Abstract
Organophosphorus anticholinesterases (OPs) elicit acute toxicity by inhibiting acetylcholinesterase (AChE), leading to acetylcholine accumulation and overstimulation of cholinergic receptors. Endocannabinoids (eCBs, e.g., arachidonoyl ethanolamide [AEA] and 2-arachidonoyl glycerol [2-AG]) are neuromodulators that regulate neurotransmission by reducing neurotransmitter release. The eCBs are degraded by the enzymes fatty acid amide hydrolase (FAAH, primarily involved in hydrolysis of AEA) and monoacylglycerol lipase (MAGL, primarily responsible for metabolism of 2-AG). We previously reported that the cannabinoid receptor agonist WIN 55,212-2 reduced cholinergic toxicity after paraoxon exposure. This study compared the effects of the cannabinoid receptor antagonist AM251 on acute toxicity following either paraoxon (PO) or chlorpyrifos oxon (CPO). CPO was more potent in vitro than PO at inhibiting AChE (≈ 2 fold), FAAH (≈ 8 fold), and MAGL (≈ 19 fold). Rats were treated with vehicle, PO (0.3 and 0.6 mg/kg, sc.) or CPO (6 and 12 mg/kg, sc.) and subsets treated with AM251 (3 mg/kg, ip; 30 min after OP). Signs of toxicity were recorded for four hours and rats were then sacrificed. OP-treated rats showed dose-related involuntary movements, with AM251 increasing signs of toxicity with the lower dosages. PO and CPO elicited excessive secretions, but AM251 had no apparent effect with either OP. Lethality was increased by AM251 with the higher dosage of PO, but no lethality was noted with either dosage of CPO, with or without AM251. Both OPs caused extensive inhibition of hippocampal AChE and FAAH (>80–90%), but only CPO inhibited MAGL (37–50%). These results provide further evidence that eCB signaling can influence acute OP toxicity. The selective in vivo inhibition of MAGL by CPO may be important in the differential lethality noted between PO and CPO with AM251 co-administration.
Keywords: anticholinesterase, organophosphate, acetylcholinesterase, fatty acid amide hydrolase, monoacylglycerol lipase, endocannabinoids
INTRODUCTION
Organophosphorus toxicants (OPs) elicit acute toxicity primarily via inhibition of the enzyme acetylcholinesterase (AChE, see Banks and Lein 2012). Extensive AChE inhibition leads to elevated levels of the neurotransmitter acetylcholine at cholinergic synapses throughout the central and peripheral nervous systems, which in turn leads to widespread overstimulation of cholinergic receptors. Acute OP toxicity can manifest as classic cholinergic signs including involuntary movements (e.g., tremors and seizures) and autonomic dysfunction (typically expressed as excessive secretions [salivation, lacrimation, urination, and defecation]) as well as others (e.g., miosis, changes in heart rate). The autonomic signs are due to prolonged activation of muscarinic receptors at parasympathetic innervated end organs, while tremors and seizures are likely the consequence of enhanced muscarinic receptor activation in the central nervous system (Espinola et al. 1999). Lethality is typically due to depression of brainstem respiratory control centers, compounded by excessive airway secretions and dysfunction of diaphragm and intercostal muscles (see Pope et al., 2005).
Endocannabinoids (eCBs, e.g., arachidonoyl ethanolamide, also known as anandamide [AEA] and 2-arachidonoylglycerol [2-AG]) are neuromodulators that mediate a retrograde signaling pathway to modulate neurotransmitter release at the presynaptic terminal (Castillo et al. 2012). The synthesis and release of eCBs in postsynaptic neurons can be elicited “on demand” by depolarization or via receptor-mediated pathways involving muscarinic M1 and M3, metabotropic glutamate (mGluR), 5-HT2, and other types of receptors (Maejima et al. 2001; Kim et al. 2002; Ohno-Shosaku et al. 2012, 2014). Once released into the synapse, eCB signaling is affected primarily by activation of presynaptic G-protein coupled cannabinoid CB1 receptors, with modulation of neurotransmitter release coupled to inhibition of calcium influx or facilitation of potassium efflux. Other signal transduction pathways may also play a role (Maingret et al. 2001; Brown et al. 2004; van der Steldt and Di Marzo 2005; Yoshihara et al. 2006).
Endocannabinoids inhibit the release of a number of neurotransmitters including acetylcholine (Gifford and Ashby 1996; Gessa et al. 1997; Sullivan 1999; Cheer et al. 2004). The synthetic cannabinoid agonists WIN 55,212-2 and CP 55,940 reduced hippocampal acetylcholine release both in vitro (Gifford and Ashby 1996; Gifford et al. 2000) and in vivo (Tzavara et. al. 2003; Degroot et al. 2006) while the CB1 antagonist SR141716A increased hippocampal acetylcholine release (Gifford and Ashby 1996; Gessa et al. 1997; Kathmann et al. 2001). Degroot and colleagues (2006) reported that both systemic and direct hippocampal infusion of the CB1 receptor antagonists SR141716A and AM251 increased acetylcholine efflux in a dose-dependent manner, a response that was absent in mice lacking the CB1 receptor. Thus a number of studies suggest that eCBs can potentially regulate cholinergic transmission by modulating acetylcholine release.
