Abstract
Cocaine-experienced Wistar and Wistar Kyoto (WKY) rats received four daily repeated forced swim stress sessions (R-FSS), each of which preceded 4-hour cocaine self-administration sessions. Twenty-four hours after the last swim stress, cocaine valuation was assessed during a single-session threshold procedure. Prior exposure to R-FSS significantly altered cocaine responding in Wistar, but not WKY, rats. Behavioral economic analysis of responding revealed that the Wistar rats that had received R-FSS exhibited an increase in the maximum price that they were willing to pay for cocaine (Pmax). Pre-treatment with the long-lasting kappa opioid receptor (KOR) antagonist norbinaltorphimine prevented the stress-induced increase in Pmax. Thus, R-FSS exposure had strain-dependent effects on cocaine responding during the threshold procedure, and the stress effects on cocaine valuation exhibited by Wistar, but not WKY, required intact KOR signaling.
Keywords: Addiction, cocaine, kappa opioid receptor, self-administration, stress
Understanding how stress affects the reward systems to influence the development of drug addiction continues to be an essential step in identifying improved treatments for addiction and relapse prevention. Endogenous dynorphin neuropeptides, which activate kappa opioid receptors (KORs), play important roles in mediating both stress responses (Land et al. 2008) and reward (Wee & Koob 2010). Additionally, it has been reported that KOR antagonists possess antidepressant-like qualities when administered prior to forced swim stress (Van’t Veer & Carlezon 2013). Finally, it has been reported that KORs are involved in repeated forced swim stress (R-FSS) potentiation of cocaine-conditioned place preference (McLaughlin, Marton-Popovici & Chavkin 2003; Schindler et al. 2012), demonstrating a role for dynorphin in stress-induced enhancement of the rewarding value of cocaine.
Recently, Oleson, Richardson & Roberts (2011) described a behavioral economics approach to the analysis of cocaine self-administration responding obtained during a within-session descending dose threshold procedure. The purpose of this study was to utilize the threshold procedure (1) to assess the effect of R-FSS exposure on cocaine valuation in two strains of rats that exhibit differences in baseline stress responses (Carr & Lucki 2010) and (2) to characterize the involvement of KOR signaling in regulating these stress effects.
Forty-six male Wistar rats (Charles River Laboratories, Hollister, CA, USA) and 21 Wistar Kyoto (WKY) rats (Taconic, Germantown, NY, USA) (275–325 g) were surgically implanted with jugular catheters as previously described (Willuhn et al. 2012). Following 10–14 days of recovery, rats were trained to self-administer cocaine [National Institute on Drug Abuse Drug (NIDA) Supply Program] at 0.5 mg/kg per infusion on a fixed-ratio 1 (FR1) schedule. Each cocaine infusion was paired with a 20-second presentation of a cue (light and tone), dimming of the house light and a concurrent 20-second time-out during which responding in the active and inactive ports was recorded but had no programmed consequence. Four hours following the last acquisition session (and 72 hours prior to the first FSS exposure), rats received 10 mg/kg (intraperitoneal) of the long-lasting KOR antagonist norbinaltorphimine (norBNI; NIDA Drug Supply Program) or saline injection.
Seventy-two hours after the last training session, rats received a 15-minute swim session (23–25°C) 1 hour prior to prolonged access (4 hours) self-administration trials on 4 consecutive days. Twenty-four hours after the last R-FSS session, cocaine valuation was assessed utilizing a single within-session threshold procedure as previously described (Oleson et al. 2011; Oleson & Roberts 2012). Pilot data revealed that threshold responding (gPmax and total infusions) was consistent over multiple days of testing (Supporting Information Fig. S1); thus, a single-threshold procedure following stress exposure was utilized. Briefly, during the threshold procedure rats received access to a series of 11 descending unit doses of cocaine available during consecutive 10-minute bins by manipulating pump duration resulting in unit doses of 421, 237, 133, 75, 41, 24, 13, 7.5, 4.1, 2.4 and 1.3 µg per infusion. An FR1 response requirement was maintained but no contingent cues were presented, and the house light was illuminated throughout the duration of the session. The only time-out imposed corresponded to the pump duration for each given dose. Although the threshold procedure has been performed with (Bentzley, Fender & Aston-Jones 2013) and without (Oleson et al. 2011) presentation of drug-paired cues, cues were withheld during the threshold procedure to reduce the influence of conditioned reinforcement on threshold responding. Both the experimental timeline and design (including group size) are shown in Supporting Information Fig. S2.
