Australian Institute of Marine Science. [Google Scholar]
- 5.Dollar SJ. 1982. Wave stress and coral community structure in Hawaii. Coral Reefs 1, 71–81. (doi:10.1007/BF00301688) [Google Scholar]
- 6.Frade PR, De Jongh F, Vermeulen F, van Bleijswijk J, Bak RPM. 2008. Variation in symbiont distribution between closely related coral species over large depth ranges. Mol. Ecol. 17, 691–703. (doi:10.1111/j.1365-294X.2007.03612.x) [DOI] [PubMed] [Google Scholar]
- 7.Lesser MP, Slattery M, Stat M, Ojimi M, Gates RD, Grottoli A. 2010. Photoacclimatization by the coral Montastraea cavernosa in the mesophotic zone: light, food, and genetics. Ecology 91, 990–1003. (doi:10.1890/09-0313.1) [DOI] [PubMed] [Google Scholar]
- 8.Trench RK. 1993. Microalgal-invertebrate symbioses—a review. Endocytobios. Cell Res. 9, 135–175. [Google Scholar]
- 9.Titlyanov EA. 1987. Structure and morphological differences of colonies of reef-building branched corals from habitats with different light conditions. Mar. Biol. 1, 32–36. [Google Scholar]
- 10.Enríquez S, Méndez ER, Iglesias-Prieto R. 2005. Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol. Oceanogr. 50, 1025–1032. (doi:10.4319/lo.2005.50.4.1025) [Google Scholar]
- 11.Salih A, Larkum A, Cox G, Kühl M, Hoegh-Guldberg O. 2000. Fluorescent pigments in corals are photoprotective. Nature 408, 850–853. (doi:10.1038/35048564) [DOI] [PubMed] [Google Scholar]
- 12.Rowan R, Knowlton N. 1995. Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc. Natl Acad. Sci. USA 92, 2850–2853. (doi:10.1073/pnas.92.7.2850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warner ME, LaJeunesse TC, Robison JD, Thur RM. 2006. The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnol. Oceanogr. 51, 1887–1897. (doi:10.4319/lo.2006.51.4.1887) [Google Scholar]
- 14.Sampayo EM, Franceschinis L, Hoegh-Guldberg O, Dove S. 2007. Niche partitioning of closely related symbiotic dinoflagellates. Mol. Ecol. 16, 3721–3733. (doi:10.1111/j.1365-294X.2007.03403.x) [DOI] [PubMed] [Google Scholar]
- 15.Iglesias-Prieto R, Beltrán VH, LaJeunesse TC, Reyes-Bonilla H, Thomé PE. 2004. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc. R. Soc. Lond. B 271, 1757–1763. (doi:10.1098/rspb.2004.2757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frade PR, Bongaerts P, Winkelhagen AJS, Tonk L, Bak RPM. 2008. In situ photobiology of corals over large depth ranges: a multivariate analysis on the roles of environment, host, and algal symbiont. Limnol. Oceanogr. 53, 2711 (doi:10.4319/lo.2008.53.6.2711) [Google Scholar]
- 17.Bongaerts P, Ridgway T, Sampayo EM, Hoegh-Guldberg O. 2010. Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 29, 309–327. (doi:10.1007/s00338-009-0581-x) [Google Scholar]
- 18.LaJeunesse T. 2002. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar. Biol. 141, 387–400. (doi:10.1007/s00227-002-0829-2) [Google Scholar]
- 19.LaJeunesse TC, Loh WK, van Woesik R, Hoegh-Guldberg O, Schmidt GW, Fitt WK. 2003. Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol. Oceanogr. 48, 2046–2054. (doi:10.4319/lo.2003.48.5.2046) [Google Scholar]
- 20.LaJeunesse TC, Pettay DT, Sampayo EM, Phongsuwan N, Brown B, Obura DO, Hoegh-Guldberg O, Fitt WK. 2010. Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J. Biogeogr. 37, 785–800. (doi:10.1111/j.1365-2699.2010.02273.x) [Google Scholar]
- 21.Finney JC, Pettay DT, Sampayo EM, Warner ME, Oxenford HA, LaJeunesse TC. 2010. The relative significance of host-habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microb. Ecol. 60, 250–263. (doi:10.1007/s00248-010-9681-y) [DOI] [PubMed] [Google Scholar]
- 22.Cooper TF, Ulstrup KE, Dandan SS, Heyward AJ, Kühl M, Muirhead A, O'Leary RA, Ziersen BE, Van Oppen MJ. 2011. Niche specialization of reef-building corals in the mesophotic zone: metabolic trade-offs between divergent Symbiodinium types. Proc. R. Soc. B 278, 1840–1850. (doi:10.1098/rspb.2010.2321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Oppen MJ, Bongaerts P, Underwood JN, Peplow LM, Cooper TF. 2011. The role of deep reefs in shallow reef recovery: an assessment of vertical connectivity in a brooding coral from west and east Australia. Mol. Ecol. 20, 1647–1660. (doi:10.1111/j.1365-294X.2011.05050.x) [DOI] [PubMed] [Google Scholar]
