Abstract
Tissue kallikrein (KLK-1), a serine protease, initiates the release of bradykinin (BK)-related peptides from low-molecular weight kininogen. KLK-1 and the BK B2 receptor (B2R) mediate beneficial effects on the progression of type 2 diabetes and renal disease, but the precise role of KLK-1 independent of its kinin-forming activity remains unclear. We used DM199, a recombinant form of human KLK-1, along with the isolated human umbilical vein, a robust bioassay of the B2R, to address the previous claims that KLK-1 directly binds to and activates the human B2R, with possible receptor cleavage. DM199 (1–10 nmol/L) contracted the isolated vein via the B2R, but in a tachyphylactic, kinin-dependent manner, without desensitization of the tissue to exogenously added BK. In binding experiments with recombinant N-terminally tagged myc-B2Rs expressed in HEK 293a cells, DM199 displaced [3H]BK binding from the rabbit myc-B2R, but not from the human or rat myc-B2Rs. No evidence of myc-B2R degradation by immunoblot analysis was apparent following treatment of these 3 myc-B2R constructs with DM199 (30 min, ≤10 nmol/L). In HEK 293 cells stably expressing rabbit B2R-GFP, DM199 (11–108 pmol/L) elicited signaling-dependent endocytosis and reexpression, while a higher concentration (1.1 nmol/L) induced a partially irreversible endocytosis of the construct (microscopy), paralleled by the appearance of free GFP in cells (immunoblotting, indicative of incomplete receptor down-regulation). The pharmacology of DM199 at relevant concentrations (<10 nmol/L) is essentially based on the activity of locally generated kinins. Binding to and mild down-regulation of the B2R is possibly a species-dependent idiosyncratic response to DM199.
Keywords: Bradykinin, bradykinin B2 receptor, human isolated umbilical vein, radioligand binding, receptor internalization, tissue kallikrein
Introduction
Several in vitro and in vivo small animal studies have demonstrated beneficial metabolic, cardiovascular, and renoprotective effects following stimulation of the endogenous bradykinin (BK) B2 receptor (B2R) (e.g., Henriksen et al. 1998; Duka et al. 2001; Kakoki et al. 2007; Yuan et al. 2007; Potier et al. 2013). The glycosylated serine protease, tissue kallikrein-1 (KLK-1), generates kallidin (Lys-BK) from circulating low-molecular (LMW)-kininogen (KNG); BK is generated following aminopeptidase cleavage of Lys-BK. Both peptides are equipotent agonists of the B2R (Leeb-Lundberg et al. 2005). In various rodent models, ectopic expression of the KLK-1 gene attenuates hypertension, ischemic renal injury, and insulin resistance (Wolf et al. 2000; Tu et al. 2008), while the administration of purified KLK-1 protein confers renoprotective (Uehara et al. 1994) and antidiabetic effects (Kolodka et al. 2014). The availability of purified, recombinant human KLK-1 of high specific activity (DM199) has prompted us to reevaluate the pharmacology of this enzyme with the following objectives:
To assess the claim that KLK-1 directly binds to and activates the human B2R in the absence of LMW-KNG (Hecquet et al., 2000; Biyashev et al. 2006; Chao et al. 2008). The alternative, supported by a previous study, is that residual KNG present in cultured cells or isolated tissues allows kinin generation at the vicinity of B2Rs, eliciting conventional but tachyphylactic signaling, as the substrate is rapidly consumed in vitro (Houle et al. 2003).
To examine whether clinically reached levels of DM199 (<10 nmol/L) cleave the B2R, leading to its rapid internalization and down-regulation as previously suggested in a study with human urinary KLK-1 (Houle et al. 2003). By contrast, B2R-induced stimulation of the B2R results in a cycle of desensitization-endocytosis, followed by essentially complete recycling of the receptor to the plasma membrane (Leeb-Lundberg et al. 2005; Charest-Morin et al. 2013). The study of the effect of KLK-1 on the B2R is facilitated by the systematic definition of the amino acid sequences that can be cleaved by the protease, revealing its dual tryptic and chymotryptic actions (Li et al. 2008). The species of origin of the B2Rs is a significant parameter in this line of investigation, because extracellular domains of the receptor where KLK-1 may bind consist of sequences that are less conserved than other receptor sequences (e.g., transmembrane or intracellular phosphorylable domains).
DM199 may possess unexpected actions at other receptors, on the model of the G protein-coupled receptors (GPCRs) activated by thrombin and other serine proteases. Indeed, four related protease activated receptors (PAR-1 to -4) are cleavable by such proteases that exist in the extracellular space (Vergnolle 2009); for instance thrombin cleaves PAR-1, -3 and -4 at consensus cleavage sites found at the receptor extracellular N-terminus, thus revealing a new amino terminus that behaves as a tethered peptide agonist. KLK-1-induced cleavage and activation of PAR-1 has been proposed (Gao et al. 2010a, b). This study exploits a robust contractile bioassay of the human B2R ligands, the isolated human umbilical vein preparation, which expresses many types of endogenous receptors (Altura et al. 1972; Marceau et al. 2010; Gera et al. 2013), including some of the PARs (Tay-Uyboco et al. 1995; Saifeddine et al. 1998), and is therefore suitable to address the open question about unconventional effects of DM199.
Other complementary assays based on recombinant receptors from human and animal species supported molecular investigations. It was found that the most plausible pharmacologic actions of DM199 at relevant nanomolar concentrations are essentially based on the generation of kinins.
Materials and Methods
Vascular smooth muscle contractility
The institutional research ethics board (CHU de Québec) approved the anonymous use of human umbilical cord segments obtained after elective cesarean section deliveries. Informed consent was obtained from mothers. Umbilical vein rings, used in the contractile bioassay for the BK B2R, were prepared and suspended in organ baths and submitted to equilibration in Krebs' solution as described (Marceau et al. 1994; Charest-Morin et al. 2014). The vascular preparation was used to assess the effect of DM199 on vascular smooth muscle. The concentration–effect relationship of DM199, the tachyphylactic nature of the responses, and effects of a BK receptor antagonist or of an inhibitor of the KLK-1 enzymatic activity (aprotinin) were addressed using this assay, as described in Results.
Drugs and reagents
DM199 (recombinant human KLK-1), was obtained from DiaMedica. Briefly, DM199 was produced from Chinese hamster ovary (CHO) cells expressing a gene encoding the full length pre-pro-protein for human KLK-1 (NP_002248.1). Following harvest and clarification, the supernatant containing secreted pro-KLK-1 was treated with recombinant trypsin (Roche Diagnostics, Mannheim, Germany) to generate active KLK-1. The active KLK-1 was further purified under aseptic conditions by column chromatography and filtration essentially as described (Lu et al. 1996a,b), and was >95% pure as determined by SDS-PAGE analysis. N-terminal Edman sequencing of purified DM199 confirmed that the purified protein was exclusively active KLK-1, free of the pro-KLK-1 heptapeptide. The specific activity of DM199 was measured in vitro by cleavage of the substrate D-Val-Leu-Arg-7 amido-4-trifluoromethylcoumarin (D-VLR-AFC, FW 597.6; Sigma, St. Louis, MO, USA, Cat # V2888 or AnaSpec Inc, Fremont, CA, USA, Cat # 24137). When D-VLR-AFC was hydrolyzed, the free AFC produced in the reaction was quantified by fluorometric detection (excitation 360 nm, emission 460 nm). DM199 activity was determined by comparing the relative activity of a DM199 sample to the porcine kininogenase standard acquired from the National Institute for Biological Standards and Control (NIBSC Product No. 78/543). For this standard, the assigned potency is 22.5 international units (IU) per 20 μg ampoule of porcine pancreatic kininogenase. DM199 used in this study had a specific activity 359 EU/mg or 9473 EU/mL. Anatibant (LF 16-0687; Pruneau et al. 1999) was a gift from Laboratoire Fournier (Daix, France). BK was from Bachem (Torrance, CA). Other drugs, low-molecular weight-kininogen (LMW-KNG) and human serum albumin were from Sigma-Aldrich (St. Louis, MO).
