Abstract
Expanding knowledge about the crucial roles of microRNAs (miRNAs) in human diseases has led to the idea that miRNAs may be novel, promising therapeutic targets against various pathological conditions. The recent success of a human clinical trial using anti-miR-122 oligonucleotides against chronic hepatitis C virus has paved the way for this approach. In this review, we summarize briefly the current status of clinical trials of miRNA-targeting therapy and several representative preclinical trials against hepato-gastrointestinal carcinoma. In addition, we describe the currently available technologies for modification and delivery of oligonucleotides, which are essential in providing efficient, specific and safe approaches to targeting miRNAs.
Keywords: Locked nucleic acids, Miravirsen, MicroRNA, Therapeutics
Introduction
The expression and functional importance of non-coding RNAs (ncRNAs), such as long ncRNAs and microRNAs, in various human diseases has been reported extensively [1–3]. Accordingly, clinical applications of ncRNAs are highly anticipated [4].
MicroRNAs (miRNAs) are small non-coding RNAs first discovered in C. elegans[5]. They are now known to be expressed in most organisms from plants to vertebrates [6]. Many miRNAs are functionally important, acting as oncogenes, tumor suppressors and crucial modulators in intracellular pathways [7].
miRNAs are generated from endogenous transcripts through maturation processing. Long primary miRNAs (pri-miRNAs) ranging in size from several hundred nucleotides (nt) to several kilobases are transcribed from the genome [8]. These are processed into stem-loop precursor miRNAs (pre-miRNAs) of ~70 nt by a ribonuclease III (RNase III) known as Drosha and DGCR8/Pasha in the nucleus [8–11]. After these processing steps, pre-miRNAs are transported to the cytoplasm via exportin-5 [12], where they are recognized by an RNA-induced silencing complex (RISC). This complex is composed of the RNase III Dicer, a double-strand RNA binding protein TRBP, and Argonaute2 (Ago2). Pre-miRNAs are cleaved into mature miRNAs of ~22 nt by Dicer [13]. The two RNA strands are separated, and the guide strand for the target mRNA remains associated with Ago2. RISC recognizes the target mRNA based on the complementarity between the guide miRNA and the mRNA transcript [14] within the 3′ untranslated region (UTR) [15]; the target mRNA is subsequently degraded or translationally inhibited [14, 16], resulting in post-transcriptional gene silencing [14]. While the molecular mechanisms for gene silencing by miRNA-mRNA targeting require further elucidation, some reports have suggested that miRNAs may sequester target mRNAs into P-bodies [17], where mRNA decay occurs through the initiation of rapid deadenylation [18] or translational repression occurs by inhibiting the binding of ribosomes to the 5′ caps of miRNAs [19].
Alterations in miRNA expression levels contribute to the pathogenesis of human malignancies. The changes result from various mechanisms, including deletions, amplifications or mutations at miRNA loci, epigenetic silencing, dysregulation of transcription factors that are related to the transcription of specific miRNAs, environmental factors such as cigarette smoke and infection, and gene polymorphisms [20–22]. Describing specific patterns of miRNA expression levels may be useful for diagnosis, prognosis or evaluating therapeutic response, or in miRNA-targeted therapies that repress or facilitate expression of specific miRNAs.
Many excellent reviews have discussed the aberrant expression and potential biological roles of miRNAs in gastroenterological diseases [23–28]. Here, we focus on the recent progress of clinical trials of miRNA-target therapies and representative preclinical trials against gastroenterological carcinoma; additionally, we outline the future work required for clinical utilization of miRNAs.
Review
Current clinical applications of miRNA-targeting therapeutics
Anti-miR-122 therapy against chronic hepatitis C
Experiments in vitro and in vivo have led to the development of potential new therapies targeting miRNAs. While targeting miRNAs in human clinical trials has focused largely on miRNA signatures as biomarkers for the diagnosis, prognosis, or therapeutic response to traditional treatment [22], two human clinical trials have assessed directed miRNA-targeting as therapeutics, according to ClinicalTrials.gov (http://clinicaltrials.gov) (Table 1). Both trials are related to gastroenterological diseases.
Table 1.
