Abstract
Acute leukaemias (AL) correspond to 25–35% of all cancer cases in children. The aetiology is still sheltered, although several factors are implicated in causality of AL subtypes. Childhood acute leukaemias are associated with genetic syndromes (5%) and ionising radiation as risk factors. Somatic genomic alterations occur during fetal life and are initiating events to childhood leukaemia. Genetic susceptibility has been explored as a risk factor, since environmental exposure of the child to xenobiotics, direct or indirectly, can contribute to the accumulation of somatic mutations. Hence, a systematic review was conducted in order to understand the association between gene polymorphisms and childhood leukaemia risk. The search was performed in the electronic databases PubMed, Lilacs, and Scielo, selecting articles published between 1995 and 2013. This review included 90 case-control publications, which were classified into four groups: xenobiotic system (n = 50), DNA repair (n = 16), regulatory genes (n = 15), and genome wide association studies (GWAS) (n = 9). We observed that the most frequently investigated genes were: NQO1, GSTM1, GSTT1, GSTP1, CYP1A1, NAT2, CYP2D6, CYP2E1, MDR1 (ABCB1), XRCC1, ARID5B, and IKZF1. The collected evidence suggests that genetic polymorphisms in CYP2E1, GSTM1, NQO1, NAT2, MDR1, and XRCC1 are capable of modulating leukaemia risk, mainly when associated with environmental exposures, such as domestic pesticides and insecticides, smoking, trihalomethanes, alcohol consumption, and x-rays. More recently, genome wide association studies identified significant associations between genetic polymorphisms in ARID5B e IKZF1 and acute lymphoblastic leukaemia, but only a few studies have replicated these results until now. In conclusion, genetic susceptibility contributes to the risk of childhood leukaemia through the effects of gene–gene and gene–environment interactions.
Keywords: leukaemia, genetic polymorphism, genetic predisposition to disease, environmental exposure
Introduction
Childhood acute leukaemia
Acute leukaemias (AL) are the highest incidence malignancy in children and adolescents (≤19 years of age) and as a whole, the aetiology has not yet been unveiled. There are two major groups of AL, acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML), classified according to characteristics presented by leukaemic cells, such as morphological features, surface antigens, chromosomal and molecular abnormalities [1], and gene expression profile [2].
Observational epidemiology has demonstrated that about 5 to 10% of AL are associated with ionising radiation exposure and congenital genetic syndromes (Down, neurofibromatosis, Fanconi anaemia, and Bloom syndrome) [3], that are associated with specific leukaemia subtypes. For the remaining 90% of AL, the aetiopathology is postulated to be a multistep process and somatic mutations are the start point of the pathway. When initiated during fetal life, the majority of them require postnatal events that contribute to accumulation of secondary mutations and proliferative advantage [3–5]. In this regard, the initiating event originating in cells would take advantage of the genetic predisposition conferred, in part, by genetic susceptibility and damage from exogenous exposures [6].
Genetic susceptibility
Genetic polymorphisms are defined as natural genetic variations that occur randomly in the general population. The most common type is the single nucleotide polymorphism (SNP) that consists of a variation at a single base pair [7]. Depending on where it is located, SNPs can interfere with a gene’s function, affecting metabolic pathways [8]. This review will focus on three main pathways that have been related to AL genetic susceptibility: xenobiotic system, DNA repair system, and cell regulation, which have been identified as risk factors in childhood leukaemia.
Xenobiotic system
Children are more vulnerable and susceptible to environmental toxicants than adults because of physiological immaturity, and also indirect and unintended exposures. Environmental agents such as tobacco and traffic smoke, pesticides, household chemicals, paintings, and diet are potential AL risk factors, as they may contain carcinogenic substances to humans, such as organic solvents (benzene derivatives), polycyclic aromatic hydrocarbons (PAHs), and organochloride compounds [9]. These substances, however, require metabolic activation by enzymes from the xenobiotic system to be able to interact with genetic material and eventually cause somatic mutations [10].