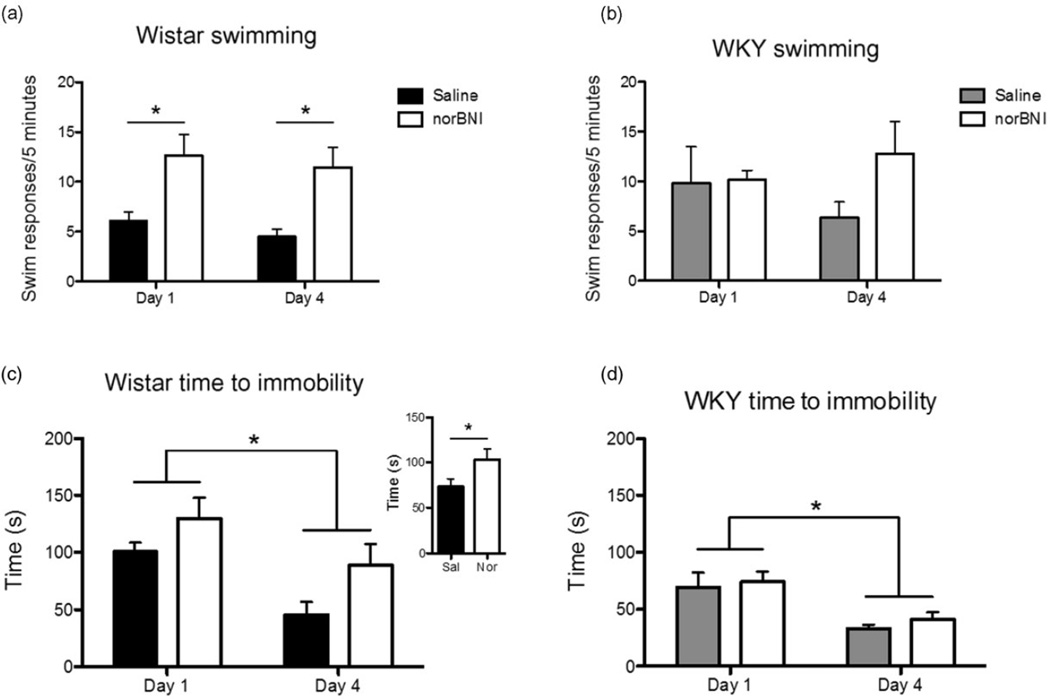
Analysis of the R-FSS data from the first and last swims (days 1 and 4) revealed that norBNI pre-treatment resulted in an increase in swimming behavior and concomitant decrease in time to immobility in Wistar but not WKY rats (Fig. 1). Specifically, analysis of the swimming behavior yielded a significant main effect of norBNI [F(1,21) = 14.0, P < 0.01; Fig. 1a and b] in Wistar, but not WKY, rats. Post hoc analysis revealed that the norBNI–pre-treated Wistar group exhibited more swimming behavior than the saline group on days 1 and 4 (P < 0.01). Additional analysis revealed that the norBNI pre-treated Wistar, but not WKY, rats showed significantly increased times to immobility as indicated by main effect of norBNI [F(1,21) = 4.9, P < 0.05; Fig. 1c and d] despite all rats showing a decrease in time over days as indicated by a main effect of day [Wistar: F(1,21) = 15.8, P < 0.001; WKY: F(1,9) = 14.4, P < 0.005]. Previous reports have established that increased swimming behavior and decreased immobility (and time to become immobile) are both indicative of depressive-like behaviors in rats (e.g. Lucki 1997; Pliakas et al. 2001). Thus, in the current experiments norBNI pre-treatment reduced depressive-like behaviors in cocaine-exposed Wistar, but not WKY.
Figure 1.
