Abstract
Objective
To determine the in vitro cytotoxicity of dental composites containing bioactive glass fillers.
Methods
Dental composites (50:50 Bis-GMA/TEGDMA resin: 72.5wt% filler, 67.5%Sr-glass and 5% OX50) containing different concentrations (0, 5, 10 and 15 wt %) of two sol-gel bioactive glasses, BAG65 (65 mole% SiO2, 31 mole% CaO, 4 mole% P2O5) and BAG62 (3 mole% F added) were evaluated for cytotoxicity using Alamar Blue assay. First, composite extracts were obtained from 7 day incubations of composite in cell culture medium at 37° C. Undifferentiated pulp cells (OD-21) were exposed to dilutions of the original extracts for 3, 5, and 7 days. Then freshly cured composite disks were incubated with OD-21 cells (n=5) for 2 days. Subsequently, fresh composite disks were incubated in culture medium at 37°C for 7 days, and then the extracted disks were incubated with OD-21 cells for 2 days. Finally, fresh composites disks were light cured for 3, 5, and 20 seconds and incubated with OD-21 cells (n=5) for 1, 3, 5, and 7 days. To verify that the three different curing modes produced different levels of degree of conversion (DC), the DC of each composite was determined by FTIR. Groups (n=5) were compared with ANOVA/Tukey’s (α≤0.05).
Results
Extracts from all composites significantly reduced cell viability until a dilution of 1:8 or lower, where the extract became equal to the control. All freshly-cured composites showed significantly reduced cell viability at two days. However, no reduction in cell viability was observed for any composite that had been previously soaked in media before exposure to the cells. Composites with reduced DC (3 s vs. 20 s cure), as verified by FTIR, showed significantly reduced cell viability.
Significance
The results show that the composites, independent of composition, had equivalent potency in terms of reducing the viability of the cells in culture. Soaking the composites for 7 days before exposing them to the cells suggested that the “toxic” components had been extracted and the materials were no longer cytotoxic. The results demonstrate that the cytotoxicity of composites with and without BAG must predominantly be attributed to the release of residual monomers, and not to the presence of the BAG.
Keywords: Cytotoxicity, Dental composites, Bioactive glass, Degree of Conversion, FTIR, Undifferentiated pulp cells (OD-21)
Introduction
The cytotoxicity of dental composite materials has been attributed to the release of residual monomers, a result of an incomplete polymerization reaction, or to the by-products of resin matrix degradation processes [1]. It has been shown that well cured resin composites are less cytotoxic than those with lower degree of conversion [2]. In fact, the elimination of leachable components from polymerized composites by the use of organic solvents completely eliminated the cytotoxicity of certain composites in one study [3].
The principal monomers used in dental resin composites are bisphenol A-glycidylmethacrylate (Bis-GMA), triethyleneglycol dimethacrylate (TEGDMA), and urethane dimethacrylate (UDMA), all of which have been identified as cytotoxic molecules [4, 5]. It has also been shown that TEGDMA may be a mutagenic compound in mammalian cells. The material induced gene mutations probably because of the covalent binding to DNA via Michael addition [6, 7]. Based on these concerns, considerable effort is being expended to develop alternative monomer systems which may have fewer negative biological consequences.
Additionally, new materials are being formulated with additives that may provide a positive biological effect, such as antimicrobial characteristics, or tooth remineralizing potential. Adhesives containing silver nanoparticles have been shown to significantly decrease bacteria compared to the adhesive alone [8]. Quaternary ammonium compounds (QACs) are a group of cationic antimicrobials which exhibit stable and long-term antibacterial effects. Imazato et al combined a QAC, with a methacrylate group to synthesize a novel dental resin monomer called MDPB (12-methacryloyloxydodecylpyridinium bromide). MDPB-containing resins can inhibit the growth of bacteria, such as S. mutans [9, 10]. In other work, it has been shown that adhesives containing S-PRG (pre-reacted glass ionomer) filler decreased demineralization of tooth structure [11]. Weir et al. found that NACP (Amorphous calcium phosphate nanoparticles) containing nanocomposites could effectively remineralize human enamel by releasing Ca and PO4 and increasing the pH [12]. While there is excitement over the potential for these potentially “bioactive” materials, there is a need to assess their biocompatibility.
Bioactive glasses (BAG) are another type of biologically active compound that have been used as a substituent of materials designed for regeneration of bone [13], orthopedic bone cements [14], dental restorative materials [15], dental cements [16] pit and fissure sealants [17], and also for treatment of hypersensitive teeth [18, 19]. The potential remineralizing effect of these materials, owing to the release of calcium and phosphate ions, has attracted much attention. The development of sol-gel processed glasses for these purposes is particularly interesting due to the high surface area attainable with the particulate material, which enhances ion release [19]. These glasses are produced with higher silica contents than typical melt derived glasses and do not contain sodium, but remain highly reactive because the processing method results in a nanoporous glass structure that increases their surface area by orders of magnitude over typical melt derived glasses. These glasses may also have antibacterial characteristics that would be beneficial when used in dental restorations where they may be placed very close to vital pulp tissues of teeth previously infected by bacteria [19, 20]. Recently, Hu et al. showed that bioactive glass 45S5 had a strong antibacterial effect against both gram-positive and gram-negative bacteria, though the sensitivity of the two different bacteria varied [21]. Waltimo et al. found that a nanometric bioactive glass 45S5 released more alkaline species due to a larger surface area, and subsequently showed a higher antimicrobial effect than melt-derived, micron-sized 45S5 bioactive glass [22].