- 24.Bongaerts P, et al. 2013. Sharing the slope: depth partitioning of agariciid corals and associated Symbiodinium across shallow and mesophotic habitats (2–60 m) on a Caribbean reef. BMC Evol. Biol. 13, 205 (doi:10.1186/1471-2148-13-205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richmond RH, Hunter CL. 1990. Reproduction and recruitment of corals: comparisons among the Caribbean, the tropical Pacific, and the Red Sea. Mar. Ecol. Prog. Ser. 60, 185–203. (doi:10.3354/meps060185) [Google Scholar]
- 26.Baird AH, Guest JR, Willis BL. 2009. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol. Evol. Syst. 40, 551–571. (doi:10.1146/annurev.ecolsys.110308.120220) [Google Scholar]
- 27.Bongaerts P, Sampayo EM, Bridge TC, Ridgway T, Vermeulen F, Englebert N, Webster JM, Hoegh-Guldberg O. 2011. Symbiodinium diversity in mesophotic coral communities on the Great Barrier Reef: a first assessment. Mar. Ecol. Prog. Ser. 439, 117–126. (doi:10.3354/meps09315) [Google Scholar]
- 28.Chan YL, Pochon X, Fisher MA, Wagner D, Concepcion GT, Kahng SE, Toonen RJ, Gates RD. 2009. Generalist dinoflagellate endosymbionts and host genotype diversity detected from mesophotic (67–100m depths) coral Leptoseris. BMC Ecol. 9, 21 (doi:10.1186/1472-6785-9-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahng E, Copus M, Wagner D. 2014. Recent advances in the ecology of mesophotic coral ecosystems (MCEs). Curr. Opin. Environ. Sustain. 7, 72–81. (doi:10.1016/j.cosust.2013.11.019) [Google Scholar]
- 30.Slattery M, Lesser MP, Brazeau D, Stokes MD, Leichter JJ. 2011. Connectivity and stability of mesophotic coral reefs. J. Exp. Mar. Biol. Ecol. 408, 32–41. (doi:10.1016/j.jembe.2011.07.024) [Google Scholar]
- 31.Bongaerts P, Riginos C, Ridgway T, Sampayo EM, van Oppen MJ, Englebert N, Vermeulen F, Hoegh-Guldberg O. 2010. Genetic divergence across habitats in the widespread coral Seriatopora hystrix and its associated Symbiodinium. PLoS ONE 5, e10871 (doi:10.1371/journal.pone.0010871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frade PR, Reyes-Nivia MC, Faria J, Kaandorp JA, Luttikhuizen PC, Bak RP. 2010. Semi-permeable species boundaries in the coral genus Madracis: introgression in a brooding coral system. Mol. Phylogenet. Evol. 57, 1072–1090. (doi:10.1016/j.ympev.2010.09.010) [DOI] [PubMed] [Google Scholar]
- 33.Brazeau DA, Lesser MP, Slattery M. 2013. Genetic structure in the coral, Montastraea cavernosa: assessing genetic differentiation among and within mesophotic reefs. PLoS ONE 8, e65845 (doi:10.1371/journal.pone.0065845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serrano X, Baums IB, O'Reilly K, Smith TB, Jones RJ, Shearer TL, Nunes FLD, Baker AC. 2014. Geographic differences in vertical connectivity in the Caribbean coral Montastraea cavernosa despite high levels of horizontal connectivity at shallow depths. Mol. Ecol. 23, 4226–4240. (doi:10.1111/mec.12861) [DOI] [PubMed] [Google Scholar]
- 35.Shearer TL, Gutiérrez-Rodríguez C, Coffroth MA. 2005. Generating molecular markers from zooxanthellate cnidarians. Coral Reefs 24, 57–66. (doi:10.1007/s00338-004-0442-6) [Google Scholar]
- 36.Wilson K, et al. 2002. Genetic mapping of the black tiger shrimp Penaeus monodon with amplified fragment length polymorphism. Aquaculture 204, 297–309. (doi:10.1016/S0044-8486(01)00842-0) [Google Scholar]
- 37.Clement M, Posada D, Crandall KA. 2000. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 9, 1657–1659. (doi:10.1046/j.1365-294x.2000.01020.x) [DOI] [PubMed] [Google Scholar]
- 38.Franklin EC, Stat M, Pochon X, Putnam HM, Gates RD. 2012. GeoSymbio: a hybrid, cloud-based web application of global geospatial bioinformatics and ecoinformatics for Symbiodinium–host symbioses. Mol. Ecol. Res. 12, 369–373. (doi:10.1111/j.1755-0998.2011.03081.x) [DOI] [PubMed] [Google Scholar]
- 39.Santos RS, LaJeunesse TC. 2006. Searchable database of symbiodinium diversity–geographic and ecological diversity (SD2-GED). Auburn, AL: Auburn University; See www.auburn.edu/~santosr/sd2_ged.htm. [Google Scholar]
- 40.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. (doi:10.1093/molbev/mst197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker AC, Rowan R, Knowlton N. 1997. Symbiosis ecology of two Caribbean acroporid corals. In Proc. 8th Int. Coral Reef Symp. 2, 1295–1300. [Google Scholar]