Current clinical applications targeting miRNAs in human
| miRNA | NIH identifier | Drug | Subjects | Outline/purposes | Reference |
|---|---|---|---|---|---|
| miR-122 | NCT01646489 | Miravirsen Telaprevir | Hapatitis C chronic hepatitis C | To assess the safety, tolerability, and affect on blood levels of miravirsen and telaprevir when co-administered miravirsen and telaprevir in healthy subjects. <Phase 1> | |
| NCT01872936 | Miravirsen Telaprevir Ribavirin | Chronic hepatitis C (genotype1) Null responders to treatment with peg IFNα/RBV therapy. | To assess the safety, tolerability, antiviral activity, genotype resistance associated with virological failure, pharmacokinetics and pharmacodynamics of two dose regimens of miravirsen in combination with telaprevir and ribavirin in subjects with hepatitis C virus genotype 1 infection. <Phase 2> | ||
| NCT01200420 | Miravirsen | Hepatitis C | 1. Determining the safety and tolerability of multiple dosing of miravirsen in subjects infected with chronic hepatitis C. | [29] | |
| Saline | |||||
| 2. Assessing of pharmacokinetics of miravirsen and assessment of miravirsen's effect on HCV viral titer. <Phase 2> | |||||
| NCT01727934 | Miravirsen | Hepatitis C (genotype1) Null responders to treatment with peg IFNα/RBV therapy. | To aseess the safety, antiviral activity, and pharmacokinetics of 9 subcutaneous injections of miravirsen monotherapy over a total of 12 weeks of treatment.<Phase 2> | ||
| NCT00688012 | SPC3649 | Hepatitis C | A placebo-controlled, double-blind, randomized, single dose, dose escalating trial in healthy men to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of SPC3649. <Phase 1> | [30] | |
| miR-34 | NCT01829971 | MRX-34 | Primary HCC metastatic liver cancer | Evaluating the safety of MRX34 in patients with primary liver cancer or those with liver metastasis from other cancers. <Phase 1> |
SPC3649: the active component of miravirsen.
One of the most extensively studied miRNAs is miR-122, an abundant liver-specific miRNA that plays a critical role in liver function, such as fatty acid and cholesterol metabolism, and in the pathophysiology of liver diseases, such as hepatitis C viral (HCV) replication [11, 31–33]. Inhibition of miR-122 with locked-nucleic-acid (LNA)-based anti-miR-122 oligonucleotides complementary to miR-122, caused a long-lasting decrease in total plasma cholesterol in mice [31] and in monkeys [34]. Lanford et al. demonstrated that LNA-based anti-miR-122 oligonucleotides led to the long-lasting suppression of HCV viremia and improvement of HCV-induced liver pathology in chimpanzees [33]. In these three cases, no LNA-associated toxicity or histopathological changes were found in mice and non-human primates after short-term administration of the oligonucleotides [31, 33, 34]. These reports indicate that LNA-based anti-miRs can achieve efficient silencing of endogenous miRNA function in mammals and primates, which supports the application of anti-miRNA therapy to other human diseases.
These preclinical results led to the development of miravirsen, a LNA-modified DNA phosphorothioate antisense oligonucleotide against miR-122, as the first miRNA-targeting drug for clinical use [29]. It was developed to target HCV since the stability and propagation of HCV is dependent on a functional interaction between the HCV genome and miR-122 [35]. miR-122 binds to two closely spaced target sites in the highly conserved 5′-untranslated region of the HCV genome, thereby forming an oligomeric miR-122-HCV complex that protects the HCV genome from nucleolytic degradation or from host innate immune responses [35]. The miR-122 binding sites are conserved across all HCV genotypes and subtypes [36]. Miravirsen, an LNA-modified DNA phosphorothioate oligonucleotide complementary to miR-122, is thought to hybridize to the 5′ region of mature miR-122, resulting in sequestration and inhibition of miR-122 [29]. Recently, it was reported that Miravirsen also binds to the stem-loop structure of pri- and pre- miR-122 and inhibits both Dicer- and Drosha- mediated processing of miR-122 precursors [30] (Figure 1).
Figure 1.
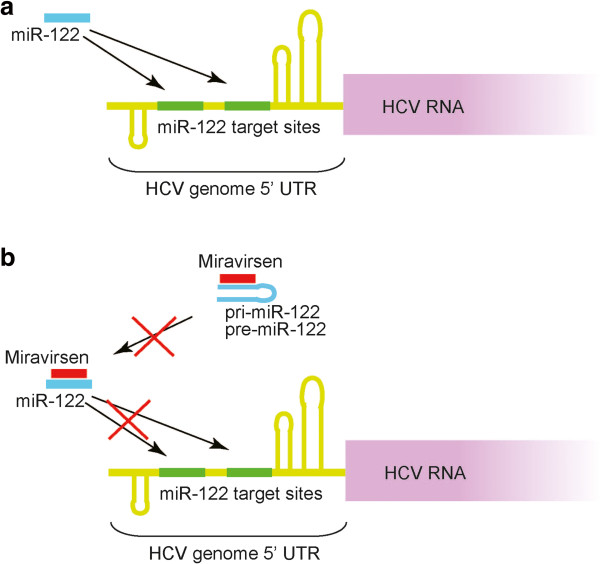
Miravirsen inhibits miR-122. a, Mir-122 binds two target sites in the HCV 5′ non-coding region and promotes HCV propagation. b, Miravirsen, a modified oligonucleotide complementary to miR-122 sequences, binds and sequesters mature miR-122, resulting in the functional inhibition of miR-122. Miravirsen also binds to the stem-loop structure of pri- and pre-miR-122 and inhibits the maturation of miR-122.