Endocannabinoid signaling is terminated by enzymatic hydrolysis of the signaling molecules AEA and 2-AG. Fatty acid amide hydrolase (FAAH) is the primary enzyme involved in degradation of AEA (Cravatt et al. 1996, 2001; Egertova et al. 2003). Greater than 80% of the enzymatic hydrolysis of 2-AG is mediated by monoacylglycerol lipase (MAGL), with the residual being metabolized by the serine hydrolases ABHD6 and ABHD12 (Hashimotodani et al. 2007; Savinainen et al. 2012). In addition to their well-known effects on AChE, a number of OPs have been shown to inhibit FAAH and MAGL (Quistad et al. 2001, 2002, 2006; Nallapaneni et al. 2006; Casida et al. 2008; Nomura et al. 2008; Nomura and Casida 2011; Liu et al. 2013). Anticholinesterases may therefore increase eCB levels through at least three different mechanisms, i.e., widespread neuron depolarization, prolonged M1/M3 receptor activation, and inhibition of FAAH and/or MAGL.
Paraoxon (PO) and chlorpyrifos oxon (CPO) are the active metabolites of the OP insecticides parathion and chlorpyrifos. Acute toxicity following exposure to these insecticides requires metabolic activation to the respective oxons, with subsequent potent effects of the metabolites on AChE and related toxicity. Thus studying the toxicity of the oxons and its modulation by eCBs allows mechanistic insight into synaptic neurochemistry without concern for differences in metabolic activation/detoxification between parathion and chlorpyrifos. We previously reported that signs of cholinergic toxicity in rats following acute PO exposure were reduced by the cannabinoid receptor agonist WIN 55,212-2 (Nallapaneni et al. 2006). Interestingly, we recently found that the signs of toxicity following exposure to the parent insecticide parathion (PO is the active metabolite) were reduced by the cannabinoid receptor antagonist/inverse agonist (AM251; Liu et al. 2013). Moreover, AM251 had no influence on responses to chlorpyrifos (the parent insecticide of CPO). The objective of this study was to evaluate the comparative effects of AM251 on functional signs of toxicity following either acute PO or CPO exposure.
METHODS AND MATERIALS
Chemicals
Paraoxon (PO) and chlorpyrifos oxon (CPO were purchased from ChemService (West Chester, PA; both >98% purity). [3H]Anandamide (ethanolamine-1-3H), specific activity 60 Ci/mmol) was purchased from American Radiochemical Company (St. Louis, MO). Arachidonoyl-1-thio-glycerol, anandamide, and AM 251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) were purchased from Cayman Chemical (Ann Arbor, MI). Acetylthiocholine iodide and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Animals and Treatments
Adult male Sprague-Dawley rats (average body weight around 300 grams) were used throughout (Harlan, Indianapolis, IN). Animals were maintained in a climate controlled room with 12 h:12 h light:dark illumination cycle in an AAALAC-accredited facility. All procedures were performed in accordance with protocols established according to the “Guide for the Care and Use of Laboratory Animals” and approved by the Institutional Animal Care and Use Committee of Oklahoma State University.
Rats were treated with either vehicle (peanut oil, 1 ml/kg, sc), PO (0.3 and 0.6 mg/kg), or CPO (6 and 12 mg/kg). Thirty minutes after the OP exposure, subsets were given either vehicle (1:1:18; ethanol: Alkamuls EL-620: saline, ip) or AM251 (3 mg/kg in vehicle, ip). Functional signs of toxicity were graded at 1, 2, 3, and 4 hours after OP dosing and cumulative lethality was recorded. Functional signs were only reported for animals that survived throughout the four hour observation period. Tissues were collected at the end of the observations and frozen at −80°C until time of assay.
Cholinergic Signs of Toxicity
Functional signs of toxicity were recorded essentially as described by Moser et al. (1988) with modifications (Liu et al. 2013) by a trained observer “blinded” to the treatment groups. SLUD signs (an acronym for salivation, lacrimation, urination and defecation) were graded as: 1 = normal (no secretions); 2 = one or multiple mild secretions; 3 = moderate degree of multiple secretions; 4 = severe degree of multiple secretions. Involuntary movements were scored as: 2 = normal quivering of vibrissae and head; 3 = fine head and neck tremors; 3.25 = more consistent tremors in head, neck and forelimbs; 3.5 = consistent tremors extending caudally from head to midbody; 3.75 = tremors extending caudally to hindlimbs; 4 = whole body tremors; 5 = myoclonic jerks.
Measurement of AChE, MAGL and FAAH Activities
For in vitro inhibition studies, OPs were dissolved in dry ethanol (10 mM stock solutions) and kept at −80°C. On the day of assay, serial dilutions in PBS for each OP were made such that ethanol (0.1% final concentration) was included in all reactions. Tissues were thawed on ice and homogenized in PBS (1:60) using a Polytron disruptor (28,000 rpm for 20 sec on ice). Tissue homogenates were pre-incubated with OP for 20 min at 37°C followed by measurement of residual AChE, MAGL and FAAH activities.
Activities of AChE and MAGL were measured by a modification of the photometric methods of Ellman and colleagues (1961), Casida and coworkers (2010) and Ulloa and Deutsch (2010). Buffer (PBS, no enzyme blanks) or homogenates in PBS (1:60, 25 µl, approximately 20 µg protein) were added to each well of a 96-well plastic plate on ice in quadruplicate. A solution (175 µl) containing either acetylthiocholine iodide (1 mM final concentration) or arachidonoyl-1-thioglycerol (100 µM final concentration) and the color reagent (5,5'-dithiobis-[2-nitrobenzoic acid]), 100 µM final concentration) in 10 mM Tris-HCl buffer, pH 7.2 containing disodium EDTA (1 mM) was then added and the plate was immediately placed into the reader (SPECTRAmax 340PC, Molecular Devices, Sunnyvale, CA). The reaction was conducted in kinetic mode at 412 nm, 30°C for 5 min, with an initial lag time of 60 sec. Data were collected each 20 sec and reaction velocity averaged (rates were linear over the entire incubation period). Rates of hydrolysis were calculated based on the molar extinction coefficient of the thiolate product, which is produced in direct proportion to either thiocholine (13,600 M−1 cm−1, Ellman et al., 1961) or thioglycerol (14,150 M−1 cm−1, Ulloa and Deutsch, 2010).
