Abstract
We characterized mice administered corticosterone (CORT) at a dose of 20 mg/kg for 3 weeks to determine their suitability as a model of mood disorders and found that the time immobilized in the tail suspension test was longer and the time spent in the open arms of the elevated plus-maze test was shorter than those of the vehicle-treated group, findings demonstrating that chronic CORT induced both depression-like and anxiety-like behaviors. Furthermore, the levels of phosphorylated extracellular signal-regulated kinase (pERK) 1/2 in the hippocampus and cerebral cortex were reduced in the CORT-treated group. Using this model, we investigated the protective effect of the ester, thioester, and amide compounds of 2-decenoic acid derivatives (termed compounds A, B, and C, respectively). The potency of the protective activity against the CORT-induced depression-like or anxiety-like behaviors and the reduction in pERK1/2 level were found to be in the following order: compound B > compound C > compound A. Therefore, we further investigated the therapeutic activity of only compound B, and its effect on depression-like behavior was observed after oral administration for 1 or 2 weeks, and its effect on anxiety-like behavior was observed after oral administration for 3 weeks. The ratios of phosphorylated ERK1/2, Akt, and cAMP-response element-binding protein to their respective nonphosphorylated forms were smaller in the CORT-treated group than in the vehicle-treated group; however, subsequent treatment with compound B at either 0.3 or 1.5 mg/kg significantly ameliorated this reduction. Compound B appeared to elicit intracellular signaling, similar to that elicited by brain-derived neurotrophic factor, and its mode of action was shown to be novel and different from that of fluvoxamine, a currently prescribed drug for mood disorders.
Keywords: 2-decenoic acid thioester, anxiety-like behavior, corticosterone, depression-like behavior, extracellular signal-regulated kinase (ERK) 1/2, fluvoxamine
Introduction
Stress is one of the most important environmental factors responsible for the pathogenesis of mood disorders having various symptoms such as depression- and anxiety-like symptoms (Paykel 2003; Charney and Manji 2004). Animal models in which the animals are subjected to repeated exposure to stress ranging from chronic mild stress exposure to repeated restrain stress are used for a wide range of stimulations to activate the hypothalamus–pituitary–adrenal axis because of the presumed etiology of the disorder (Ito et al. 2012; Makino et al. 2013). However, stress-evoked, anxiety-like, and depression-like behaviors are sometimes influenced by the procedures used in each model, such as term, frequency, and type of stress (Beck and Luine 2002; Faraday 2002; Gregus et al. 2005). Furthermore, lack of consistency of the results may be derived from habituation, generalized by the repetition of stress, as reported earlier (Luine et al. 1996; Gregus et al. 2005). These drawbacks of the stress-induced model suggest the usage of more practical animal models with reproducible behaviors and characteristic neurochemical changes. Administration of corticosterone (CORT), a prominent glucocorticoid in rodents, may help to avoid these problems by elevating CORT levels, as would be expected as a consequence of stress exposure (Sousa et al. 1998; Johnson et al. 2006).
There have been many reports of studies using CORT-induced animal models. For example, CORT has been injected at high doses (20–40 mg/kg; Kalynchuk et al. 2004; Gregus et al. 2005; Johnson et al. 2006; Zhao et al. 2008; Lee et al. 2014) or low doses (1–7 mg/kg; David et al. 2009; Gourley et al. 2008a,b; Kutiyanawalla et al. 2011) into rats or mice to prepare animal models of mood disorders. High doses of CORT-induced depression-like symptoms in all of these reports, but anxiety-like symptoms have not been studied in most cases (Johnson et al. 2006; Zhao et al. 2008; Lee et al. 2014). Other reports have demonstrated that high doses of CORT did not cause anxiety-like behaviors; however, only the open-field test was used (Kalynchuk et al. 2004; Gregus et al. 2005). However, these reports did not conclusively prove a lack of anxiety-like symptoms by some other tests, such as the elevated plus-maze test (EPMT), which is used more commonly than the open-field test. It has been difficult to determine from previous observations if high doses of CORT could induce anxiety-like symptoms. On the other hand, it has been clearly shown that low doses of CORT cause anxiety-like but not depression-like symptoms when estimated by the tail suspension test (TST), which is a standard method (David et al. 2009; Kutiyanawalla et al. 2011). Furthermore, other reports have focused on anxiety-like behavior without any investigation of depression-like behaviors (Gourley et al. 2008a,b). The appearance of depressive symptoms has apparently been infrequent in low-dose CORT-treated animals.
Because both anxiety-like and depression-like behaviors have been frequently observed at the same time in stress-induced animal models, we initially focused on chronic CORT-treatment at a high dose in the present study and found that the treatment clearly evoked both depression-like and anxiety-like symptoms, followed by decreased levels of phosphorylated mitogen-activated protein kinases (MAPK)/extracellular signal-regulated kinases (ERK) 1/2.
Earlier we found that (E)-2-ethyl dec-2-enoate (trans-2-decenoic acid ethyl ester: DAEE; see Fig.1) increases the level of phosphorylated ERK1/2 (pERK1/2) in cortical/hippocampal neurons in vitro (Makino et al. 2010) or in vivo (Makino et al. 2013). In addition, DAEE activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and increases the level of the cAMP-response element-binding protein (CREB) as well as the expression of mRNAs of neurotrophins and the content of synapse-specific proteins in vitro (Makino et al. 2010), which indicates that DAEE generates intracellular signaling, similar to that generated by neurotrophins. Furthermore, therapeutic effects of DAEE on depression-like (Furukawa 2009) and/or anxiety-like symptoms (Makino et al. 2013) have been observed. However, a serious problem has been that the biological activities of DAEE are unstable and influenced by the lot preparation used in experiments (Furukawa S., Iinuma M., Soumiya H., Fukumitsu H. and Furukawa Y. unpubl. results). A primary reason for this instability is thought to be the easy hydrolysis of the ester bond because DAEE has five times higher activity for increasing pERK1/2 levels in vitro than trans-2-decenoic acid, the nonester form of DAEE (Hirakawa et al. 2010). DAEE is thought to be a promising lead compound for development of new drugs for treatment of mood disorders; however, it is not considered to be a goal compound.
Figure 1.