Vectors and cell transfection
A pharmacologically intact rabbit B2R-green fluorescent protein (-GFP) fusion protein was exploited; this construct is stably expressed in HEK 293 cells (Bachvarov et al. 2001). The myc-tagged rabbit B2R construct was previously described (Bawolak et al. 2007).
The methods for constructing and validating novel vectors encoding for the human and rat B2Rs, including myc-tagged receptors, are presented in the Data S1. Stable transfectant HEK 293a cell lines expressing myc-B2R construct from each species (human, rabbit, rat) have been developed by growing the cells for one month in the presence of geneticin (500 μg/mL; Thermo Scientific, Ottawa, ON, Canada) and sorting based on the fluorescence of the anti-myc 4A6 monoclonal antibody conjugated to AlexaFluor-488 (Millipore Billerica, MA, USA).
Radioligand binding studies
[3H]BK ([2,3-prolyl-3,4-3H(N)]-BK, 85.4 Ci/mmol) was purchased from PerkinElmer (Boston, MA). The binding of the radioligand to adherent intact cells expressing a form of the B2R was evaluated in the following manner: cells grown in 24-well plates were washed with an assay buffer (ice-cold phosphate-buffered saline [PBS], pH 7.4, supplemented with 0.02% NaN3, 0.1% bovine serum albumin, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], and 1 μmol/L captopril). 0.5 mL of this buffer was left in each cell well, to which were optionally added cold competitors and the radioligand. After 90 min incubation at 0°C, wells were washed 3 times with 1 mL of ice-cold PBS. The well supernatants were discarded and the cells were dissolved in 1 mL 0.1 N NaOH. This suspension was transferred in a scintillation vial containing 7 mL of Ecolite Plus and counted for radioactivity.
The binding assay was exploited in 2 types of experimental situations: (1) a series of experiments involved the binding competition of a fixed concentration of the radioligand with DM199; in this case, the assay was conducted at 0°C and PMSF was omitted from the buffer to avoid inactivation of the enzyme; (2) the assay was also used to ascertain the high affinity binding of the radioligand to novel myc tagged receptor constructions and their correct cell surface expression (as in Bawolak et al. 2007).
Microscopy
To detect the effect of KLK-1 on B2R-GFP subcellular distribution, DM199 or BK were directly introduced in the existing serum-containing culture medium. Cells were further incubated for 30 min-3 h at 37°C, then observed in microscopy for the green epifluorescence, and photographed using an Olympus (Center Vally, CA, USA) BX51 microscope coupled to a CoolSnap HQ digital camera.
Immunoblots
The monoclonal antibodies to GFP (Clontech, Palo Alto, CA, USA; clone JL-8, used at dilution 1/1000) exhibited an exceptionally low background in total cell extracts of untransfected HEK 293 cells. For the analysis of B2R-GFP in total cell extracts, total cell extracts were recovered and analyzed after SDS/PAGE (9% gel) and protein transfer (Bachvarov et al. 2001) using the anti-GFP antibodies. Myc-tagged B2R constructs were detected in HEK 293a total cell extracts with the anti-myc monoclonal antibodies, clone 4A6 (Millipore, dilution 1:2000). The staining was detected with the appropriate horseradish peroxydase-conjugated secondary antibodies and a luminescent substrate used as directed (Western Lightning, PerkinElmer). Equal track loading was verified by migrating and transferring the same samples separately and immunoblotting for β-actin (monoclonal from Sigma-Aldrich).
Results
Contractile effects of DM199 on the human isolated umbilical vein
The human isolated umbilical vein preparation contains endogenous receptors for many agents that induce contractile responses, including BK (Altura et al. 1972). As a bioassay for BK B2R ligands, the vein contractility allows quantitative evaluation of agonists, antagonists and partial agonists of this pharmacological entity with good correlation with radioligand-binding competition and other assays (Marceau et al. 1994; Bawolak et al. 2007, 2009; Charest-Morin et al. 2014). The effect of BK stimulation is reproducible if sufficient time is allowed between stimulations for the vein to recover (Marceau et al. 1994): this is illustrated in Figure1A where a vein ring was contracted 3 times at 1 h intervals with 10 nmol/L BK (an EC40 approximately). The average amplitudes of these responses in replicated experiment are presented in Figure2A. DM199 at 0.1 nmol/L had no effect on the preparation, but contracted the vein at 1 or 10 nmol/L concentration levels (Fig.1B–D, statistical analysis in Fig.2B; molar concentrations calculated, using an average molecular weight of 38.5 kDa; Kolodka et al. 2014). However, the effect of DM199 is dramatically tachyphylactic, as it was not observed upon subsequent stimulations (Fig.2). At the end of the recordings of DM199 effects, BK stimulation produced a contraction, a significant finding because the direct effect of KLK-1 on the BK B2R has been proposed under different schemes in the past, and this may include B2R cleavage and destruction (Hecquet et al. 2000; Houle et al. 2003). To further ascertain that DM199 does not desensitize the BK B2R, other groups of tissues were exposed to the protease or its saline vehicle for 30 min, washed and allowed to rest for an additional 30 min period. This was followed by the construction of the full cumulative concentration-effect curve of BK. Figure3A illustrates the protocol with contraction tracings obtained with the 2 extreme pretreatments, saline or 10 nmol/L DM199. The peak contractile effects of the protease during the pretreatment period are reported in Figure3B. Pretreatment with the various concentration levels of DM199 (0–10 nmol/L) did not significantly alter the subsequently recorded maximal effect (Fig.3B) or potency of BK (Fig.3C; the 95% confidence intervals of all EC50 values overlapped). Thus, DM199, up to 10 nmol/L, did not desensitize endogenous human B2Rs in the umbilical vein.
Figure 1.
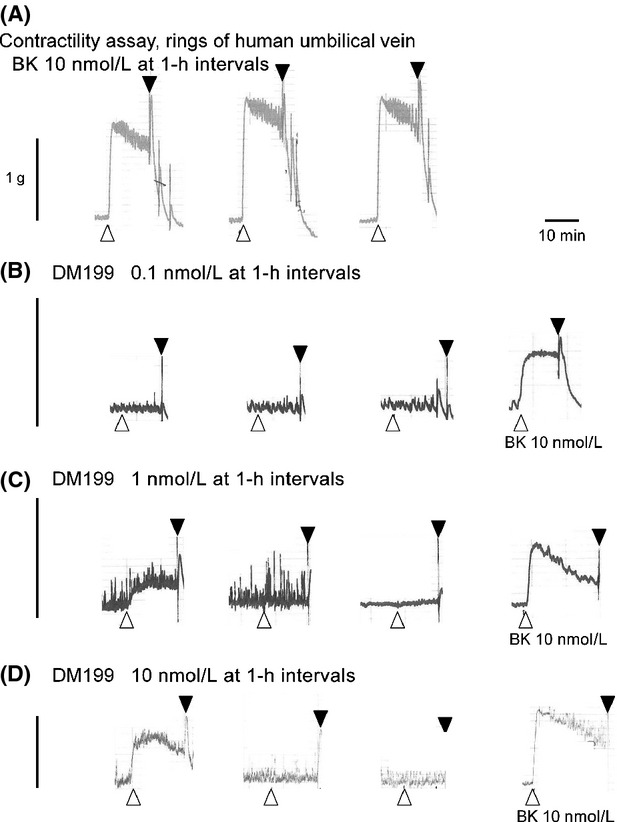
Representative tracings of the effect of DM199 and bradykinin on the human isolated umbilical vein contractility. Stimulants (10 nmol/L BK or 0.1–10 nmol/L DM199) were applied 3 times to each tissue for 12 min at 1 h intervals. Tissues exposed to DM199 did not respond at the 2nd or 3rd stimulation, but were still responsive to BK applied afterwards. Open symbols refer to stimulant applications; closed symbols, to the first of a series of tissue wash-outs with fresh Krebs' buffer.
Figure 2.