The xenobiotic system is classified into two phases: i) phase I enzymes, represented by cytochrome P450 isoenzymes (CYP), that catalyse hydrolysis, reduction, and oxidation reactions; and ii) phase II enzymes, that catalyse conjugation reactions, comprising glutathione S-transferases (GST) and N-acetyl transferases (NAT).
Interindividual genetic variations that are capable of altering the process of metabolism of pro-carcinogens, in both mother and child, may modulate the risk of developing paediatric leukaemia [10].
DNA repair
DNA repair systems play an essential role in maintaining integrity and genomic stability [11]. Ionising radiation, environmental carcinogens and their reactive intermediates, together with genomic instability and inherent errors in DNA replication process contribute to the occurrence of damage in DNA. Mutations, chromosomal breaks, and crosslinks are actively recognised and repaired by sets of enzymes that constitute the DNA repair system [12]. Three main mechanisms are responsible for single-stranded DNA damage repair: i) base excision repair (BER), ii) nucleotide excision repair (NER), iii) and mismatch repair (MMR); which comprises enzymes enconded by several genes, such as XRCC1, ERCC2, MLH1, MSH3. Meanwhile, double-stranded DNA breaks can be repaired by homologous recombination (HR) or nonhomologous end joining (NHEJ), throughout enzymes as nibrin (NBN) and others, encoded by several genes, such as ATM, BRCA2, and RAD51 [13]. Failures in those systems have been linked to birth defects, cancer, and premature ageing [12]. For instance, Fanconi anaemia, ataxia–telangiectasia, Nijmegen breakage syndrome, and Bloom syndrome, resulting from DNA repair disorders, are highly associated with childhood leukaemia [14].
Regulatory genes
Cells often present signal transduction alterations that lead to proliferation in response to external factors. Several growth factors, their receptors and effector molecules have been identified as proto-oncogenes or tumour suppressor genes. Mutations in these genes may interfere with regulatory mechanisms that control cell cycle, leading to generation of malignant clones [15]. Considering this fact, polymorphisms in genes involved in cell cycle regulation also contribute to cancer susceptibility [16]. Nevertheless, few studies to date have investigated the association of regulatory genes with paediatric leukaemia.
Considering the large amount of epidemiologic data about this subject, it becomes necessary to use systematic methods to evaluate and synthesise all the information in order to facilitate communication between molecular epidemiology and clinical practice [17]. In an attempt to clarify some issues in this field, a systematic review was conducted which aimed to add comprehensive information about genetic susceptibility in childhood leukaemia.
Methods
Publication search strategy
A literature search on genetic susceptibility and childhood leukaemia was carried out using PubMed, Lilacs, and Scielo (last updated in June 2013). The following terms were used in different combinations: ‘acute lymphoblastic leukaemia’, ‘acute myeloid leukaemia’, ‘genetic polymorphism(s)’, ‘genetic susceptibility’, ‘xenobiotic(s)’, ‘molecular epidemiology’, ‘risk factor(s)’, and ‘child* or infant or paediatric’. Also, the following MeSH terms were used: ‘precursor cell lymphoblastic leukaemia–lymphoma’, ‘leukaemia, myeloid, acute’, ‘polymorphism, genetic’, ‘genetic predisposition to disease’, ‘gene–environment interaction’, ‘case-control studies’, and ‘genetic association studies’. References were also checked in order to look for articles that were missing in the electronic databases. The search strategy was elaborated using the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement as a guideline [18].
Inclusion and exclusion criteria
The inclusion criteria for all publications were: 1) case-control genotyping studies published between 1995 and 2013 that tested the risk of genetic polymorphisms with childhood ALL and/or AML (ages ≤ 21 years); 2) studies that provided sufficient data for estimating the risk association with odds ratio (OR), relative risk (RR) or interaction odds ratio (IOR); and 3) full text available in English, Spanish, and Portuguese. The exclusion criteria were: 1) studies with a different theme from what was proposed for this review; 2) publications in different languages, otherwise the ones specified; 3) studies that specifically include leukaemia cases related to genetic syndromes—Down, neurofibromatosis, Fanconi anaemia, Bloom syndrome and ataxia–telangiectasia—or secondary leukaemia; 4) articles that include other malignancies besides leukaemia in the same cohort, avoiding extrapolation of results exclusively for leukaemias; 5) articles about family gene transmissions; 6) articles about genes and prognosis; 7) articles about folate genes and immune system; and 8) comments and editorials.