(a, b) Pre-treatment with norbinaltorphimine (norBNI) resulted in an increase in swimming behavior across the four repeated forced swim stress (R-FSS) sessions in Wistar (a), but not Wistar Kyoto (WKY), (b) rats. Behavioral counts from days 1 and 4 of the R-FSS expressed as mean [±standard error of the mean (SEM)] swimming responses observed during the first 5 minutes of each swim session. * denotes P < 0.05 for Bonferroni-corrected pairwise comparisons following a significant main effect of norBNI. (c, d) Time to immobility decreased over the course of R-FSS in all Wistar (c) and WKY (d) rats, whereas norBNI pre-treatment caused a general increase in time to immobility only in Wistar rats [mean (±SEM) seconds]. * denotes P < 0.05 for the main effect of day for both strains. Inset shows the significant main effect of norBNI pre-treatment on time to immobility in Wistar rats where * denotes P < 0.05 for the main effect of norBNI
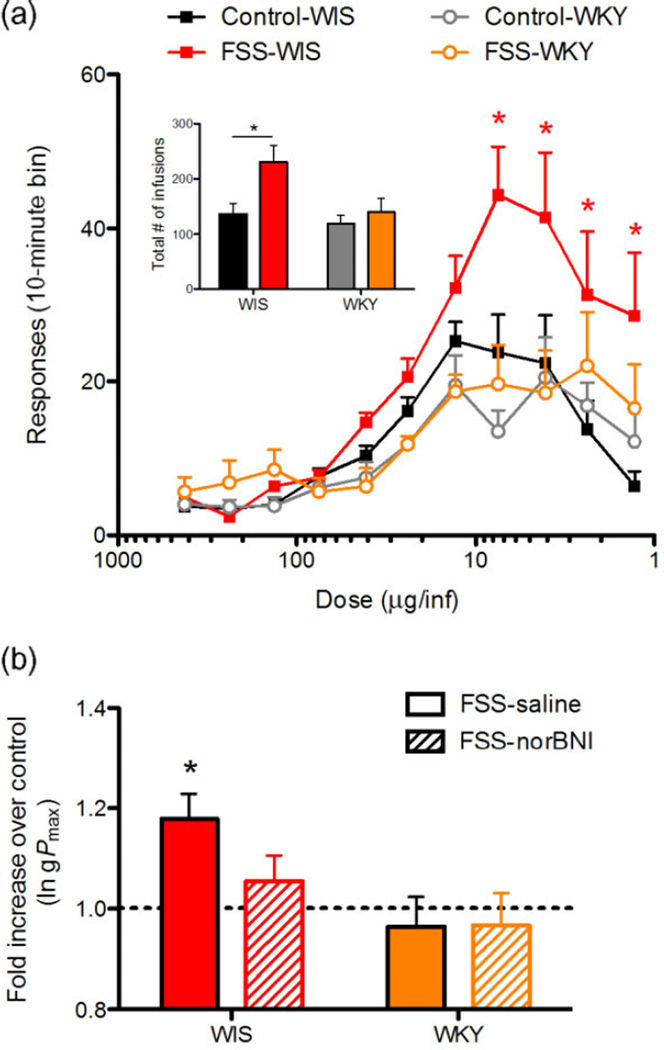
Initial analysis of responding during the threshold procedure revealed that R-FSS caused a significant increase in responding at multiple doses in saline-pretreated Wistar, but not WKY, rats. Prior exposure to R-FSS increased responding (most prominently at the lower unit doses) in only the Wistar rats (Fig. 2a). Analysis of the dose–response functions for the two Wistar groups (control and R-FSS) yielded a significant group × cocaine dose interaction [F(10,23) = 3.4, P < 0.001] and main effects of both group [F(1,23) = 7.8, P < 0.05] and cocaine dose [F(10,23) = 21.4, P < 0.0001], whereas the analysis for the two WKY groups yielded only a main effect of cocaine dose [F(10,10) = 8.3, P < 0.0001]. Post hoc comparisons of the two Wistar groups at each dose showed a significant difference at the four lowest doses (Ps < 0.05). Additionally, analysis of the total number of infusions self-administered by all four groups during the threshold procedure (Fig. 2a, inset) yielded a significant main effect of swim [F(1,33) = 8.3, P < 0.05] with a significant difference between control and R-FSS groups only in Wistar rats (P < 0.01). Together, these results demonstrate clear strain-dependent effects of R-FSS exposure on responding for diminishing doses of cocaine.
Figure 2.
(a) Repeated forced swim stress (R-FSS) exposure resulted in enhanced responding by Wistar, but not Wistar Kyoto (WKY), rats for descending unit doses of cocaine during the threshold procedure. Data expressed as mean [±standard error of the mean (SEM)] responses in each 10-minute dose bin. * denotes P < 0.05 for Bonferroni-corrected pairwise comparisons following a significant group × cocaine dose interaction. Inset shows that R-FSS exposure increased the total number of infusions in the Wistar, but not WKY, rats. Data expressed as mean (±SEM) number of total infusions during the entire threshold procedure. * denotes P < 0.05 for Bonferroni-corrected pairwise comparisons. (b) R-FSS exposure increased gPmax over controls in Wistar, but not WKY, rats and was blocked by pre-treatment of norbinaltorphimine (norBNI). Data expressed as a fold increase in gPmax over each group’s respective control group. * denotes P < 0.05 for a one-sample t-test comparing each group mean to 1.0
To assess the involvement of KOR signaling in mediating these effects of stress, a behavioral economic metric of the maximal price paid was determined graphically (gPmax) for each rat (Oleson et al. 2011; Oleson & Roberts 2012). Given that all doses were administered on an FR1 schedule, the maximal price paid (Pmax) reflects the descending dose of cocaine at which maximum responding occurs. Analysis revealed that norBNI pre-treatment significantly attenuated the R-FSS-induced increase in the gPmax of Wistar, but not WKY, rats (Fig. 2b). Specifically, when the gPmax values of each of the four R-FSS groups were normalized to their respective drug pretreatment control group, analysis revealed that only the saline pre-treated Wistar rats displayed a significant stress-induced increase in gPmax over controls [t(11) = 3.6, P < 0.005]; in contrast, norBNI–pre-treated Wistar rats did not show stress-induced increase in gPmax. Neither the saline- nor norBNI–pre-treated WKY rats showed stress-induced changes in gPmax.