The objective of this study was to determine the in vitro cytocompatibility of dental resin composites containing sol-gel derived bioactive glass fillers using undifferentiated dental pulp cells (OD-21).
The hypothesis to be tested was that any cytotoxicity of the composites would be attributable to the residual monomer in the cured materials, and not to the ions leached from the bioactive glass additive. To accomplish this, composites were directly exposed to cells immediately after being light cured to various extents, as well as after extraction of residual monomer in media. In addition, cells were exposed directly to the extracts from the variably cured composites.
Materials and methods
Preparation of experimental resin composites
Experimental resins were prepared with a mix of 50wt% BisGMA (bisphenol A-glycidyl-dimethacrylate) and 50wt% TEGDMA (triethylene glycol-dimethacrylate), both of which were graciously donated by ESSTECH Technology Inc., (Essington, PA, USA). To this mixture, 0.4wt% of Camphorquinone and 0.8 wt% of EdMAB (Ethyl 4-dimethyl aminobenzoate) were added as photoinitiator and accelerator, respectively (purchased from Sigma–Aldrich Inc., St. Louis, MO, USA). Chemicals were used as provided by the manufacturers.
Control dental composites were prepared by mixing the resin with 72.5 wt% of filler comprised of 5% silane-treated OX50 and 67.5% of a silane-treated strontium glass of approximately 2–3 μm average size (graciously supplied by Bisco, Inc., Schaumburg, IL, USA) in a mechanical mixer (SpeedMixer DAC 150 FVZ, Flacktek, Landrum, SC, USA) at 2300 rpm for two minutes. Experimental composites were made from the same resin and strontium glass filler, but without the OX50 and with the substitution of the strontium glass with 0, 5, 10 and 15 wt% of two sol-gel processed bioactive glasses: BAG65 (65 mole% SiO2, 31 mole% CaO, 4 mole% P2O5) and BAG62 (3 mole% F added with commensurate reduction of CaO). The BAG was synthesized as described previously [19]. Thus, seven composite formulations were produced, six containing bioactive glass (three of which contained fluoride) and one control without BAG.
Specimen and extract preparation
Composite extract group – Production of composite extracts
Disk-shaped specimens (D=5 mm, 2 mm thick) were made by packing uncured composite into a polyvinylsiloxane mold, sandwiched between two glass slides and clamped with a paper clip for 20 s to extrude the excess material. The specimens where then light cured using a dental curing unit (Demi, Kerr, Danbury, CT, USA) with a 11 mm diameter light tip and measured exitance irradiance of approximately 600 mW/cm2 (PM1 Laser Power Meter, Molectron, Portland, OR, USA). The materials were light-cured for 20 s (~12 J/cm2) from the top surface. All samples were stored dry for 24 hours and then the top surface was finished with sequential SiC sandpapers (600, 1200, 4000 grit) under water cooling. All the specimens were sterilized by UV radiations [23] for 15 min each side in cell culture hood. Extracts of these samples were prepared following ISO 10993-12. Extracts were prepared from 15 samples (each of 70.68 mm2 surface area) in 5 ml cell culture medium. After a 7 day soaking period, these original extracts were then serially diluted in cell culture medium as follows: 1:1, 1:2, 1:4, 1:8, 1:16, and 1:32 [24]. Five replicate cell cultures were exposed to serial dilutions (at least six dilutions down to 1:32) of the original extracts of the materials.
Fresh composite-direct extract group – Production of fresh-cured composites for direct exposure to cells
Disk-shaped specimens (D=9 mm, 2 mm thick, n= 5 per composite/BAG formulation) were made as described in Composite extract group, and exposed directly to cultured cells as described below.
Previously extracted composite-direct extract group – Production of previously extracted composites for direct exposure to cells
Disk-shaped specimens (D=9 mm, 2 mm thick, n= 5 per composite/BAG formulation) were made as described in Composite extract group and subjected to the same extraction protocol before the composite disks were then exposed directly to cultured cells as described below.
Reduced cure composite group – Production of composites with reduced radiant exposure for curing
Three different light curing modes were used to produce different levels of degree of conversion (DC). Specimens with and without BAG 65 and BAG 62 were prepared as in Composite extract group. The DC was evaluated by near-FTIR spectrometry (Nicolet 6700 FTIR, Thermo Scientific, Madison, WI, USA). The uncured composites were placed in silicone rubber molds (n=5; D=5 mm; 2 mm thick) sandwiched between glass slides separated by spacers to collect the FTIR spectra (32 scans per spectrum, 4 cm−1 resolution; KBr beam splitter; InGaAs detector). The specimens were then light-cured with three different curing protocols: 360 mW/cm2 for 3 s (1 J); 360 mW/cm2 for 5 s (1.8 J) and 600 mW/cm2 for 20 s (12 J). The lower irradiance (360 mW/cm2) was obtained by placing a 0.2 absorptive neutral density filter (Thorlabs Inc, Newton, NJ) inside the light curing unit. Another spectrum of all the specimens was obtained after 24 h of storage in dry conditions. The peak area corresponding to vinyl stretching (6165 cm−1) was used before and after curing to calculate DC as follows: DC = [1 – (area of peak cured/area of peak uncured)] x 100%. After determining the conversion, the specimens were weighed prior to immersion in 8 mL of methylene chloride containing 0.01 wt% BHT (2,6-di-tert-butyl-4-methylphenol, Sigma Aldrich) for 1 week. The use of BHT is intended to avoid further conversion due to the increased molecular mobility produced in solvent-swollen networks. The solvent from the extraction solutions was removed under rotary evaporation to constant mass and 1H-NMR (600 MHz, Varian, Palo Alto, CA) spectra were obtained from the extracts to determine the composition on the sol fraction, according to previously described methods [25–27]. The remaining discs were dried to a constant mass and re-weighed after extraction to determine mass loss (%).