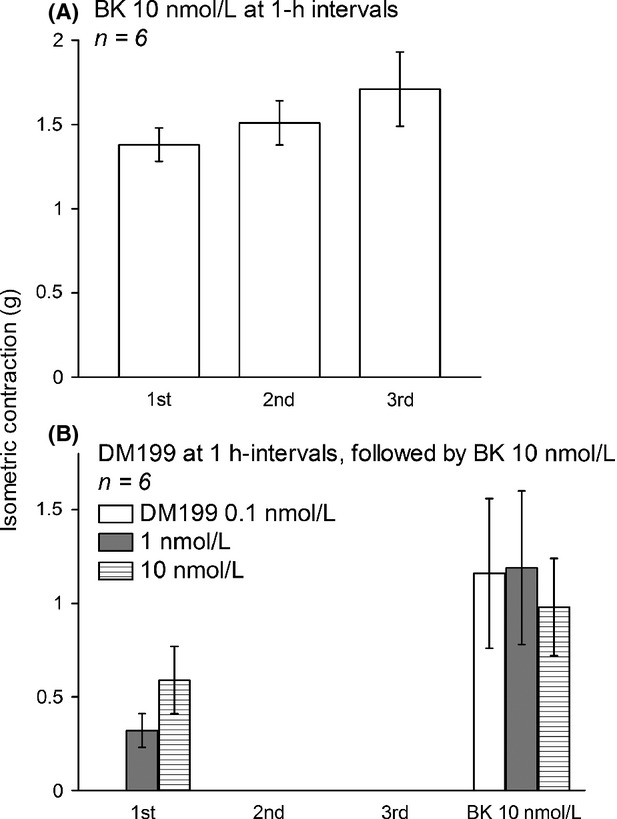
Maximal effects of repeated DM199 and bradykinin administration in the human isolated umbilical vein contractility assay. (A) Repeated effect of 10 nmol/L BK at 1 h intervals. ANOVA indicated that values were homogeneous (P > 0.05). (B) Repeated effect of DM199, followed by BK stimulation. The 2nd and 3rd effects of the protease at 1 or 10 nmol/L were significantly inferior to the 1st effect (ANOVA followed by Dunnett's test, P < 0.01).
Figure 3.
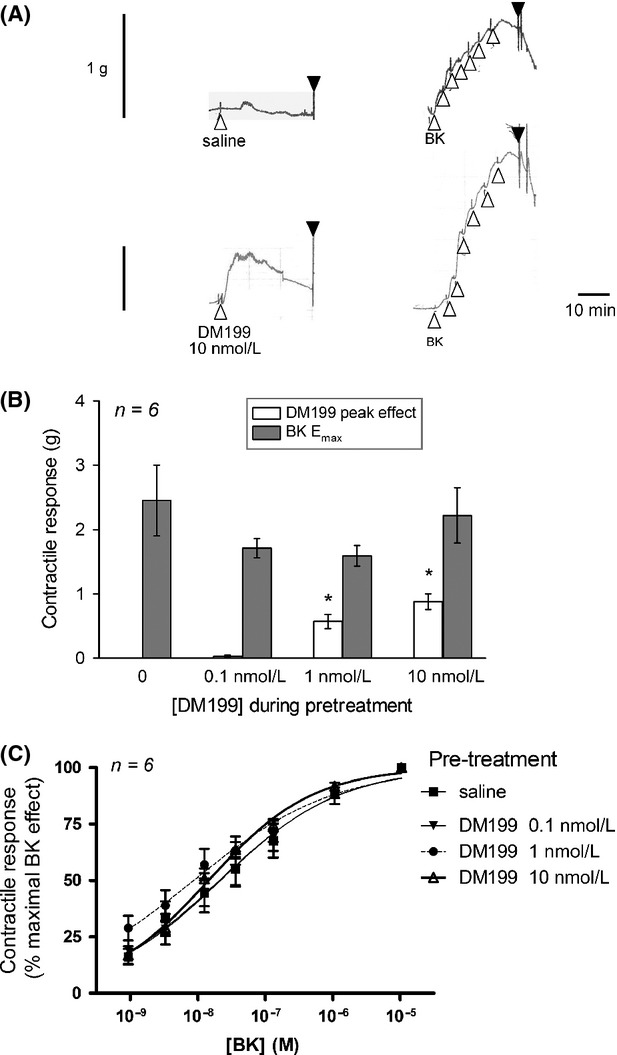
Effect of pretreatment with DM199 (0.1–10 nmol/L) or its saline vehicle on the subsequent full cumulative concentration-effect curve of BK. (A) Representative tracings of the two extreme conditions, 10 nmol/L DM199 and saline vehicle. Presentation as in Figure1. The concentration levels reached after successive additions of BK are 0.94, 3.3, 12.7 36.2, 131, 1074, and 10,500 nmol/L. (B) Amplitude of the maximal DM199-induced contraction and that of later recorded BK-induced maximal contraction (g). The latter set of values did not differ between groups, but the former did (P < 10−4, ANOVA). *P < 0.01 vs. control (Dunnett's test). (C) Concentration-effect curves of BK-induced contraction as a function of the pretreatment (expressed as a percent of the maximal effect). The 95% confidence limits of BK EC50 values overlapped under all experimental conditions.
The mechanism of DM199-induced contraction was further assessed in response to inhibitory drugs (applied 30 min before DM199 and maintained thereafter). DM199 (10 nmol/L) was applied only once due to the tachyphylactic nature of the response and control responses are derived from a paired set of control tissues derived from the same veins. The second contraction was induced by BK, and the third, by histamine (Fig.4A). The specific nonpeptide B2R antagonist anatibant (1 μmol/L) virtually abolished the effects of either DM199 or BK, leaving that of histamine unaffected. The KLK-1 inhibitor aprotinin (10 μmol/L) prevented only the effect of DM199, whereas the H1 receptor antagonist pyrilamine (1 μmol/L) abated only the contractile effect of histamine (Fig.4A). Thus, DM199-induced contraction depends on its catalytic activity and is ultimately mediated by the B2R. These observations, along with the tachyphylactic nature of the responses to DM199, are compatible with the cleavage of a limited and nonrenewable tissue reservoir of KNG in isolated tissues. Another hypothetical tachyphylactic mechanism, the degranulation of mast cells possibly present in the tissue, seems unlikely as the antihistamine did not inhibit the effect of DM199. The effect of LMW-KNG replenishment on DM199-induced contractility in veins desensitized by two applications of the protease (10 nmol/L) is illustrated in Figure4B. In these experiments, tissues were randomized to 3 groups. The first two responses to DM199 treatment at 1 h interval were similar across the groups, with confirmation that the vein rings were desensitized at the second stimulation. Then, 30 min before the third stimulation, one group of tissues was replenished with 9.4 nmol/L LMW-KNG, partially restoring the contractile response to DM199 (10 nmol/L). In a second group of vein rings, the restored response provided by LMW-KNG was abolished by co-treatment with the B2R antagonist anatibant (Fig.4B), a finding compatible with an effect dependent on the kinin-dependent effect on the B2R. Treatment of a third group of vein rings with an irrelevant protein (human serum albumin, 19 nmol/L) 30 min prior to the third stimulation did not restore the responsiveness to the protease.
Figure 4.