For FAAH, the 1:60 homogenates in PBS were further diluted (1:120 final dilution) and assayed essentially by the method of Long and coworkers (2009) with modifications. Briefly, tissues (approximately 30 µg protein) were incubated at 37°C for 20 min with [3H]anandamide (10 µM final concentration) in a final reaction volume of 0.4 ml. The reaction was stopped by adding 0.4 ml chloroform:methanol (1:1) and vortexing. Tubes were then centrifuged in a microcentrifuge (3,300 rpm for 10 min, Marathon 13K/M, Fisher, Chicago, IL). An aliquot of the upper aqueous phase (200 µl) was removed, added to 4 ml Scintisafe® scintillation fluid, and counted.
In all cases, protein concentration was estimated using the Bio-Rad protein assay method (with bovine serum albumin as the standard).
Data Analysis
Data were analyzed using GraphPad Prism 5.0 software (La Jolla, CA). Concentrations of OP that inhibited 50% of the total activity (IC50) were calculated using the nonlinear fit program, log (inhibitor) vs. normalized response (variable parameter). Parametric data were analyzed by one-way ANOVA followed by Bonferroni’s post-tests. Repeated measures, nonparametric data (functional signs) were first transformed (square root) and then analyzed using repeated measures two-way ANOVA (treatment vs time). Lethality data were analyzed using the Fisher’s exact test.
RESULTS
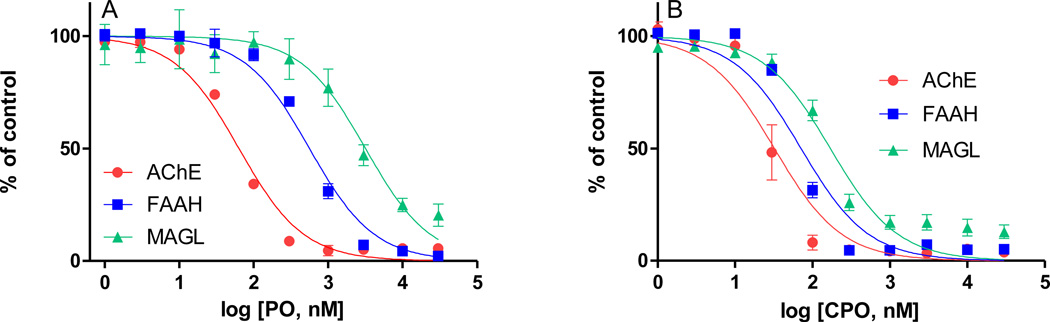
Figure 1 shows the comparative in vitro inhibitory potencies of A) paraoxon and B) chlorpyrifos oxon against rat hippocampal AChE, FAAH, and MAGL activities. Chlorpyrifos oxon was a potent in vitro inhibitor of all three enzymes with IC50 values (95% confidence intervals in parentheses) for AChE = 32 nM (23 to 46), FAAH = 71 nM (53 to 96), and MAGL = 170 nM (128 to 227). Paraoxon was a relatively less potent inhibitor compared to chlorpyrifos oxon, in particular against FAAH and MAGL, with IC50 values (95% confidence intervals in parentheses) for AChE = 64 nM (54 to 75), FAAH = 562 nM (481 to 656), and MAGL = 3,175 nM (2,472 to 4,077).
Figure 1. In vitro inhibition of hippocampal acetylcholinesterase (AChE), fatty acid amide hydrolase (FAAH), and monoacylglycerol lipase (MAGL) by paraoxon (PO) and chlorpyrifos oxon (CPO).
Tissues were homogenized in PBS and pre-incubated (20 min, 37°C) with vehicle or one of a range of PO or CPO concentrations (all in 0.1% ethanol final). Residual activity was measured by a radiometric (FAAH: [3H]AEA as substrate, 10 µM) or photometric method (AChE: acetylthiocholine as substrate, 1 mM; MAGL: arachidonoyl thioglycerol as substrate, 100 µM) as described in Methods and Materials. IC50 values (nM, with 95% confidence intervals) with CPO for AChE, FAAH, and MAGL are: 32 (23 – 46), 71 (53 – 96), 170 (128 – 227), respectively. IC50 values (nM, with 95% confidence intervals) with PO for AChE, FAAH, and MAGL are: 64 (54 – 75), 562 (481 – 656), 3175 (2472 – 4077), respectively.