No harmful events were observed in phase I studies of miravirsen in healthy volunteers; therefore, phase II studies were initiated to evaluate the safety and efficacy of miravirsen in 36 patients with chronic HCV genotype 1 infection. The patients were randomly assigned to receive 5-week subcutaneous injections of placebo or doses of miravirsen at 3, 5 or 7 mg per kilogram of body weight over a 29-day period. Patients who received miravirsen showed a dose-dependent reduction in HCV levels, without major adverse events and with no escape mutations in the miR-122 binding sites of the HCV genome [29].
The success of miravirsen is promising, not only as a novel anti-HCV drug, but also as the first trial of miRNA-targeting therapy. However, caution should be used since miR-122 is generally known as a tumor-suppressive miRNA. Downregulation of miR-122 expression in hepatocellular carcinoma (HCC) is associated with a poor prognosis [37–39], and liver tumorigenesis is facilitated in mice lacking miR-122 [40, 41]. Nonetheless, as an anti-HCV drug, short-term administration of miravirsen with a 4-week regimen was reversible, and the effects of a 12-week regimen were tested from November 2012–May 2013 (ClinicalTrials.gov Identifier: NCT01727934). Although miravirsen also showed promise for decreasing serum cholesterol levels, we cannot conclude that miravirsen remains free of adverse effects for long-term administration until a long-term trial is completed.
miR-34 mimics as a therapeutic against primary and metastatic liver cancer
In addition to miravirsen, a clinical trial of MRX34 as a mimic of miR-34 is ongoing. MRX34 is a liposome-formulated mimic of the tumor suppressor miR-34 (Mirna Therapeutics, Austin, TX). The expression levels of miR-34 are decreased in most human cancers [42–44], including several epithelial cancers, melanomas, neuroblastomas, leukemias and sarcoma [45]. miR-34 is involved in regulating the p53 pathway and inhibits cancer cell growth by directly targeting oncogenes such as Myc, c-Met, Bcl-2, CDK4, CDK6, Cyclin D1, and Cyclin E2 [42, 43, 46]. Liu et al. evaluated the survival of mice with established prostate tumors that received a miR-34 injection [43]. The authors reported that miR-34a extended the survival of tumor-bearing mice compared to mice that did not receive a miR-34 injection. Hu et al. demonstrated that systemic administration of a miR-34a delivery system in a pancreatic xenograft cancer model significantly inhibited tumor growth and induced cancer cell apoptosis [47]. Further study of MRX34 is being conducted by Mirna Therapeutics, which initiated a Phase I study in May 2013 to examine the effects of MRX34 on unresectable primary liver cancer or advanced or metastatic cancer with liver involvement (ClinicalTrials.gov Identifier: NCT01829971).
Representative preclinical in vivo experiments
Since their discovery, many novel miRNAs have been identified. As of September 2013, 2,578 mature human miRNA sequences were deposited in miRBase, a public repository hosted by the Sanger Institute (Cambridge, UK). miRNAs have diverse biological functions as they can target mRNAs with weak complementarity. Thus, it is not surprising that most miRNAs are associated with facilitating or suppressing tumors via modulating the expression levels of various tumor-related genes. The PubMed database contains nearly 25,000 miRNA-related articles, many of which aimed to identify novel targets of miRNAs, novel miRNAs, or novel functions of miRNAs in oncogenic/tumor suppressive function or stabilization. The majority of these in vitro studies need to be confirmed in mice or in non-human primates before their use in clinical trials. In the following section, we describe representative in vivo approaches to targeting miRNAs in hepato-gastroenterological cancers, which have evaluated the effects on the initiation, proliferation or growth of tumors in transgenic mice or an in vivo xenograft cancer model (Figure 2 and Table 2).
Figure 2.
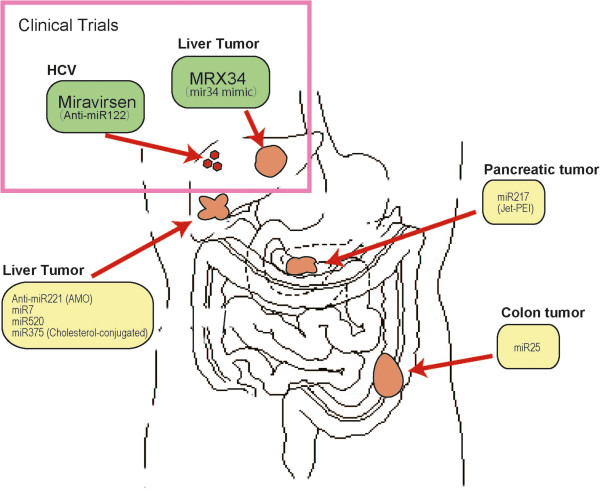
Representative clinical and preclinical trials targeting miRNAs. Currently on-going clinical trials and representative preclinical studies targeting miRNAs against cancers in the gastroenterological field.
Table 2.