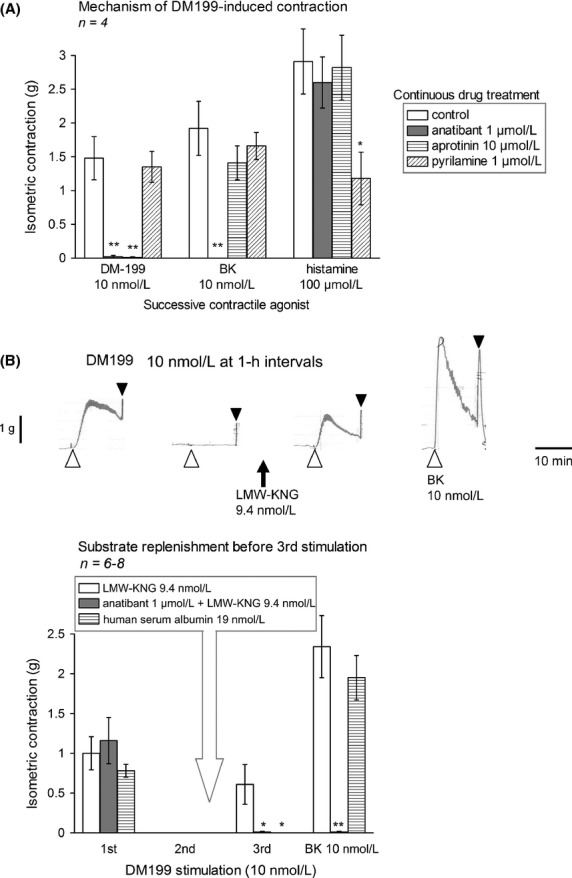
Mechanism of DM199-induced contraction in the human umbilical vein. (A) Effect of continuous drug treatments on the contractile response to DM199 (10 nmol/L, recorded at the 3-h equilibration time point), BK (10 nmol/L, 4 h) and histamine (100 μmol/L, 4.5 h). Tissues were randomly assigned to one of the inhibitory drug treatment, as indicated, from the equilibration time point 2.5 h; this treatment was maintained for all subsequent recordings. ANOVA showed that the response to each stimulant significantly differed across treatments with inhibitory drugs (DM199 P < 10−4; BK P < 0.001; histamine P < 0.05). The effect of each drug versus control responses was further tested using Dunnett's test. *P < 0.05; **P < 0.01. (B) Effect of LMW-KNG replenishment (9.4 nmol/L applied between the second and 3rd stimulation) on DM199-induced contraction. Top: representative tracing showing the partial recovery of the response to DM199. Bottom: replicated experiments with groups of randomized tissues stimulated thrice with 10 nmol/L DM199 and treated with LMW-KNG, anatibant and LMW-KNG, or human serum albumin. The indicated treatments were introduced only between the 2nd and 3rd stimulation with DM199. ANOVA showed significant differences between groups in the 3rd DM199 stimulation (P < 0.05; Dunnett's test: *P < 0.05 vs. replenishment with LMW-KNG alone). ANOVA also showed significant differences between groups in the final recording of BK effect (P < 10−4; Dunnett's test: **P < 0.01 vs. replenishment with LMW-KNG alone).
Experiments based on myc-tagged B2Rs from 3 species
It has been previously claimed that tissue kallikreins compete for [3H]BK binding at the human B2R with nanomolar affinity (Hecquet et al. 2000). Further, since a putative KLK-1-binding/cleavage site(s) in the B2R sequence has not been identified, the species of B2R origin may be a significant parameter due to limited sequence conservation in the extracellular domains. We have previously reported an myc-tagged version of the rabbit B2R (Bawolak et al. 2007) and herein we report construction of the human and rat versions of the myc-B2R. Vectors harboring these constructs were sequenced and proven to encode for high affinity BK receptors, based on [3H]BK binding (Figs. S1, S2). In a modified assay of [3H]BK binding to transiently expressed myc-B2R constructs (intact HEK 293a cells, assay run at 0°C in the absence of PMSF to avoid KLK-1 inactivation), we have found that DM199 (up to 1 μmol/L) did not compete for the radioligand binding to receptors that possessed the human or rat sequences; however, a form of partial competition with little concentration dependence was observed at the rabbit myc-B2R, but with reproducible effect at and above 1 nmol/L (Fig.5A).
Figure 5.
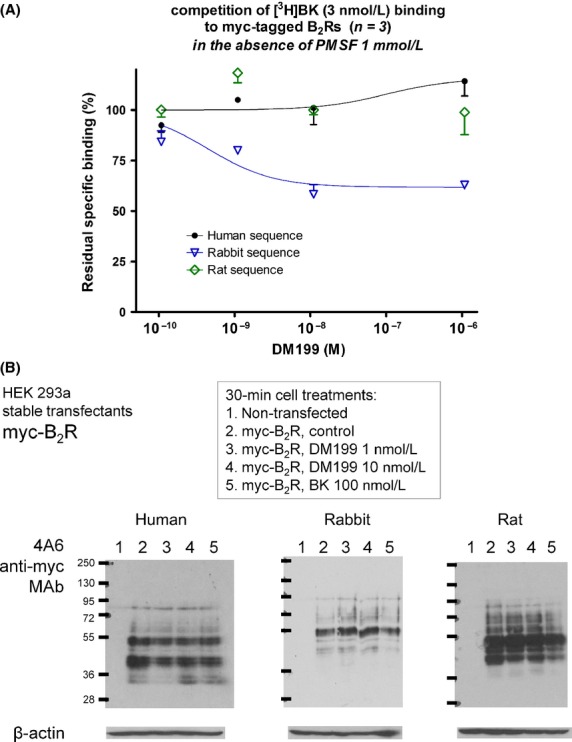
Effect of DM199 on myc-tagged B2R constructs from 3 mammalian species. (A) Competition of [3H]BK (3 nmol/L) binding to transiently expressed myc-B2Rs from 3 species. Results are expressed as the residual-specific bindings (means ± SEM of 3 duplicate determinations). The absolute specific binding was 60–70 fmol/well in these experiments and the nonspecific binding, recorded in the presence of 1 μmol/L unlabeled BK, was consistently inferior to 3% of the specific binding. (B) Immunoblot of HEK 293a cell extracts that stably expressed one of the myc-B2Rs: effect of 30 min treatments (37°C) with putative ligands added to the serum-containing culture medium. Anti-myc tag monoclonal antibodies were exploited in these experiments. Representative of 3 experiments.
The antigenic tag in the myc-B2R constructs supports immunoblotting (Bawolak et al. 2007). In experiments reported in Figure5B, stably expressed myc-B2Rs were exploited. Nontransfected cells reacted minimally with the anti-myc antibody, supporting that the bands in receptor-expressing cells originate from the transgenes. In transfected cells, rather complex “fingerprints” were observed, which may correspond to glycosylated and immature nonglycosylated receptors along with possible spontaneous degradation products in the total cell extracts. We have previously argued that bands in the 60–70 kDa range are more probably mature glycosylated rabbit myc-B2R (Bawolak et al. 2007). The 30-min treatment with BK (100 nmol/L), that determines the rapid endocytosis of rabbit myc-B2R without degradation (Bawolak et al. 2007, 2011; Charest-Morin et al. 2013), did not modify the immunoblot fingerprints of the 3 myc-B2R constructs (Fig.5B). DM199 (1 or 10 nmol/L, 30 min) was also inactive in this respect. If KLK-1-induced partial cleavage of the receptor constructs occurred at all under the tested conditions, metabolite(s) that included the N-terminal tag might not accumulate sufficiently for detection.
Effect of DM199 on the subcellular distribution of B2R-GFP
The competition of [3H]BK binding to the rabbit myc-B2R (Fig.5) may be compatible with the previously reported limited but rapid (10 min) down-regulation of the rabbit B2R-GFP construct exposed to KLK-1 (Houle et al. 2003). As DM199 is a recombinant KLK-1 preparation possessing a very high specific activity, we have reassessed its effects on B2R-GFP construct using a longer incubation periods, notably to test the extent of the mature receptor destruction. The rabbit B2R-GFP fusion protein stably expressed in HEK 293 cells is visualized as a sharply defined fluorescence associated with the plasma membrane in the vast majority of cells (Fig.6). As previously documented, the agonist BK (100 nmol/L) translocates a large fraction of these fluorescent receptors into more or less well-defined endosomal structures in 30 min (Bachvarov et al. 2001; Fig.6A). However, a 3-h treatment with this concentration of BK in live cells that are maintained in their serum-containing culture medium at 37°C evidences a recycling of the receptor to the plasma membrane (Charest-Morin et al. 2013; Fig.6B), which derives in part from the fact that BK has a half-life of ∼8 min in this medium (Bachvarov et al. 2001). In previous experiments, receptor recycling is parallel to BK degradation in both the extracellular and endosomal compartments while the total amount of B2R-GFP in cells remains stable (immunoblots, [3H]BK-binding assay; Bachvarov et al. 2001; Bawolak et al. 2012). Figure6A also shows that BK-induced endocytosis of B2R-GFP is partially inhibited by a nonpeptide B2R antagonist, anatibant, but not by the protease inhibitor aprotinin (anatibant has a lower affinity for the rabbit B2R than for the human B2R, a likely explanation for the partial inhibition of the maximal BK concentration; Pruneau et al. 1999; Houle et al. 2000).