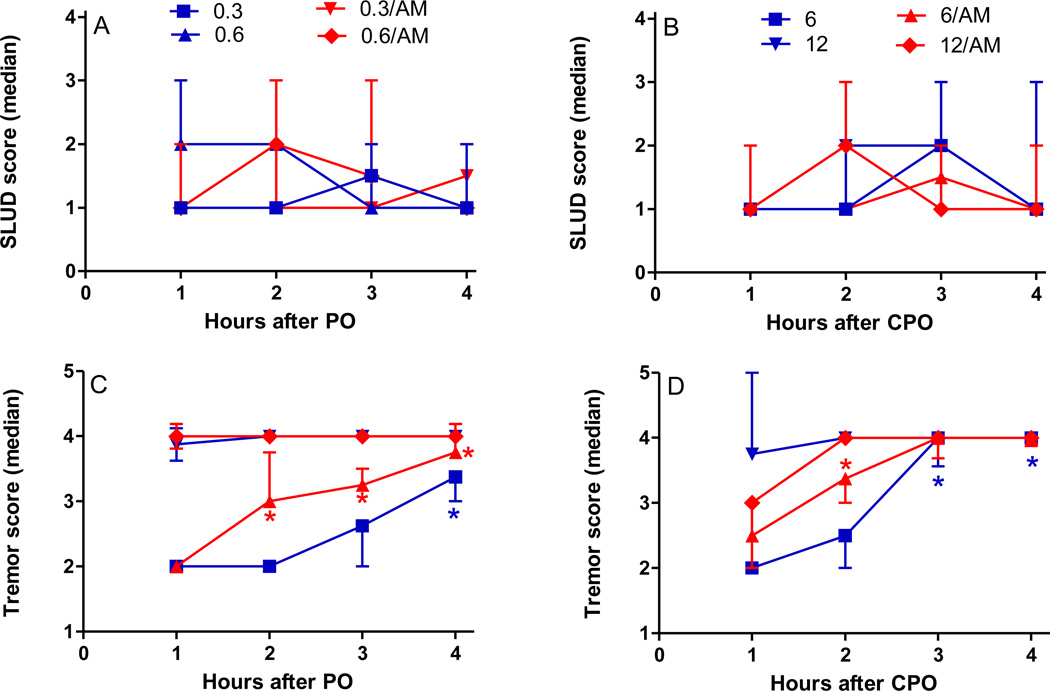
Figure 2 shows functional signs of toxicity following either paraoxon or chlopyrifos oxon exposure, with or without AM251 post-treatment. SLUD signs (primarily defecation and lacrimation) were slightly increased following exposure to the high dosages of either paraoxon (0.6 mg/kg, sc) or chlorpyrifos oxon (12 mg/kg, sc). Post-treatment with AM251 had relatively little effect on SLUD signs following either OP (Figure 2A, B). In contrast, more extensive dose-related involuntary movements (tremors) were noted with both OPs (Figure 2C, D). More importantly, AM251 post-treatment increased the extent of involuntary movements elicited by the lower dosages of either chlorpyrifos oxon or paraoxon (6 mg/kg and 0.3 mg/kg, respectively).
Figure 2. Functional signs of toxicity of paraoxon (PO) or chlorpyrifos oxon (CPO), with or without CB1 receptor antagonist/inverse agonist AM251 post-treatment.
Male SD rats were treated with either vehicle (peanut oil, 1 ml/kg), PO (0.3 or 0.6 mg/kg, sc) or CPO (6 or 12 mg/kg, sc) and then given either vehicle (1:1:18, ethanol:Alkamuls EL-620:saline) or AM 251 (AM, 3 mg/kg, ip) 30 min later. Rats were evaluated for functional signs (SLUD and involuntary movements) at 1, 2, 3, and 4 hours after OP as described in Methods and Materials. Values are reported as median ± quartile.
Table 1 shows the incidence of lethality in rats treated with high dosages of either paraoxon (0.6 mg/kg) or chlorpyrifos oxon (12 mg/kg), and the influence of AM251 post-treatment. While chlorpyrifos oxon elicited no lethality, whether or not AM251 was given 30 minutes later, lethality with paraoxon was significantly increased by AM251 post-treatment (paraoxon alone, 2/14; paraoxon plus AM251, 7/17).
Table 1.
Effects of AM251 on acute lethality following either paraoxon (PO) or chlorpyrifos oxon (CPO). Rats were treated with OP and then given vehicle or AM251 (3 mg/kg, ip) 30 minutes later. Cumulative lethality was recorded for 4 hours after OP exposure.
| OP | Lethality |
|---|---|
| PO (0.6 mg/kg, sc) | 2/14 |
| PO + AM 251 | 7/17* |
| CPO (12 mg/kg, sc) | 0/6 |
| CPO + AM 251 | 0/7 |
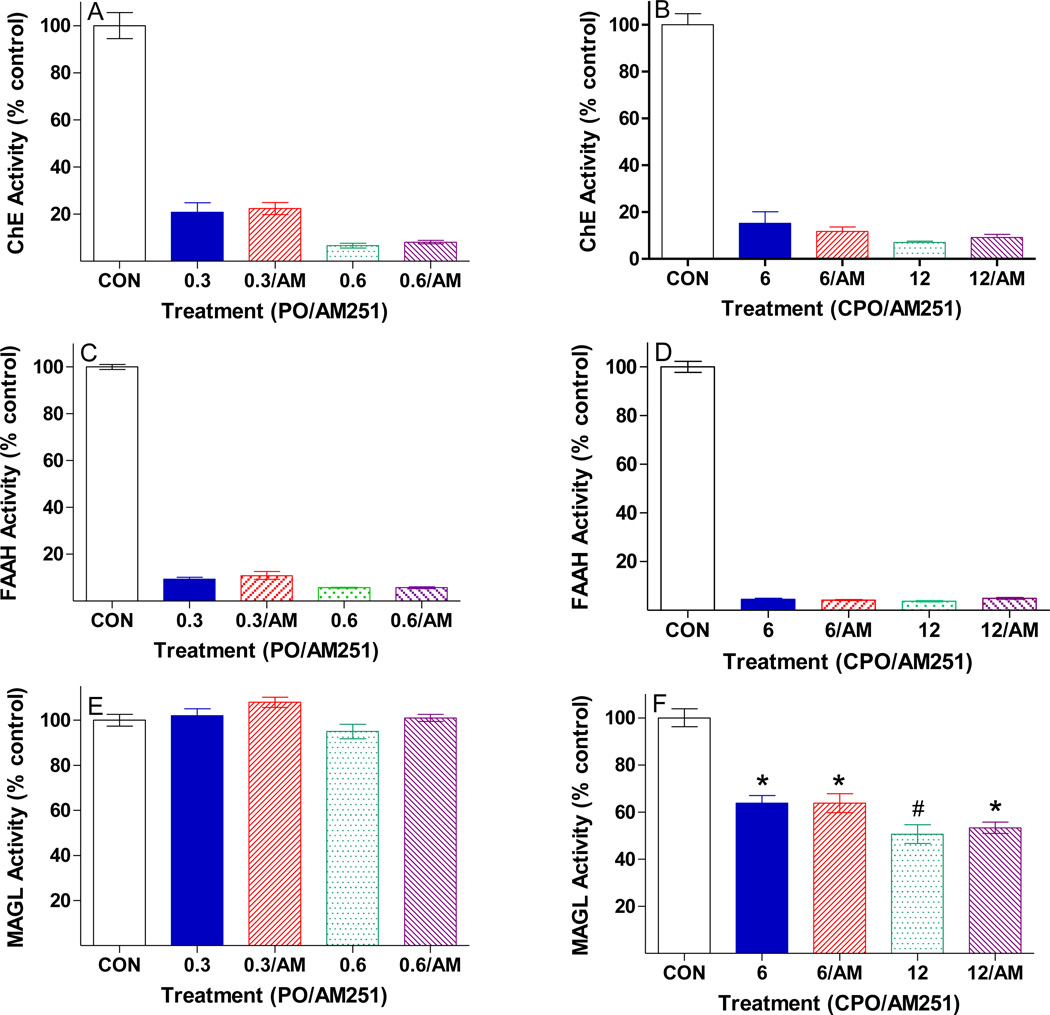
Figure 3 shows the comparative in vivo effects of paraoxon and chlorpyrifos oxon, with or without AM251 post-treatment, on hippocampal AChE, FAAH, and MAGL activities. Four hours after dosing, about 80–90% inhibition of AChE activity was noted with both OPs, with generally more extensive reduction with the higher dosages (Figure 3A). FAAH was somewhat more extensively inhibited compared to AChE by both OPs (≈ 90–95%, Figure 3B). Interestingly, neither dosage of paraoxon affected MAGL activity in vivo, while chlorpyrifos oxon reduced MAGL activity in a dose-related manner (37–50%, Figure 3C). In all cases, AM251 post-treatment had no apparent influence on OP-induced enzyme inhibition.
Figure 3. In vivo inhibition of hippocampal acetylcholinesterase (AChE), fatty acid amide hydrolase (FAAH), and monoacylglycerol lipase (MAGL) four hours after either paraoxon (PO) or chlorpyrifos oxon (CPO), with or without AM251.