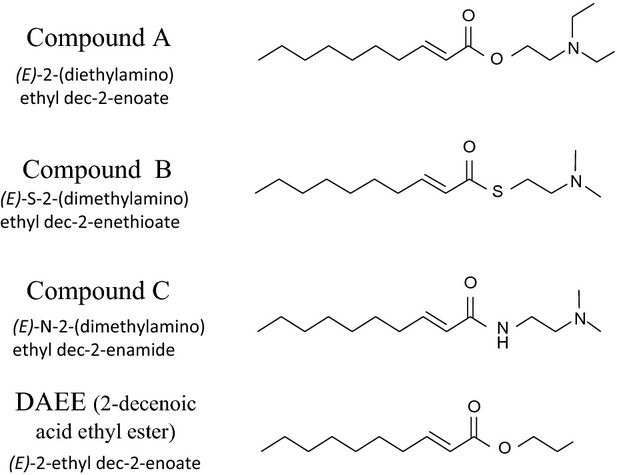
Chemical structures of 2-decenoic acid derivatives (compounds A, B, and C) and 2-decenoic acid ethyl ester (DAEE).
In the present study, we newly synthesized three types of novel 2-decenoic acid derivatives, which we expected to stabilize the biological activities of 2-decenoic acid. Using these derivatives in a CORT-induced mouse model of anxiety/depression, we assessed their antidepressant-like and/or anxiolytic-like activities, as well as the phosphorylation of molecules, such as ERK1/2, Akt, and CREB, of the hippocampal signaling pathway.
Materials and Methods
Synthesis of 2-decenoic acid derivatives
We synthesized three types of novel 2-decenoic acid derivatives, which we expected to have higher biological activities than those of DAEE. For this purpose, we tried to stabilize 2-decenoic acid by adding an ethyl moiety composed of an N,N-dimethylamino or N,N-diethylamino group through an ester, thioester, or amide bond.
1. dec-2-enoate (compound A)
Trans-2-decenoic acid chloride (3.4 g, 20 mmol), which was obtained by treatment of 2-decenoic acid with thioyl chloride in the usual manner, 2-diethylamino ethanol (2.3 g, 20 mmol), and pyridine (1.6 g, 20 mmol) were dissolved in tetrahydrofuran (20 mL). The reactant mixture was boiled under reflux in a water bath for 3 h and then poured into a solution of dilute hydrochloric acid and extracted into ethyl acetate. The desired substance was obtained after purification by using silica-gel chromatography and elution with hexane-ethyl acetate: Colorless oil, C16H32NO2, molecular weight (MW) 270, positive-ion high-resolution (HR)-fast atom bombardment (FAB) mass spectrum (MS) m/z: 271.2424 [M+H]+ (calculated for C16H33NO2: 271.2433), positive-ion FABMS m/z: 271 [M+H]+, 1H-nuclear magnetic resonance (NMR) (400 MHz, CDCl3): 0.87 (3H, t, J = 7.2 Hz), 1.04 (6H, t, J = 7.3 Hz), 1.29 (8H, br s), 1.45 (2H, m), 2.19 (2H, m), 2.60 (4H, q, J = 7.3 Hz), 2.74 (2H, t, J = 6.3 Hz), 4.2-(2H, t, J = 6.3 Hz), 5.83 (1H, d, J = 16.0 Hz), 6.97 (1H, dt, J = 16.0, 7.8 Hz).
2. ethyl dec-2-enethioate (compound B)
Trans-2-decenoic acid chloride and 2-dimethylamino ethanethiol were condensed in the same manner as (E)-2-(diethylamino) ethyl dec-2-enoate: Colorless oil, C14H27NOS, MW 257, positive-ion HR-FABMS m/z: 258.1895 [M+H]+ (calculated for C14H28NOS: 258.1892), positive-ion FABMS m/z: 258 [M+H]+, 1H-NMR (400 MHz, CDCl3) δ: 0.88 (3H, t, J = 6.4 Hz), 1.29 (8H, br s), 1.45 (2H, m), 2.19 (2H, m), 2.34 (6H, s), 2.59 (2H, t, J = 7.5 Hz), 3.09 (2H, t, J = 7.5 Hz), 6.10 (1H, d, J = 15.6 H), 6.90 (1H, dt, J = 15.6, 7.6 Hz).
3. ethyl dec-2-enamide (compound C)
Trans-2-decenoic acid chloride and N,N-dimethyl ethylene diamine were used as starting materials. Treatment was as described above: Colorless oil, C14H28N2O, MW 240, positive- ion HR-FABMS m/z: 241.2284 [M+H]+ (calculated for C14H29N2O: 241.2280), positive-ion FABMS m/z: 241 [M+H]+, direct analysis in real-time MS m/z: 241.2 [M+H]+, 1H-NMR (400 MHz, CDCl3) δ: 0.88 (3H, t, J = 6.8 Hz), 1.28 (8H, br s), 1.43 (2H, m), 2.14 (2H, m), 2.23 (6H, s), 2.43 (2H, t, J = 5.8 Hz), 3.40 (2H, t, J = 5.8 Hz), 5.79 (1H, d, J = 15.6 Hz), 6.17 (1H of NH), 6.82 (1H, dt, J = 15.6, 7.2 Hz).
Chemical structures of compounds A, B, and C are indicated in Figure1, as is the structure of (DAEE). DAEE was purchased from Sigma-Aldrich (St. Louis, MO).
Animals
Male ddY mice (7 weeks old, weighing 35–40 g) were purchased from Japan SLC (Hamamatsu, Japan) and maintained under constant temperature (23 ± 2°C), humidity (55 ± 10%), and a 12-h light/12-h dark cycle with food and water freely available. The ddY strain is an outbred one and has been maintained as a closed colony with good reproductive performance and superior growth. In Japan, this strain has been widely used in various fields of research. All animal experiments were performed according to the Guideline for Care and Use of Laboratory Animals of Gifu Pharmaceutical University.
Administration of CORT and test drugs
CORT (Sigma-Aldrich) was suspended in phosphate-buffered saline (PBS) containing 0.1% Tween-80 and subcutaneously injected into mice at a dose of 20 mg/kg body weight in a volume of 5 mL/kg body weight during the light phase. Each compound (A, B, or C) was dissolved in PBS and orally administered to the mice once a day at a dose of 0.3 or 1.5 mg/kg through a stomach tube. The volume of solution was 0.25 mL/mouse. Fluvoxamine (Flv) was orally injected (1.0 mg/kg) as a currently prescribed antidepressant or anxiolytic to evaluate the validity and usefulness of the CORT-treated mouse model. As shown in Figure2, the mice were exposed to CORT and/or drug in two different manners. To evaluate the protective activity against depression-like and anxiety-like symptoms, we administered each drug simultaneously with the CORT injection once a day for 21 days from experimental day 1 (Fig.2A). On the other hand, for the experiments to test therapeutic activity, CORT was injected once a day for 21 days prior to administration of each drug. The drug was then administered once a day for 7, 14, or 21 days (n = 6/group), starting the next day after the end of the CORT injection (Fig.2B). Behavioral tests and measurement of body weight were performed at the desired times. After these tests, the animals were sacrificed, and the hippocampi and/or cerebral cortices were dissected out for western immunoblotting.
