‘Supersystem’ is a designation given to a highly integrated life system, which was coined by the late Tomio Tada nearly a decade ago to describe both immune and nervous systems [1]. Even decades earlier, however, were scientists already appreciating the level of interplay that exists between these two supersystems. Work by Paunicka et al now highlight the importance of this relationship in corneal allograft rejection.
The cornea is a fascinating model site to explore how the nervous and immune systems interface, as it is arguably the most densely innervated site in mammals. Furthermore, the cornea enjoys immune privilege status, which is a feature that permits spontaneous acceptance in approximately 50% of corneal allografts in certain mouse strain combinations [2]. Paunicka et al now show that the severing of corneal nerves in the graft bed, an unavoidable consequence of penetrating keratoplasty, abolishes immune privilege that would otherwise be enjoyed by subsequent allografts, even in the fellow eye. This perhaps explains why a markedly increased incidence of failure of second grafts is seen in the clinic.
The authors came to this conclusion with elegant experimentation. They initially found that placement of an allograft led to the increased tempo and incidence of rejection to a subsequently placed corneal allograft in the same, or fellow eye. Importantly, this pattern was not due to a second-set rejection, as the same result was observed when the subsequent allograft was unrelated to the first. However, this antigen nonspecific increase in rejection was indeed T cell mediated, as subsequent syngeneic grafts survived indefinitely. The root cause of increased rejection turned out to be the nerve injury response, originating from the first transplantation. The same results were recapitulated by simply severing of nerves 360 degrees around the cornea. Remarkably, an allograft placed 60 days after such injury also succumbed to increased immune rejection. Authors conclusively demonstrated that a burst of the neuropeptide substance P, produced as an effect of such nerve severing, caused this heightened state of rejection. Furthermore, they show that substance P disables T regulatory (Treg) suppression, and associated this impairment with increased rejection.
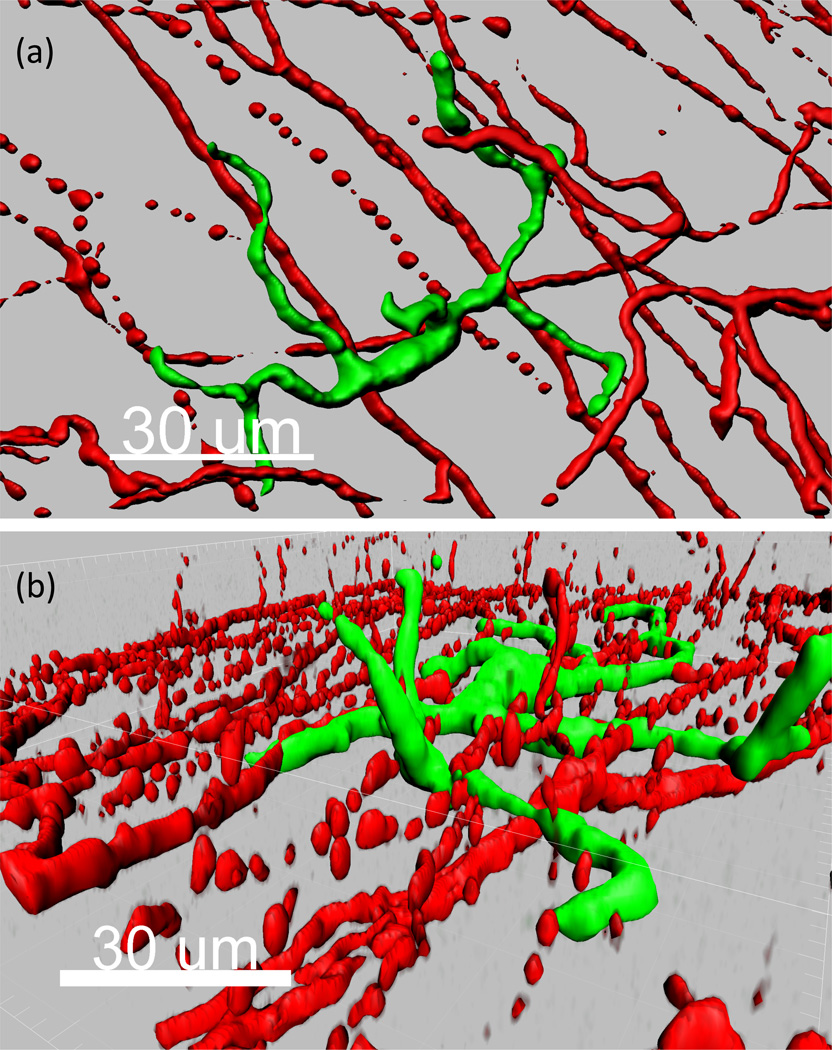
How could Tregs be impaired and augment immune rejection to a substance P burst that occurred 60 days prior? The authors are unsure, particularly given a serum half-life of only several minutes. Perhaps one could point to a role for tissue resident macrophages as a possible explanation. Indeed, these mononuclear phagocytes have a long life span of many months to even a year. Furthermore, macrophages express substance P receptors, produce substance P, and are altered by ligation of this neuropeptide [3]. Additionally, macrophages