Male SD rats were treated as in Figure 2 above and sacrificed 4 hours later. AChE and MAGL activities were assayed by photometric methods, while FAAH activity was measured by a radiometric method, as described in Methods and Materials. AChE and FAAH inhibition were significantly different from control in all treatment groups. An asterisk indicates a significant difference in MAGL activity compared to control, while a pound sign indicates a significant difference in rats treated with 6 mg/kg CPO compared to 12 mg/kg CPO. Data were calculated as nmol substrate hydrolyzed/min/mg protein and plotted as percent of control values (mean ± SEM). Control values for AChE, FAAH, and MAGL were 116 ± 3.6, 0.8 ± 0.03, and 24.7 ± 1.2 nmol/min/mg protein.
DISCUSSION
Paraoxon and chlorpyrifos oxon are both potent inhibitors of acetylcholinesterase (AChE). With dosages of either that lead to extensive AChE inhibition, cholinergic signs of toxicity including lethality can be elicited. Paraoxon and chlorpyrifos oxon are also inhibitors of fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL, Quistad et al. 2001, 2006), the two enzymes primarily responsible for degradation of the endocannabinoids (eCBs) anandamide and 2-arachidonoylglycerol. In rat hippocampal homogenates, paraoxon and chlorpyrifos oxon inhibited AChE, FAAH, and MAGL activities in vitro, with AChE being most and MAGL least sensitive (Figure 1). Chlorpyrifos oxon was markedly more potent in vitro than paraoxon at inhibiting FAAH (≈ 8-fold) and MAGL (≈ 19-fold). Relatively similar in vitro differences in inhibitory potency against FAAH and MAGL in mouse brain homogenates were previously reported between these two OPs (Quistad et al. 2001, 2006; Casida et al. 2010). Thus chlorpyrifos oxon may elicit more extensive inhibition of these enzymes in vivo compared to paraoxon.
As noted before, the “on demand” synthesis and release of eCBs can be triggered by both neuronal depolarization and through receptor-mediated signaling, e.g., via muscarinic M1 or M3 receptor activation (Maejima et al. 2001; Kim et al. 2002; Fukudome et al. 2004). With extensive AChE inhibition by either paraoxon or chlorpyrifos oxon, an increase in neuronal depolarization and muscarinic M1 and/or M3 receptor activation would lead to an increase in eCB synthesis and release. Moreover, the concurrent inhibition of eCB-degrading enzymes (FAAH and/or MAGL) could further increase/prolong eCB signaling, with subsequent enhancement of eCB-mediated inhibition of neurotransmitter release. Thus we hypothesized that interrupting eCB signaling by blocking the CB1 receptor with AM251 would disinhibit acetylcholine release and lead to a net increase in signs of toxicity following either paraoxon or chlorpyrifos oxon exposure. Moreover, relative differences in the extent of in vivo inhibition of eCB-degrading enzymes could potentially lead to selective differences in response between the two OPs.