Cell culture experiments
Undifferentiated dental pulp cells, OD-21, were maintained in Dulbecco’s modified Eagle’s medium (DMEM from Lonza Walkersville, MD, USA), containing 4.5 g/L glucose, 10% fetal bovine serum (FBS), and supplemented with an antibiotic solution (100 U/ml penicillin-G and 100 μg/ml streptomycin) and 0.594 g/l L-glutamine (Acros Organics, New Jersey, USA) at 37°C in a humidified atmosphere of 5% CO2 in air. These cells, graciously supplied by Dr. Tania Botero of the University of Michigan, were chosen due to their relevance to restorative dentistry. Several studies have shown that the use of resins as restorative materials is associated with irritation and necrosis of the pulp tissue. In addition, undifferentiated dental pulp cells might differentiate into odontoblasts to replace the cells that are destroyed by caries or dental restorative procedures [28–30].
To study the effect of the extracts from the BAG65 composites (Composite extract group) on cell viability, the OD-21 cells were cultured in 24-well plates, seeded at 5 K cells/well [24]. After 24 hrs, the cells were treated with serial dilutions of each of the extracts and allowed to incubate in humidified 5% CO2 and air at 37°C for up to 7 days, with media exchanges containing the appropriate extract every other day. After 3, 5 and 7 days, a 1ml solution of 10% Alamar Blue (Biosource, Camarillo, CA, USA) in DMEM was added to each well. Since Alamar Blue is a non-toxic aqueous dye, it is possible to assess cell viability over several time points with the same culture [31–33]. After 1 hour of incubation, 200 μl of each medium was placed into black 96-well plates in triplicate, and fluorescence intensity was measured using a plate reader (Fluoroskan Ascent FL Labsystems) with excitation at 530 nm and emission at 580 nm.
To study the effect of the BAG65 composites when directly exposed to the cells, OD-21 cells were directly cultured with freshly cured composite (Fresh composite-direct extract group) as well as composite that had been preincubated in media to extract residual monomers (Previously extracted composite-direct extract group) in 24-well plates at a seeding of 10 K cells/well. The composites were introduced after the cells were allowed to grow for 24 hrs, and then the composite/cell combination was allowed to incubate for 2 days.
To study the effect of total light exposure of BAG62 and BAG65 composites on cell viability, the OD-21 cells (n=5) were cultured in 96-well plates, seeded at 5 K cells/well. After 24 hours, the disks from Reduced cure composite group were placed in the wells containing the cells and incubated for 1, 3, 5, and 7 days. The cytotoxicity was evaluated by Alamar blue assay (n=3).
Statistical Analysis
Cell viability data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test (α ≤ 0.05). DC and solubility results were analyzed by to-way analysis of variance followed by Tukey’s test (α ≤ 0.05).
Results
Cytotoxicity of extracts of composite materials with and without bioactive glasses (Composite extract group)
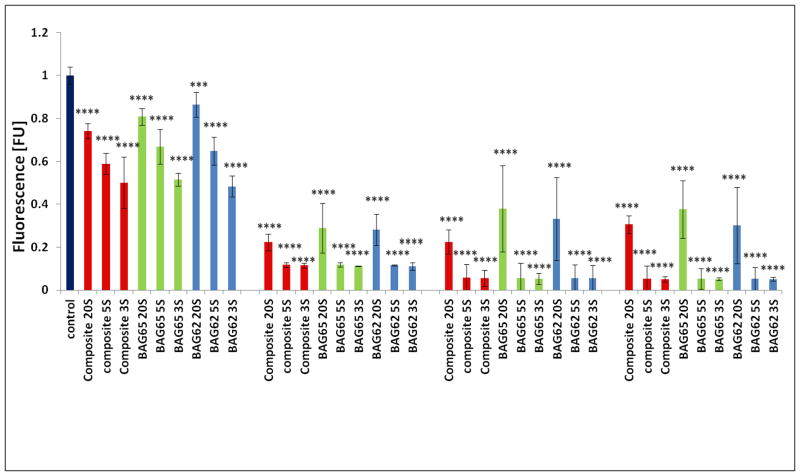
Incubation of the OD-21 cells with the extracts of different composites (Composite extract group) led to differing results of cell vitality in a dose-dependent response (Figure 1). Extracts from all composites significantly reduced cell viability until a dilution of 1:8 or lower, where the extract became equal to the control (Alamar Blue fluorescent normalized to control). The effect was similar at 3 days (Fig. 1a), 5 days (Fig. 1b) and 7 days (Fig. 1c). There was no statistical difference between the composites with various amounts of BAG 65.
Fig. 1.