Figure 2.

Protocols for CORT-treatment and drug administration. (A) Protocol for evaluation of protective activity. Mice were given CORT (20 mg/kg) by the subcutaneous route and/or each drug orally once a day for 21 days (n = 6/group). Behavioral tests and measurement of body weight were performed 1 day after the final CORT administration (on experimental day 22, indicated by the arrow). Then, the hippocampus was dissected out from the anesthetized animals for western immunoblotting. (B) Protocol for evaluation of therapeutic activity. Mice were intraperitoneally injected with CORT once a day for 21 days. Then, each drug was orally administered once a day for 7, 14, or 21 days starting 1 day after the final CORT administration (n = 6/group); and the behavioral tests and measurement of body weight were then performed 1 day after the final drug administration (on experimental days 23, 30, 37, or 43 indicated by the arrows). Then, the hippocampus was dissected out for western blotting.
Tail suspension test
Depression-like symptoms were evaluated as a decrease in motivation by performing the TST (Cryan et al. 2002). In brief, mice were suspended by their tails from a hanger and measured for the immobility time during the last 6 min of the 7-min suspension period because all mice were uniformly active for the first min (Ito et al. 2012).
Elevated plus-maze test
Anxiety-like symptoms were evaluated by use of the EPMT, as previously described (Makino et al. 2013). The animals were placed in the center of a 4-arm maze (30 × 5 cm/arm) elevated to a height of 50 cm, in which two arms were open and two were closed. The number of times the animal entered each of the arms and the time spent in each arm were recorded during a 5-min test period. The procedure was performed in a sound-attenuated room.
Western immunoblotting
Experimental procedures were previously described (Makino et al. 2013). In brief, the hippocampi and cerebral cortices were homogenized in lysis buffer (20 mmol/L Tris-HCl, pH 7.4, containing 150 mmol/L NaCl, 2 mol/L EDTA, 1% nonidet P-40, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 50 mmol/L NaVO4, 1 mmol/L phenylmethylsulfonyl fluoride, 0.1% sodium dodecyl sulfate (SDS), and 1% Na deoxycholate) with an ultrasonic cell disruptor and then centrifuged. The protein (5 μg each) of the tissue extracts was subjected to SDS-polyacrylamide gel electrophoresis. Six samples of each group were run in two or more gels while ensuring that all gels were treated under similar conditions. However, because small differences in the conditions may occur, we ran the standard sample in all gels and corrected the experimental values on the basis of a value obtained for the standard sample in each gel. Proteins in the gel were transferred to a polyvinylidene difluoride membrane, and the membrane was reacted with rabbit IgG antibody against protein or phosphorylated protein of ERK1/2, CREB, or Akt (Cell Signaling, Beverly, MA), followed by incubation with alkaline phosphatase-conjugated antirabbit IgG antibody (Promega, Madison, WI). Enzyme activity was visualized using nitro blue tetrazolium/bromochloroindolyl phosphate color substrate. Immunoreactive bands were analyzed using ImageJ analysis software (NIH, Bethesda, MD).
Results
Healthy animals were not used in the experiments to evaluate the activities of the compounds because (1) 2-decenoic acid derivatives, which have structures and activities similar to those of compound B, were earlier shown not to affect healthy mice in terms of their depressive or anxious state or level of phosphorylated ERK1/2 in the hippocampus (Furukawa 2009; Makino et al. 2013), (2) the most important point was the efficacy of the compounds toward CORT-induced changes, and (3) unnecessary animal experiments should be avoided from the point of view of animal protection.
Characterization of CORT-treated mouse model and its use for evaluation of 2-decenoic acid derivatives
CORT was subcutaneously injected into mice at a dose of 20 mg/kg body weight. To evaluate the protective activity against depression-like and anxiety-like symptoms, we administered each drug simultaneously with the CORT injection once a day for 21 days from the experimental day 1 (Fig.2A). Each compound (A, B, or C) was orally administered to the mice once a day at a dose of 0.3 or 1.5 mg/kg through a stomach tube. Other CORT-treated mice were orally treated (1.0 mg/kg) with Flv once a day for comparison of its effects with those of compound A, B, or C.
The immobilization times in the TST of non-CORT animals treated with vehicle (non-CORT/vehicle group) and CORT animals with vehicle (CORT/vehicle group) were 59.2 ± 9.5 and 124.9 ± 15.5 sec, respectively, which showed that chronic CORT administration significantly increased the immobilization time almost twofold. This increase was attenuated by administration of Flv (1.0 mg/kg), which demonstrated that this chronic CORT-treatment model was responsive to a currently prescribed antidepressant effective against chronic mild stress. Among the three types of trans-2-decenoic acid derivatives, compounds B and C, but not compound A, attenuated extension of the time immobilized in the TST (Fig.3), which suggested that compounds B and C behaved like antidepressants.
Figure 3.
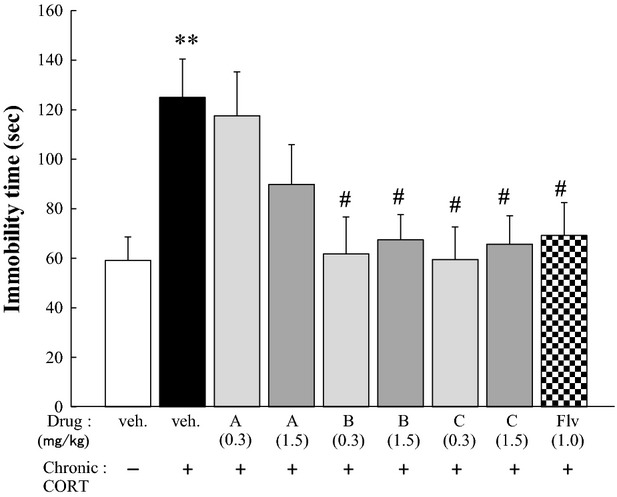
Evaluation of protective effects against CORT-induced depression-like behavior. Mice were treated orally once a day with vehicle or compound A, B, or C (0.3 or 1.5 mg/kg body weight) for 21 days while being treated daily with vehicle or CORT (see protocol A in Fig.2). Then, the time immobilized was measured by performing the TST. The significance of the difference between the value of the CORT-treated group and that of the vehicle-treated group was determined by one-way ANOVA with Tukey's post hoc test, as **P < 0.01 (n = 6/group). Among the CORT-treated groups, significant differences from the value of the vehicle-treated group were also determined by performing one-way ANOVA with Tukey's test, as #P < 0.05 (n = 6/group). TST, tail suspension test. ANOVA, analysis of variance.