Representative preclinical in vivo experiments
| miRNA | Cancer | Target | Result | Reference |
|---|---|---|---|---|
| miR-221 | HCC | CDKN1B/p27, CDKN1C/p57, Bmf, PTEN, TIMP3, DDIT4, mTOR | A transgenic mouse model of miR-221 overexpression in the liver was established, which is characterized by the inevitable appearance of spontaneous liver tumors with diethylnitrosamine. When received an in vivo intravenous injection of anti-miR-221 oligonucleotides exhibited a significant reduction in the number and size of liver tumor nodules. | [48] |
| miR-7 | HCC | PIK3CD | In a xenograft model, overexpressed miR-7 effectively repressed tumor growth and decreased metastasis to the lung. | [49] |
| miR-520e | HCC | NIK | HepG2 cells transfected with miR-520e or a negative control were injected subcutaneously into nude mice. The introduction of miR-520e led to a significant reduction in both the size of tumor volume and the frequency of tumor formation. In addition, direct intratumoral injection with miR-520e oligonucleotides repressed the growth of HCC cells in an in vivo xenograft model. | [50] |
| miR-375 | HCC | AEG-1 | Overexpression of miR-375 in liver cancer cells decreased cell proliferation, clonogenicity, migration, and invasion, and induced G1 cell cycle arrest and apoptosis. Direct administration of cholesterol-conjugated 2’-O-methyl-modified miR375 mimics significantly affected the growth of HCC xenografts. | [51] |
| miR-25 | Colon cancer | Smad7 | In a xenograft model study, stable overexpression of miR25 in colon cancer cells suppressed tumor growth. | [52] |
| miR-217 | PDCA | KRAS | Xenograft tumors of PDAC cells were directly injected with miR217-expressing plasmids or a control vector using in vivo-jet PEI. The results from these assays indicated that miR-217 suppresses tumor cell growth in vivo. | [53] |
Oncogenic miRNA: miR-221.
Tumor suppressive miRNA: miR-7, 520, 375, 25,217.
miR-221 as an oncogenic miRNA
miR-221 is one of the most frequently and consistently upregulated miRNAs in human cancer, including in HCC, pancreatic, colon, stomach, glioblastoma, kidney, bladder, prostate and thyroid cancer, which indicates its importance in tumorigenesis [48]. miR-221 has multiple gene targets, such as the cyclin-dependent kinase inhibitors p27Kip1 (CDKN1B/p27) [54, 55] and CDKN1C/p57 [55], the pro-apoptotic protein B-cell lymphoma 2-modifying factor (Bmf) [56], the inhibitor of the phosphoinositide 3-kinase pathway phosphatase and tensin homolog (PTEN) [57], the tissue inhibitor of metalloproteinase 3 (TIMP3) [57], the DNA damage- inducible transcript 4 (DDIT4), and a tumor suppressor that modulates the kinase activity of mammalian target of rapamycin (mTOR) [58].
Callegari et al. demonstrated that miR-221 could promote liver tumorigenicity in a transgenic mouse model of miR-221 overexpression in the liver. This model is characterized by the appearance of spontaneous liver tumors in a fraction of male mice and a strong acceleration of tumor development in 100% of mice treated with diethylnitrosamine (DEN). Ten-day old mice received one intraperitoneal injection of DEN, followed 2 months later by a single intravenous dose of anti-miR-221 oligonucleotide (AMO) diluted in saline solution every 15 days, for a total of three injections. Similar to human HCC, tumors in these mice were characterized by an increase in miR-221 expression and a concomitant inhibition of its target protein-coding genes (CDKN1B/p27 and Bmf, not CDKN1C/p57). As expected, mice that received an in vivo intravenous injection of anti-miR-221 oligonucleotides exhibited a significant reduction in the number and size of liver tumor nodules. This study not only shows that miR-221 can promote liver tumorigenicity, but it also establishes a valuable animal model for preclinical investigations of the use of anti-miRNA approaches to liver cancer therapy [48].
Tumor-suppressive miRNAs
Many studies have used the nude mouse xenograft model to evaluate the effects of a specific miRNA on tumorigenesis, tumor growth or metastasis in vivo. In particular, this model is commonly used to assess xenograft growth or progression after transplantation of cancer cells transfected with specific miRNA-expressing plasmids or empty vectors.
miR-7 in hepatocellular carcinoma (HCC) cells
miR-7 inhibits HCC cell growth and metastasis in vitro and in vivo. Phosphoinositide 3-kinase catalytic subunit delta (PIK3CD) was first identified as a miR-7 target, and further study suggested that miR-7 might be a key regulator of the PI3K/Akt/mTOR signaling pathway. In a xenograft model, overexpressed miR-7 effectively repressed tumor growth and decreased metastasis to the lung. These findings indicate that miR-7 functions as a tumor suppressor and plays a substantial role in inhibiting the tumorigenesis and reversing the metastasis of HCC through the PI3K/Akt/mTOR-signaling pathway. Given these results, miR-7 may be a potential therapeutic or diagnostic/prognostic target for treating HCC [49].
