Abstract
Marble-burying behavior in rodents commonly has been used as an animal model of compulsive and/or anxiety behavior. The purpose of this study was to further assess the adequacy of marble burying as a preclinical animal model of compulsive behaviors using pharmacological tools. In particular, we were interested in whether dopamine D2/D3 agonists (e.g., pramipexole) known to produce compulsive behaviors in humans would increase marble burying. The effects of pramipexole on marble-burying behavior and locomotor activity were compared to those of diazepam, a drug known to decrease marble burying; d-amphetamine, a stimulant that produces increases in locomotor activity; and methyl beta-carboline-3-carboxylate (βCCM), a beta carboline previously shown to produce anxiogenic effects in rodents. All drugs produced dose-dependent decreases in marble burying, which were not always related to the locomotor effects of these drugs. The inability of pramipexole and βCCM to increase marble burying questions the validity of this assay as an adequate animal model of compulsive and/or anxiety behavior.
Keywords: marble burying, compulsion, anxiety, animal model, pramipexole, dopamine agonist, mouse
Introduction
Compulsive behavior is a common characteristic of a variety of behavioral disorders (e.g., drug abuse, obsessive-compulsive disorder). Although there is no single widely accepted animal model of compulsive behavior, several preclinical models have been proposed (Korff and Harvey, 2006). One such model is marble burying in rodents. Marble burying originally was proposed as a preclinical model of anxiety (Broekkamp et al., 1986). Evidence supporting the use of marble burying as an animal model of anxiety mainly came from studies reporting a decrease in marble burying produced by benzodiazepines (e.g., diazepam; Broekkamp et al., 1986) and serotonin reuptake inhibitors (SSRIs, e.g., citalopram; Njung'e and Hadley, 1991a), which also decrease anxiety in humans.
More recently, however, Thomas et al. (2009) suggested that marble burying was more adequately conceptualized as an animal model of compulsive or repetitive behavior. Not only did marble burying behavior not correlate with other behavioral measures of anxiety (e.g., open-field activity), but the most relevant aspect of marble burying appeared to be its repetitive nature that persisted across multiple exposures and despite environmental changes (e.g., novelty of item to be buried). Marble burying has yet to be pharmacologically validated as an adequate model of compulsive behavior.
Given that dopamine D2/D3 receptor agonists produce dose-dependent increases in compulsive behavior in a subset of individuals (e.g., pathological gambling, compulsive shopping, compulsive sexual behavior, and compulsive eating, among others; Salas et al., 2009; Weintraub et al., 2006, 2010), these compounds are useful pharmacological tools for evaluating marble burying as a model of compulsive behavior. The present study was designed to assess whether, as observed with humans (e.g., Weintraub et al., 2006), pramipexole would produce dose-dependent increases in compulsive behavior. More broadly, the present study sought to further evaluate the adequacy of marble burying as a preclinical animal model of compulsive behavior by assessing the effects of pramipexole on marble burying behavior and comparing it with the effects produced by other drugs.
Methods
Subjects
Male NIH Swiss mice (25-30 g) were obtained from Harlan Laboratories (Indianapolis, IN, USA). Mice were group housed 10 mice per Plexiglass cage (44.5 × 22.3 × 17.8 cm) in a climate-controlled room with a 12-h light/dark cycle, lights on at 7 a.m. Food and water were freely available in the home cage. Experiments were conducted in AAALAC accredited facilities and approved by the University of Michigan Committee for Use and Care of Animals.
Apparatus
A standard Plexiglas mouse cage, measuring approximately 26.7 × 17.8 × 12.7 cm, was used for experimental observations. The bottom of the cage was covered with 3 cm of corncob bedding, which was manually pressed to ensure even distribution. Black glass marbles, measuring approximately 1.27 cm in diameter, were arranged in two rows of five for a total of 10 marbles.
Experimental sessions were recorded using a video camera placed directly above the test cage.
Procedure
Mice were weighed and marked before testing sessions. Cages were prepared for testing by placing the bedding and arranging the marbles. Two mice were tested simultaneously in adjacent cages. Drugs were injected immediately prior to placing the mouse inside the test cage. Test sessions lasted 20 min.
During preliminary tests (not shown), informal observations suggested that doses that produced decreases in marble burying also produced a decrease in locomotor activity. To control for the possibility that locomotor impairment was interfering with marble-burying behavior, locomotor activity was recorded with a video camera during all tests. A sheet of Plexiglas with a marked 6-square grid was placed above the experimental cage. Each instance of crossing from one square to the next was counted as locomotor activity. Videos were coded at a later time by a research assistant blind to the experimental conditions. A marble was counted as buried if at least 2/3 of the marble was covered by bedding.