- 42.Toller WW, Rowan R, Knowlton N. 2001. Zooxanthellae of the Montastraea annularis species complex: patterns of distribution of four taxa of Symbiodinium on different reefs and across depths. Biol. Bull. 201, 348–359. (doi:10.2307/1543613) [DOI] [PubMed] [Google Scholar]
- 43.Tonk L, Sampayo EM, LaJeunesse TC, Schrameyer V, Hoegh-Guldberg O. 2014. Symbiodinium (Dinophyceae) diversity in reef-invertebrates along an offshore to inshore reef gradient near lizard island, great barrier reef. J. Phycol. 50, 552–563. (doi:10.1111/jpy.12185) [DOI] [PubMed] [Google Scholar]
- 44.Rowan R. 2004. Coral bleaching: thermal adaptation in reef coral symbionts. Nature 430, 742 (doi:10.1038/430742a) [DOI] [PubMed] [Google Scholar]
- 45.Tchernov D, Gorbunov MY, de Vargas C, Narayan Yadav S, Milligan AJ, Häggblom M, Falkowski PG. 2004. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl Acad. Sci. USA 101, 13531–13535. (doi:10.1073/pnas.0402907101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornhill DJ, LaJeunesse TC, Kemp DW, Fitt WK, Schmidt GW. 2006. Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar. Biol. 148, 711–722. (doi:10.1007/s00227-005-0114-2) [Google Scholar]
- 47.Willis BL, van Oppen MJ, Miller DJ, Vollmer SV, Ayre DJ. 2006. The role of hybridization in the evolution of reef corals. Annu. Rev. Ecol. Evol. Syst. 37, 489–517. (doi:10.1146/annurev.ecolsys.37.091305.110136) [Google Scholar]
- 48.Loh WKW, Loi T, Carter D, Hoegh-Guldberg O. 2001. Genetic variability of the symbiotic dinoflagellates from the wide ranging coral species Seriatopora hystrix and Acropora longicyathus in the Indo-West Pacific. Mar. Ecol. Prog. Ser. 222, 97–107. (doi:10.3354/meps222097) [Google Scholar]
- 49.LaJeunesse TC, Thornhill DJ, Cox EF, Stanton FG, Fitt WK, Schmidt GW. 2004. High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs 23, 596–603. [Google Scholar]
- 50.Byler KA, Carmi-Veal M, Fine M, Goulet TL. 2013. Multiple symbiont acquisition strategies as an adaptive mechanism in the coral Stylophora pistillata. PLoS ONE 8, e59596 (doi:10.1371/journal.pone.0059596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Oppen JH. 2004. Mode of zooxanthella transmission does not affect zooxanthella diversity in acroporid corals. Mar. Biol. 144, 1–7. (doi:10.1007/s00227-003-1187-4) [Google Scholar]
- 52.Stat M, Loh WKW, Hoegh-Guldberg O, Carter DA. 2008. Symbiont acquisition strategy drives host–symbiont associations in the southern Great Barrier Reef. Coral Reefs 27, 763–772. (doi:10.1007/s00338-008-0412-5) [Google Scholar]
- 53.Schmidt-Roach S, Miller KJ, Lundgren P, Andreakis N. 2014. With eyes wide open: a revision of species within and closely related to the Pocillopora damicornis species complex (Scleractinia; Pocilloporidae) using morphology and genetics. Zool. J. Linn. Soc. 170, 1–33. (doi:10.1111/zoj.12092) [Google Scholar]
- 54.Fabina NS, Putnam HM, Franklin EC, Stat M, Gates RD. 2012. Transmission mode predicts specificity and interaction patterns in coral–Symbiodinium networks. PLoS ONE 7, e44970 (doi:10.1371/journal.pone.0044970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chornesky EA, Peters EC. 1987. Sexual reproduction and colony growth in the scleractinian coral Porites astreoides. Biol. Bull. 172, 161–177. (doi:10.2307/1541790) [Google Scholar]
- 56.LaJeunesse TC. 2005. Sexual reproduction and colony growth in the scleractinian coral Porites astreoides. Biol. Bull. 22, 570–581. (doi:10.1093/molbev/msi042) [Google Scholar]
- 57.Howells EJ, Beltran VH, Larsen NW, Bay LK, Willis BL, Van Oppen MJH. 2012. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Change 2, 116–120. (doi:10.1038/nclimate1330) [Google Scholar]
- 58.Barshis DJ, Stillman JH, Gates RD, Toonen RJ, Smith LW, Birkeland C. 2010. Protein expression and genetic structure of the coral Porites lobata in an environmentally extreme Samoan back reef: does host genotype limit phenotypic plasticity? Mol. Ecol. 19, 1705–1720. (doi:10.1111/j.1365-294X.2010.04574.x) [DOI] [PubMed] [Google Scholar]
- 59.Serrano X. 2013. Horizontal versus vertical connectivity in Caribbean reef corals: identifying potential sources of recruitment following disturbance. PhD thesis, University of Miami, Coral Gables, FL, USA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data have been deposited in GenBank under the following accession nos. KP178779–KP178809, KP178822–KP178900.