miR-520 in hepatocellular carcinoma (HCC) cells
The expression levels of miR-520e were decreased dramatically in HCC cells and clinical HCC tissues resulting from DNA hypermethylation in the upstream region of miR-520e locus, whereas silencing of the expression of miR-520e promoted cell proliferation [50]. Introduction of miR-520e suppresses the growth of HCC cells in vitro by targeting NF-κB-inducing kinase (NIK), which is involved in NIK/ERK/NF-κB signaling. To determine the effect of miR-520e on HCC cell growth in vivo, HepG2 cells transfected with miR-520e or a negative control were injected subcutaneously into nude mice. The introduction of miR-520e led to a significant reduction in both the size of tumor volume and the frequency of tumor formation. In addition, direct intratumoral injection with miR-520e oligonucleotides repressed the growth of HCC cells in an in vivo xenograft model. This finding provides new insight into the mechanism of hepatocarcinogenesis, indicating the therapeutic potential of miR-520e in the treatment of HCC [50].
miR-375 in hepatocellular carcinoma (HCC) cells
He et al. reported that miR-375 targets astrocyte elevated gene-1 (AEG-1) in HCC and suppresses liver cancer cell growth in vitro and in vivo. Overexpression of miR-375 in liver cancer cells decreased cell proliferation, clonogenicity, migration, and invasion, and induced G1 cell cycle arrest and apoptosis. Direct administration of cholesterol-conjugated 2’-O-methyl-modified miR375 mimics significantly affected the growth of HCC xenografts. These findings indicate that miR-375 targets AEG-1 in HCC and suppresses liver cancer cell growth [51].
miR-25 in colon cancer cells
Li et al. reported that miR-25 was significantly down-regulated in human colon cancer tissues, and identified Smad7 as its direct target. In a xenograft model study, stable overexpression of miR25 in colon cancer cells suppressed tumor growth [52]. These results suggest that miR-25 functions as a tumor suppressor by targeting Smad7 in colon cancer, suggesting that miR-25 may serve as a potential therapeutic target for colon cancer therapy.
miR-217 in pancreatic cancer cells
In most studies, cells overexpressing miRNAs or miRNA antagonists are used for xenografts and subsequent analyses. However, Zhao et al. reported another method of evaluating the function of miRNAs in vivo. They investigated the biological role of miR-217 in PDAC cells in vitro and in vivo since miR-217 is frequently down-regulated in pancreatic ductal adenocarcinoma (PDCA) [59, 60]. KRAS was identified as a direct target of miR-217 and, concordantly, up-regulation of miR-217 decreased KRAS protein expression and subsequently reduced the constitutive phosphorylation of downstream Akt. To confirm the function of miR-217 in vivo, xenograft tumors of PDAC cells were directly injected with miR217-expressing plasmids or a control vector using in vivo-jet PEI (Polyplus Transfection, Illkirch, France). The results from these assays indicated that miR-217 suppresses tumor cell growth in vivo. Therefore, miR-217 may serve as a therapeutic target for miRNA-based PDAC therapy [53].
Development of effective delivery methods for miRNA-targeting oligonucleotides
The development of effective and safe delivery methods of miRNA-targeting molecules is critical to the success of miRNA-targeting drugs. Currently, modified oligonucleotides and various delivery particles are being used for these purposes (Table 3).
Table 3.
Comparison of systemic delivery methods
| Delivery method | Features | Advantage and disadvantage | Reference |
|---|---|---|---|
| AMOs | Complementary to mature miRNAs | AMOs are widely used to inhibit miRNAs in vitro and in vivo. | |
| Modified AMOs | |||
| -OMe | 2′-O-methyl modification | Modified AMOs have more stability and efficiency than AMOs. | [22], |
| [61–63] | |||
| -MOE | 2′-O-methoxyethyl modification | Especially LNA increases the stability, efficiency and specificity. | |
| -LNA | 2′,4′-methylene modification | ||
| Sponges | Competitive inhibitors which are transcripts expressed from plasmid with strong promoters, containing multiple, tandem binding sites to the miRNAs of interest. | Sponges can block a whole family of related miRNAs. Selectable marker or reporter gene in the vector allows to isolate a fraction of cells in which the family of miRNAs is strongly inhibited. | [64–67] |
| AAV | Adenovirus- associated vectors | AAV are also widely used for systemic delivery. While the toxicity of viral mediated delivery is rarely reported, it remains controversial. | [47], [62], |
| [68] | |||
| (PEI/miR complex) | (Intratumoral injection) | PEI/miR complex, plasmid and CC9 are probably useful for delivery. However we cannot assure their utility because few experiments using them for delivery have been performed. | [69] |
| Plasmid | miRNA-expressing plasmids encapsulated in small multilamellar cationic liposome (DOTAP/cholesterol) | [70] | |
| CC9 | A specific tumor-homing and -penetrating bifunctional peptide conjugated with oligonucleotides. | [47] |
AMOs: anti-miRNA oligonucleotides.
LNA: locked nucleic acid.