The times that the non-CORT/vehicle group and CORT/vehicle group spent in the open arms of the EPMT were 62.3 ± 11.1 and 27.0 ± 5.0 sec, respectively, which demonstrated that chronic CORT treatment shortened by almost half the time spent in the open arms (Fig.4A). This decrease was attenuated by administration of Flv, which indicated that Flv behaved like an anxiolytic in this chronic CORT-treatment model, behavior that was similar to the behavior in the chronic stress-treatment model. Among the three types of trans-2-decenoic acid derivatives, compound B at both 0.3 and 1.5 mg/kg and compound C at 1.5 mg/kg attenuated shortening of the time spent in the open arms of the EPMT; however, compound A at either 0.3 or 1.5 mg/kg or compound C at 0.3 mg/kg did not cause attenuation (Fig.4A). The total number of entries into the arms was unchanged irrespective of CORT/drug administration, which demonstrated that the locomotor activity was constant in all groups (Fig.4B).
Figure 4.
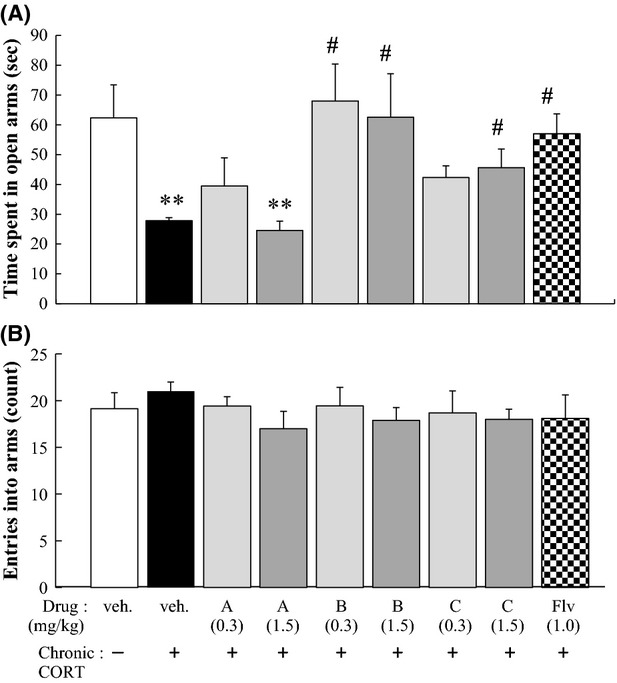
Evaluation of protective effects against CORT-induced anxiety-like behavior. Mice were processed in the same manner as described in the legend of Figure3 and subjected to the EPMT 1 day after the final CORT administration (see protocol A in Fig.2). The time spent in open arms (A) and the number of entries into the arms (B) were evaluated. The significance of differences between the CORT-treated mice and the non-CORT-treated (vehicle-treated) mice was determined by using one-way ANOVA with Tukey's post hoc test, as *P < 0.05, **P < 0.01 (n = 6/group). Significant differences between the CORT-treated groups and the vehicle-treated mice were also determined by one-way ANOVA with Tukey's test as, ##P < 0.01 (n = 6/group). EPMT, elevated plus-maze test. ANOVA, analysis of variance.
Compound B at doses of either 0.3 or 1.5 mg/kg significantly suppressed CORT-induced reduction in the pERK1/2 level in the hippocampus and cerebral cortex; however, compound C at either 0.3 or 1.5 mg/kg was active only in the cerebral cortex (Fig.5). Compound A and Flv were completely inactive in affecting the pERK1/2 level in either brain tissue.
Figure 5.

Evaluation of protective effects of compounds A, B, or C against CORT-induced reduction in the levels of pERK1/2 in the hippocampus and cerebral cortex. Mice were processed as described in the legend of Figure3 (n = 6/group). The hippocampus (A) and cerebral cortex (B) were dissected out 1 day after the final CORT administration and used for western immunoblot analysis. The ratio of the intensity of the pERK1/2 band to that of the total ERK1/2 band was calculated, and the values were expressed as fold-increase over the value of the non-CORT-treated and vehicle-administered group taken as “1.” The significance of differences between the values of the CORT-treated and the non-CORT-treated mice was determined by one-way ANOVA with Tukey's post hoc test, as *P < 0.05 or **P < 0.01 (n = 6/group). For differences among values of CORT-treated groups, the significance was similarly determined by the same statistical treatment as #P < 0.05 (n = 6/group). ANOVA, analysis of variance.
These results demonstrated that chronic CORT-induced depression-like and anxiety-like behaviors could be attenuated by oral administration of Flv (Figs.3 and 4) but that CORT-induced reductions in the pERK1/2 level in the hippocampus and cerebral cortex were unchanged by Flv (Fig.5), which suggested that the mechanisms of action of compounds B and C differed from that of Flv.
These observations (Figs.5) indicated that the potency of the protective activities against CORT-induced depression-like or anxiety-like behaviors and reduction in pERK1/2 level were in the order of compound B > compound C > compound A. Therefore, we further investigated the therapeutic activity of only compound B toward the CORT-induced symptoms.
Activities of compound B toward CORT-induced depression-like and anxiety-like symptoms
Therapeutic activities of compound B were evaluated according to protocol B (Fig.2B). Chronic administration of CORT injections increased the immobility time in the TST 1 day after the final CORT administration, and the increase lasted for the subsequent 3 weeks when the animals were treated with the vehicle (Fig.6). However, this increase was significantly attenuated by oral administration of compound B: Compound B at 0.3 or 1.5 mg/kg significantly shortened the immobility time from onset at 2 or 1 week, respectively (Fig.6), which indicated that the shortening occurred earlier at the high dose than at the lower dose. Chronic administration of CORT significantly reduced the time spent in the open arms in the EPMT 1 day after the final CORT administration, and the reduction lasted for the subsequent 3 weeks in the case of vehicle treatment (Fig.7A). However, the time spent in the open arms was significantly restored 3 weeks later by compound B at 0.3 or 1.5 mg/kg (Fig.7A). The total number of entries into the arms was unchanged irrespective of CORT/drug administration, which demonstrated that the locomotor activity was constant in all groups (Fig.7B). Therefore, we considered the difference in the period of time spent in the open arms to reflect anxiety-like symptoms.
Figure 6.
