Abstract
Plant-derived chemicals including aroma oil compounds have an ability to inhibit nerve conduction and modulate transient receptor potential (TRP) channels. Although applying aroma oils to the skin produces a local anesthetic effect, this has not been yet examined throughly. The aim of the present study was to know how nerve conduction inhibitions by aroma oil compounds are related to their chemical structures and whether these activities are mediated by TRP activation. Compound action potentials (CAPs) were recorded from the frog sciatic nerve by using the air-gap method. Citral (aldehyde), which activates various types of TRP channels, attenuated the peak amplitude of CAP with the half-maximal inhibitory concentration (IC50) value of 0.46 mmol/L. Another aldehyde (citronellal), alcohol (citronellol, geraniol, (±)-linalool, (−)-linalool, (+)-borneol, (−)-borneol, α-terpineol), ester (geranyl acetate, linalyl acetate, bornyl acetate), and oxide (rose oxide) compounds also reduced CAP peak amplitudes (IC50: 0.50, 0.35, 0.53, 1.7, 2.0, 1.5, 2.3, 2.7, 0.51, 0.71, 0.44, and 2.6 mmol/L, respectively). On the other hand, the amplitudes were reduced by a small extent by hydrocarbons (myrcene and p-cymene) and ketone (camphor) at high concentrations (2–5 mmol/L). The activities of citral and other TRP agonists ((+)-borneol and camphor) were resistant to TRP antagonist ruthenium red. An efficacy sequence for the CAP inhibitions was generally aldehydes ≥ esters ≥ alcohols > oxides >> hydrocarbons. The CAP inhibition by the aroma oil compound was not related to its octanol–water partition coefficient. It is suggested that aroma oil compounds inhibit nerve conduction in a manner specific to their chemical structures without TRP activation.
Keywords: Aroma oil compound, compound action potential, drug discovery, frog, local anesthetic, nerve conduction, pain, plant, sciatic nerve, TRP agonist
Introduction
A red pepper component capsaicin, which activates transient receptor potential (TRP) vanilloid-1 (TRPV1) channels, reportedly inhibits voltage-gated Na+ channels in a manner dependent on or independent of TRPV1 channels (Cao et al. 2007). We have recently reported that capsaicin inhibits fast-conducting and voltage-gated Na+-channel blocker tetrodotoxin (TTX)-sensitive compound action potential (CAP) in a manner resistant to a nonselective TRP antagonist ruthenium red in the frog sciatic nerve (Tomohiro et al. 2013). A similar CAP inhibition was produced by a peppermint component menthol (Kawasaki et al. 2013), that activates TRP melastatin-8 (TRPM8) channels, and also by a wasabi component allyl isothiocyanate and a cinnamon component cinnamaldehyde (Matsushita et al. 2013), both of which are TRP ankyrin-1 (TRPA1) agonists. Although some of plant-derived aroma oil compounds, such as citral, camphor, and (+)-borneol, activate TRP channels (Moqrich et al. 2005; Xu et al. 2005; Vogt-Eisele et al. 2007; Stotz et al. 2008), it has not been examined whether their TRP activations affect nerve conduction.
Aroma oil compounds have various therapeutic effects including anticonvulsion and antinociception through an action on the central nervous system (Buchbauer and Jirovetz 1994; Almeida et al. 2001; Quintans-Júnior et al. 2008). When administrated to the skin, some of the compounds modulate muscle contractions (Lis-Balchin and Hart 1997) and have a local anesthetic effect through an action on the peripheral nervous system (Ghelardini et al. 1999; Zalachoras et al. 2010; Guimarães et al. 2013). We have previously demonstrated that various vanilloid compounds and also menthol and its related chemicals, all of which are derived from plants, inhibit frog CAPs in a manner dependent on their chemical structures (Kawasaki et al. 2013; Tomohiro et al. 2013). A similar CAP inhibition has been shown as a difference among cocaine-related chemicals (Tokuno et al. 2004), between tramadol and mono-O-demethyl tramadol (Katsuki et al. 2006), among morphine, ethylmorphine, and codeine (Mizuta et al. 2008), among adrenoceptor agonists including dexmedetomidine (Kosugi et al. 2010), or between carbamazepine and oxcarbazepine (Uemura et al. 2014; for review see Kumamoto et al. 2011). To our knowledge, it has not yet been systematically addressed how various aroma oil compounds act on nerve conduction and thus whether there is a chemical structure–activity relationship for their actions on nerve conduction. We investigated the actions of aroma oil compounds on CAPs, which were recorded by applying the air-gap method to the frog sciatic nerve, by focusing on the structure–activity relationship and an involvement of TRP channels.
Materials and Methods
Animals
This study was approved by the Animal Care and Use Committee of Saga University, and was conducted in accordance with the Guiding Principles for the Care and Use of Animals in the Field of Physiological Science of the Physiological Society of Japan. All efforts were made to minimize animal suffering and the number of animals used.
Preparation of frog sciatic nerves
The method used for obtaining frog sciatic nerve preparation has been described previously (Kumamoto et al. 2011; Matsushita et al. 2013). In brief, either sex of frogs (Rana nigromaculata) was decapitated and then pithed; thereafter the sciatic nerve was dissected from the lumbar plexus to the knee in Ringer solution. The isolated sciatic nerve was carefully desheathed under a binocular microscope and then loosely placed in five platinum wires that were glued to a Lucite plate, where the two ends of the nerve were tied to the wires by using threads. The plate was put on a beaker having Ringer solution in which the sciatic nerve was soaked. The composition of Ringer solution used was (mmol/L): NaCl, 115.5; KCl, 2.0; CaCl2, 1.8; Na2HPO4, 1.3; and NaH2PO4, 0.7 (pH = 7.0).
Recordings of CAPs from frog sciatic nerve fibers
As performed previously (Kumamoto et al. 2011; Matsushita et al. 2013), the Lucite plate having platinum wires attached with the sciatic nerve was moved from the beaker containing Ringer solution to a vacant one and then CAPs were recorded in air using a preamplifier. Here, two of the platinum wires were used to record CAPs, and the other two were for stimulating the sciatic nerve. The stimulation was performed at a frequency of 1 Hz with a stimulator, where rectangular pulses having 0.1 msec duration and various strengths were used. In order not to dry the sciatic nerve in air, this procedure was quickly performed at a time interval of 2 min. When the effects of drugs on CAPs were examined, the nerve was put back into the soaking solution with drugs in between two measures. The data were monitored on a storage oscilloscope while being recorded on a thermal array recorder having a wave form storage module and stored on USB flash memory (ELECOM, Osaka, Japan) with a Data logger (mini LOGGER GL900; GRAPHTEC, Yokohama, Japan) for later analyses. Stimulating the sciatic nerve produced a CAP following a stimulus artifact. The peak amplitude of the CAP was measured as a difference between baseline and CAP peak level, as done previously (Kumamoto et al. 2011; Matsushita et al. 2013). The peak amplitude of the CAP depended on the strength of the stimulus given to the sciatic nerve as such that the CAP peak amplitude enhanced with an increase in stimulus strength and attained a maximal value. As done previously (Kumamoto et al. 2011; Matsushita et al. 2013), we analyzed the peak amplitude of the maximal CAP. A conduction velocity value was determined by using the fifth electrode as an additional stimulation site. All experiments were carried out at room temperature (22–27°C).
Data analysis
The concentration-dependence curve for the reduction of the peak amplitude of CAP in the sciatic nerve soaked with a drug was analyzed using the following Hill equation:
where [Drug] is drug concentration, IC50 is the concentration of drug for half-maximal inhibition, and nH is the Hill coefficient.
Data were indicated as mean ± SEM and statistical significance was set at P < 0.05 using a paired or unpaired Student's t-test. In all cases n refers to the number of sciatic nerves studied. The peak amplitude of CAP before drug application was denoted as control.
Materials
Drugs used were citral, citronellal, citronellol, geraniol, (−)-linalool, (±)-linalool, (+)-borneol, (−)-borneol, geranyl acetate, camphor, rose oxide, myrcene, α-terpineol, ruthenium red (Sigma-Aldrich, St. Louis, MO), bornyl acetate (Funakoshi, Tokyo, Japan), p-cymene, and linalyl acetate (Tokyo Kasei, Tokyo, Japan). All of the drugs except for ruthenium red were first dissolved in dimethyl sulfoxide (DMSO) and then diluted to the final concentration in Ringer solution, where the concentration of DMSO was less than 1%. Ruthenium red was dissolved in distilled water at 10 mmol/L and then stored at −25°C. This drug was then diluted to the final concentration in Ringer solution immediately before use. DMSO at 1% did not affect CAPs. The pH of Ringer solution containing drugs was adjusted to 7.0 with 1 mmol/L NaOH. Drugs at concentrations larger than 10 mmol/L were not tested, because a change in osmotic pressure may affect CAPs. Nomenclature of all receptors, drugs, enzymes, and ion channels is according to the Guide to Receptors and Channels (Alexander et al. 2011).