The effects of pramipexole were compared to the effects of diazepam, which previously has been shown to decrease marble burying in mice (Broekkamp et al., 1986). D-amphetamine was used as a pretreatment to assess whether drug-induced increases in locomotor activity would be related to increases in marble-burying behavior. Finally, the beta carboline βCCM, an anxiogenic compound, was used to assess whether marble-burying behavior, a purported animal model of anxiety, was as sensitive to pharmacologically induced increases by an anxiogenic compound as it is sensitive to decreases induced by anxiolytic compounds (e.g., benzodiazepines).
Drugs
Pramipexole was provided by Drs. Jianyong Chen and Shaomeng Wang (University of Michigan, Ann Arbor, MI, USA). Diazepam was purchased from Henry Schein Medical Supplies (henryschein.com) in a solution containing 40% propylene glycol, 10% alcohol, 5% sodium benzoate and benzoic acid, and 1.5% benzyl alcohol. D-amphetamine sulfate was obtained from Abbott Laboratories (Chicago, IL, USA). Methyl beta-carboline-3-carboxylate (βCCM) was provided by Dr. James M. Cook (University of Wisconsin, Milwaukee, WI, USA). All drugs were dissolved in sterile saline except βCCM, which was suspended in dimethyl sulfoxide (50% of final volume) and a drop of Tween 80 before diluting in saline. All drugs were administered intraperitoneally in a volume of 0.1ml/10g.
Data analysis
A one-way analysis of variance was used to evaluate the statistical significance of the drug effects on marble burying and locomotor activity. A Bonferroni test was used for pairwise comparisons. Statistical analyses were considered significant when p < 0.05.
Results
The number of marbles buried and locomotor activity during the 20-min observation period are displayed in the upper and lower panels of Figure 1, respectively. Consistent with previous findings, diazepam dose-dependently decreased marble-burying behavior. This decrease was not related to locomotor impairments, as the doses of diazepam used did not produce significant changes in locomotor activity. Pramipexole also produced dose-dependent decreases in marble burying, which were accompanied by decreases in locomotor activity. Similar effects were obtained with the anxiogenic βCCM, which decreased both marble burying and locomotor activity. Amphetamine also decreased marble burying, but increased locomotor activity.
Figure 1.
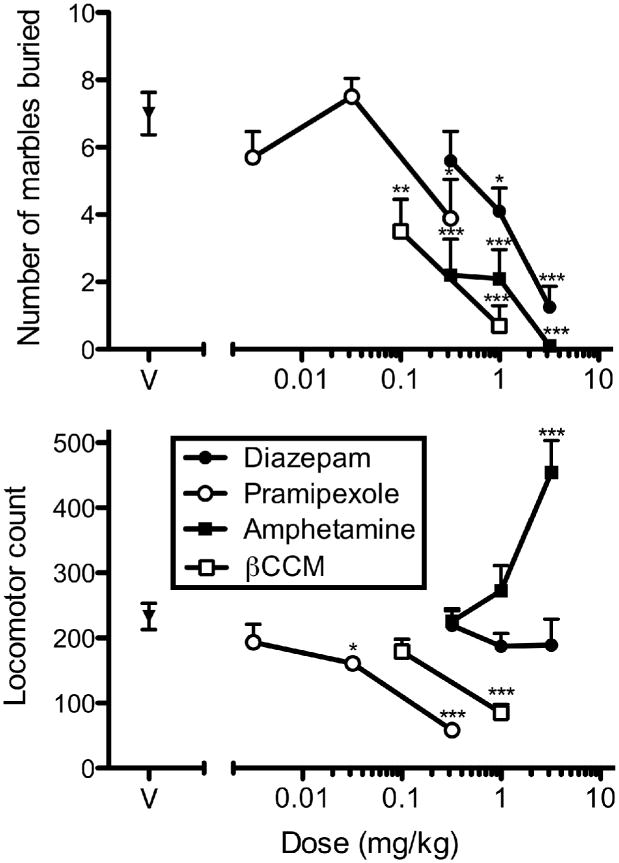
Number of marbles buried (upper panel) and locomotor counts (lower panel) after treatment with diazepam (closed circles), pramipexole (open circles), d-amphetamine (closed squares), or βCCM (open squares). Each data point represents mean values + SEM. Asterisks above data points denote the level of statistical significance relative to vehicle (* p=0.01-0.05; ** p=0.001-0.01; *** p<0.001).