Chemically modified anti-miRNA oligonucleotides (AMOs) complementary to mature miRNAs are widely used to inhibit miRNAs in vitro and in vivo. Effective AMOs typically have proprietary modifications such as 2′-O-methyl, 2′-O-methoxyethyl or 2′,4′-methylene (LNA) [61], which increases their stability, efficacy [62, 63], and specificity [22].
In addition to LNA modification, other oligonucleotide modifications to increase effectiveness and tolerability are under development. These include cholesterol-conjugated modified “antagomirs” [71, 72] and “miRNA sponges”, which are competitive inhibitors of small RNAs [64–67].
“miRNA sponges” are transcripts that contain multiple, tandem binding sites to the miRNAs of interest. When vectors encoding these sponges are transfected into cells, multiple miRNA targets can be suppressed simultaneously [64]; however, further experiments are necessary to demonstrate the utility of the sponge method in vivo.
In terms of delivery methods, adenovirus-associated vectors are used widely for systemic delivery [47, 62]. However, while the toxicity of viral-mediated delivery is rarely reported in in vivo studies [62, 68], it remains controversial. Novel nanoparticle-based delivery systems, which may be safer and more amenable [47] than viruses, are being developed. As mentioned earlier, the systemic injection of low-molecular-weight PEI/miRNA mimic complexes has proved useful for intratumoral delivery in xenograft models [69]. Additionally, miRNA-expressing plasmids encapsulated in small multilamellar cationic liposomes (DOTAP/cholesterol), have been reported as a useful delivery method in the mouse xenograft model [70].
AMOs such as LNA are currently the most promising targeted therapy method in terms of safety, stability and prominence. Continuing studies in vitro and in vivo are undoubtedly critical to discovery of simple, efficient, and safe delivery methods; this will facilitate the development of miRNA-targeting therapies for human disease.
Conclusions
Along with recent discoveries of the diverse effects of miRNAs in biological systems, miRNA-mediated intervention is a promising avenue for the development of novel therapeutics against human diseases. In addition to the current success of anti-miR122 therapy against chronic hepatitis C and the ongoing studies of miR-34 mimics against liver cancers in human clinical trials, the results of preclinical studies will likely lead to human clinical trials in the near future. However, several important issues must be addressed if this knowledge is to be used effectively in clinical trials. These include the delivery method, improved oligonucleotide modification for delivery, and safety. Because of its relative novelty, the safety of oligonucleotide therapy is an important consideration. Since miRNAs generally have diverse effects by targeting multiple mRNAs, undesired outcomes, so called “off-target effects”, may be encountered even when a specific miRNA is targeted. Effectiveness should also be considered. Although most current trials target individual miRNAs, targeting multiple miRNAs simultaneously may be necessary because most are considered to function cooperatively [73]. Therefore, although our understanding of miRNAs has increased, further research is needed to transform this knowledge into effective therapeutics against human diseases.
Acknowledgements
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan (#22390058 and #20390204) (M.O. and K.K.), by Health Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan (Research on Hepatitis) (to K.K.), and a grant from the Japanese Society of Gastroenterology (to M.O.).
Footnotes
Competing interests
The authors declare no competing interests.
Authors’ contributions
CS wrote the draft. TK, TY, and MOh prepared the tables. TA prepared the figures. MOt and KK wrote and summarized the entire manuscript. All authors read and approved the final manuscript.
Contributor Information
Chikako Shibata, Email: cshibata-tky@umin.ac.jp.
Motoyuki Otsuka, Email: otsukamo-tky@umin.ac.jp.
Takahiro Kishikawa, Email: kishikawat-int@h.u-tokyo.ac.jp.
Takeshi Yoshikawa, Email: yoshikawat-int@h.u-tokyo.ac.jp.
Motoko Ohno, Email: onom-int@h.u-tokyo.ac.jp.
Akemi Takata, Email: atakata-tky@umin.ac.jp.
Kazuhiko Koike, Email: kkoike-tky@umin.ac.jp.