Figure 6.
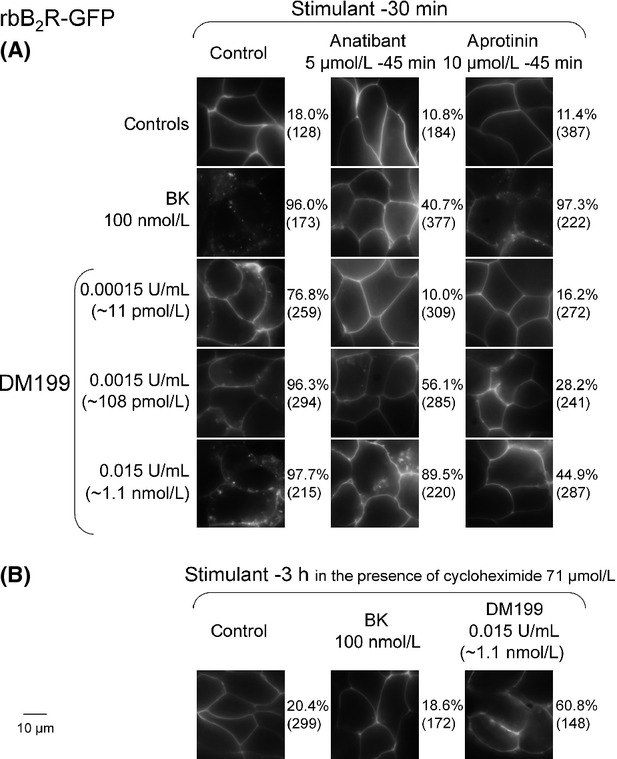
Endocytosis of B2R-GFP in response to BK or DM199. (A) Effect of 30-min treatments. Antagonist drugs (anatibant or aprotinin) were introduced 15 min before the stimulant. (B) Effect of 3 h treatments. Green epifluorescence (1000×) of HEK 293 cells stably expressing the fusion protein. The percentage of cells exhibiting endocytosis, defined as a loss of continuity of the plasma membrane labeling and as the presence of fluorescent endosomal structures, is indicated at the right of each typical photomicrograph; the number of evaluated cells in several fields is indicated between parentheses.
DM199 also induced the endocytosis of B2R-GFP at very low concentrations. The effect is already measurable at 11 pmol/L (Fig.6A). In the 11 pmol/L–1.1 nmol/L range illustrated in Figure6A, all effects were abated by aprotinin (10 μmol/L), but the B2R antagonist anatibant was active only in the lower part of this range, suggesting that the 1.1 nmol/L concentration of DM199 exerted a signaling-independent form of receptor internalization. The long 3-h treatment with DM199 was not associated with a complete return of fluorescence from endosomal structures to sharply defined plasma membrane (Fig.6B). The protein synthesis inhibitor cycloheximide was applied for this longer treatment period in order to avoid interference from newly synthesized B2R-GFP molecules; the blockade of protein synthesis did not inhibit the essentially complete recycling of internalized fluorescent receptors in response to BK (present Fig.6B and Bachvarov et al. 2001).
Effect of DM199 on B2R-GFP integrity
An immunoblot study is presented in Figures7, 8. In resting HEK 293 cells that stably express rabbit B2R-GFP, this construct is seen as a band with an average molecular weight 101 kDa with little free GFP in the total cell extract. A 3-h treatment with BK, while promoting a spectacular cycle of endocytosis and recycling (microscopy), usually does not alter the quantity and integrity of B2R-GFP in cells. Treatments with increasing concentrations of DM199 did not induce a progressive loss of the 101 kDa band, but the parallel accumulation of the reaction product, free GFP, may be a more sensitive indicator of B2R-GFP proteolysis (significant for 1 nmol/L DM199 vs. the amount of free GFP in control cells; Fig.7). Indeed, GFP is very stable in mammalian cells (Corish and Tyler-Smith 1999) and is a likely final C-terminal reaction product if the receptor construct is degraded. The digestion of B2R-GFP by 1.1 nmol/L DM199 was not inhibited by the nonpeptide B2R antagonist anatibant, but may have been reduced by co-treatment with aprotinin (Fig.8).
Figure 7.
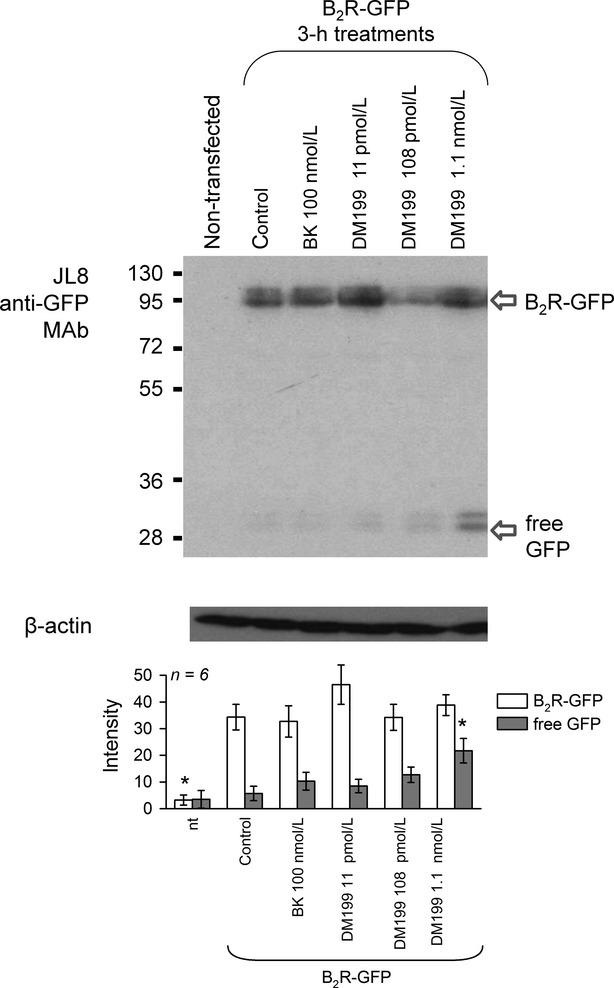
Immunoblot of GFP or GFP-tagged B2R in total extracts of HEK 293 cells that stably express B2R-GFP. Cells maintained in their serum-containing culture medium were treated for 3 h as indicated. Bottom: Scanning intensity of relevant bands in replicated experiments. ANOVA showed that B2R-GFP bands significantly varied among groups (P < 10-4) and also that free GFP values were significantly heterogeneous (P < 0.01). *P < 0.01 versus values of control cells (second set of histograms from the left; Dunnett's test).
Figure 8.
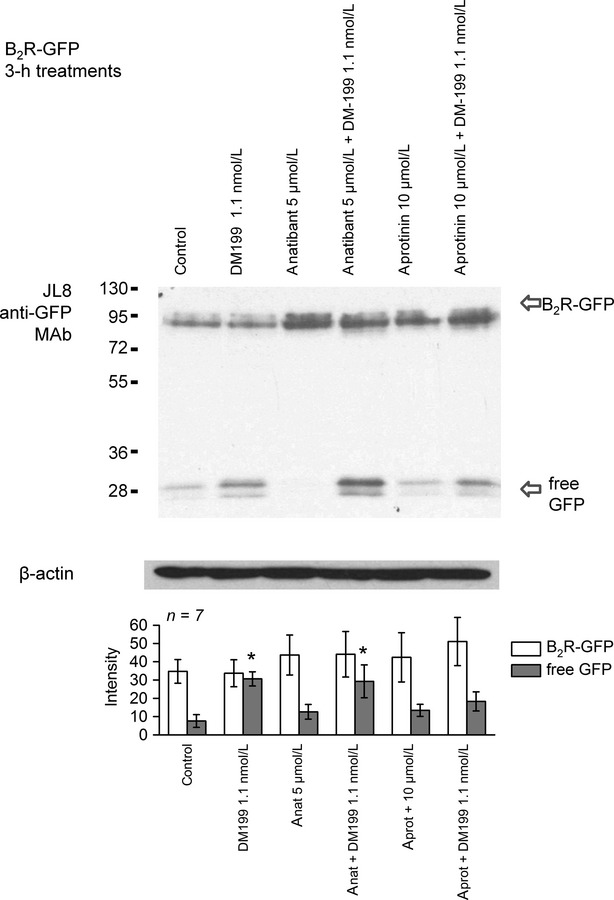
Effect of inhibitory drugs on the digestion of B2R-GFP by DM199 (1.1 nmol/L). HEK 293 cells maintained in their serum-containing culture medium were treated for 3 h as indicated (anatibant or aprotinin were applied 15 min before DM199). Presentation as in Figure7. ANOVA showed that B2R-GFP bands did not significantly vary among groups, but that free GFP values were significantly heterogeneous (P < 0.02). *P < 0.05 versus values of control cells (left-most set of histograms from the left; Dunnett's test).