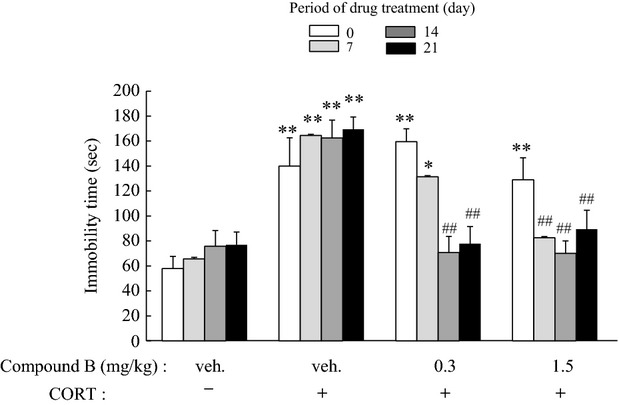
Therapeutic activity of compound B toward the depression-like symptom in the TST. Mice were treated with CORT or vehicle for 21 days and subsequently with compound B (0.3 or 1.5 mg/kg) or vehicle for 7, 14, or 21 days (see protocol B in Fig.2). Then, the time immobilized in the TST was measured. The significance of the difference between the value of the CORT-treated group and that of the vehicle-treated group was determined by one-way ANOVA with Tukey's post hoc test, as *P < 0.05, **P < 0.01 (n = 6/group). Among the CORT-treated groups, significant differences from the value of the vehicle-treated group were also determined by one-way ANOVA with Tukey's test, as ##P < 0.01 (n = 6/group). TST, tail suspension test. ANOVA, analysis of variance.
Figure 7.
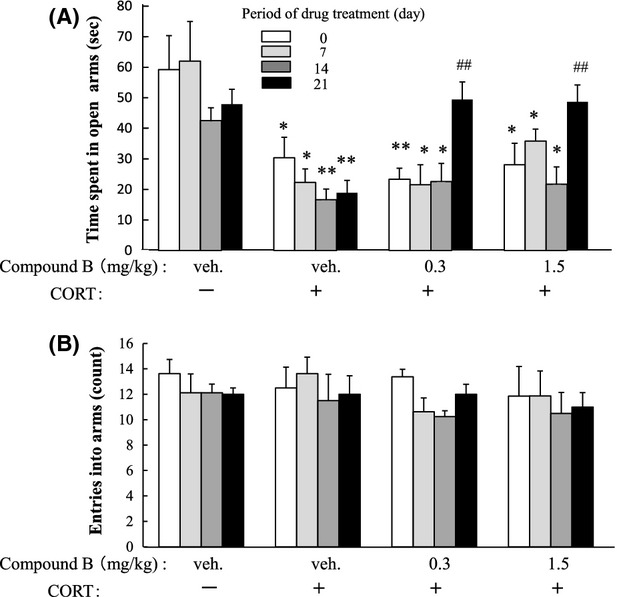
Therapeutic activity of compound B toward anxiety-like symptoms in the EPMT. Mice were treated as described in the legend of Figure6. Furthermore, they were tested for the time spent in the open arms in the EPMT (A) and the number of entries into the arms (B) 1 day after the final drug administration. The significance of the difference between the value of the CORT-treated mice and that of the vehicle-treated mice was determined by one-way ANOVA with Tukey's post hoc test, as *P < 0.05, **P < 0.01 (n = 6/group). Furthermore, among the CORT-treated groups, a significant difference from the value of the CORT-treated vehicle-administered mice was examined by performing one-way ANOVA with Tukey's post hoc test, as ##P < 0.01 (n = 6/group). EPMT, elevated plus-maze test. ANOVA, analysis of variance.
Effects of compound B on the CORT-influenced phosphorylated proteins
Mice were treated with CORT or vehicle for 3 weeks and subsequently with vehicle or compound B (0.3 or 1.5 mg/kg) for the subsequent 3 weeks according to protocol B (Fig.2B). The hippocampi were dissected out 1 day after the final administration of vehicle or compound B and then used for western immunoblotting. All ratios of the phosphorylated forms of ERK1/2, Akt, and CREB to their respective nonphosphorylated forms were significantly smaller in the CORT-treated group than in the vehicle-treated group; however, subsequent treatment with compound B at either 0.3 or 1.5 mg/kg significantly ameliorated this reduction in ratio (Fig.8). Because coadministration of Flv with CORT for 3 weeks could not suppress the CORT-induced reduction in the ERK1/2 level, a similar experiment using non-CORT animals was not considered to be necessary (Fig.5). The difference in mechanism of action between compound B and Flv should be clarified by comparison of the exact time course of activation of signal transduction pathways, based on a paradigm different from that used in the present study.
Figure 8.
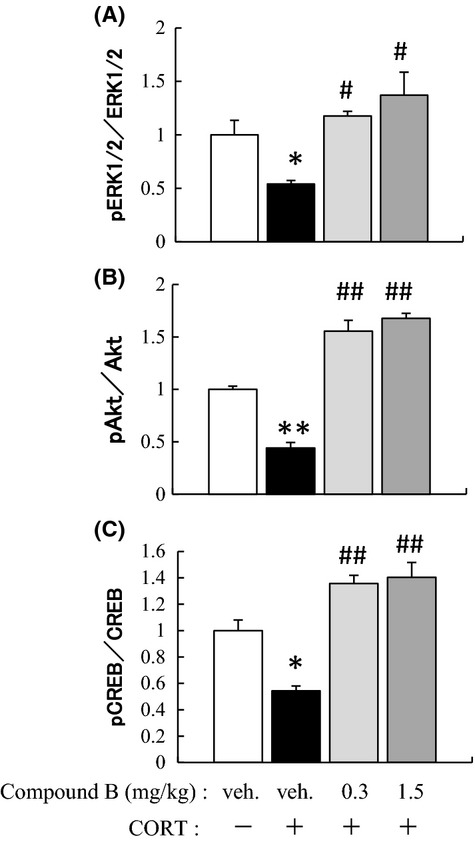
Ameliorative activity of compound B toward the reduced levels of pERK1/2, pAkt, and pCREB in the hippocampus of the CORT-treated mice. Mice were treated with CORT or vehicle for 21 days and subsequently with compound B (0.3 or 1.5 mg/kg) or vehicle for 21 days (see protocol B in Fig.2). Then, the significance of the difference between the value of the CORT-treated mice and that of the vehicle-treated mice was determined by one-way ANOVA with Tukey's post hoc test, as *P < 0.05, **P < 0.01 (n = 6/group). Among the CORT-treated groups, a significant difference from the value of the CORT-treated mice with vehicle administration was examined by using one-way ANOVA with Tukey's post hoc test, as #P < 0.05 or ##P < 0.01 (n = 6/group). ANOVA, analysis of variance.