Data extraction
Information was extracted from each eligible article supervised by two investigators (LRA and MSPO), according to the inclusion criteria listed above. The following variables were collected from eligible studies: geographical origin, first author’s name, year of publication, leukaemia subtype, number of cases and controls, age, candidate genes investigated, and significant genotyping results.
Statistical analysis
The strength of association between different genetic polymorphisms through the case-control method was evaluated by analysis of OR, RR, or IOR, with 95% confidence interval (95% CI), that were collected from the studies. Risk associations were considered significant when the P-value was ≤0.05.
Results and discussion
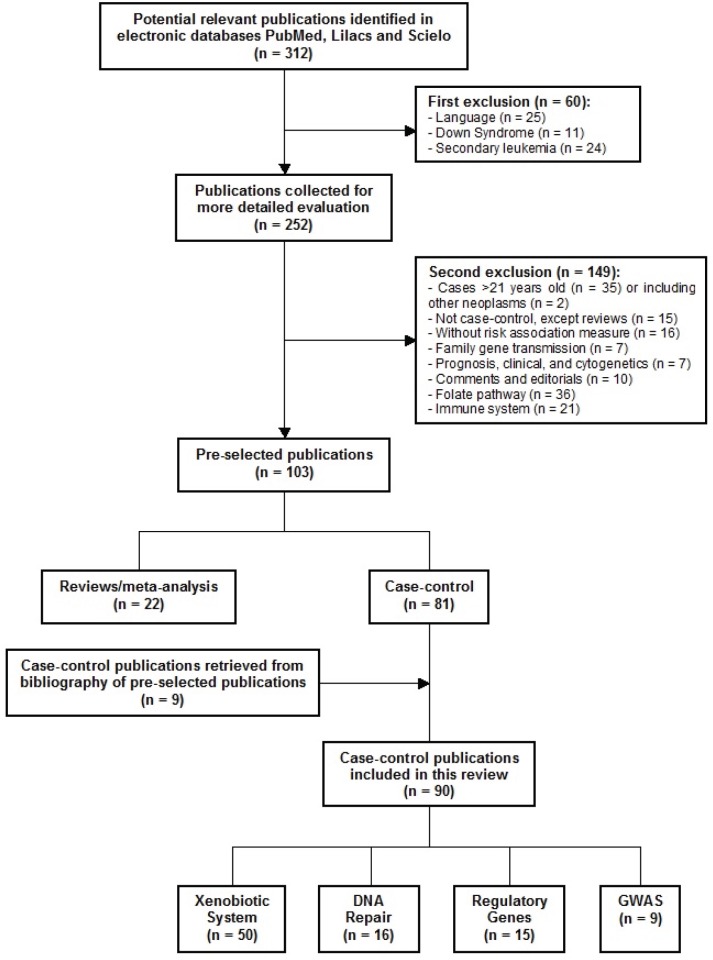
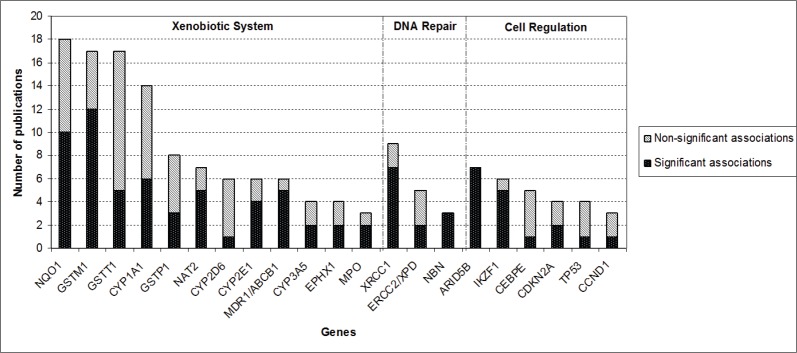
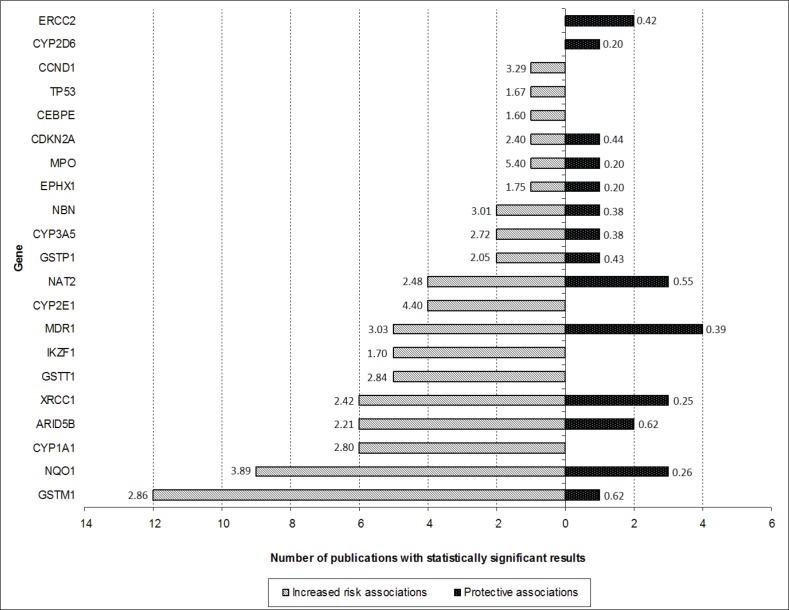
After screening of the retrieved titles, 312 publications were identified as potentially relevant to this review (Figure 1). After application of exclusion criteria, 103 publications were pre-selected, of which 22 were reviews, and 81 were case-control studies. After checking for bibliographies of pre-selected publications, nine papers were added, giving a total of 90 case-control publications included in this review. The majority of publications addressed polymorphisms in genes related to xenobiotic system (n = 50), followed by DNA repair genes (n = 16), regulatory genes (n = 15), and genome wide association studies (GWAS) (n = 9). The most frequently analysed gene polymorphisms, presented by at least three papers (Figure 2), were located in genes