Discussion
We have investigated the effects of KLK-1 on the binding, activation and internalization of the BK B2R under a variety of assay formats using DM199, a pharmaceutically refined, catalytically active form of recombinant human KLK-1. Previous reports have suggested that KLK-1 elicits activation of the B2R even in the absence of kinins, possibly through a direct interaction with the receptor (Hecquet et al. 2000; Biyashev et al., 2006; Chao et al. 2008).
The contractile response of isolated human umbilical vein provides a pharmacologically relevant and robust assay for agonist, partial agonist and antagonist activities at the B2R and other receptors (Altura et al. 1972; Marceau et al. 2010; Gera et al. 2013). The assay demonstrates that the vascular pharmacology of DM199 is dependent on kinin release from an exhaustible supply of LMW-KNG, as shown by the loss of contractility following kininogen depletion and restoration of contractility following exogenous LMW-KNG addition (Figs.4). The tachyphylactic response to DM199 is similar to the one recorded using the rabbit jugular vein in a previous study with purified urinary KLK-1 (Houle et al. 2003). No unexpected effects have been observed on the umbilical vein, and there was no evidence of a persistent desensitization of the preparation after pretreatment with DM199 (Fig.3).
We assessed whether DM199 directly binds to the B2R in a [3H]BK-binding competition assay conducted on ice (Fig.5). About 97% or more of the binding was displaceable by an excess of unlabeled BK in these assays; further, [3H]BK binding from rabbit myc-B2R was also extensively displaced by several relevant ligands like the alternate agonist Lys-BK or the antagonists, anatibant, icatibant, and bradyzide (Bawolak et al. 2007). DM199 did not displace [3H]BK binding from the myc-B2R constructs possessing the rat or human sequences (at odds with Hecquet et al. 2000; in the latter case). However, a species-dependent competition was observed with the rabbit receptor construct, with an irregularly shaped curve that does not conform to one-site competition equation. This may not be entirely surprising since extracellular receptor domains are not as highly conserved as other receptors sequences, like the transmembrane domains or the C-terminal phosphorylable site of the B2R (Bachvarov et al. 1995). In addition, the protease docking site may not coincide well with the BK docking sites in the rabbit B2R, for which a model has been proposed (Leeb-Lundberg et al. 2005; Fig.9A). Such species-specific findings tend to disprove that a direct molecular interaction of physiological importance exists between KLK-1 and the B2R.
Figure 9.
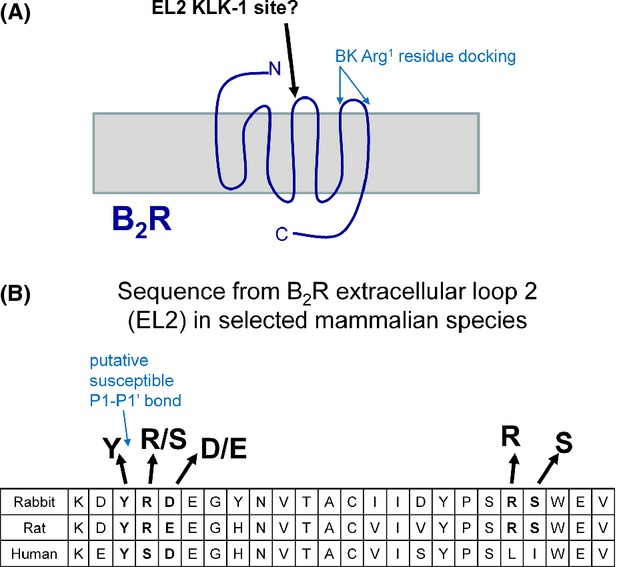
Schematic representation of a possible KLK-1 cleavage site in the rabbit BK B2R. (A) General topography. The regions where the two positive charges of the BK Arg1 residue are believed to interact with the receptor are shown; the C-terminus of bound BK rather interacts deeper in the rosette formed by the transmembrane domains (Leeb-Lundberg et al. 2005). (B) Sequence of the N-terminal part of the extracellular loop 2 in selected mammalian species. From Genkank sequences AAA96149.1 (rabbit), AAB23467.1 (human), and AAA62492.1 (rat). The cited sequences start at residues 175 (rabbit, rat) or 171 (human) in the Genbank records.
However, the findings are compatible with past studies where a KLK-1 preparation purified from human urine was shown to promote the partial degradation of the rabbit B2R-GFP construct expressed in intact cells (Houle et al. 2003). These results were confirmed and extended in the present study using DM199, a preparation possessing a much higher specific activity (Figs.8). Again, lower concentrations of DM199 (≤0.1 nmol/L) seem to stimulate a reversible B2R-GFP endocytosis via local kinin generation, based on the inhibitory effect of anatibant, but a higher concentration level degraded some of the fusion protein (as judged from free GFP in cells, Figs.8) and leads to the apparent disruption of the receptor recycling, based on microscopy and immunoblotting (Figs.8). However, this may not apply to human or rat B2Rs, compatible with the facts that DM199 did not displace [3H]BK from the B2Rs in these species and that a high concentration (10 nmol/L) of the protease did not desensitize the human umbilical vein smooth muscle to BK. In replicated experiments, densitometry suggests a high ratio of free GFP to B2R-GFP in the absence of a significant decrease of the fusion protein band cells treated with 1 nmol/L DM199 (Figs.8); this may be due to a better affinity of the antibody for free GFP. Of note, DM199 is much less efficacious in down-regulating either B2R-GFP or rabbit myc-B2R compared to BK analogs that are resistant to endosomal inactivation, because the consumption of the mature receptor bands were observed in these systems (Bawolak et al. 2007, 2012). Proteases released by activated neutrophils also completely digest B2R-GFP (Marceau et al. 2002). These findings put in physiological perspective the partial cleavage of B2R-GFP induced by DM199.
Li et al. (2008) have exploited a phage display technology to define amino acid sequences that could be cleaved by KLK-1. In addition to a specialized tryptic activity (ability to cleave on the C-terminal side of Arg in the Arg↓Ser sequence), KLK-1 possesses a chymotryptic activity (ability to cleave at the C-terminal side of an aromatic residue) that particularly concerns the sequences Tyr↓Ser-polar or Tyr↓Arg-polar (Li et al. 2008). While an Arg-Ser sequence is found in the C-terminal part of the extracellular loop 2 (EL2) of the B2R, the flanking sequences are identical in the 3 examined species, and this site is not likely to support the species-dependent effect of KLK-1 on the rabbit B2R. In the N-terminal part of EL2 of the BK B2R in various mammalian species, the 3 mammalian species possess variations of a possible chymotryptic KLK-1 site that may be the basis of a species-dependent variation of substrate affinity (Fig.9B). In any event, such potential cleavages sites must be widely distributed in cell surface proteins and may account for the KLK-1-induced activation of PAR-1 or the cleavage of the renal epithelial Na channel (Gao et al. 2010a,b; Patel et al. 2012). However, all these effects were recorded at KLK-1 concentrations of 100 nmol/L or above, casting doubts on their physiological relevance. KLK-1 administration, notably via expression vectors, exerts various beneficial cardiovascular effects in animals models (for instance, Chao et al. 2008; Yao et al. 2013), but it is difficult to determine the tissue concentration and molecular mechanism of action of the protease in such complex models.