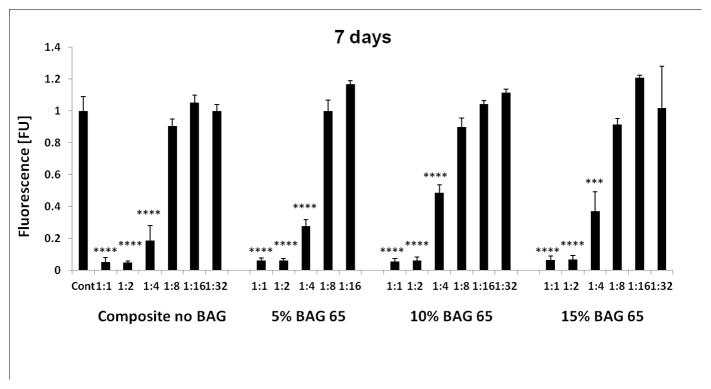
The effect of the various dilutions of the 7-day extracts of the BAG65 composites on proliferation of OD-21 cells evaluated at 3 days (1a), 5 days (1b) and 7 days (1c) as determined by Alamar blue fluorescent assay (n=5; normalized to control). *p<0.05, **p<0.01, *** p<0.001, and **** p<0.0001 vs. control (no composite).
Cytotoxicity of fresh and pre-incubated composites with and without bioactive glasses (Fresh composite-direct extract group, and Previously extracted composite-direct extract group)
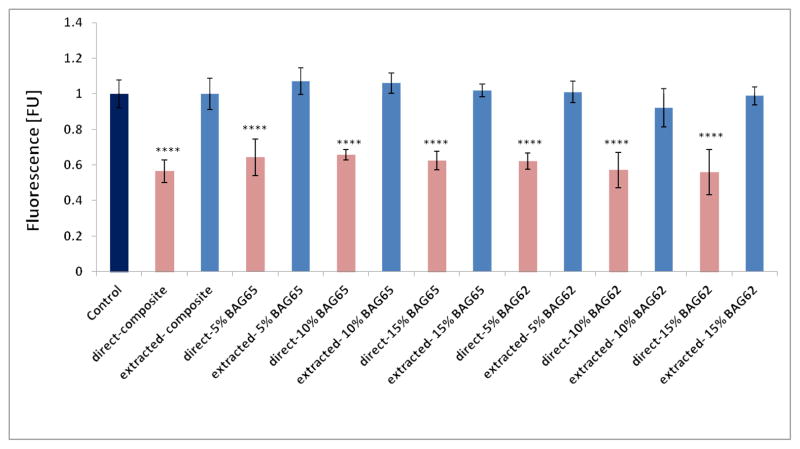
Figure 2 indicates that the cell viability was reduced by exposure to freshly cured composite (Fresh composite-direct extract group) compared to the control (no composite), but that the metabolic activity of the cells was similar to the control for composites pre-incubated for 7 days before being exposed to the cells (Previously extracted composite-direct extract group). Also, there was no statistical difference between the composites with various amounts of BAG with or without fluoride.
Fig. 2.
The effect of the “fresh” cured (direct) and pre-incubated (residual monomer extracted) BAG65 and BAG62 composites on proliferation of OD-21 cells evaluated at 2 days as determined by Alamar blue fluorescent assay (n=5; normalized to control). **** p<0.0001 vs. control (no composite).
Cytotoxicity of fresh composites cured with reduced radiant exposure (Reduced cure composite group)
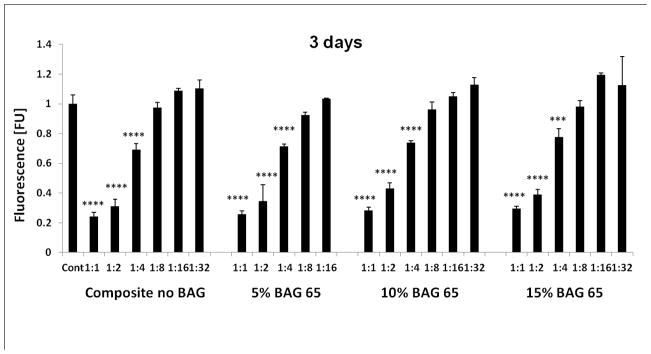
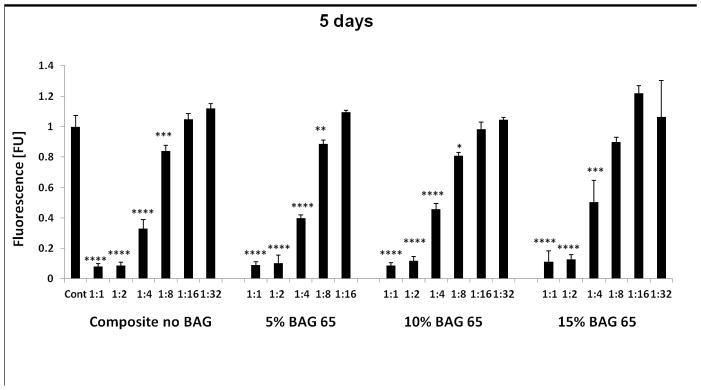
Figure 3 shows that composites exposed to a lower radiant exposure (less time or less irradiance) were more cytotoxic compared to the more fully cured specimens (20 second groups). This effect was greater for the longer incubation periods. There was no statistical difference between the composites with or without various amounts of BAG, or between the BAG with or without fluoride. The FTIR results revealed the DC increased with increased radiant exposure for the three composites (Table 1). For the BAG-modified composites, the DC determined by FTIR was statistically higher for composites irradiated for 20 s in comparison with composites irradiated for 3 s and 5 s, which were identical to each other. Composites in the control group showed the same conversion if irradiated for 5 or 20 s, which was statistically higher than the values obtained at 3 s. However, all BAG-modified materials showed conversion identical to that obtained for the control at 5 s.