In the present study, we designed our western blot analysis such that the values would not be biased by the differences in lanes and gels used for the electrophoresis. For example, individual samples of each group were separately run in different lane numbers in different gels. Therefore, we could not prepare a typical electrophoretic pattern to match the figures without cutting and pasting the bands, which is why we did not show the pattern of raw bands in Figure5.
Effect of CORT and compound B on body weight
Mice were treated with CORT or vehicle for 3 weeks and subsequently with vehicle or compound B (0.3 or 1.5 mg/kg) for the next 1, 2, or 3 weeks according to protocol B (Fig.2B). Chronic administration of CORT for 3 weeks slightly but significantly reduced body weight, and this decrease was maintained at least for the next 2 weeks (Fig.9). Compound B had no influence on CORT-induced reduction in body weight (Fig.9), which suggested that the pharmacological effects of compound B were not responsible for the CORT-induced reduction in body weight.
Figure 9.
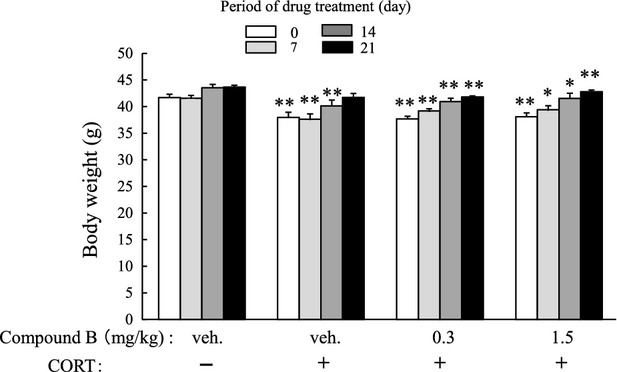
Change in body weight during CORT treatment and/or compound B administration. Mice were treated as described in the legend of Figure6, and body weight was measured (see protocol B in Fig.2B). The significance of the difference between the value of the CORT-treated group and that of the vehicle-treated group was determined by performing one-way ANOVA with Tukey's post hoc test, as *P < 0.05, **P < 0.01 (n = 6/group). Among the CORT-treated groups, significant differences from the value of the vehicle-treated group were examined by one-way ANOVA with Tukey's test, but no group values were significantly different. ANOVA, analysis of variance.
Discussion
Anxiety and depression are stress-induced diseases in modern society that anyone may suffer from during their lifetime. Substantial numbers of antidepressants and anxiolytics have been available for clinical use; however, patients are not always satisfied with these drugs. Delayed actions and substantial side effects are major drawbacks of these drugs (Quitkin et al. 1984; Anderson et al. 2000); consequently, development of new drugs based on novel mechanisms of action is an important goal.
It is well known that glucocorticoid hormones can readily enter the brain where they bind directly to mineralocorticoid receptors and glucocorticoid receptors (Reul and de Kloet 1985; De Kloet and Derijk 2004). These two receptor types differ in their affinity for CORT. Glucocorticoid receptors have a low affinity for CORT and become occupied only during stress and at the circadian peak, when circulating levels of glucocorticoids are high. In contrast, mineralocorticoid receptors have a 10-fold higher affinity for CORT and are almost saturated under basal conditions (Reul and de Kloet 1985). Thus, it is likely that the negative effects of CORT on animal behavior are mediated by activation of glucocorticoid receptors (Radeley and Morrison 2005), which is postulated to suppress neurogenesis by reducing the synthesis of neurotrophins, such as brain-derived neurotrophic factor (BDNF) (Henn et al. 2004). Evidence supporting this postulation includes the findings that glucocorticoid receptor gene-disrupted mice showed reduced sensitivity to chronic stress (Gass et al. 2001; Urani and Gass 2003) and RU-43044, an antagonist of glucocorticoid receptors, reduced depression-like symptoms in a chronic CORT-induced mouse model of depression (Ago et al. 2008).
In the present study, we characterized mice treated chronically with CORT to address their suitability as an animal model for the study of mood disorders. Such mice expressed both depression-like and anxiety-like behaviors (Figs.3 and 4), which appeared to be similar or identical to those induced by chronic stress exposure because they could be ameliorated by Flv (Furukawa 2009; Makino et al. 2013), a currently prescribed antidepressant or anxiolytic. These observations suggested that compared with the stress-exposed model the chronic CORT-treated mice can be used as a reliable and labor-saving model for the study of mood disorders. Furthermore, the effect of chronic CORT injection on depression-like and anxiety-like behaviors continued for at least 3 weeks after the final CORT injection, which indicated that this model was suitable for both investigating actions of CORT and screening of antidepressants or anxiolytics. On the other hand, ERK1/2, an intracellular signal transduction molecule that is regulated by neurotrophins or neurotransmitters, has an important role in neuronal function and is hypothesized to be responsible for depression-like symptoms elicited by stress-related insults and to be associated with the efficacy of antidepressants (Shirayama et al. 2002; Duman et al. 2007; Gourley et al. 2008a,b). In the CORT-treated mice, the pERK1/2 level decreased in the hippocampus and cerebral cortex (Fig.5), as was also observed in the hippocampus of chronic stress-exposed mice (Ito et al. 2012). These previous observations taken together with our present results showing that compound B ameliorated both depression-like and anxiety-like behaviors (Figs.3 and 4) suggest that the pERK1/2 level may be associated with both symptoms in CORT-treated mice.
There is growing evidence showing the importance of BDNF in the etiology and/or pathology of mood disorders. BDNF binds to a specific Trk receptor tyrosine kinase, which triggers signal transduction pathways of MAPK/ERK1/2, P13K/Akt, and phospholipase C γ (Kaplan and Miller 2000). Activated ERK1/2 then passes into the nucleus to activate transcription factors, such as CREB, which then regulate expression of various genes involved in neuronal differentiation, learning, and memory (Huang and Reichardt 2001; Lu et al. 2003). Analysis of gene-disrupted animals has clarified that ERK1/2 activity may govern neurogenesis to ensure proper brain development (Satoh et al. 2011). BDNF gene disruption causes behavioral abnormalities consistent with serotonergic dysfunction (Calabrese et al. 2010), and delivery of the BDNF gene in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities (Quesseveur et al. 2013). Further, stress downregulates the level of BDNF mRNA in the hippocampus in mice (Nibuya et al. 1995), whereas antidepressants increase BDNF transcription (Russo-Neustadt et al. 1991). BDNF thus induced by antidepressants takes a longer time to exert its subsequent biological actions resulting in a delay in the onset of the therapeutic effects of antidepressants (Nibuya et al. 1995). Thus, BDNF is a key prospective target molecule of novel drugs.