In summary, in the human isolated umbilical vein, the pharmacology of DM199 at relevant concentrations (<10 nmol/L) is essentially based on the local generation of kinins. There is no evidence for a direct action of DM199 on the human B2R, indirectly supporting that circulating LMW-KNG may ultimately explain the beneficial effects of KLK-1/DM199 via the generation of kinins. The significance of the findings for the therapeutic application of DM199 is unknown. Whether endogenous protease inhibitor(s) limit kinin generation or interaction of DM199 with other proteins in vivo is unclear. An alternate possibility, suggested by the idiosyncratic susceptibility of the rabbit B2R to 1 nmol/L of DM199, is that KLK-1 cleavage sites are present in one or more proteins that are not members of the kallikrein-kinin system.
Acknowledgments
This work was supported by the grant MOP-93773 from the Canadian Institutes of Health Research and a research contract from DiaMedica, Inc. (Minneapolis, MN). The latter organization also supplied DM199. We thank Marc Pouliot for facilitating the access to microscopic equipment.
Glossary
- B2R
B2 receptor
- BK
bradykinin
- D-VLR-AFC
D-Val-Leu-Arg-7 amido-4-trifluoromethylcoumarin
- EL2
extracellular loop 2
- GFP
green fluorescent protein
- GPCR
G protein-coupled receptor
- KLK-1
tissue kallikrein (1)
- KNG
kininogen
- LMW
low-molecular
- myc-B2R
myc-tagged B2 receptor
- PAR
protease-activated receptor
- PBS
phosphate-buffered saline
- PMSF
phenylmethylsulfonyl fluoride
Disclosures
The authors are either employed by DiaMedica, Inc. (A. R., M. L. C., T. K., M. S. R.), or members of an academic laboratory partly supported by DiaMedica, Inc. (X. C.-M., J. B., M. J., F. M.).
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. Supplementary Materials and Methods.
Figure S1. Validation of novel human B2R vectors. (A) Coding DNA sequence inserted into the hu B2R vector based on the pcDNA3.1 vector, as determined by post hoc sequencing. The covered sequence is identical to the NCBI Reference Sequence: NP_000614.1. (B) Translation of the DNA sequence into an amino acid sequence. The myc-B2R construct is made by inserting 9 residues in the N-terminal region to reconstitute the 10-residue myc tag (underlined). (C) Expression of hu B2R and of its myc-tagged variant in HEK 293a cells as measured by saturation curves of [3H]BK in cells (transfected with the indicated vector). Values are the mean of 2–3 values, each composed of duplicate determinations.
Figure S2. Validation of novel rat B2R vectors. (A) Coding DNA sequence inserted into the rat B2R vector based on the pcDNA3.1 vector, as determined by post hoc sequencing. The covered sequence is identical to the NCBI Reference Sequence: NP_001257642. (B) Translation of the DNA sequence into an amino acid sequence. Predicted transmembrane domains in boldface. The myc-B2R construct was made by inserting the 10-residue myc tag (underlined). (C) Expression of rat B2R and of its myc-tagged variant in HEK 293a cells as measured by saturation curves of [3H]BK in cells (transfected with the indicated vector). Presentation as in Figure S1.
References
- Altura BM, Malaviya D, Reich CF, Orkin LR. Effects of vasoactive agents on isolated human umbilical arteries and veins. Am J Physiol. 1972;222:345–355. doi: 10.1152/ajplegacy.1972.222.2.345. [DOI] [PubMed] [Google Scholar]
- Bachvarov DR, Saint-Jacques E, Larrivée JF, Levesque L, Rioux F, Drapeau G, et al. Cloning and pharmacological characterization of the rabbit bradykinin B2 receptor. J Pharmacol Exp Ther. 1995;275:1623–1630. [PubMed] [Google Scholar]
- Bachvarov DR, Houle S, Bachvarova M, Bouthillier J, Adam A, Marceau F. Bradykinin B2 receptor endocytosis, recycling, and down-regulation assessed using green fluorescent protein conjugates. J Pharmacol Exp Ther. 2001;297:19–26. [PubMed] [Google Scholar]
- Bawolak MT, Gera L, Morissette G, Stewart JM, Marceau F. B-9972 (D-Arg-[Hyp3, Igl5, Oic7, Igl8]-bradykinin) is an inactivation-resistant agonist of the bradykinin B2 receptor derived from the peptide antagonist B-9430 (D-Arg-[Hyp3, Igl5, D-Igl7, Oic8]-bradykinin): pharmacologic profile and effective induction of receptor degradation. J Pharmacol Exp Ther. 2007;323:534–546. doi: 10.1124/jpet.107.123422. [DOI] [PubMed] [Google Scholar]
- Bawolak MT, Fortin S, Bouthillier J, Adam A, Gera L, C-Gaudreault R, et al. Effects of inactivation-resistant agonists on the signalling, desensitization and down-regulation of bradykinin B2 receptors. Br J Pharmacol. 2009;158:1375–1386. doi: 10.1111/j.1476-5381.2009.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawolak MT, Lodge R, Morissette G, Marceau F. Bradykinin B₂ receptor-mediated transport into intact cells: anti-receptor antibody-based cargoes. Eur J Pharmacol. 2011;668:107–114. doi: 10.1016/j.ejphar.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Bawolak MT, Roy C, Gera L, Marceau F. Prolonged signalling and trafficking of the bradykinin B2 receptor stimulated with the amphibian peptide maximakinin: insight into the endosomal inactivation of kinins. Pharmacol Res. 2012;65:247–253. doi: 10.1016/j.phrs.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Biyashev D, Tan F, Chen Z, Zhang K, Deddish PA, Erdös EG, et al. Kallikrein activates bradykinin B2 receptors in the absence of kininogen. Am J Physiol Heart Circ Physiol. 2006;290:H1244–H1250. doi: 10.1152/ajpheart.00934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Yin H, Gao L, Hagiwara M, Shen B, Yang ZR, et al. Tissue kallikrein elicits cardioprotection by direct kinin B2 receptor activation independent of kinin formation. Hypertension. 2008;52:715–720. doi: 10.1161/HYPERTENSIONAHA.108.114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest-Morin X, Fortin S, Lodge R, Roy C, Gera L, C-Gaudreault R, et al. Inhibitory effects of cytoskeleton disrupting drugs and GDP-locked Rab mutants on bradykinin B2 receptor cycling. Pharmacol Res. 2013;71:44–52. doi: 10.1016/j.phrs.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Charest-Morin X, Roy C, Fortin EJ, Bouthillier J, Marceau F. Pharmacological evidence of bradykinin regeneration from extended sequences that behave as peptidase-activated B2 receptor agonists. Front Pharmacol. 2014;5:32. doi: 10.3389/fphar.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corish P, Tyler-Smith C. Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng. 1999;12:1035–1040. doi: 10.1093/protein/12.12.1035. [DOI] [PubMed] [Google Scholar]
- Duka I, Shenouda S, Johns C, Kintsurashvili E, Gavras I, Gavras H. Role of the B2 receptor of bradykinin in insulin sensitivity. Hypertension. 2001;38:1355–1360. doi: 10.1161/hy1201.096574. [DOI] [PubMed] [Google Scholar]
- Gao L, Chao L, Chao J. A novel signaling pathway of tissue kallikrein in promoting keratinocyte migration: activation of proteinase-activated receptor 1 and epidermal growth factor receptor. Exp Cell Res. 2010a;316:376–389. doi: 10.1016/j.yexcr.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Smith RS, Chen LM, Chai KX, Chao L, Chao J. Tissue kallikrein promotes prostate cancer cell migration and invasion via a protease-activated receptor-1-dependent signaling pathway. Biol Chem. 2010b;391:803–812. doi: 10.1515/BC.2010.084. [DOI] [PubMed] [Google Scholar]