Fig. 3.
The effect of different exposure levels of the “fresh” cured BAG65 and BAG62 composites on the proliferation of OD-21 cells evaluated at 1day, 3 days, 5 days, and 7 days as determined by Alamar blue fluorescent assay (n=5; normalized to control). **** p<0.0001 vs. control (no composite).
Table 1.
Degree of conversion (average/standard deviation) for materials containing no BAG (control) or 15 wt% BAG62 or BAG65, photoactivated for 3, 5 or 20 s (1, 1.8 and 15 J/cm2, respectively). Values followed by the same superscript are statistically similar (α=5%).
| DC | Control | 15%BAG 62 | 15%BAGF 65 |
|---|---|---|---|
| 3s (1J) | 0.67 (0.015) c | 0.71 (0.011) b | 0.71 (0.01) b |
| 5s (1.8J) | 0.76 (0.028) ab | 0.74 (0.01) b | 0.74 (0.005) b |
| 20s (12J) | 0.79 (0.015) a | 0.79 (0.005) a | 0.78 (0.015) a |
P values: BAG type=0.102; exposure=0.000; interaction=0.002
As for total mass of residual monomers extracted, in general, shorter exposures led to greater amount of leachables, as expected. Without exception, the groups irradiated with 20 s presented at least 90% lower amounts of leachables compared with the shorter exposures. The highest amount of leachates was presented by the material modified with BAG62 (Table 2).
Table 2.
Total mass loss (average/standard deviation) for materials containing no BAG (control) or 15 wt% BAG62 or BAG65, photoactivated for 3, 5 or 20 s (1, 1.8 and 15 J/cm2, respectively). Values followed by the same superscript are statistically similar (α=5%).
| Total Mass Loss% | Control | 15%BAG 62 | 15%BAGF 65 |
|---|---|---|---|
| 3s (1J) | 4.36 (1.61) b | 6.75 (1.29) a | 4.75 (0.395) ab |
| 5s (1.8J) | 4.04 (0.868) bc | 4.35 (0.149) b | 3.17 (0.408) c |
| 20s (12J) | 0.77 (0.372) d | 0.62 (0.743) d | 0.35 (0.226) d |
P values: BAG type=0.022; exposure=0.000; interaction=0.111
The results for the release of residual monomers (total mass % loss) vs. cell viability show that, there was an inverse correlation between the released residual monomer and the viability of OD-21 cells after 24 hours (Fig 4). The NMR data showed that the proportion of monomers released was generally around 40% Bis-GMA and 60% TEGDMA, but a slightly higher amount of Bis-GMA from the more fully cured specimens (20 s) especially for the control and BAG65-modified groups (Figure 5).
Fig. 4.
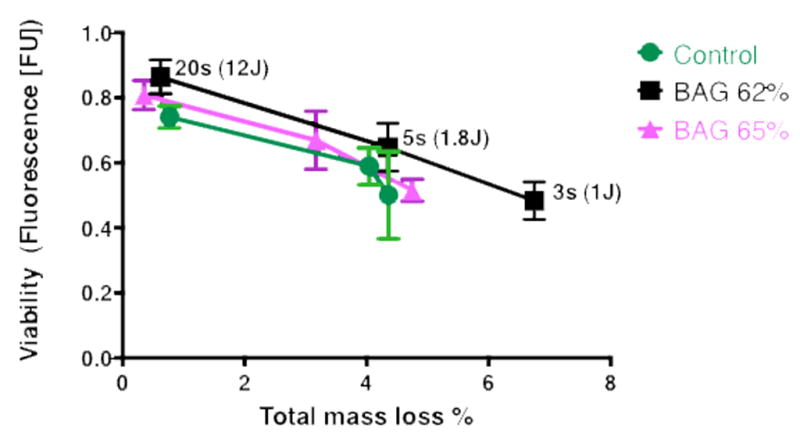
orrelation between viability of OD-21 cells and released residual monomer after 24 hours. R2=0.9163 for composite without BAG, R2=0.9971 for composite with BAG 65, and R2=0.9643 for composite with BAG 62.
Fig. 5.
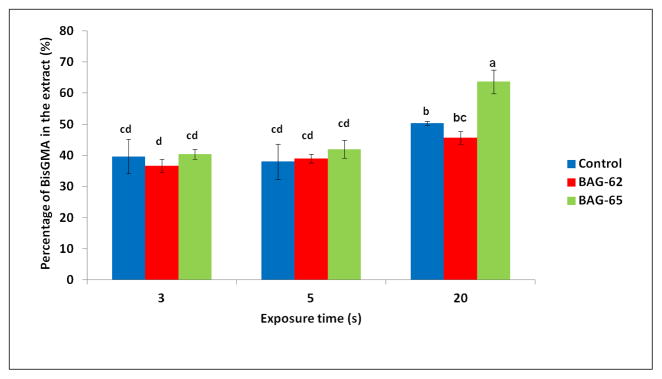
Percentage BisGMA in extract (average/standard deviation) for materials containing no BAG (control) or 15 wt% BAG62 or BAG65, photoactivated for 3, 5 or 20 s (1, 1.8 and 15 J/cm2, respectively). Values followed by the same superscript are statistically similar (p=0.05). P values: BAG type=0.000; exposure=0.000; interaction=0.001
There were significant differences in the DC determined by FTIR for 3 s composite without BAG groups compare to 5 s and 20 s of composite without BAG groups as well as composites with different types of BAG. Also, there were significant differences in the DC of 3 s composite with BAG62 compare to 20 s composite with BAG62 as well as between 3 s composite with BAG 65 compare to 20 s composite with BAG 65.