How did 2-decenoic acid derivatives, including compound B, generate a BDNF-like signal? Earlier we observed that one of the active 2-decenoic acid derivatives, DAEE, started increasing the level of phosphorylated ERK1/2 at 15 min after the addition and attained the maximal value at the 2-h time point when tested on cultured cortical/hippocampal neurons (Makino et al. 2010). Such a gradual increase in activation is unlikely to indicate molecular kinetics produced by a specific interaction between ligand and its specific receptor. We presume that the active compound was incorporated passively into the lipid bilayer of neurons and then interacted with and activated some “protein X” in the neurons. This activated protein X may then have mediated the phosphorylation of particular tyrosine kinases of high-affinity neurotrophin receptors such as TrkB and TrkC. We have found a candidate of protein X predominantly expressed in neuronal cells and are currently investigating its involvement in the DAEE-mediated mechanism of actions.
Our present results (Figs.5) indicated that the protective activities against CORT-induced depression-like or anxiety-like behaviors and reduction in the pERK1/2 level ranged in potency in the order of compound B > compound C > compound A. This order of activity may reflect the order of stability of the bond links between 2-enoate, 2-enethioate or 2-enamide, and dimethylamino ethyl or diethylamino ethyl (see Fig.1). Compound A is an ester generally labile in tissues in vivo because of the presence of numerous ester hydrolases in the tissues; however, compound B and compound C are a thioester and amide, respectively, and not as labile. The difference in the in vivo activity between compound B and C may be explained by the differences in their content in the tissues and tissue distribution between fatty acid amide hydrolase (McKinney and Cravatt, 2005) and thioesterase (Naggert et al., 1991). In particular, the physiological actions of thioesters are quite limited. For example, in animal tissues, medium- and long-chain fatty acids are assembled on the pantetheine thiol group of the multifunctional fatty acid synthase, and the intervention of a thioesterase terminates growth of the acyl chain (Naggert et al. 1991). Furthermore, compounds A, B, and C have a dimethyl amino ethyl or diethyl amino ethyl group, in which the methyl or ethyl group may push the electron pair on the nitrogen and result in strengthening of the minus charge of the nitrogen to interact with some plus-charged compounds. Therefore, such groups may stabilize the entire compound by interactions with some other molecules. The difference between compound A and DAEE is limited in this respect. Therefore, compound A is considered to be more stable and to have more potent activity than DAEE; however, DAEE is more potent than compound A, probably because the plus charge of the nitrogen of compound A interfered with the transition into the cell. In the case of compound B or C, a very low or lack of hydrolysis may negate such interference and allow entry into the cell.
It should be noticed that the dose of Flv sufficient to elicit the behaviors was unable to ameliorate reduction in the pERK1/2 level (Fig.5), which suggested that the mechanism of action of compound B was likely different from that of Flv. In terms of the protective activities against CORT-induced deleterious effects, compound B was the strongest among the compounds tested to date, and its activities were more stable and reproducible than those of DAEE (data not shown). CREB is a transcription factor that regulates neuronal function, including synapse plasticity (Tao et al. 1998), and Akt is a critical factor in the regulation of cell survival and apoptosis (Burgering and Coffer 1995; Franke et al. 1997). Compound B ameliorated CORT-evoked reduction in not only the pERK1/2 level but also in the pAkt and pCREB levels (Fig.8). Therefore, as supported by previous observations and considerations (Furukawa 2009; Makino et al. 2010, 2013), compound B seems to have elicited intracellular signaling similar to that of BDNF. This mode of action of compound B is totally novel and different from that of Flv or other antidepressants and reflects direct enhancement of phosphorylation of ERK1/2 (Figs.5 and 8).
Furthermore, it is unclear at present why the onset of the efficacy of compound B toward the depression-like behavior in the TST was 1 or 2 weeks earlier than that toward the anxiety-like behavior in the EPMT (Figs.6 and 7). For instance, compound B at 1.5 mg/kg required 7 days to significantly expand the time immobilized in the TST but required 21 days to increase the time spent in the open arms in the EPMT. Differences in the pathology, etiology, and/or focus of brain regions between the two disorders may be involved in the reason. In addition, which receptor on the cell membrane of neurons is bound by this compound and how this compound would be metabolized after orally dosing is unknown. Further investigations to unravel the details of the mechanism of action are needed to develop this compound as a novel drug for the treatment of mood disorders.
In conclusion, we confirmed that chronic CORT-treatment at a high dose in mice clearly evoked both depression-like and anxiety-like symptoms and decreased the level of phosphorylated ERK1/2, which suggested that compared with the chronic stress-exposed model, CORT-treated mice is a labor-saving and reliable model for the study of mood disorders. Furthermore, using this model, we showed that a novel trans-2-decenoic acid thioester exhibited therapeutic activity for the treatment of mood disorders.
Acknowledgments
The authors thank Enago (www.enago.jp) for the English language review.
Glossary
- ANOVA
analysis of variance
- BDNF
brain-derived neurotrophic factor
- CORT
corticosterone
- CREB
cAMP-response element-binding protein
- DAEE
trans-2-decenoic acid ethyl ester
- EPMT
elevated plus maze test
- ERK1/2
extracellular signal-regulated kinase 1/2
- Flv
fluvoxamine
- HR-FABMS
positive-ion high-resolution FAB mass spectrum
- MAPK
mitogen-activated protein kinase
- PBS
phosphate-buffered saline
- pERK1/2
phosphorylated ERK1/2
- PI3K
phosphatidylinositol 3-kinase
- SDS
sodium dodecyl sulfate
- TST
tail suspension test
Author Contributions
S. Shibata conducted experiments. M. Iinuma synthesized the compounds. S. Shibata and S. Furukawa participated in research design. S. Shibata, H. Fukumitsu, and H. Soumiya performed data analysis. S. Shibata, S. Furukawa, and Y. Furukawa wrote or contributed to writing of the manuscript.
Disclosure
None declared.