Results
Effects of aroma oil compounds on fast-conducting CAPs were examined in a total of 308 sciatic nerves, and the peak amplitudes of the CAPs averaged to be 26.2 ± 0.4 mV (n = 308). When measured in some of the nerves, the CAPs had the averaged conduction velocity value of 27.4 ± 0.9 m/sec (n = 220), a value comparable to those reported previously (Katsuki et al. 2006; Mizuta et al. 2008; Kosugi et al. 2010; Kawasaki et al. 2013; Matsushita et al. 2013; Tomohiro et al. 2013; Uemura et al. 2014).
Effects of citral, camphor, and (+)-borneol on frog sciatic nerve CAPs
At first, we examined the effect of an aldehyde citral (contained in lemongrass, having an antinociceptive effect; Viana et al. 2000; Fig.1A), that has an ability to activate TRPV1, TRPM8, TRPA1, and TRP vanilloid-3 (TRPV3) channels (Stotz et al. 2008), on CAPs recorded from the frog sciatic nerve. Soaking the sciatic nerve into citral (0.5 mmol/L)-containing Ringer solution reduced the peak amplitude of the CAP, as seen from Figure1Ba. Figure1Bb demonstrates an average of the time courses of a change in CAP peak amplitude following soaking into citral (0.5 mmol/L), relative to control, which are obtained from six sciatic nerves. The citral-induced reduction in CAP peak amplitude attained an almost maximal effect at 20 min of the soaking, where the peak amplitude of the CAP was 45 ± 6% (n = 6; P < 0.05) of control. In nerves treated with citral and then returned to drug-free Ringer solution (washout) for up to 50 min, the CAP amplitude recovered to about 70% of control level. Figure1C shows the averaged time courses of changes in CAP peak amplitude with an increase in time after soaking the sciatic nerve into citral at various concentrations ranging from 0.01 to 2 mmol/L. The rate of the CAP peak amplitude reduction produced by citral was enhanced in extent with an increase in its concentration. CAP amplitude reduction at 20 min of the soaking increased in magnitude with an increase in citral concentration. The concentration–response curve for the citral-induced CAP amplitude reduction obtained from many nerve trunks is given in Figure1D. Table1 shows IC50 and nH values obtained from analysis based on the Hill equation.
Figure 1.
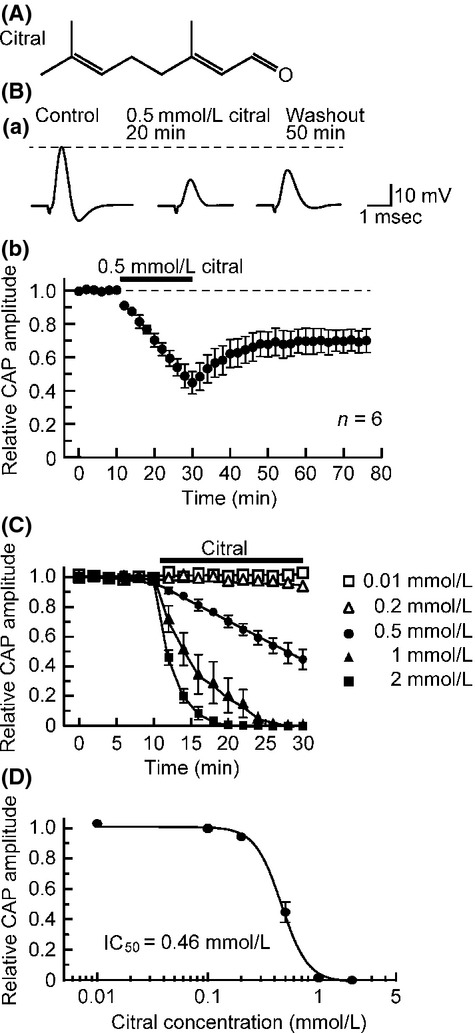
Effect of citral on compound action potentials (CAPs) recorded from frog sciatic nerve fibers. (A) The chemical structure of citral. (B) Citral at a concentration of 0.5 mmol/L reduces CAP peak amplitudes. (a) Recordings of CAPs in the control, at 20 min after exposure to citral, and thereafter 50 min in the absence of citral. (b) Average time course of changes in CAP peak amplitudes following exposure to citral for 20 min, relative to control. In this and subsequent figures, each point with vertical bars represents the mean and SEM, and the dotted line denotes the control value. (C) Average time courses of CAP peak amplitude reductions produced by citral at 0.01–2 mmol/L; data at each concentration were obtained from 3 to 4 sciatic nerves. Solid lines in this graph were arbitrarily drawn. (D) The peak amplitudes of CAPs recorded from sciatic nerve fibers treated with citral at various concentrations for 20 min, relative to control, which were plotted against citral concentration. Each of the data points was obtained from 3 to 4 sciatic nerves. This concentration–response curve was drawn according to the Hill equation (IC50 = 0.46 mmol/L; nH = 3.6).
Table 1.
Values of IC50 for frog sciatic nerve CAP peak amplitude reductions by aroma oil compounds and of the logarithum of their Kow.
| Group | Compound | IC50 (mmol/L) | log Kow |
|---|---|---|---|
| Phenols | Carvacrol*1 | 0.34 | 3.49 |
| Thymol*1 | 0.34 | 3.30 | |
| Eugenol*2 | 0.81 | 2.49 | |
| Aldehydes | Citral | 0.46 (3.6) | 3.45 |
| Citronellal | 0.50 (3.8) | 3.53 | |
| Esters | Linalyl acetate | 0.71 (1.6) | 3.93 |
| Geranyl acetate | 0.51 (1.1) | 4.04 | |
| Bornyl acetate | 0.44 (2.2) | 3.86 | |
| Alcohols | Citronellol | 0.35 (3.1) | 3.91 |
| Geraniol | 0.53 (3.3) | 3.56 | |
| (±)-Linalool | 1.7 (1.8) | 2.97 | |
| (−)-Linalool | 2.0 (2.0) | − | |
| (+)-Borneol | 1.5 (3.0) | 2.85 | |
| (−)-Borneol | 2.3 (2.9) | 3.01 | |
| α-Terpineol | 2.7 (4.0) | 2.98 | |
| (−)-Menthol*1 | 1.1 | 3.3 | |
| (+)-Menthol*1 | 0.93 | − | |
| Ketones | (+)-Pulegone*1 | 1.4 | 3.08 |
| (−)-Carvone*1 | 1.4 | 2.71 | |
| (+)-Carvone*1 | 2.0 | 3.07 | |
| (−)-Menthone*1 | 1.5 | 3.05 | |
| (+)-Menthone*1 | 2.2 | − | |
| Oxides | Rose oxide | 2.6 (2.8) | − |
| 1,8-Cineole*1 | 5.7 | 2.74 | |
| 1,4-Cineole*1 | 7.2 | 2.97 |
Value in parentheses, next to IC50, indicates nH used to obtain the IC50 value in the present work. Data for the compounds denoted as *1 and *2, and of log Kow were taken from Kawasaki et al. (2013), Tomohiro et al. (2013), and ChemlDplus (2014), respectively. CAP, compound action potential; nH, Hill coefficient.
We next examined the effects on frog CAPs of an alcohol (+)-borneol (contained in rosemary; Fig.2Aa) which activates TRPV3 channels (Vogt-Eisele et al. 2007) and has an antinociceptive effect (da Silva Almeida et al. 2013) and also of a ketone camphor (contained in camphor tree; Fig.2Ba), which activates TRPV1 and TRPV3 channels (Moqrich et al. 2005; Xu et al. 2005) while being a topical analgesic (Buckingham 1994). As seen from Figure2Ab, (+)-borneol (3 mmol/L) reduced CAP peak amplitudes in a reversible manner. The (+)-borneol-induced CAP amplitude reduction attained a maximal effect at 20 min of the soaking, where this amplitude decreased to 2 ± 1% of control (P < 0.05; n = 4; Fig.2Ab). The (+)-borneol-induced CAP inhibition was concentration-dependent in extent in the range of 0.2–3 mmol/L (see Table1 for IC50 and nH values obtained). On the other hand, camphor at 5 mmol/L, a maximal concentration tested, reduced CAP peak amplitudes in a partially reversible manner (see Fig.2Bb). The camphor (5 mmol/L)-induced CAP amplitude reduction attained a maximal effect at 20 min of the soaking, where this amplitude reduced to 67 ± 4% of control (P < 0.05; n = 4; Fig.2Bb). The camphor-induced CAP inhibition was concentration-dependent in extent in the range of 0.5–5 mmol/L.