This set of experiments is the first to utilize the threshold procedure to characterize the effects of R-FSS on cocaine valuation in two different strains of drug-experienced rats. Furthermore, this study provides novel insight into the role of KOR signaling in regulating the effects of R-FSS on the behavioral economics of cocaine self-administration. Analysis of self-administration responding from the threshold procedure provides a unique means with which to assess multiple aspects of the characteristics of human drug dependence including an increase in energy and time devoted to acquiring drug and an increase in drug intake over time (Oleson et al. 2011). However, as this procedure has only recently been described, the mechanisms responsible for responding during the threshold procedure remain unknown. It is possible that components of behavioral extinction, learning, drug reinforcement/reward and drug sensitivity contribute to changes in responding. As such, future studies are required to further characterize the underlying mechanisms regulating the strain- and KOR-dependent effects of R-FSS reported here. However, irrespective of the specific behavioral component affected, we identified a stress effect on cocaine valuation that is relevant to the development of drug addiction.
Administration of the long-lasting KOR antagonist norBNI prior to stress exposure (but after stable acquisition of self-administration behavior) showed that blockade of KORs reduced depressive-like behaviors in Wistar, but not WKY, rats during swim stress sessions. It has been reported that although WKY rats display increased basal depressive-like behaviors, they remain sensitive to the antidepressive effects of norBNI (Carr & Lucki 2010). Similar to Carr & Lucki (2010), the current data show that WKY rats display decreased times to immobility but the effects of norBNI in WKY rats did not attain significance despite showing a similar trend as that reported by Carr & Lucki (2010). As suggested in previous reports (e.g. Chartoff et al. 2012), it is possible that treatment history (cocaine self-administration training) may have influenced the expected antidepressant effects of norBNI in the WKY rats.
In conclusion, these experiments are the first to report the ability of prior R-FSS to significantly alter cocaine responding during the threshold procedure in a strain-dependent manner. Specifically, R-FSS exposure caused a significant KOR-dependent increase in the maximum price that the Wistar, but not WKY, rats were willing to pay for cocaine (gPmax). These experiments suggest that exposure to stress followed by long periods of drug access could lead to alterations in subjective drug value that may cause a recreational drug user to exhibit increased motivation to obtain drug despite heightened risk and/or cost. Additionally, these studies demonstrate that the susceptibility to stress effects on cocaine responding are influenced by genetic factors and, in rodents, are strain dependent. Furthermore, this study supports the notion that KOR antagonists may promote stress resilience and could therefore be a valuable clinical target for helping to dampen the effects of stress on the development of, and relapse to, drug addiction.
Supplementary Material
Acknowledgements
We would like to thank Scott Ng-Evans, PhD, for his assistance with programming and data analysis. We also thank Dr. Gary Aston-Jones for initially suggesting the utility of the Oleson-Roberts threshold procedure. Additionally, this work was supported by grants from the NIH (DA033004, DA030074 and DA020570) and the University of Washington Alcohol & Drug Abuse Institute.
Footnotes
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
Authors Contributions
PAG and CZ performed all experiments; PAG, CZ, IW, PEMP and CC designed experiments; PAG and CC prepared the manuscript, and IW and PEMP provided comments on the manuscript.
References
- Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 2013;226:113–125. doi: 10.1007/s00213-012-2899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Lucki I. Comparison of the kappa-opioid receptor antagonist DIPPA in tests of anxiety-like behavior between Wistar Kyoto and Sprague Dawley rats. Psychopharmacology (Berl) 2010;210:295–302. doi: 10.1007/s00213-010-1832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC. Cocaine self-administration in rats: threshold procedures. Methods Mol Biol. 2012;829:303–319. doi: 10.1007/978-1-61779-458-2_20. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DC. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pretreatments on cocaine consumption and maximal price paid. Psychopharmacology (Berl) 2011;214:567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Messinger DI, Smith JS, Shankar H, Gustin RM, Schattauer SS, Lemos JC, Chavkin NW, Hagan CE, Neumaier JF, Chavkin C. Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake. J Neurosci. 2012;32:17582–17596. doi: 10.1523/JNEUROSCI.3220-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, Phillips PE. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci U S A. 2012;109:20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.