- Gera L, Roy C, Marceau F. Vasopeptidase-activated latent ligands of the histamine receptor-1. Int Immunopharmacol. 2013;17:677–683. doi: 10.1016/j.intimp.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Hecquet C, Tan F, Marcic BM, Erdös EG. Human bradykinin B2 receptor is activated by kallikrein and other serine proteases. Mol Pharmacol. 2000;58:828–836. doi: 10.1124/mol.58.4.828. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Jacob S, Fogt DL, Dietze GJ. Effect of chronic bradykinin administration on insulin action in an animal model of insulin resistance. Am J Physiol Reg Int Comp Physiol. 1998;275:R40–R45. doi: 10.1152/ajpregu.1998.275.1.R40. [DOI] [PubMed] [Google Scholar]
- Houle S, Larrivée JF, Bachvarova M, Bouthillier J, Bachvarov DR, Marceau F. Antagonist-induced intracellular sequestration of rabbit bradykinin B2 receptor. Hypertension. 2000;35:1319–1325. doi: 10.1161/01.hyp.35.6.1319. [DOI] [PubMed] [Google Scholar]
- Houle S, Molinaro G, Adam A, Marceau F. Tissue kallikrein actions at the rabbit natural or recombinant kinin B2 receptors. Hypertension. 2003;41:611–617. doi: 10.1161/01.HYP.0000054971.03046.9B. [DOI] [PubMed] [Google Scholar]
- Kakoki M, McGarrah RW, Kim H-S, Smithies O. Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2007;104:7576–7581. doi: 10.1073/pnas.0701617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodka T, Charles ML, Raghavan A, Radichev IA, Amatya C, Ellefson J, et al. Preclinical characterization of recombinant human tissue kallikrein-1 as a novel treatment for type 2 diabetes mellitus. PLoS One. 2014;9:e103981. doi: 10.1371/journal.pone.0103981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Li HX, Hwang BY, Laxmikanthan G, Blaber SI, Blaber M, Golubkov PA, et al. Substrate specificity of human kallikreins 1 and 6 determined by phage display. Protein Sci. 2008;17:664–672. doi: 10.1110/ps.073333208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HS, Hsu YR, Lu LI, Ruff D, Lyons D, Lin FK. Isolation and characterization of human tissue kallikrein produced in Escherichia coli: biochemical comparison to the enzymatically inactive prokallikrein and methionyl kallikrein. Protein Expr Purif. 1996a;8:215–226. doi: 10.1006/prep.1996.0094. [DOI] [PubMed] [Google Scholar]
- Lu HS, Hsu YR, Narhi LO, Karkare S, Lin FK. Purification and characterization of human tissue prokallikrein and kallikrein isoforms expressed in Chinese hamster ovary cells. Protein Expr Purif. 1996b;8:227–237. doi: 10.1006/prep.1996.0095. [DOI] [PubMed] [Google Scholar]
- Marceau F, Levesque L, Drapeau G, Rioux F, Salvino JM, Wolfe HR, et al. Effects of peptide and nonpeptide antagonists of bradykinin B2 receptors on the venoconstrictor action of bradykinin. J Pharmacol Exp Ther. 1994;269:1136–1143. [PubMed] [Google Scholar]
- Marceau F, Sabourin T, Houle S, Fortin JP, Petitclerc E, Molinaro G, et al. Kinin receptors: functional aspects. Int Immunopharmacol. 2002;2:1729–1739. doi: 10.1016/s1567-5769(02)00189-3. [DOI] [PubMed] [Google Scholar]
- Marceau F, deBlois D, Petitclerc E, Levesque L, Drapeau G, Audet R, et al. Vascular smooth muscle contractility assays for inflammatory and immunological mediators. Int Immunopharmacol. 2010;10:1344–1353. doi: 10.1016/j.intimp.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Patel AB, Chao J, Palmer LG. Tissue kallikrein activation of the epithelial Na channel. Am J Physiol Renal Physiol. 2012;303:F540–F550. doi: 10.1152/ajprenal.00133.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier L, Waeckel L, Vincent MP, Chollet C, Gobeil F, Marre M, et al. Selective kinin receptor agonists as cardioprotective agents in myocardial ischemia and diabetes. J Pharmacol Exp Ther. 2013;346:23–30. doi: 10.1124/jpet.113.203927. [DOI] [PubMed] [Google Scholar]
- Pruneau D, Paquet JL, Luccarini JM, Defrêne E, Fouchet C, Franck RM, et al. Pharmacological profile of LF 16-0687, a new potent non peptide bradykinin B2 receptor antagonist. Immunopharmacology. 1999;43:187–194. doi: 10.1016/s0162-3109(99)00128-9. [DOI] [PubMed] [Google Scholar]
- Saifeddine M, Roy SS, Al-Ani B, Triggle CR, Hollenberg MD. Endeothelium-dependent contractile actions of proteinase-activated receptor-2-activating peptides in human umbilical veins: release of a contracting factor via a novel receptor. Br J Pharmacol. 1998;125:1445–1454. doi: 10.1038/sj.bjp.0702213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay-Uyboco J, Poon MC, Ahmad S, Hollenberg MD. Contractile actions of thrombin receptor-derived polypeptides in human umbilical and placental vasculature: evidence for distinct receptor systems. Br J Pharmacol. 1995;115:569–578. doi: 10.1111/j.1476-5381.1995.tb14970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L, Xu X, Wan H, Zhou C, Deng J, Xu G, et al. Hum Gene Ther. 2008;19:318–330. doi: 10.1089/hum.2007.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Hirawa N, Kawabata Y, Suzuki T, Ohshima N, Oka K, et al. Long-term infusion of kallikrein attenuates renal injury in Dahl salt-sensitive rats. Hypertension. 1994;24:770–778. doi: 10.1161/01.hyp.24.6.770. [DOI] [PubMed] [Google Scholar]
- Vergnolle N. Protease-activated receptors as drug targets in inflammation and pain. Pharmacol Ther. 2009;123:292–309. doi: 10.1016/j.pharmthera.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Wolf WC, Yoshida H, Agata J, Chao L, Chao J. Human tissue kallikrein gene delivery attenuates hypertension, renal injury, and cardiac remodeling in chronic renal failure. Kidney Int. 2000;58:730–739. doi: 10.1046/j.1523-1755.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Yao Y, Sheng Z, Li Y, Fu C, Ma G, Liu N, et al. Tissue kallikrein-modified human endothelial progenitor cell implantation improves cardiac function via enhanced activation of akt and increased angiogenesis. Lab Invest. 2013;93:577–591. doi: 10.1038/labinvest.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Deng J, Wang T, Zhao C, Xu X, Wang P, et al. Tissue kallikrein reverses insulin resistance and attenuates nephropathy in diabetic rats by activation of phosphatidylinositol 3-kinase/protein kinase B and adenosine 5'-monophosphate-activated protein kinase signaling pathways. Endocrinology. 2007;148:2016–2026. doi: 10.1210/en.2006-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary Materials and Methods.
Figure S1. Validation of novel human B2R vectors. (A) Coding DNA sequence inserted into the hu B2R vector based on the pcDNA3.1 vector, as determined by post hoc sequencing. The covered sequence is identical to the NCBI Reference Sequence: NP_000614.1. (B) Translation of the DNA sequence into an amino acid sequence. The myc-B2R construct is made by inserting 9 residues in the N-terminal region to reconstitute the 10-residue myc tag (underlined). (C) Expression of hu B2R and of its myc-tagged variant in HEK 293a cells as measured by saturation curves of [3H]BK in cells (transfected with the indicated vector). Values are the mean of 2–3 values, each composed of duplicate determinations.
Figure S2. Validation of novel rat B2R vectors. (A) Coding DNA sequence inserted into the rat B2R vector based on the pcDNA3.1 vector, as determined by post hoc sequencing. The covered sequence is identical to the NCBI Reference Sequence: NP_001257642. (B) Translation of the DNA sequence into an amino acid sequence. Predicted transmembrane domains in boldface. The myc-B2R construct was made by inserting the 10-residue myc tag (underlined). (C) Expression of rat B2R and of its myc-tagged variant in HEK 293a cells as measured by saturation curves of [3H]BK in cells (transfected with the indicated vector). Presentation as in Figure S1.