Figure 2.

Citral, (+)-borneol, and camphor inhibit frog sciatic nerve CAPs in a manner resistant to a nonselective TRP antagonist ruthenium red (RR). (A and B) Effects of (+)-borneol (3 mmol/L) and camphor (5 mmol/L) on CAPs. (Aa and Ba) The chemical structures of (+)-borneol (Aa) and camphor (Ba). (Ab and Bb) Average time course of changes in CAP peak amplitudes following exposure to (+)-borneol (Ab) or camphor (Bb) for 20 min, relative to control. (Ca, Cb, and Cc) Average time course of changes in CAP peak amplitudes following treatment with RR (0.3 mmol/L) and then with citral (1 mmol/L; Ca), (+)-borneol (3 mmol/L; Cb) or camphor (5 mmol/L; Cc) together with RR (0.3 mmol/L), relative to that before drug treatment. In each of (Ca), (Cb), and (Cc), recordings in the right-hand side indicate a superimposition of CAPs in the control and in the presence of RR without (RR) and with citral (Ca), (+)-borneol (Cb) or camphor (Cc). (D) CAP peak amplitudes under the action of citral (1 mmol/L), (+)-borneol (3 mmol/L) or camphor (5 mmol/L) in the absence and presence of RR (0.3 mmol/L), relative to that just before the application of the TRP agonist. Value in parentheses denotes the number of nerve trunk examined. n.s., not significant; TRP, transient receptor potential; CAP, compound action potential.
Effects of TRP antagonist on the citral-, (+)-borneol, and camphor-induced CAP inhibitions
Figure2C and D demonstrates how the effect of citral (1 mmol/L), (+)-borneol (3 mmol/L) or camphor (5 mmol/L) on sciatic nerve CAPs is affected by a nonselective TRP antagonist ruthenium red (0.3 mmol/L). Pretreatment with ruthenium red for 20 min resulted in a slight reduction in the amplitude of the CAP, albeit its duration was largely prolonged (possibly by inhibiting voltage-gated Na+-channel inactivation; Neumcke et al. 1987), as reported previously (Kawasaki et al. 2013; Matsushita et al. 2013). Adding citral, (+)-borneol or camphor to the ruthenium red-containing Ringer solution markedly inhibited the CAP, as summarized in Figure2Ca, Cb, and Cc. The peak amplitude after 20 min treatment with citral, (+)-borneol or camphor together with ruthenium red, relative to that just before the co-treatment, was not significantly different from that obtained with citral, (+)-borneol or camphor only without ruthenium red (P > 0.05; Fig.2D).
Effects of aroma oil compounds having various chemical structures on frog sciatic nerve CAPs
Since citral (aldehyde), (+)-borneol (alcohol), and camphor (ketone) reduced CAP peak amplitudes with a different extent, we next examined in a quantitative manner how aroma oil compounds having various chemical structures affect CAPs in the frog sciatic nerve.
Effects of aroma oil compounds belonging to alcohols
As with menthol (Kawasaki et al. 2013), aroma oil alcohols inhibited CAPs. Citronellol (contained in rose; Fig.3Aa) having an antinociceptive effect (Brito et al. 2012) reduced CAP peak amplitudes in a partially reversible manner, as seen in Figure3Ab. The citronellol-induced CAP amplitude reduction attained a maximal effect at 20 min of the soaking, where this amplitude decreased to 6 ± 2% of control (P < 0.05; n = 4; Fig.3Ab). On the other hand, geraniol (where one of the single bonds of carbon of citronellol changes to a double bond; Fig.3Ba) contained in rose and geranium reversibly reduced CAP peak amplitudes (Fig.3Bb). The geraniol-induced CAP amplitude reduction attained an almost maximal effect at 20 min of the soaking, where this amplitude reduced to 5 ± 3% of control (P < 0.05; n = 6; Fig.3Bb). The CAP inhibitions produced by citronellol and geraniol were concentration-dependent in extent in the range of 0.05–1 mmol/L (see Table1 for IC50 and nH values obtained).
Figure 3.
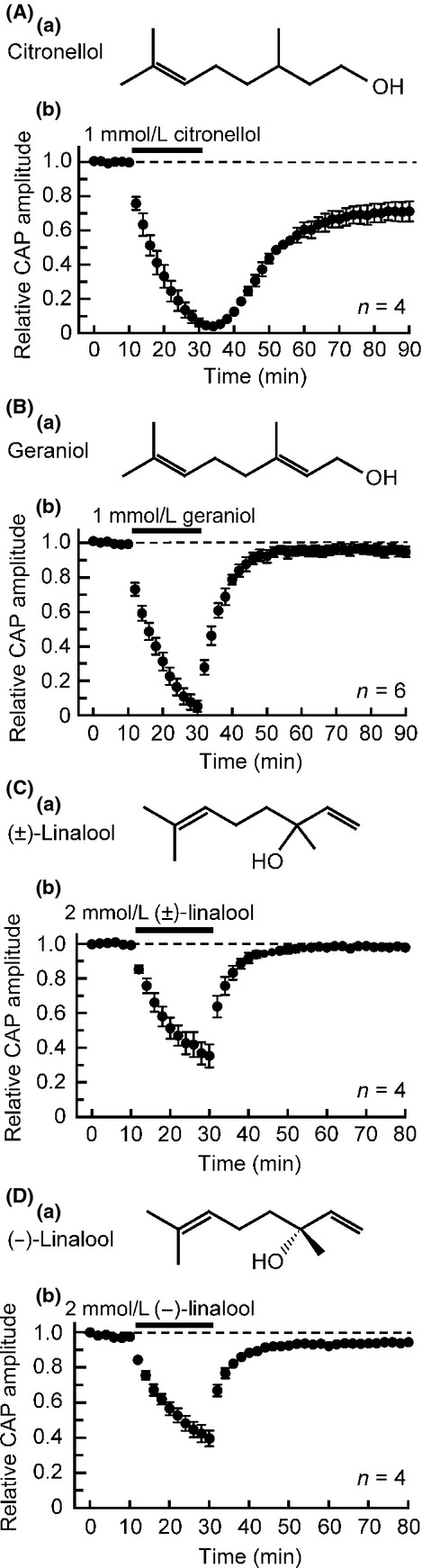
Effects of aroma oil compounds, chain alcohols (citronellol, geraniol, (±)-linalool, and (−)-linalool), on frog sciatic nerve CAPs. (Aa, Ba, Ca, and Da) The chemical structures of citronellol (Aa), geraniol (Ba), (±)-linalool (Ca), and (−)-linalool (Da). (Ab, Bb, Cb, and Db) Average time course of changes in CAP peak amplitudes following exposure to citronellol (1 mmol/L; Ab), geraniol (1 mmol/L; Bb), (±)-linalool (2 mmol/L; Cb) or (−)-linalool (2 mmol/L; Db) for 20 min, relative to those before the soaking. CAP, compound action potential.
There is linalool (Fig.3Ca) that is one of the aroma oil components contained in lavender essential oil having a local anesthetic effect (Ghelardini et al. 1999). As seen in Figure3Cb, (±)-linalool (2 mmol/L) reduced CAP peak amplitudes in a reversible manner. The (±)-linalool-induced CAP amplitude reduction attained a maximal effect at 20 min of the soaking, where this amplitude decreased to 35 ± 7% of control (P < 0.05; n = 4; Fig.3Cb). A similar inhibition was produced by (–)-linalool, albeit this action was incomplete in reversibility (Fig.3Db). The (−)-linalool-induced CAP amplitude reduction attained a maximal effect at 20 min of the soaking, where this amplitude reduced to 42 ± 5% of control (P < 0.05; n = 4; Fig.3Db). The CAP inhibitions produced by (±)-linalool and (−)-linalool were concentration-dependent in extent in the range of 0.1–5 mmol/L (see Table1 for IC50 and nH values obtained).