References
- 1.Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153(3):516–519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338(6113):1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 3.Dethoff EA, Chugh J, Mustoe AM, Al-Hashimi HM. Functional complexity and regulation through RNA dynamics. Nature. 2012;482(7385):322–330. doi: 10.1038/nature10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 6.Carrington J, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301(5631):336–338. doi: 10.1126/science.1085242. [DOI] [PubMed] [Google Scholar]
- 7.Cho WC. MicroRNAs: potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int J Biochem Cell Biol. 2010;42(8):1273–1281. doi: 10.1016/j.biocel.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432(7014):231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 10.Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18(24):3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takata A, Otsuka M, Yoshikawa T, Kishikawa T, Ohno M, Koike K. MicroRNAs and liver function. Minerva gastroenterologica e dietologica. 2013;59(2):187–203. [PubMed] [Google Scholar]
- 12.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303(5654):95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 13.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 14.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123(4):631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol. 2005;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A. 2006;103(11):4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathonnet G, Fabian MR, Svitkin YV, Parsyan A, Huck L, Murata T, Biffo S, Merrick WC, Darzynkiewicz E, Pillai RS, et al. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science. 2007;317(5845):1764–1767. doi: 10.1126/science.1146067. [DOI] [PubMed] [Google Scholar]
- 20.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10):704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pappas TC, Bader AG, Andruss BF, Brown D, Ford LP. Applying small RNA molecules to the directed treatment of human diseases: realizing the potential. Expert Opin Ther Targets. 2008;12(1):115–127. doi: 10.1517/14728222.12.1.115. [DOI] [PubMed] [Google Scholar]
- 22.Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93(1):98–104. doi: 10.1038/clpt.2012.192. [DOI] [PubMed] [Google Scholar]
- 23.Wang XW, Heegaard NH, Orum H. MicroRNAs in liver disease. Gastroenterology. 2012;142(7):1431–1443. doi: 10.1053/j.gastro.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song JH, Meltzer SJ. MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology. 2012;143(1):e32–e47. doi: 10.1053/j.gastro.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Wu WK, Lee CW, Cho CH, Fan D, Wu K, Yu J, Sung JJ. MicroRNA dysregulation in gastric cancer: a new player enters the game. Oncogene. 2010;29(43):5761–5771. doi: 10.1038/onc.2010.352. [DOI] [PubMed] [Google Scholar]
- 26.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 27.Mott JL. MicroRNAs involved in tumor suppressor and oncogene pathways: implications for hepatobiliary neoplasia. Hepatology. 2009;50(2):630–637. doi: 10.1002/hep.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visone R, Petrocca F, Croce CM. Micro-RNAs in gastrointestinal and liver disease. Gastroenterology. 2008;135(6):1866–1869. doi: 10.1053/j.gastro.2008.10.074. [DOI] [PubMed] [Google Scholar]
- 29.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, et al. Treatment of HCV infection by targeting MicroRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 30.Gebert LF, Rebhan MA, Crivelli SE, Denzler R, Stoffel M, Hall J. Nucleic Acids Res. 2013. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elmén J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjärn M, Hansen JB, Hansen HF, Straarup EM, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008;36(4):1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeisel MB, Pfeffer S, Baumert TF. miR-122 acts as a tumor suppressor in hepatocarcinogenesis in vivo. J Hepatol. 2013;58(4):821–823. doi: 10.1016/j.jhep.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327(5962):198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elmén J, Lindow M, Schütz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjärn M, Hansen HF, Berger U, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452(7189):896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 35.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309(5740):1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Zhao H, Zhang X, Wood LD, Anders RA, Choti MA, Pawlik TM, Daniel HD, Kannangai R, Offerhaus GJ, et al. Inactivating mutations of the chromatin remodeling gene ARID2 in hepatocellular carcinoma. Nat Genet. 2011;43(9):828–829. doi: 10.1038/ng.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28(40):3526–3536. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai S, Nasser MW, Wang B, Hsu SH, Datta J, Kutay H, Yadav A, Nuovo G, Kumar P, Ghoshal K. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284(46):32015–32027. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kojima K, Takata A, Vadnais C, Otsuka M, Yoshikawa T, Akanuma M, Kondo Y, Kang YJ, Kishikawa T, Kato N, et al. MicroRNA122 is a key regulator of α-fetoprotein expression and influences the aggressiveness of hepatocellular carcinoma. Nat Commun. 2011;2:338. doi: 10.1038/ncomms1345. [DOI] [PubMed] [Google Scholar]
- 40.Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, Yu L, Bai S, La Perle K, Chivukula RR, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122(8):2871–2883. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122(8):2884–2897. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447(7148):1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17(2):211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy S, Levi E, Majumdar AP, Sarkar FH. Expression of miR-34 is lost in colon cancer which can be re-expressed by a novel agent CDF. J Hematol Oncol. 2012;5:58. doi: 10.1186/1756-8722-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong MY, Yu Y, Walsh WR, Yang JL. microRNA-34 family and treatment of cancers with mutant or wild-type p53 (Review) Int J Oncol. 2011;38(5):1189–1195. doi: 10.3892/ijo.2011.970. [DOI] [PubMed] [Google Scholar]