There was a difference in the amount of residual monomers released (total mass % loss) from composites exposed to a lower radiant exposure (less time or less irradiance) compared to the more fully cured specimens (20 second groups) during 24 hours of incubation (Table 1).
The results for the release of residual monomers (total mass % loss) vs. cell viability show that, there was an inverse correlation between the released residual monomer and the viability of OD-21 cells after 24 hours (Fig 4). The NMR data showed that the proportion of monomers released was generally around 40% Bis-GMA and 60% TEGDMA (Figure 5). There were significant differences between the concentrations of released Bis-GMA monomer from lower radiant exposure specimens and well-cured specimens during 24 hours of incubation, with a higher proportion of Bis-GMA being released for the longest exposure time.
Discussion
It is well known that unreacted monomers are capable of being leached from dental resin-based composites over time, with the majority being extracted within one week [34]. It is also known that the monomers may produce cytotoxic effects when they are released from the bulk of the material, or from the surface, as a result of incomplete cure [35, 36]. The results in this study for the extracts on the viability of the OD-21 cells confirm that there are components of the experimental composites that are toxic to cells, and that the response is dose-dependent. This was demonstrated in this study by the correlation between cytotoxicity and mass loss and/or conversion, with lower conversion specimens presenting greater mass loss and greater cytotoxicity.
Both Bis-GMA and TEGDMA were released from the composites, as demonstrated by the NMR data, in basically a 40:60 proportion at 3 and 5 seconds of curing, and at a slightly higher ratio for the specimens with 20 seconds of curing. The slight change in proportion of Bis-GMA and TEGDMA released as a function of DC may be due to a more complete reaction of the TEGDMA with extended curing time, leaving a relatively higher proportion of the Bis-GMA molecules unreacted and susceptible to extraction. This compositional shift at higher conversions is in accordance with previously reported data [25].
In any case, the removal of the leachable components caused a significant decrease in toxicity, consistent with previous work. Rathbun (1991) has shown that the primary leachable component from a commercial BIS-GMA-based composite was unreacted BIS-GMA [36]. The removal of these leachable components reduced toxicity by 90% compared to the non-extracted BIS-GMA composites. A similar result was shown in the present study as the composites subjected to an extraction protocol showed no significant cytotoxicity compared to the negative control. Others have shown that cytotoxicity is increased with the increase of unreacted substance in the cured material and decreased by increasing the light-curing time [37]. This also was confirmed in the present study.
The results of this study also show that the composites, independent of composition, had equivalent potency in terms of reducing the viability of the cells in culture. All freshly prepared composites, with and without the BAG additive, were cytotoxic (Fresh composite-direct extract group). These effects diminished to the point of the composites not being cytotoxic after pre-incubation in media for 7 days to extract the majority of the unreacted monomers (Previously extracted composite-direct extract group). This suggests that incubation at 37° C in cell-culture medium for 7 days is sufficient to extract the vast majority of the cytotoxic components, and that dental composite itself is unlikely to be a chronic source of unreacted components that produce cytotoxic effects. However, long-term degradation of the resin matrix can produce potentially cytotoxic compounds, though these are likely present in low concentrations at any given time.
The results of this study suggest that the most “toxic” components of the composites had been extracted in media, either during or after exposure to cells. While a potential cytotoxic response to ions leached from the composite fillers, including the strontium glass and the BAG, cannot be completely ruled out, it appears from the data presented that the effect of the unreacted monomers was the primary cause of the cytotoxicity. This latter observation is made based on the fact that after fully extracting the unreacted monomers, the composites were no longer toxic to the cells (i.e. they were equivalent to the control culture without composite). In other work, we have verified that these composite formulations do release calcium and fluoride ions, but apparently they did not contribute significantly to the cell response [38]. Further evidence that the BAG itself was not cytotoxic was apparent when comparing the three different BAG-containing formulations, which showed no statistical difference in cytotoxicity despite the composites having 5, 10 or 15% BAG. This result is also consistent with another study showing bioactive glass to be non-cytotoxic [39], though that study tested melt-derived rather than a sol-gel processed glass like the one used in the present study.
Finally, the cytotoxic effects of the composite with and without BAG 65 or BAG 62 increased significantly for the lower radiant exposures compared to the series cured with 12 J/cm2/s (group 4, Figure 4). It is known that the total curing energy can significantly influence polymerization, and that when inadequate energy is applied, the resulting is reduced degree of conversion and a higher residual monomer content. This not only has an undesirable effect on the properties of the materials, but also increases the cytotoxicity of the composites [40–42], as was confirmed in this study by the inverse correlation between the quantity of extractable monomers and the cell viability.
Altogether, these results show that cytotoxicity of composites with and without BAG must primarily be attributed to the release of residual monomers. Furthermore, the presence of fluoride in the BAG formulation did not cause any increased concern. In addition, though the materials did demonstrate cytotoxicity, the results confirm that dental composites themselves do not provide a chronic source of unreacted monomers that demonstrate cytotoxic effects, and that relatively short term exposure to fluids will lead to extraction of unreacted monomers, at least to the point of being not detectable by cell viability assays.