Although aroma oil compounds tested in Figure3 are chain alcohols, we next examined the effects of cyclic alcohols on frog sciatic nerve CAPs. Like (+)-borneol, a stereoisomer (−)-borneol (Fig.4Aa) inhibited CAPs, albeit this action was not reversible (Fig.4Ab). The (−)-borneol (3 mmol/L)-induced CAP amplitude reduction attained a maximal effect at 20 min of the soaking, where this amplitude decreased to 2 ± 2% of control (P < 0.05; n = 4; Fig.4Ab). α-Terpineol (contained in eucalyptus; Fig.4Ba), which has an antinociceptive effect (Quintans-Júnior et al. 2011b), also inhibited CAPs in a reversible manner (Fig.4Bb). The α-terpineol (2 mmol/L)-induced CAP amplitude reduction attained a maximal effect at 20 min of the soaking, where this amplitude reduced to 79 ± 4% of control (P < 0.05; n = 4; Fig.4Bb). The CAP inhibitions produced by (−)-borneol and α-terpineol were concentration-dependent in extent in the range of 0.1–5 mmol/L (see Table1 for IC50 and nH values obtained).
Figure 4.
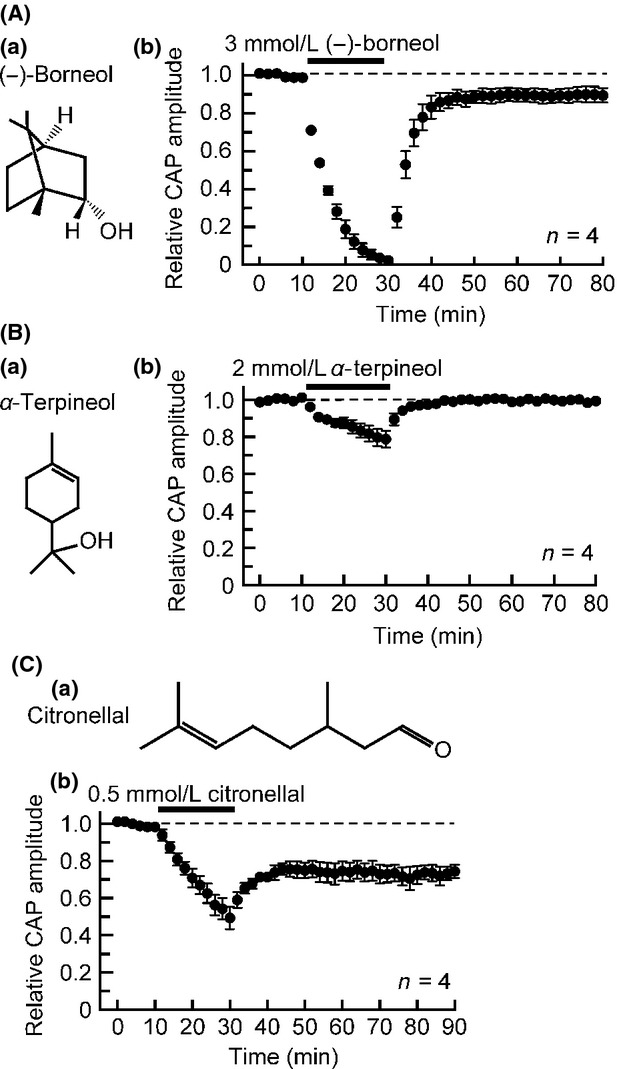
Effects of aroma oil compounds, cyclic alcohols ((−)-borneol and α-terpineol), and aldehyde (citronellal), on frog sciatic nerve CAPs. (Aa, Ba, and Ca) The chemical structures of (−)-borneol (Aa), α-terpineol (Ba), and citronellal (Ca). (Ab, Bb, and Cb) Average time course of changes in CAP peak amplitudes following exposure to (−)-borneol (3 mmol/L; Ab), α-terpineol (2 mmol/L; Bb) or citronellal (0.5 mmol/L; Cb) for 20 min, relative to those before the soaking. CAP, compound action potential.
Effect of an aroma oil compound citronellal belonging to aldehydes
Citronellal (where one of the double bonds of carbon in citral is altered to a single bond; contained in lemongrass; Fig.4Ca) having an antinociceptive effect (Quintans-Júnior et al. 2011a) inhibited CAPs in an irreversible manner (Fig.4Cb), as seen for citral. The citronellal (0.5 mmol/L)-induced CAP amplitude reduction attained a maximal effect at 20 min of soaking, where this amplitude decreased to 49 ± 6% of control (P < 0.05; n = 4; Fig.4Cb). The citronellal-induced CAP inhibition was concentration-dependent in extent in the range of 0.02–1 mmol/L (see Table1 for IC50 and nH values obtained).
Effects of aroma oil compounds belonging to esters
Figure5 demonstrates the effects on CAPs of linalyl acetate (Fig.5Aa), geranyl acetate (Fig.5Ba), and bornyl acetate (Fig.5Ca), all of which are esters. Linalyl acetate contained in lavender essential oil has a local anesthetic activity (Ghelardini et al. 1999), geranyl acetate existing in ylang ylang has an antinociceptive effect (Quintans-Júnior et al. 2013), and bornyl acetate contained in conifer leaf oil produces autonomic nerve relaxation (Matsubara et al. 2011). As seen in Figure5Ab, Bb, and Cb, all of them inhibited CAPs in a partially reversible manner. The CAP amplitude reductions by them somewhat persisted after 20 min of the soaking. The CAP peak amplitude at 20 min under the action of linalyl acetate (0.5 mmol/L), geranyl acetate (0.5 mmol/L) or bornyl acetate (1 mmol/L), respectively, reduced to 45 ± 3% (P < 0.05; n = 5), 49 ± 2% (P < 0.05; n = 4) or 36 ± 1% (P < 0.05; n = 4) of control. When estimated from CAP inhibitions at 20 min soaking, the linalyl acetate, geranyl acatate, and bornyl acetate activities were concentration-dependent in extent in the range of 0.01–1 mmol/L (see Table1 for IC50 and nH values obtained).
Figure 5.
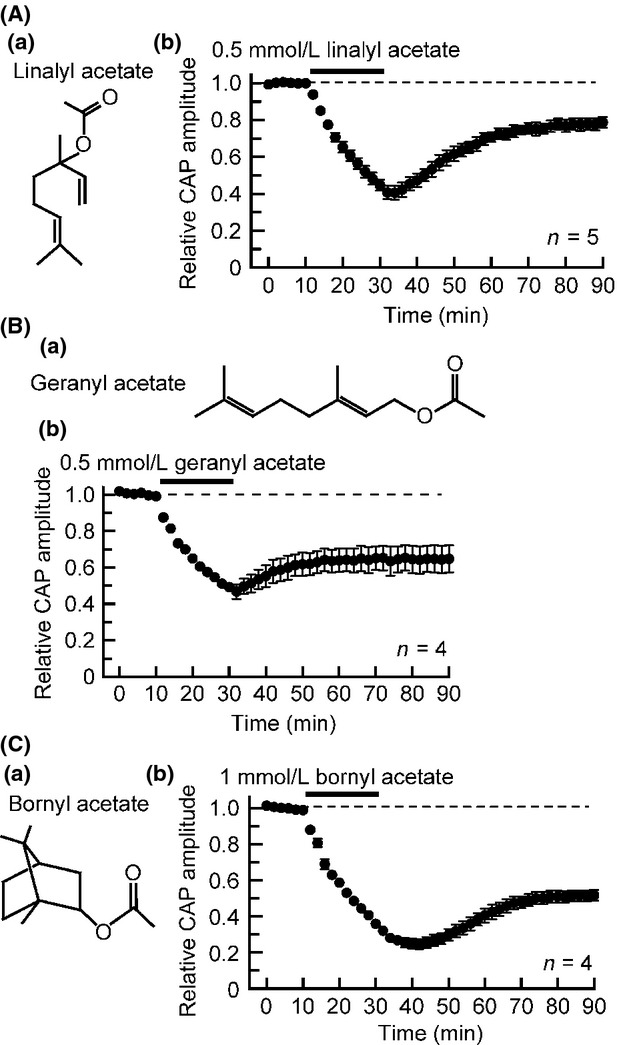
Effects of aroma oil compounds, esters (linalyl acetate, geranyl acetate, and bornyl acetate), on frog sciatic nerve CAPs. (Aa, Ba, and Ca) The chemical structures of linalyl acetate (Aa), geranyl acetate (Ba), and bornyl acetate (Ca). (Ab, Bb, and Cb) Average time course of changes in CAP peak amplitudes following exposure to linalyl acetate (0.5 mmol/L; Ab), geranyl acetate (0.5 mmol/L; Bb) or bornyl acetate (1 mmol/L; Cb) for 20 min, relative to those before the soaking. CAP, compound action potential.