- 46.Kim NH, Kim HS, Kim NG, Lee I, Choi HS, Li XY, Kang SE, Cha SY, Ryu JK, Na JM, et al. p53 and microRNA-34 are suppressors of canonical Wnt signaling. Sci Signal. 2011;4(197):ra71. doi: 10.1126/scisignal.2001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu QL, Jiang QY, Jin X, Shen J, Wang K, Li YB, Xu FJ, Tang GP, Li ZH. Cationic microRNA-delivering nanovectors with bifunctional peptides for efficient treatment of PANC-1 xenograft model. Biomaterials. 2013;34(9):2265–2276. doi: 10.1016/j.biomaterials.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Callegari E, Elamin BK, Giannone F, Milazzo M, Altavilla G, Fornari F, Giacomelli L, D'Abundo L, Ferracin M, Bassi C, et al. Liver tumorigenicity promoted by microRNA-221 in a mouse transgenic model. Hepatology. 2012;56(3):1025–1033. doi: 10.1002/hep.25747. [DOI] [PubMed] [Google Scholar]
- 49.Fang Y, Xue JL, Shen Q, Chen J, Tian L. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology. 2012;55(6):1852–1862. doi: 10.1002/hep.25576. [DOI] [PubMed] [Google Scholar]
- 50.Zhang S, Shan C, Kong G, Du Y, Ye L, Zhang X. MicroRNA-520e suppresses growth of hepatoma cells by targeting the NF-κB-inducing kinase (NIK) Oncogene. 2012;31(31):3607–3620. doi: 10.1038/onc.2011.523. [DOI] [PubMed] [Google Scholar]
- 51.He XX, Chang Y, Meng FY, Wang MY, Xie QH, Tang F, Li PY, Song YH, Lin JS. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31(28):3357–3369. doi: 10.1038/onc.2011.500. [DOI] [PubMed] [Google Scholar]
- 52.Li Q, Zou C, Han Z, Xiao H, Wei H, Wang W, Zhang L, Zhang X, Tang Q, Zhang C, et al. MicroRNA-25 functions as a potential tumor suppressor in colon cancer by targeting Smad7. Cancer Lett. 2013;335(1):168–174. doi: 10.1016/j.canlet.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 53.Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM, Chen J. The miR-217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis. 2010;31(10):1726–1733. doi: 10.1093/carcin/bgq160. [DOI] [PubMed] [Google Scholar]
- 54.Galardi S, Mercatelli N, Giorda E, Massalini S, Frajese GV, Ciafrè SA, Farace MG. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem. 2007;282(32):23716–23724. doi: 10.1074/jbc.M701805200. [DOI] [PubMed] [Google Scholar]
- 55.Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, Calin GA, Grazi GL, Giovannini C, Croce CM, Bolondi L, et al. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene. 2008;27(43):5651–5661. doi: 10.1038/onc.2008.178. [DOI] [PubMed] [Google Scholar]
- 56.Gramantieri L, Fornari F, Ferracin M, Veronese A, Sabbioni S, Calin GA, Grazi GL, Croce CM, Bolondi L, Negrini M. MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin Cancer Res. 2009;15(16):5073–5081. doi: 10.1158/1078-0432.CCR-09-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P, et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16(6):498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM, Dejean A. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107(1):264–269. doi: 10.1073/pnas.0907904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szafranska AE, Davison TS, John J, Cannon T, Sipos B, Maghnouj A, Labourier E, Hahn SA. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26(30):4442–4452. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- 60.Yu J, Li A, Hong SM, Hruban RH, Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Res. 2012;18(4):981–992. doi: 10.1158/1078-0432.CCR-11-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Válóczi A, Hornyik C, Varga N, Burgyán J, Kauppinen S, Havelda Z. Sensitive and specific detection of microRNAs by northern blot analysis using LNA-modified oligonucleotide probes. Nucleic Acids Res. 2004;32(22):e175. doi: 10.1093/nar/gnh171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie J, Ameres SL, Friedline R, Hung JH, Zhang Y, Xie Q, Zhong L, Su Q, He R, Li M, et al. Long-term, efficient inhibition of microRNA function in mice using rAAV vectors. Nat Methods. 2012;9(4):403–409. doi: 10.1038/nmeth.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chistiakov DA, Sobenin IA, Orekhov AN. Strategies to deliver microRNAs as potential therapeutics in the treatment of cardiovascular pathology. Drug Deliv. 2012;19(8):392–405. doi: 10.3109/10717544.2012.738436. [DOI] [PubMed] [Google Scholar]
- 64.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4(9):721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szász AM, Wang ZC, Brock JE, Richardson AL, Weinberg RA. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137(6):1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J, et al. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell. 2011;147(2):370–381. doi: 10.1016/j.cell.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ebert MS, Sharp PA. Emerging roles for natural microRNA sponges. Curr Biol. 2010;20(19):R858–861. doi: 10.1016/j.cub.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ibrahim AF, Weirauch U, Thomas M, Grünweller A, Hartmann RK, Aigner A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011;71(15):5214–5224. doi: 10.1158/0008-5472.CAN-10-4645. [DOI] [PubMed] [Google Scholar]
- 70.Dai L, Wang W, Zhang S, Jiang Q, Wang R, Cheng L, Yang Y, Wei YQ, Deng HX. Vector-based miR-15a/16-1 plasmid inhibits colon cancer growth in vivo. Cell Biol Int. 2012;36(8):765–770. doi: 10.1042/CBI20110404. [DOI] [PubMed] [Google Scholar]
- 71.Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438(7068):685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 72.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 73.Ivanovska I, Cleary MA. Combinatorial microRNAs: working together to make a difference. Cell Cycle. 2008;7(20):3137–3142. doi: 10.4161/cc.7.20.6923. [DOI] [PubMed] [Google Scholar]