are antigen-presenting cells and can migrate to lymphoid organs, likely in a CCR7 mediated fashion [4]. Importantly, it has been long appreciated that many macrophages reside in close proximity to peripheral nerves [5], consistent in the cornea as well [6]. One can appreciate this in Figure 1, which is an unpublished micrograph generated by our lab demonstrating the proximity of a mononuclear phagocyte with up to 10 distinct nerves in the corneal epithelium. Perhaps one plausible hypothesis to explain the fascinating findings by Paunicka et al is that the unilateral severing of corneal nerves that causes a bilateral substance P burst has a pathogenic imprinting effect on such long-lived macrophages residing proximal to nerves. Thus, upon transplantation, either immediately or long after the substance P burst, perhaps this imprint licenses macrophages to produce additional substance P. If these antigen-presenting cells, laden with alloantigen, could migrate to the lymphoid organ, their substance P may therefore subvert Treg activity and in turn may lead to increased immune rejection.
Figure 1. Mononuclear phagocytes are in contact with corneal nerves.
Micrograph of normal cornea excised from CX3CR1-Cre; Rosa-fGFP mice and stained for neuron-specific class III beta-tubulin (Tuj 1). (a, b) Mononuclear phagocyte (green) and nerves (red) shown in an (a) en face and (b) side view image of the corneal epithelium.
Irrespective of how substance P produces such a long-lasting ability to augment immune rejection, Paunicka et al forces transplantation immunologists, particularly those studying the cornea, to now consider the interplay of both supersystems as yet another piece of the perplexing puzzle that is immune rejection.
Acknowledgments
Funded by:
R01EY021798 (Saban)
Research to Prevent Blindness, Career Development Award (Saban)
WORKS CITED
- 1.Tada T. The immune system as a supersystem. Annu Rev Immunol. 1997;15:1–13. doi: 10.1146/annurev.immunol.15.1.1. Review. PMID: 9143679. [DOI] [PubMed] [Google Scholar]
- 2.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003 Nov;3(11):879–889. doi: 10.1038/nri1224. Review. PMID: 14668804. [DOI] [PubMed] [Google Scholar]
- 3.Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997 Dec 1;159(11):5654–5660. [PubMed] [Google Scholar]
- 4.Saban DR. The chemokine receptor CCR7 expressed by dendritic cells: a key player in corneal and ocular surface inflammation. Ocul Surf. 2014 Apr;12(2):87–99. doi: 10.1016/j.jtos.2013.10.007. Epub 2013 Dec 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987 Apr 1;165(4):1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seyed-Razavi Y, Chinnery HR, McMenamin PG. A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea. Invest Ophthalmol Vis Sci. 2014 Mar 3;55(3):1313–1320. doi: 10.1167/iovs.13-12995. [DOI] [PubMed] [Google Scholar]