Effect of rose oxide belonging to oxides
With respect to aroma oil compounds belonging to oxides, we have already reported the actions of 1,8-cineole and 1,4-cineole on frog sciaitc nerve CAPs (Kawasaki et al. 2013). The present study examined the effect of rose oxide (contained in rose, albeit by a small extent; Fig.6Aa) having an anti-inflammatory effect (Nonato et al. 2012) on CAPs. As with 1,8-cineole and 1,4-cineole (Kawasaki et al. 2013), rose oxide inhibited CAPs in a partially reversible manner (Fig.6Ab). The rose oxide (2 mmol/L)-induced CAP amplitude reduction attained an almost maximal effect at 20 min of the soaking, where this amplitude decreased to 46 ± 7% of control (P < 0.05; n = 5; Fig.6Ab). The rose oxide-induced CAP inhibition was concentration-dependent in extent in the range of 0.1–5 mmol/L (see Table1 for IC50 and nH values obtained).
Figure 6.
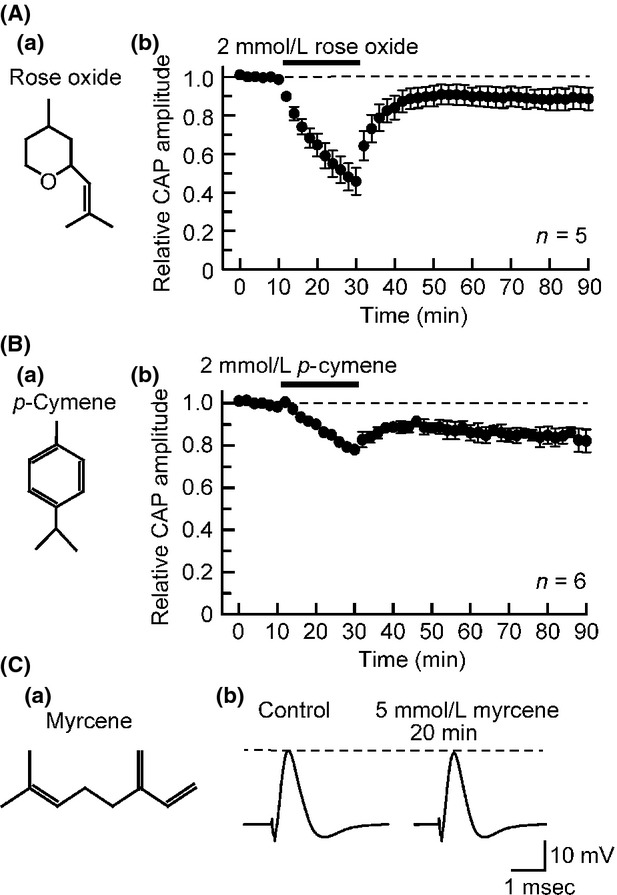
Effects of aroma oil compounds, oxide (rose oxide) and hydrocarbons (p-cymene and myrcene), on frog sciatic nerve CAPs. (Aa, Ba, and Ca) The chemical structures of rose oxide (Aa), p-cymene (Ba), and myrcene (Ca). (Ab and Bb) Average time course of changes in CAP peak amplitudes following exposure to rose oxide (2 mmol/L; Ab) or p-cymene (2 mmol/L; Bb) for 20 min, relative to those before the soaking. (Cb) Recordings of CAPs in the absence and presence of myrcene (5 mmol/L). CAP, compound action potential.
Effects of p-cymene and myrcene which belong to hydrocarbons
p-Cymene (contained in amaranthacease in an appreciable quantity; Fig.6Ba) having an antinociceptive effect (Quintans-Júnior et al. 2013) inhibited CAPs by a small extent in a partially reversible manner. CAP peak amplitude reduction produced by p-cymene at a maximal concentration (2 mmol/L) tested attained a maximal effect at 20 min of the soaking, where this amplitude was 78 ± 2% (P < 0.05) of control (n = 6; Fig.6Bb). The p-cymene-induced CAP inhibition was concentration-dependent in extent in the range of 0.5–2 mmol/L. Another hydrocarbon myrcene (contained in pimenta racemota; Fig.6Ca) exhibiting an antinociception (Rao et al. 1990) at 5 mmol/L, a maximal concentration examined, minimally inhibited CAPs, as seen in Figure6Cb. CAP peak amplitude at 20 min of the soaking with myrcene (5 mmol/L), relative to control, was 93 ± 4% (P > 0.05; n = 4). A similar small inhibition was obtained in the action of a hydrocarbon (+)-limonene on frog CAPs (Kawasaki et al. 2013).
Discussion and Conclusions
The present study demonstrated that various aroma oil chemicals, many of which have antinociceptive effects, reduce the peak amplitudes of fast-conducting CAPs, recorded from frog sciatic nerve fibers by using the air-gap method, in a concentration-dependent manner. This action was either reversible or irreversible and the extent of its reduction was variable, depending on the chemicals tested.
No involvement of TRP channels in the CAP inhibition by aroma oil chemicals
We have previously reported that a TRPV1 agonist capsaicin, a TRPM8 agonist menthol and TRPA1 agonists, AITC and cinnamaldehyde, inhibit CAPs in a manner independent of TRP channels (Kawasaki et al. 2013; Matsushita et al. 2013; Tomohiro et al. 2013). Thus, the CAP inhibitions produced by capsaicin and AITC were, respectively, resistant to a specific TRPV1 antagonist capsazepine and a specific TRPA1 antagonist HC-030031 (Matsushita et al. 2013; Tomohiro et al. 2013). In the present study, citral, which has an ability to activate TRPV1, TRPM8, TRPA1, and TRPV3 channels (Stotz et al. 2008), attenuated CAP peak amplitudes with the IC50 value of 0.46 mmol/L; this action was resistant to a nonselective TRP antagonist ruthenium red. Camphor (TRPV1 and TRPV3 agonist; Moqrich et al. 2005; Xu et al. 2005) at 5 mmol/L reduced CAP amplitudes by 33% and (+)-borneol (TRPV3 agonist; Vogt-Eisele et al. 2007) inhibited CAPs with the IC50 value of 1.5 mmol/L; these actions were insensitive to ruthenium red. Thus, the aroma oil compounds having an ability to activate TRP channels inhibited CAPs without TRP activation. This result obtained by using ruthenium red which prolonged CAP durations was consistent with one obtained by use of capsazepine and HC-030031 which by themselves did not affect the CAPs.
Like (+)-borneol, (−)-borneol inhibited CAPs with the IC50 value of 2.3 mmol/L, a value similar to that of (+)-borneol, indicating that there is almost no difference in the extent of CAP inhibition between the stereoisomers, as seen in the actions of (+)-menthol and (−)-menthol on frog CAPs (Kawasaki et al. 2013).
Chemical structure–activity relationship for CAP inhibition by aroma oil compounds
The components of lavender oil exhibiting a local anesthetic action, linalyl acetate, (±)-linalool, and (−)-linalool reduced CAP peak amplitudes with the IC50 values of 0.71, 1.7, and 2.0 mmol/L, respectively. The lack of difference in efficacy between (±)-linalool and (−)-linalool suggests that (+)-linalool and (−)-linalool have almost the same efficacy in inhibiting CAPs. This IC50 value was similar to that (1.85 mmol/L) in inhibiting action potentials (APs) recorded intracellularly from rat dorsal root ganglion neurons (Leal-Cardoso et al. 2010) while being somewhat larger than that (0.56 mmol/L) for inhibition of voltage-gated Na+ channels expressed in newt olfactory receptor cells (Narusuye et al. 2005).