Discussion
Dopamine D2/D3 receptor agonists, such as pramipexole, produce increases in compulsive behavior in humans (e.g., Weintraub et al., 2010), and thus can be useful pharmacological tools for assessing the adequacy of marble burying as a preclinical animal model of compulsive behavior. In the present study, the effects of pramipexole were compared to those of diazepam, a drug known to decrease marble burying, βCCM, an anxiogenic expected to increase marble burying if this behavior reflects anxiety, and amphetamine, a stimulant that increases locomotor activity. Pramipexole produced decreases in marble burying comparable to those produced by diazepam, amphetamine, and βCCM. It is important to note that these decreases in marble burying were not necessarily a result of locomotor impairment because diazepam decreased marble burying at doses that did not affect locomotor activity and amphetamine produced increases in locomotor activity that accompanied the decrease in marble burying.
Support for marble burying as a model of anxiety and/or compulsive behavior mainly derives from decreases in the behavior as a result of treatment with drugs used to treat the human condition (e.g., benzodiazepines, SSRIs; Broekkamp et al., 1986; Bruins Slot et al., 2008). In the animal literature there is no evidence that drugs that produce anxiety or compulsion increase marble burying. Njung'e and Hadley (1991b) assessed the effects of yohimbine, a purported anxiogenic compound, and found decreases in marble burying behavior, as in the present study with βCCM. It is possible, however, that marble-burying behavior was at an asymptote and could not be increased further or that the treatment regimen used in the present study is not adequate for testing marble burying as a model of compulsive behavior.
The possibility of burying behavior being at asymptote does not seem a likely explanation because increases in marble burying have been reported previously. Umathe et al. (2006) showed increases in marble burying during ethanol withdrawal, which they suggest could be reflecting the tendency for compulsive behavior seen in alcoholic individuals during abstinence periods. An important aspect to note is that in the study of Umathe et al. (2006), mice were exposed to ethanol for 7 consecutive days prior to testing. This chronic exposure to ethanol differs from the acute treatment regimen used in the present study and previous studies that failed to find increases in marble burying (e.g., Njung'e and Hadley, 1991b). Compulsive behaviors in humans are dose-dependent effects observed as a result of chronic treatment with dopamine agonists, such as pramipexole. Thus, it is possible that extended exposure to these compounds is necessary for compulsive behaviors to emerge in this behavioral assay. It also is possible that the relatively small number of mice used in the present study was insufficient to detect this side effect of pramipexole, which has been reported to occur in a small subset of individuals treated with dopamine agonists (6-15%; Weintraub et al., 2010). Finally, it is possible that marble burying does not model compulsive behavior per se, but serves to identify drugs with therapeutic potential for the treatment of anxiety and compulsive disorders, much in the same way as the forced swim test is used to identify drugs with anti-depressant properties.
Acknowledgments
CJ was supported at the University of Michigan by the National Institutes of Health under Ruth L. Kirschstein National Service Research Service Awards T32 DA007267 and DA007268. The authors thank Emily Jutkiewicz for help setting up the marble burying assay.
References
- Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL. Major tranquilizers can be distinguished from minor tranquilizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur J Pharmacol. 1986;126:223–229. doi: 10.1016/0014-2999(86)90051-8. [DOI] [PubMed] [Google Scholar]
- Bruins Slot LA, Bardin L, Auclair AL, Depoortere R, Newman-Tancredi A. Effects of antipsychotics and reference monoaminergic ligands on marble burying behavior in mice. Behav Pharmacol. 2008;19:145–152. doi: 10.1097/FBP.0b013e3282f62cb2. [DOI] [PubMed] [Google Scholar]
- Korff S, Harvey BH. Animal models of obsessive-compulsive disorder: Rationale to understanding psychobiology and pharmacology. Psychiatr Clin N Am. 2006;29:371–390. doi: 10.1016/j.psc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Njung'e K, Hadley SL. Effects of 5-HT uptake inhibitors, agonists and antagonists on the burying of harmless objects by mice; a putative test for anxiolytic agents. Br J Pharmacol. 1991a;104:105–112. doi: 10.1111/j.1476-5381.1991.tb12392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njung'e K, Hadley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991b;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- Salas RA, Allen RP, Earley CJ, Gamaldo CE. A case of compulsive behaviors observed in a Restless Legs Syndrome patient treated with a dopamine agonist. Sleep. 2009;32:587–588. doi: 10.1093/sleep/32.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacol. 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umathe S, Bhutada P, Dixit P, Shende V. Increased marble-burying behavior in ethanol-withdrawal state: Modulation by gonadotropin-releasing hormone agonist. Eur J Pharmacol. 2006;587:175–180. doi: 10.1016/j.ejphar.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, Moberg PJ, Stern MB. Association of dopamine agonist use with impulse control disorders in Parkinson Disease. Arch Neurol. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE. Impulse control disorders in Parkinson Disease: A cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]