While autonomic signs of toxicity appeared relatively unaffected by AM251 post-treatment with either OP (Figure 2A, B), involuntary movements (tremors) elicited by the lower dosages of both chlorpyrifos oxon and paraoxon were increased by AM251 (Figure 2C, D). There was no apparent increase in involuntary movements with AM251 treatment in rats given the higher dosages of either OP, however. Together with previous findings that the cannabinoid receptor agonist WIN 55,212-2 blocked toxicity following either paraoxon (Nallapaneni et al. 2006) or diisopropylfluorophosphate (Nallapaneni et al. 2008; Wright et al. 2010), these results provide further support for a role of eCB signaling in the expression of acute toxicity associated with extensive AChE inhibition.
With dosages of paraoxon and chlorpyrifos oxon that elicited relatively similar degrees of toxicity (Figure 2), one would expect similar degrees of AChE inhibition. Indeed, we noted relatively similar AChE inhibition in hippocampus four hours after exposure to either paraoxon (0.3 and 0.6 mg/kg) or chlorpyrifos oxon (6 and 12 mg/kg; Figure 3A). Even though there was about an 8-fold difference in in vitro inhibitory potency towards FAAH between paraoxon and chlorpyrifos oxon (and 20-fold higher dosages of CPO in vivo), relatively similar levels of extensive FAAH inhibition were noted in vivo with these two OPs (Figure 3B). Chlorpyrifos oxon was a markedly more potent in vitro inhibitor of MAGL compared to paraoxon, and indeed it also inhibited hippocampal MAGL in vivo (37–50%) while paraoxon had no effect (Figure 3C). Thus in vivo, these two OPs have some degree of selectivity towards eCB degrading enzymes (Figure 3 C–F) at dosages eliciting relatively similar degrees of AChE inhibition (Figures 3A, B) and cholinergic signs (Figure 2). We recently reported greater inhibition of hippocampal FAAH and a higher increase in extracellular levels of anandamide, relative to MAGL activity and extracellular 2-arachidonoylglycerol levels, following exposure to the parent insecticides (parathion and chlorpyrifos, 27 mg/kg and 280 mg/kg, respectively) in adult rats (Liu et al. 2013). Carr and coworkers (2013) also reported relatively greater effects of chlorpyrifos on FAAH activity and brain tissue anandamide levels with repeated exposures in postnatal rats (postnatal day 10–16; 1–5 mg/kg, po), compared to MAGL and 2-AG levels.
While dose-related functional signs (involuntary movements) were relatively similar following paraoxon or chlorpyrifos oxon exposure, and while AM251 increased the severity of tremors in rats treated with the lower dosages of either OP, AM251 only increased acute lethality in paraoxon-treated rats (0.6 mg/kg, Table 1). These OP-related differences may be due to some degree of lethality with paraoxon alone (0.6 mg/kg) but not with chlorpyrifos oxon alone (12 mg/kg, Table 1). However, OP-related differences in lethality with AM251 co-administration could also be due to the differential in vivo effects of the oxons on MAGL, i.e., only chlorpyrifos oxon inhibited hippocampal MAGL (Figure 3 E, F). Thus some aspect of OP-induced lethality following high-dose exposure may be sensitive to 2-AG signaling. Under these conditions, selective inhibition of MAGL by chlorpyrifos oxon may enhance 2-AG levels, leading to more effective inhibition of acetylcholine release.
We previously studied the effects of AM251 on the expression of cholinergic toxicity with exposures to the parent insecticides, parathion and chlorpyrifos (Liu et al. 2013). Interestingly, AM251 reduced cholinergic toxicity when given after parathion, but had no effect on functional signs following chlorpyrifos. Thus AM251 appears to have different effects on the expression of toxicity between parathion and chlorpyrifos, and between the oxons and their respective parent compounds: AM251 increased toxicity with both paraoxon and chlorpyrifos oxon, decreased toxicity with parathion, and had no influence on signs of toxicity after chlorpyrifos. It must be noted however that few overt signs of cholinergic toxicity are elicited in adult male Sprague Dawley rats treated with a high subcutaneous dosage of chlorpyrifos, even with extensive AChE inhibition (Pope et al., 1991; Pope et al., 1992; Liu et al., 2013).
An underlying mechanism(s) for the qualitatively different effects of AM251 on the toxicity of the parent compound parathion compared to its active metabolite paraoxon remains unclear. One possible explanation could be differential involvement of non-cholinergic neurotransmission. The delayed “recruitment” of non-cholinergic signaling (e.g., glutamate, GABA, or dopamine) following an initial cholinergic “surge” has been recognized for decades with severe intoxications with OP nerve agents (Shih et al. 1991; Jacobsson et al. 1997; Bourne et al. 2001; Dekundy et al. 2001, 2007). With the rapid onset (within 30 minutes) and recovery (by 24 hours) of acute toxicity associated with paraoxon exposure, hypercholinergic signaling may be the primary driving force in the expression of toxicity. Endocannabinoids may act to inhibit acetylcholine release, with a net decrease in cholinergic signs. Blocking cannabinoid receptors with AM251 may therefore increase paraoxon (and chlorpyrifos oxon) toxicity. With parathion, the relatively slow metabolic activation of the parent insecticide to its active metabolite leads to a relatively delayed onset (about 2 days after exposure) and protracted signs lasting for days (Karanth et al., 2007; Liu et al. 2013). Such conditions may favor “recruitment” of non-cholinergic signaling pathways. If eCB signaling modulates the release of a non-cholinergic neurotransmitter(s) that lessens the toxic impact of hypercholinergic signaling, blocking the effects of eCBs with AM251 could reduce toxicity, as seen with parathion (Liu et al., 2013). The parent insecticide parathion also inhibited hippocampal MAGL in vivo at both 2 and 4 days after exposure (Liu et al. 2013), while in the present studies paraoxon did not (Figure 3E). Thus differences in MAGL inhibition and possibly 2-AG clearance elicited by paraoxon and parathion under these conditions could contribute to the qualitatively different effects of AM251 on paraoxon- and parathion-induced toxicity noted (even though paraoxon is the ultimate toxicant in each case).