With respect to other aroma oil compounds, aldehyde (citronellal), alcohols (citronellol, geraniol, and α-terpineol), esters (geranyl acetate and bornyl acetate), and oxide (rose oxide) reduced CAP peak amplitudes with the IC50 values of 0.50, 0.35, 0.53, 2.7, 0.51, 0.44, and 2.6 mmol/L, respectively. The IC50 value (0.35 mmol/L) of citronellol was somewhat smaller than that (2.2 mmol/L) obtained for CAPs recorded from the rat sciatic nerve by using the sucrose-gap method (de Sousa et al. 2006). The CAP inhibitory action of α-terpineol may be consistent with its ability to inhibit convulsive seizures produced by pentylenetetrazole in the mouse (de Sousa et al. 2007). On the other hand, myrcene (5 mmol/L) and p-cymene (2 mmol/L) reduced CAP amplitudes by 7 and 22%, respectively.
Table1 summarizes the effects of aroma oil compounds on frog sciatic nerve CAPs together with data reported previously (Kawasaki et al. 2013; Tomohiro et al. 2013). An efficacy sequence of aroma oil compounds for the CAP inhibitions was phenols (carvacrol, thymol, and eugenol) ≥ aldehydes (citral and citronellal) ≥ esters (linalyl acetate, geranyl acetate, and bornyl acetate) ≥ alcohols (citronellol, geraniol, (±)-linalool, (−)-linalool, (+)-borneol, (−)-borneol, α-terpineol, (−)-menthol, and (+)-menthol) ≥ ketones ((+)-pulegone, (−)-carbone, (+)-carbone, (−)-menthone, and (+)-menthone) > oxides (rose oxide, 1,8-cineole, and 1,4-cineole) >> hydrocarbons (p-cymene, myrcene, and limonene), except for a ketone camphor that was less effective than oxides. The aroma oil esters may have been more effective than obtained in the present study, because 20 min of soaking with the drugs appear to be somewhat insufficient to attain a steady CAP inhibition (see Fig.5). Consistent with our results, Zalachoras et al. (2010) have reported that linalool is more effective than 1,8-cineole and p-cymene in inhibiting frog sciatic nerve CAPs. Rat sciatic nerve CAPs were inhibited by aroma oil compounds with an efficacy sequence of carvacrol > carbone > limonene (Gonçalves et al. 2010). The sequence results demonstrate that there is a relationship between nerve conduction inhibitions by aroma oil compounds and their chemical structures. This result may be consistent with our previous observation that −OH or =O group in menthol-related compounds plays an important role in inhibiting frog CAPs (Kawasaki et al. 2013).
With respect to recovery from CAP inhibition, aroma oil compounds having =O group (aldehydes and esters) appeared to exhibit a less reversibility, as noted from Figures1Bb, 4Cb, and 5. Since menthol-related chemicals having =O group in a six-membered ring (menthone, pulegone, and carvone) exhibited an almost reversible CAP inhibition (see Fig.5 in Kawasaki et al. 2013), it appeared to be necessary for this group to be attached to a ring structure for its reversibility. This issue remains to be further examined.
There was a variation among IC50 values of the aroma oil alcohols; chain alcohols (citronellol, geraniol, (−)-linalool, and (±)-linalool) were generally more effective than cyclic ones ((−)-borneol, (+)-borneol, α-terpineol, (−)-menthol, and (+)-menthol; Fig.7A). Moreover, in the chain alcohols, it appeared to be important for the −OH group to exist at the end of the chemical structure in inhibiting CAPs, because citronellol and geraniol were more effective than linalool (Fig.7A). Such a result may be consistent with our previous observation that menthol was less effective by about three-fold in inhibiting CAPs than tetrahydrolavandulol (IC50 = 0.38 mmol/L) in which the six-membered ring of menthol is opened and −OH group is located at the end of the chemical structure (Matsushita et al. 2013). In aroma oil esters, on the other hand, there appeared to be no difference in inhibiting CAPs between chain (linalyl acetate and geranyl acetate) and cyclic ones (bornyl acetate).
Figure 7.
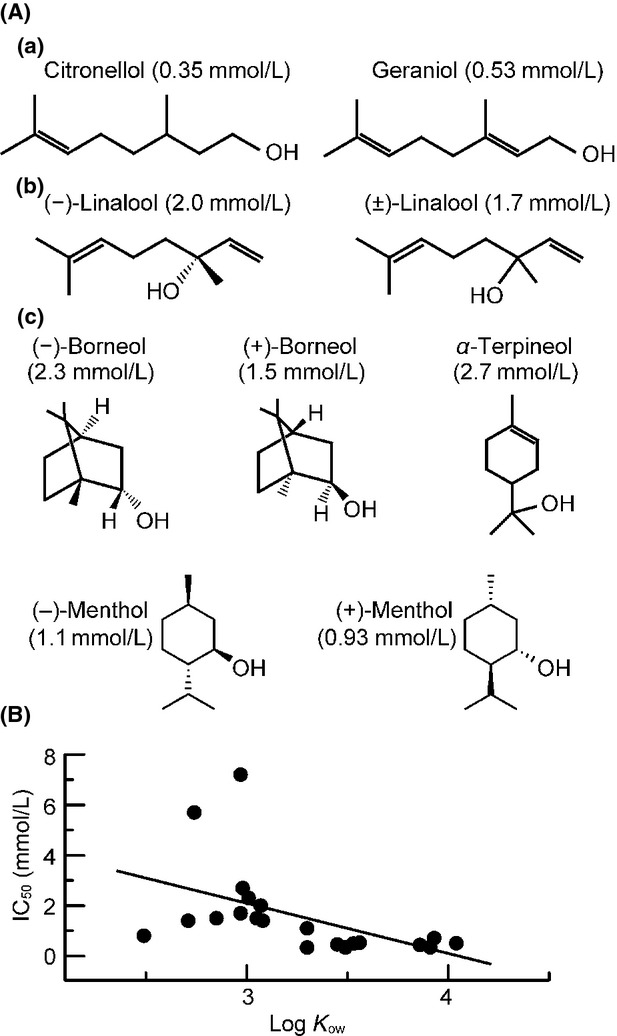
The CAP inhibitions produced by aroma oil compounds are specific to their chemical structures while being not related to their octanol–water partition coefficients (Kows). (A) The chemical structures of aroma oil alcohols (a, b: chain alcohols having −OH at the end of the structure and at the other positions, respectively; c: cyclic alcohols) and their IC50 values (as shown in parentheses) for CAP inhibitions. (B) A relationship between the IC50 of aroma oil compound for CAP inhibition and the logarithum of its Kow. Here, are plotted data of aroma oil compounds whose Kows are available from ChemlDplus (see Table1). The straight line drawn through the data points is the least squares regression line (correlation coefficient: 0.24). CAP, compound action potential.
The CAP inhibition produced by aroma oil compounds would be due to an inhibition of TTX-sensitive voltage-gated Na+ channels involved in producing frog CAPs (Katsuki et al. 2006; Mizuta et al. 2008). In support of this idea, various aroma oil compounds including linalool, menthol, carvacrol, thymol, and eugenol have been reported to inhibit voltage-gated Na+ channels (Haeseler et al. 2002; Cho et al. 2008; Leal-Cardoso et al. 2010; de Araújo et al. 2011; Joca et al. 2012). Their IC50 values for CAP amplitude reductions were similar to those for Na+-channel current amplitude reductions. The IC50 values of menthol, carvacrol, thymol, and eugenol for CAP inhibition were 0.93–1.1, 0.34, 0.34, and 0.81 mmol/L, respectively (see Table1), while their corresponding ones for Na+-channel current inhibition were 0.571 mmol/L (Haeseler et al. 2002), 0.37 mmol/L (Joca et al. 2012), 0.149 mmol/L (Haeseler et al. 2002), and 0.308 mmol/L (Cho et al. 2008), respectively (see above for the linalool actions).
The Na+-channel inhibition may be mediated not only through the actions of aroma oil chemicals on Na+-channel proteins themselves but also through a change in lipid bilayer elasticity by the chemicals, as seen in an inhibition by capsaicin of voltage-gated Na+ channels (Lundbæk et al. 2005). Although aroma oil compounds may have produced intracellular second messengers which inhibit voltage-gated Na+ channels, as shown for capsaicin-induced Na+-channel inhibition (Liu et al. 2001), this possibility appears to be unlikely because the dissected sciatic nerve used in the present study lacks the neuronal cell body and the neuronal terminals.