The results show that the composites, independent of composition, had equivalent potency in terms of reducing the viability of the cells in culture. Soaking the composites for 7 days before exposing them to the cells suggested that the “toxic” components had been extracted and the materials were no longer cytotoxic. The results demonstrate that the cytotoxicity of composites with and without BAG must predominantly be attributed to the release of residual monomers, and not to the presence of the BAG.
Acknowledgments
We wish to thank Dr. Tania Botero of the University of Michigan for supplying the OD-21 cells. This work was funded by NIH/NIDCR grant 1R01 DE021372.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldberg M. In vitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Invest. 2008;12:1–8. doi: 10.1007/s00784-007-0162-8. [DOI] [PubMed] [Google Scholar]
- 2.Caughman WF, Caughman GB, Shiflet RA, Rueggeberg F, Schuster GS. Correlation of cytotoxicity, filler loading and curing time of dental composites. Biomater. 1991;12:737–40. doi: 10.1016/0142-9612(91)90022-3. [DOI] [PubMed] [Google Scholar]
- 3.Rathbun MA, Craig RG, Hanks CT, Filisko FE. Cytotoxicity of a BIS-GMA dental composite before and after leaching in organic solvents. J Biomed Mater Res. 1991;25:443–57. doi: 10.1002/jbm.820250403. [DOI] [PubMed] [Google Scholar]
- 4.Moharamzadeh K, Van Noort R, Brook IM, Scutt AM. Cytotoxicity of resin monomers on human gingival fibroblasts and HaCaT keratinocytes. Dent Mater. 2007;23(1):40–4. doi: 10.1016/j.dental.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Hanks CT, Strawn SE, Wataha JC, Craig RG. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res. 1991;70:1450–5. doi: 10.1177/00220345910700111201. [DOI] [PubMed] [Google Scholar]
- 6.Schweikl H, Schmalz G, Spruss T. The induction of micronuclei in vitro by unpolymerized resin monomers. J Dent Res. 2001;80:1615–20. doi: 10.1177/00220345010800070401. [DOI] [PubMed] [Google Scholar]
- 7.Schweikl H, Schmalz G. Triethylene glycol dimethacrylate induces large deletions in the hprt gene of V79 cells. Mutat Res. 1999;438:71–8. doi: 10.1016/s1383-5718(98)00164-8. [DOI] [PubMed] [Google Scholar]
- 8.Cheng YJ, Zeiger DN, Howarter JA, Zhang X, Lin NJ, Antonucci JM, Lin-Gibson S. In situ formation of silver nanoparticles in photocrosslinking polymers. J Biomed Mater Res Part B: Appl Biomater. 2011;97:124–131. doi: 10.1002/jbm.b.31793. [DOI] [PubMed] [Google Scholar]
- 9.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RRB. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–43. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 10.Imazato S, Ma S, Chen J, Xu HK. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dent Mater. 2014;30:97–104. doi: 10.1016/j.dental.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han L, Okamoto A, Fukushima M, Okiji T. Evaluation of anew fluoride-releasing one-step adhesive. Dent Mater J. 2006;25:509–15. doi: 10.4012/dmj.25.509. [DOI] [PubMed] [Google Scholar]
- 12.Weir MD, Chow LC, Xu HH. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J Dent Res. 2012;91:979–84. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poh PSP, Hutmacher DW, Stevens MM, Woodruff MA. Fabrication and in vitro characterization of bioactive glass composite scaffolds for bone regeneration. Biofabrication. 2013;5:045005. doi: 10.1088/1758-5082/5/4/045005. [DOI] [PubMed] [Google Scholar]
- 14.Kawanabe K, Tamura J, Yamamuro T, Nakamura T, Kokubo T, Satoru Yoshihara S. A new bioactive bone cement consisting of BIS-GMA resin and bioactive glass powder. J Appl Biomater. 1993;4:135–41. doi: 10.1002/jab.770040204. [DOI] [PubMed] [Google Scholar]
- 15.Khoroushi M, Mousavinasab SM, Keshani F, Hashemi S. Effect of Resin-Modified Glass Ionomer Containing Bioactive Glass on the Flexural Strength and Morphology of Demineralized Dentin. Oper Dent. 2013;38:E1–10. doi: 10.2341/11-325-L. [DOI] [PubMed] [Google Scholar]
- 16.Xie D, Zhao J, Weng Y, Park JG, Jiang H, Platt JA. Bioactive glass-ionomer cement with potential therapeutic function to dentin capping mineralization. Eur J Oral Sci. 2008;116:479–87. doi: 10.1111/j.1600-0722.2008.00562.x. [DOI] [PubMed] [Google Scholar]
- 17.Yang SY, Piao YZ, Kim SM, Lee YK, Kim KN, Kim KM. Acid neutralizing, mechanical and physical properties of pit and fissure sealants containing melt-derived 45S5 bioactive glass. Dent Mater. 2013;29:1228–35. doi: 10.1016/j.dental.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Tirapelli C, Panzeri H, Soares RG, Peitl O, Zanotto ED. A novel bioactive glass-ceramic for treating dentin hypersensitivity. Braz Oral Res. 2010;24:381–7. doi: 10.1590/s1806-83242010000400002. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JC, Musanje L, Ferracane JL. Biomimetic dentin desensitizer based on nano-structured bioactive glass. Dent Mater. 2011;27:386–393. doi: 10.1016/j.dental.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JC. Thesis. The Ohio State University; 1999. A bioactive glass material for the delivery of Bone Morphogenetic Proteins: synthesis by the solution sol–gel method, physical and chemical analyses, and in-vitro testing. OCLC# 43272687. [Google Scholar]