In summary, our findings suggest that eCBs can modulate the expression of acute OP toxicity. The cannabinoid CB1 receptor antagonist/inverse agonist AM251 increased involuntary movements elicited by either paraoxon or chlorpyrifos oxon at dosages causing relatively similar degrees of brain AChE and FAAH inhibition. Moreover, AM251 increased acute lethality, but only in paraoxon-treated rats. Together with data from studies on the AM251-mediated modulation of toxicity following exposure to the parent insecticides parathion and chlorpyrifos, these results suggest a complex role of eCBs and cannabinergic signaling in the expression of OP toxicity.
Highlights.
Both paraoxon and chlorpyrifos oxon inhibited monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH) activities in rat hippocampal homogenates in vitro.
At dosages of paraoxon and chlorpyrifos oxon leading to similar degrees of acetylcholinesterase inhibition and cholinergic signs, marked hippocampal FAAH inhibition was noted with both, but only chlorpyrifos oxon inhibited MAGL.
The cannabinoid CB1 receptor antagonist/inverse agonist AM251 increased cholinergic toxicity of paraoxon and chlorpyrifos oxon, and also increased acute lethality but only in paraoxon-treated rats.
Differences in MAGL inhibition may lead to selective toxicity with these two organophosphorus toxicants.
ACKNOWLEDGEMENTS
This research was supported by grant R01ES009119 from NIEHS and by the Oklahoma State University Board of Regents. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are no conflicts of interest.
References
- Banks CN, Lein PJ. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology. 2012;33:575–584. doi: 10.1016/j.neuro.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JA, Fosbraey P, Halliday J. SCH 23390 affords protection against soman-evoked seizures in the freely moving guinea-pig: a concomitant neurochemical, electrophysiological and behavioural study. Neuropharmacology. 2001;40:279–288. doi: 10.1016/s0028-3908(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J. Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Adams AL, Kepler DR, Ward AB, Ross MK. Induction of endocannabinoid levels in juvenile rat brain following developmental chlorpyrifos exposure. Toxicol. Sci. 2013;135:193–201. doi: 10.1093/toxsci/kft126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Gulevich AG, Sarpong R, Bunnelle EM. S-Arachidonoyl-2-thioglycerol synthesis and use for fluorimetric and colorimetric assays of monoacylglycerol lipase. Bioorg. Med. Chem. 2010;18:1942–1947. doi: 10.1016/j.bmc.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Casida JE, Nomura DK, Vose SC, Fujioka K. Organophosphate-sensitive lipases modulate brain lysophospholipids, ether lipids and endocannabinoids. Chem. Biol. Interact. 2008;175:355–364. doi: 10.1016/j.cbi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J. Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot A, Köfalvi A, Wade MR, Davis RJ, Rodrigues RJ, Rebola N, Cunha RA, Nomikos GG. CB1 receptor antagonism increases hippocampal acetylcholine release: site and mechanism of action. Mol. Pharmacol. 2006;70:1236–1245. doi: 10.1124/mol.106.024661. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Blaszczak P, Kaminski R, Turski WA. On the interactions between antimuscarinic atropine and NMDA receptor antagonists in anticholinesterase-treated mice. Arch. Toxicol. 2001;74:702–708. doi: 10.1007/s002040000189. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Kaminski RM, Zielinska E, Turski WA. NMDA antagonists exert distinct effects in experimental organophosphate or carbamate poisoning in mice. Toxicol. Appl. Pharmacol. 2007;219:114–121. doi: 10.1016/j.taap.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 2003;119:481–496. doi: 10.1016/s0306-4522(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–90. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Espinola EB, Oliveira MG, Carlini EA. Differences in central and peripheral responses to oxotremorine in young and aged rats. Pharmacol. Biochem. Behav. 1999;62:419–423. doi: 10.1016/s0091-3057(98)00192-0. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur. J. Neurosci. 2004;19:2682–2892. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Mascia MS, Casu MA, Carta G. Inhibition of hippocampal acetylcholine release by cannabinoids: reversal by SR 141716A. Eur. J. Pharmacol. 1997;327:R1–R2. doi: 10.1016/s0014-2999(97)89683-5. [DOI] [PubMed] [Google Scholar]
- Gifford AN, Ashby CR., Jr Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid antagonist, SR 141716A. J. Pharmacol. Exp. Ther. 1996;277:1431–1436. [PubMed] [Google Scholar]
- Gifford AN, Bruneus M, Gatley SJ, Volkow ND. Cannabinoid receptor-mediated inhibition of acetylcholine release from hippocampal and cortical synaptosomes. Br. J. Pharmacol. 2000;131:645–650. doi: 10.1038/sj.bjp.0703599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J. Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson SO, Sellström A, Persson SA, Cassel GE. Correlation between cortical EEG and striatal microdialysis in soman-intoxicated rats. Neurosci. Lett. 1997;231:155–158. doi: 10.1016/s0304-3940(97)00552-1. [DOI] [PubMed] [Google Scholar]