Since aroma oil compounds are lipophilic, the CAP inhibition in the present study may be related to their lipophilicity. Figure7B demonstrates IC50 value for CAP inhibition by aroma oil compounds, which is plotted against the logarithm of its octanol–water partition coefficient value (a measure of lipophilicity), taken from ChemlDplus (2014) (see Table1). There was no correlation between the two values and thus a lipophilicity of aroma oil compounds appeared to be unimportant in their CAP inhibitory actions. Therefore, a possibility appeared to be unlikely that aroma oil compounds act on hydrophobic amino acid residues located in voltage-gated Na+-channel protein (Catterall and Mackie 2011) or the lipid phase of cell membrane around the protein, resulting in an inhibition of the channel.
Clinical significance of aroma oil compound-induced CAP inhibition
When compared with local anesthetics’ activities, the IC50 values of aroma oil components (linalool: 1.7–2.0 mmol/L; linalyl acetate: 0.71 mmol/L) contained in lavender essential oil having a local anesthetic activity (Ghelardini et al. 1999), obtained in the present study, were almost comparable to those of levobupivacaine (0.23 mmol/L), ropivacaine (0.34 mmol/L), lidocaine (0.74 mmol/L), cocaine (0.80 mmol/L), and procaine (2.2 mmol/L; Katsuki et al. 2006; Mizuta et al. 2008; Tomohiro et al. 2013; Uemura et al. 2014), while being larger than that of tetracaine (0.013 mmol/L; Kosugi et al. 2010) in frog sciatic nerves. This result suggests that lavender essential oil has almost the same anesthetic effect as those of levobupivacaine, ropivacaine, lidocaine, cocaine, and procaine.
Linalool has not only a local anesthetic effect but also antinociceptive actions in the spinal and supraspinal level (Peana et al. 2004). This antinociception has been possibly attributed to an inhibition of glutamatergic transmission (Batista et al. 2008) or of voltage-gated Ca2+ channel (Narusuye et al. 2005), a potentiation of GABAergic transmission (Hossain et al. 2002), and a modulation of adenosine A1 and A2A receptors (Peana et al. 2006) in addition to nerve conduction inhibition. Myrcene that had a much smaller effect on frog sciatic nerve CAPs than other aroma oil chemicals had an antinociceptive effect in mice (Rao et al. 1990). Although frog sciatic nerve CAP inhibition by geranyl acetate was much larger than that of p-cymene, geranyl acetate exhibited a less antinociceptive effect than p-cymene in the formalin test in mice (Quintans-Júnior et al. 2013). Even if so at least a part of antinociception produced by various aroma oil compounds could be attributed to their inhibitory actions on nerve conduction, as revealed in the present study.
Although sensory information is transmitted by not only fast but also slow AP-conducting fibers in sciatic nerves, the present study does not examine the effects of aroma oil compounds on slow-conducting APs. In order to more firmly establish a clinical significance of CAP amplitude reductions produced by aroma oil compounds, their effects on slow-conducting CAPs such as TTX-resistant ones (for instance see Kobayashi et al. 1993) remain to be examined.
In conclution, aroma oil compounds reduced CAP peak amplitudes in a manner specific to their chemical structures, possibly without TRP activation. Aroma oil compounds belonging to aldehydes, esters, and alcohols were more effective in inhibiting CAPs than those of ketones, oxides, and hydrocarbons. The structure–activity relationship revealed in the present study may provide information about aroma oil compounds having a local anesthetic or anticonvulsive effect.
Author Contributions
S. Ohtsubo and A. Matsushita performed the research; S. Ohtsubo, T. Fujita and E. Kumamoto designed the research study; S. Ohtsubo and T. Fujita analyzed the data; E. Kumamoto wrote the paper.
Disclosure
None declared.
Glossary
- AP
action potential
- CAP
compound action potential
- DMSO
dimethyl sulfoxide
- IC50
half-maximal inhibitory concentration
- nH
Hill coefficient
- TRP
transient receptor potential
- TRPA1
transient receptor potential ankyrin-1
- TRPM8
transient receptor potential melastatin-8
- TRPV1
transient receptor potential vanilloid-1
- TRPV3
transient receptor potential vanilloid-3
- TTX
tetrodotoxin
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edn. Br J Pharmacol. 2011;164(Suppl 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RN, Navarro DS, Barbosa-Filho JM. Plants with central analgesic activity. Phytomedicine. 2001;8:310–322. doi: 10.1078/0944-7113-00050. [DOI] [PubMed] [Google Scholar]
- de Araújo DAM, Freitas C, Cruz JS. Essential oils components as a new path to understand ion channel molecular pharmacology. Life Sci. 2011;89:540–544. doi: 10.1016/j.lfs.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Batista PA, de Paula Werner MF, Oliveira EC, Burgos L, Pereira P, da Silva Brum LF, et al. Evidence for the involvement of ionotropic glutamatergic receptors on the antinociceptive effect of (-)-linalool in mice. Neurosci Lett. 2008;440:299–303. doi: 10.1016/j.neulet.2008.05.092. [DOI] [PubMed] [Google Scholar]
- Brito RG, Guimarães AG, Quintans JSS, Santos MRV, de Sousa DP, Badaue-Passos D, Jr, et al. Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activities in rodents. J Nat Med. 2012;66:637–644. doi: 10.1007/s11418-012-0632-4. [DOI] [PubMed] [Google Scholar]
- Buchbauer G, Jirovetz L. Aromatherapy – use of fragrances and essential oils as medicaments. Flavour Frag J. 1994;9:217–222. [Google Scholar]
- Buckingham J. Dictionary of natural products. Cambridge, UK: Chapman & Hall, University Press; 1994. p. 828. Vol. 1. [Google Scholar]
- Cao X, Cao X, Xie H, Yang R, Lei G, Li F, et al. Effects of capsaicin on VGSCs in TRPV1−/− mice. Brain Res. 2007;1163:33–43. doi: 10.1016/j.brainres.2007.04.085. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Mackie K. Local anesthetics. In: Brunton LL, Chabner BA, editors; Knollmann BC, editor. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw-Hill, Medical Publishing Division; 2011. pp. 565–582. [Google Scholar]
- ChemlDplus. Bethesda, MD: National Library of Medicine (US); 2014. ; [updated daily; accessed 2014 May 27]. Available at: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp. [Google Scholar]
- Cho JS, Kim TH, Lim J-M, Song J-H. Effects of eugenol on Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2008;1243:53–62. doi: 10.1016/j.brainres.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Ghelardini C, Galeotti N, Salvatore G, Mazzanti G. Local anaesthetic activity of the essential oil of Lavandula angustifolia. Planta Med. 1999;65:700–703. doi: 10.1055/s-1999-14045. [DOI] [PubMed] [Google Scholar]
- Gonçalves JCR, Alves AMH, de Araújo AEV, Cruz JS, Araújo DAM. Distinct effects of carvone analogues on the isolated nerve of rats. Eur J Pharmacol. 2010;645:108–112. doi: 10.1016/j.ejphar.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Guimarães AG, Quintans JSS, Quintans-Júnior LJ. Monoterpenes with analgesic activity – a systematic review. Phytother Res. 2013;27:1–15. doi: 10.1002/ptr.4686. [DOI] [PubMed] [Google Scholar]
- Haeseler G, Maue D, Grosskreutz J, Bufler J, Nentwig B, Piepenbrock S, et al. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur J Anaesthesiol. 2002;19:571–579. doi: 10.1017/s0265021502000923. [DOI] [PubMed] [Google Scholar]
- Hossain SJ, Hamamoto K, Aoshima H, Hara Y. Effects of tea components on the response of GABAA receptors expressed in Xenopus oocytes. J Agric Food Chem. 2002;50:3954–3960. doi: 10.1021/jf011607h. [DOI] [PubMed] [Google Scholar]
- Joca HC, Cruz-Mendes Y, Oliveira-Abreu K, Maia-Joca RPM, Barbosa R, Lemos TL, et al. Carvacrol decreases neuronal excitability by inhibition of voltage-gated sodium channels. J Nat Prod. 2012;75:1511–1517. doi: 10.1021/np300050g. [DOI] [PubMed] [Google Scholar]