- 21.Hu Sh, Chang J, Liu M, Ning C. Study on antibacterial effect of 45S5 Bioglass. J Mater Sci: Mater Med. 2009;20:281–6. doi: 10.1007/s10856-008-3564-5. [DOI] [PubMed] [Google Scholar]
- 22.Waltimo T, Brunner TJ, Vollenweider M, Stark WJ, Zehnder M. Antimicrobial Effect of Nanometric Bioactive Glass 45S5. J Dent Res. 2007;86:754–7. doi: 10.1177/154405910708600813. [DOI] [PubMed] [Google Scholar]
- 23.Franz A, Konig F, Anglmayer M, Rausch-Fan X, Gille G, Rausch WD, et al. Cytotoxic effects of packable and nonpackable dental composites. Dent Mater. 2003;19(5):382–92. doi: 10.1016/s0109-5641(02)00081-7. [DOI] [PubMed] [Google Scholar]
- 24.Schweikl H, Hiller KA, Bolay C, Kreissl M, Kreismann W, Nusser A, Steinhauser S, Wieczorek J, Vasold R, Schmalz G. Cytotoxic and mutagenic effects of dental composite materials. Biomaterials. 2005;26:1713–9. doi: 10.1016/j.biomaterials.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater. 2001;17:71–9. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 26.Stansbury JW, Dickens SH. Network formation and compositional drift during photo-initiated co-polymerization of dimethacrylate monomers. Polymer. 2001;42:6363–9. [Google Scholar]
- 27.Pfeifer CS, Shelton ZR, Braga RR, Machado JC, Windmoller D, Stansbury JW. Characterization of dimethacrylate polymeric networks: a study of the crosslinked structure formed by monomers used in dental composites. Eur Pol J. 2011;47:162–70. doi: 10.1016/j.eurpolymj.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballini A, De Frenza G, Cantore S, Papa F, Grano M, Mastrangelo F, Tete S, Grassi FR. In vitro stem cell cultures from human dental pulp and periodontal ligament: new prospects in dentistry. Int J Immunopathol Pharmacol. 2007;20:9–16. doi: 10.1177/039463200702000102. [DOI] [PubMed] [Google Scholar]
- 29.Modena KC, Casas-Apayco LC, Atta MT, Costa CA, Hebling J, Sipert CR, Navarro MF, Santos CF. Cytotoxicity and biocompatibility of direct and indirect pulp capping materials. J Appl Oral Sci. 2009;17(6):544–54. doi: 10.1590/S1678-77572009000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semeghini MS, Fernandes RR, Chimello DT, Oliveira FS, Bombonato-Prado KF. In Vitro Evaluation of the Odontogenic Potential of Mouse Undifferentiated Pulp Cells. Braz Dent J. 2012;23(4):328–336. doi: 10.1590/s0103-64402012000400004. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–6. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 32.Nociari MM, Shalev A, Benias P, Russo C. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J Immunol Methods. 1998;213:157–67. doi: 10.1016/s0022-1759(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 33.Fields RD, Lancaster MV. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am Biotechnol Lab. 1993;11:48–50. [PubMed] [Google Scholar]
- 34.Ferracane JL. Elution of leachable components from composites. J Oral Rehabil. 1994;21:441–52. doi: 10.1111/j.1365-2842.1994.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 35.Gupta KS, Saxena P, Pant AV, Pant BA. Release and toxicity of dental resin composite. Toxicol Int. 2012;19:225–34. doi: 10.4103/0971-6580.103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wataha JC, Rueggeberg FA, Lapp CA, Lewis JB, Lockwood PE, Ergle JW, Mettenburg DJ. In vitro cytotoxicity of resin-containing restorative materials after aging in artificial saliva. Clin Oral Invest. 1999;3:144–9. doi: 10.1007/s007840050093. [DOI] [PubMed] [Google Scholar]
- 37.Mohsen NM, Craig RG, Hanks CT. Cytotoxicity of urethane dimethacrylate composites before and after aging and leaching. J Biomed Mater Res. 1998;39:252–60. doi: 10.1002/(sici)1097-4636(199802)39:2<252::aid-jbm12>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 38.Davis H, Ferracane JL, Mitchell JC. Ion release from, and fluoride recharge of a composite with a fluoride-containing bioactive glass. Dent Mater. doi: 10.1016/j.dental.2014.07.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mortazavi V, Nahrkhalaji MM, Fathi MH, Mousavi SB, Esfahani BN. Antibacterial effects of sol-gel-derived bioactive glass nanoparticle on aerobic bacteria. J Biomed Mater Res. 2010;94:160–8. doi: 10.1002/jbm.a.32678. [DOI] [PubMed] [Google Scholar]
- 40.Yap AU, Seneviratne C. Influence of light energy density on effectiveness of composite cure. Oper Dent. 2001;26:460–6. [PubMed] [Google Scholar]
- 41.Ak AT, Alpoz AR, Bayraktar O, Ertugrul F. Monomer release from resin based dental materials cured with LED and halogen lights. Eur J Dent. 2010;4:34–40. [PMC free article] [PubMed] [Google Scholar]
- 42.Sigusch BW, Pflauma T, Völpela A, Gretscha K, Hoya S, Wattsb DC, Jandt KD. Resin- composite cytotoxicity varies with shade and irradiance. Dent Mater. 2012;28:312–19. doi: 10.1016/j.dental.2011.12.007. [DOI] [PubMed] [Google Scholar]