- Karanth S, Liu J, Ray A, Pope C. Comparative in vivo effects of parathion on striatal acetylcholine accumulation in adult and aged rats. Toxicology. 2007;239:167–179. doi: 10.1016/j.tox.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Weber B, Zimmer A, Schlicker E. Enhanced acetylcholine release in the hippocampus of cannabinoid CB(1) receptor-deficient mice. Br. J. Pharmacol. 2001;132:1169–1173. doi: 10.1038/sj.bjp.0703987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J. Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Parsons L, Pope C. Comparative effects of parathion and chlorpyrifos on extracellular endocannabinoid levels in rat hippocampus: influence on cholinergic toxicity. Toxicol. Appl. Pharmacol. 2013;272:608–615. doi: 10.1016/j.taap.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF. Dula blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. PNAS. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honoré E. The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. EMBO. J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VC, McCormick JP, Creason JP, MacPhail RC. Comparison of chlordimeform and carbaryl using a functional observational battery. Fundam. Appl. Toxicol. 1988;11:189–206. doi: 10.1016/0272-0590(88)90144-3. [DOI] [PubMed] [Google Scholar]
- Nallapaneni A, Liu J, Karanth S, Pope C. Modulation of paraoxon toxicity by the cannabinoid receptor agonist WIN 55,212-2. Toxicology. 2006;227:173–183. doi: 10.1016/j.tox.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Nallapaneni A, Liu J, Karanth S, Pope C. Pharmacological enhancement of endocannabinoid signaling reduces the cholinergic toxicity of diisopropylfluorophosphate. Neurotoxicology. 2008;29:1037–1043. doi: 10.1016/j.neuro.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Casida JE. Activity-based protein profiling of organophosphorus and thiocarbamate pesticides reveals multiple serine hydrolase targets in mouse brain. J. Agric. Food. Chem. 2011;59:2808–2015. doi: 10.1021/jf101747r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Blankman JL, Simon GM, Fujioka K, Issa RS, Ward AM, Cravatt BF, Casida JE. Activation of the endocannabinoid system by organophosphorus nerve agents. Nat. Chem. Biol. 2008;4:373–378. doi: 10.1038/nchembio.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tanimura A, Hashimotodani Y, Kano M. Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist. 2012;18:119–132. doi: 10.1177/1073858410397377. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Kano M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr. Opin. Neurobiol. 2014;29:1–8. doi: 10.1016/j.conb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Pope CN, Chakraborti TK, Chapman ML, Farrar JD, Arthun D. Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides. Toxicology. 1991;68:51–61. doi: 10.1016/0300-483x(91)90061-5. [DOI] [PubMed] [Google Scholar]
- Pope CN, Chakraborti TK, Chapman ML, Farrar JD. Long-term neurochemical and behavioral effects induced by acute chlorpyrifos treatment. Pharmacol Biochem Behav. 1992;42:251–256. doi: 10.1016/0091-3057(92)90523-i. [DOI] [PubMed] [Google Scholar]
- Pope C, Karanth S, Liu J. Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environ. Toxicol. Pharmacol. 2005;19:433–446. doi: 10.1016/j.etap.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE. Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides. Toxicol. Appl. Pharmacol. 2001;173:48–55. doi: 10.1006/taap.2001.9175. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Segall Y, Nomura DK, Casida JE. Selective inhibitors of fatty acid amide hydrolase relative to neuropathy target esterase and acetylcholinesterase: toxicological implications. Toxicol. Appl. Pharmacol. 2002;179:57–63. doi: 10.1006/taap.2001.9342. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Caboni P, Liang SN, Casida JE. Monoacylglycerol lipase inhibition by organophosphorus compounds leads to elevation of brain 2-arachidonoylglycerol and the associated hypomotility in mice. Toxicol. Appl. Pharmacol. 2006;211:78–83. doi: 10.1016/j.taap.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Savinainen JR, Saario SM, Laitinen JT. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta. Physiol. (Oxf) 2012;204:267–276. doi: 10.1111/j.1748-1716.2011.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TM, Koviak TA, Capacio BR. Anticonvulsants for poisoning by the organophosphorus compound soman: pharmacological mechanisms. Neurosci. Biobehav. Rev. 1991;15:349–362. doi: 10.1016/s0149-7634(05)80028-4. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit. Rev. Neurobiol. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- Sullivan JM. Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J. Neurophysiol. 1999;82:1286–1294. doi: 10.1152/jn.1999.82.3.1286. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Wade M, Nomikos GG. Biphasic effects of cannabinoids on acetylcholine release in the hippocampus: site and mechanism of action. J. Neurosci. 2003;23:9374–9384. doi: 10.1523/JNEUROSCI.23-28-09374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa NM, Deutsch DG. Assessment of a spectrophotometric assay for monoacylglycerol lipase activity. AAPS. J. 2010;12:197–201. doi: 10.1208/s12248-010-9180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. Cannabinoid receptors and their role in neuroprotection. Neuromolecular Med. 2005;7:37–50. doi: 10.1385/NMM:7:1-2:037. [DOI] [PubMed] [Google Scholar]
- Wright LK, Liu J, Nallapaneni A, Pope CN. Behavioral sequelae following acute diisopropylfluorophosphate intoxication in rats: comparative effects of atropine and cannabinomimetics. Neurotoxicol. Teratol. 2010;32:329–335. doi: 10.1016/j.ntt.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara S, Morimoto H, Ohori M, Yamada Y, Abe T, Arisaka O. Cannabinoid receptor agonists inhibit Ca(2+) influx to synaptosomes from rat brain. Pharmacology. 2006;76:157–162. doi: 10.1159/000091228. [DOI] [PubMed] [Google Scholar]