References
- Ago Y, Arikawa S, Yata M, Yano K, Abe M, Takuma K, et al. Antidepressant-like effects of the glucocorticoid receptor antagonist RU-43044 are associated with changes in prefrontal dopamine in mouse models of depression. Neuropharmacology. 2008;55:1355–1363. doi: 10.1016/j.neuropharm.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Anderson IM, Nutt DJ, Deakin JF. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 1993 British Association of Psychopharmacology guidelines. J Psychopharmacol. 2000;14:3–20. doi: 10.1177/026988110001400101. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Cattaneo A, Macchi F, Racagni G, Gennarelli M, et al. Long-Term duloxetine treatment normalizes altered brain-derived neurotrophic factor expression in serotonin transporter knockout rats through the modulation of specific neurotrophin isoforms. Mol Pharmacol. 2010;77:846–853. doi: 10.1124/mol.109.063081. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, gene, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004 doi: 10.1126/stke.2252004re5. 2004:re5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Derijk R. Signaling pathways in brain involved in predisposition and pathogenesis of stress-related disease: genetic and kinetic factors affecting the MR/GR balance. Ann N Y Acad Sci. 2004;1032:14–34. doi: 10.1196/annals.1314.003. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Faraday MM. Rat sex and strain differences in responses to stress. Physiol Behav. 2002;75:507–522. doi: 10.1016/s0031-9384(02)00645-5. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- Furukawa S. Development of therapeutic drugs for depression and Alzheimer's disease. Chem Eng. 2009;54:18–24. [Google Scholar]
- Gass P, Reichardt HM, Strekalova T, Henn F, Tronche F. Mice with targeted mutations of glucocorticoid and mineralocorticoid receptors: models for depression and anxiety? Physiol Behav. 2001;73:811–825. doi: 10.1016/s0031-9384(01)00518-2. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Taylor JR. Corticosterone regulates pERK1/2 map kinase in a chronic depression model. Ann N Y Acad Sci. 2008a;1148:509–514. doi: 10.1196/annals.1410.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, et al. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008b;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res. 2005;156:105–114. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Henn F, Vollmayr B, Sartorius A. Mechanisms of depression: the role of neurogenesis. Drug Discovery Today. 2004;1:407–411. [Google Scholar]
- Hirakawa A, Shimizu K, Fukumitsu H, Soumiya H, Iinuma M, Furukawa S. 2-Decenoic acid ethyl ester, a derivative of unsaturated medium-chain fatty acids, facilitates functional recovery of locomotor activity after spinal cord injury. Neuroscience. 2010;171:1377–1385. doi: 10.1016/j.neuroscience.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Ann Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Nitta Y, Fukumitsu H, Soumiya H, Ikeno K, Nakamura T, et al. Antidepressant-like activity of 10-hydroxy-trans-2-decenoic Acid, a unique unsaturated Fatty Acid of royal jelly, in stress-inducible depression-like mouse model. Evid Based Complement Alternat Med. 2012;2012:139140. doi: 10.1155/2012/139140. doi: 10.1155/2012/139140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Fournier NM, Kalynchuk LE. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res. 2006;168:280–288. doi: 10.1016/j.bbr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Kalynchuk LE, Gregus A, Boudreau D, Perrot-Sinal TS. Corticosterone increases depression-like behavior, with some effects on predator odor-induced defensive behavior, in male and female rats. Behav Neurosci. 2004;118:1365–1377. doi: 10.1037/0735-7044.118.6.1365. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kutiyanawalla A, Terry AV, Jr, Pillai A. Cysteamine attenuates the decreases in TrkB protein levels and the anxiety/depression-like behaviors in mice induced by corticosterone treatment. PLoS ONE. 2011;6:e26153. doi: 10.1371/journal.pone.0026153. . doi: 10.1371/journal.pone.0026153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. Alpha-asarone, a major component of acorus gramineus, Attenuates corticosterone-induced anxiety-like behaviours via modulating TrkB signaling process. Korean J Physiol Pharmacol. 2014;18:191–200. doi: 10.4196/kjpp.2014.18.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Hutchins AE, Doyle CM, Lundblad JR, Kwok DRPS. Acetylation of cAMP-responsive element-binding protein (CREB) by CREB-binding protein enhances CREB dependent transcription. J Biol Chem. 2003;278:15727–15734. doi: 10.1074/jbc.M300546200. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magariños AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiol Behav. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Makino A, Iinuma M, Fukumitsu H, Soumiya H, Furukawa Y, Furukawa S. 2-Decenoic acid ethyl ester possesses neurotrophin-like activities to facilitate intracellular signals and increase synapse-specific proteins in neurons cultured from embryonic rat brain. Biomed Res. 2010;31:379–386. doi: 10.2220/biomedres.31.379. [DOI] [PubMed] [Google Scholar]
- Makino A, Iinuma M, Fukumitsu H, Soumiya H, Furukawa Y, Furukawa S. Anxiolytic-like effect of trans-2-decenoic acid ethyl ester in stress-induced anxiety-like model mice. Biomed Res. 2013;34:259–267. doi: 10.2220/biomedres.34.259. [DOI] [PubMed] [Google Scholar]
- McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- Naggert J, Witkowski A, Wessa B, Smith S. Expression in Escherichia coli, purification and characterization of two mammalian thioesterases involved in fatty acid synthesis. Biochem J. 1991;273(Pt 3):787–790. doi: 10.1042/bj2730787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and TrkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paykel ES. Life events and affective disorders. Acta Psychiatr Scand Suppl. 2003;418:61–66. doi: 10.1034/j.1600-0447.108.s418.13.x. [DOI] [PubMed] [Google Scholar]
- Quesseveur G, David DJ, Gaillard MC, Pla P, Wu MV, Nguyen HT, et al. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl Psychiatry. 2013;3:e253. doi: 10.1038/tp.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitkin FM, Rabkin JG, Ross D, Stewart JW. Identification of true drug response to antidepressants. Use of pattern analysis. Arch Gen Psychiatry. 1984;41:782–786. doi: 10.1001/archpsyc.1984.01790190056007. [DOI] [PubMed] [Google Scholar]
- Radeley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4:271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Reul JMHM, de Kloet ER. Two receptor systems for corticosterone in the rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2512. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1991;21:679–682. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Kobayashi Y, Takeuchi A, Pagès G, Pouysségur J, Kazama T. Deletion of ERK1 and ERK2 in the CNS causes cortical abnormalities and neonatal lethality: Erk1 deficiency enhances the impairment of neurogenesis in Erk2-deficient mice. J Neurosci. 2011;31:1149–1155. doi: 10.1523/JNEUROSCI.2243-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC-H, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Madeira MD, Paula-Barbosa MM. Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain Res. 1998;794:199–210. doi: 10.1016/s0006-8993(98)00218-2. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Urani A, Gass P. Corticosteroid receptor transgenic mice: models for depression? Ann N Y Acad Sci. 2003;1007:379–393. doi: 10.1196/annals.1286.037. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ma R, Shen J, Su H, Xing D, Du L. A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol. 2008;581:113–120. doi: 10.1016/j.ejphar.2007.12.005. [DOI] [PubMed] [Google Scholar]