- Katsuki R, Fujita T, Koga A, Liu T, Nakatsuka T, Nakashima M, et al. Tramadol, but not its major metabolite (mono-O-demethyl tramadol) depresses compound action potentials in frog sciatic nerves. Br J Pharmacol. 2006;149:319–327. doi: 10.1038/sj.bjp.0706868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Mizuta K, Fujita T, Kumamoto E. Inhibition by menthol and its related chemicals of compound action potentials in frog sciatic nerves. Life Sci. 2013;92:359–367. doi: 10.1016/j.lfs.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Ohta M, Terada Y. C fiber generates a slow Na+ spike in the frog sciatic nerve. Neurosci Lett. 1993;162:93–96. doi: 10.1016/0304-3940(93)90568-6. [DOI] [PubMed] [Google Scholar]
- Kosugi T, Mizuta K, Fujita T, Nakashima M, Kumamoto E. High concentrations of dexmedetomidine inhibit compound action potentials in frog sciatic nerves without α2 adrenoceptor activation. Br J Pharmacol. 2010;160:1662–1676. doi: 10.1111/j.1476-5381.2010.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto E, Mizuta K, Fujita T, Kosugi T, Katsuki R. Neurotransmitter receptor agonists having the inhibitory actions of nerve conduction – structure-function relationship. In: Pandalai SG, editor. Recent research developments in pharmacology. Kerala, India: Research Signpost; 2011. pp. 1–26. Vol. 2. [Google Scholar]
- Leal-Cardoso JH, da Silva-Alves KS, Ferreira-da-Silva FW, dos Santos-Nascimento T, Joca HC, de Macedo FHP, et al. Linalool blocks excitability in peripheral nerves and voltage-dependent Na+ current in dissociated dorsal root ganglia neurons. Eur J Pharmacol. 2010;645:86–93. doi: 10.1016/j.ejphar.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Lis-Balchin M, Hart S. A preliminary study of the effect of essential oils on skeletal and smooth muscle in vitro. J Ethnopharmacol. 1997;58:183–187. doi: 10.1016/s0378-8741(97)00103-7. [DOI] [PubMed] [Google Scholar]
- Liu L, Oortgiesen M, Li L, Simon SA. Capsaicin inhibits activation of voltage-gated sodium currents in capsaicin-sensitive trigeminal ganglion neurons. J Neurophysiol. 2001;85:745–758. doi: 10.1152/jn.2001.85.2.745. [DOI] [PubMed] [Google Scholar]
- Lundbæk JA, Birn P, Tape SE, Toombes GES, Søgaard R, Koeppe RE, II, et al. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol Pharmacol. 2005;68:680–689. doi: 10.1124/mol.105.013573. [DOI] [PubMed] [Google Scholar]
- Matsubara E, Fukagawa M, Okamoto T, Ohnuki K, Shimizu K, Kondo R. (-)-Bornyl acetate induces autonomic relaxation and reduces arousal level after visual display terminal work without any influences of task performance in low-dose condition. Biomed Res. 2011;32:151–157. doi: 10.2220/biomedres.32.151. [DOI] [PubMed] [Google Scholar]
- Matsushita A, Ohtsubo S, Fujita T, Kumamoto E. Inhibition by TRPA1 agonists of compound action potentials in the frog sciatic nerve. Biochem Biophys Res Commun. 2013;434:179–184. doi: 10.1016/j.bbrc.2013.02.127. [DOI] [PubMed] [Google Scholar]
- Mizuta K, Fujita T, Nakatsuka T, Kumamoto E. Inhibitory effects of opioids on compound action potentials in frog sciatic nerves and their chemical structures. Life Sci. 2008;83:198–207. doi: 10.1016/j.lfs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KSR, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Narusuye K, Kawai F, Matsuzaki K, Miyachi E. Linalool suppresses voltage-gated currents in sensory neurons and cerebellar Purkinje cells. J Neural Transm. 2005;112:193–203. doi: 10.1007/s00702-004-0187-y. [DOI] [PubMed] [Google Scholar]
- Neumcke B, Schwarz JR, Stämpfli R. A comparison of sodium currents in rat and frog myelinated nerve: normal and modified sodium inactivation. J Physiol. 1987;382:175–191. doi: 10.1113/jphysiol.1987.sp016362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonato FR, Santana DG, de Melo FM, dos Santos GGL, Brustolim D, Camargo EA, et al. Anti-inflammatory properties of rose oxide. Int Immunopharmacol. 2012;14:779–784. doi: 10.1016/j.intimp.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Peana AT, de Montis MG, Nieddu E, Spano MT, D'Aquila PS, Pippia P. Profile of spinal and supra-spinal antinociception of (-)-linalool. Eur J Pharmacol. 2004;485:165–174. doi: 10.1016/j.ejphar.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Peana AT, Rubattu P, Piga GG, Fumagalli S, Boatto G, Pippia P, et al. Involvement of adenosine A1 and A2A receptors in (-)-linalool-induced antinociception. Life Sci. 2006;78:2471–2474. doi: 10.1016/j.lfs.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Quintans-Júnior LJ, Almeida JRGS, Lima JT, Nunes XP, Siqueira JS, de Oliveira LEG, et al. Plants with anticonvulsant properties – a review. Braz J Pharmacogn. 2008;18(Suppl):798–819. [Google Scholar]
- Quintans-Júnior LJ, da Rocha RF, Caregnato FF, Fonseca Moreira JC, da Silva FA, de Souza Araújo AA, et al. Antinociceptive action and redox properties of citronellal, an essential oil present in lemongrass. J Med Food. 2011a;14:630–639. doi: 10.1089/jmf.2010.0125. [DOI] [PubMed] [Google Scholar]
- Quintans-Júnior LJ, Oliveira MGB, Santana MF, Santana MT, Guimarães AG, Siqueira JS, et al. α-Terpineol reduces nociceptive behavior in mice. Pharm Biol. 2011b;49:583–586. doi: 10.3109/13880209.2010.529616. [DOI] [PubMed] [Google Scholar]
- Quintans-Júnior LJ, Moreira JCF, Pasquali MAB, Rabie SMS, Pires AS, Schröder R, et al. Antinociceptive activity and redox profile of the monoterpenes (+)-camphene, p-cymene, and geranyl acetate in experimental models. ISRN Toxicol. 2013;2013:459530. doi: 10.1155/2013/459530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VSN, Menezes AMS, Viana GSB. Effect of myrcene on nociception in mice. J Pharm Pharmacol. 1990;42:877–878. doi: 10.1111/j.2042-7158.1990.tb07046.x. [DOI] [PubMed] [Google Scholar]
- da Silva Almeida JRG, Souza GR, Silva JC, de Lima Saraiva SRG, de Oliveira Júnior RG, de Souza Siqueira Quintans J, et al. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci World J. 2013;2013:808460. doi: 10.1155/2013/808460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa DP, Gonçalves JCR, Quintans-Júnior L, Cruz JS, Araújo DAM, de Almeida RN. Study of anticonvulsant effect of citronellol, a monoterpene alcohol, in rodents. Neurosci Lett. 2006;401:231–235. doi: 10.1016/j.neulet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- de Sousa DP, Quintans-Júnior L, de Almeida RN. Evolution of the anticonvulsant activity of α-terpineol. Pharm Biol. 2007;45:69–70. [Google Scholar]
- Stotz SC, Vriens J, Martyn D, Clardy J, Clapham DE. Citral sensing by transient receptor potential channels in dorsal root ganglion neurons. PLoS One. 2008;3:e2082. doi: 10.1371/journal.pone.0002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuno HA, Bradberry CW, Everill B, Agulian SK, Wilkes S, Baldwin RM, et al. Local anesthetic effects of cocaethylene and isopropylcocaine on rat peripheral nerves. Brain Res. 2004;996:159–167. doi: 10.1016/j.brainres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Tomohiro D, Mizuta K, Fujita T, Nishikubo Y, Kumamoto E. Inhibition by capsaicin and its related vanilloids of compound action potentials in frog sciatic nerves. Life Sci. 2013;92:368–378. doi: 10.1016/j.lfs.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Fujita T, Ohtsubo S, Hirakawa N, Sakaguchi Y, Kumamoto E. Effects of various antiepileptics used to alleviate neuropathic pain on compound action potential in frog sciatic nerves: comparison with those of local anesthetics. Biomed Res Int. 2014;2014:540238. doi: 10.1155/2014/540238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana GSB, Vale TG, Pinho RSN, Matos FJA. Antinociceptive effect of the essential oil from Cymbopogon citratus in mice. J Ethnopharmacol. 2000;70:323–327. doi: 10.1016/s0378-8741(99)00168-3. [DOI] [PubMed] [Google Scholar]
- Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, et al. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 2005;25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalachoras I, Kagiava A, Vokou D, Theophilidis G. Assessing the local anesthetic effect of five essential oil constituents. Planta Med. 2010;76:1647–1653. doi: 10.1055/s-0030-1249956. [DOI] [PubMed] [Google Scholar]