CYP1A1, CYP2D6, CYP2E1, CYP3A5, EPHX1, GSTM1, GSTT1, GSTP1, MPO, NAT2, NQO1, MDR1 (ABCB1), XRCC1, ERCC2, NBN, ARID5B, and IKZF1. Figure 2 also shows the proportion of publications that showed statistically significant associations for each gene, among the total. Considering only statistically significant results, median ORs for increased risk or protective associations for those genes were calculated, and are demonstrated in Figure 3.
Figure 1. Flow diagram of included and excluded publications.
Figure 2. Proportion of publications that reported statistically significant associations between genetic polymorphisms and CAL risk for each of the genes that had polymorphism analysis reported by at least three publications.
Figure 3. Number of publications that have shown statistically significant protective (black bar) or increased risk (white bar) associations for each gene (only genes with polymorphism analysis reported by at least three publications are shown). The numbers presented in the extremities of each bar represent the median ORs of risk associations found for polymorphisms involving each gene.
Xenobiotic system
Genetic susceptibility studies related to xenobiotic system are presented in Table 1. The main investigated gene polymorphisms comprised the genes CYP1A1, CYP2D6, CYP2E1, CYP3A4, CYP3A5, EPHX1, GSTM1, GSTP1, GSTT1, MDR1, MPO, NAT1, NAT2, and NQO1. Most publications are from Asia (39.6%), followed by North America (25%), Europe (20.8%), and South America (14.6%). The great disparity between ethnic groups is remarkable, since the Asian continent includes a wide range of people with distinct genetic backgrounds, such as Caucasians, Turkish, Indians, Japanese, Chinese, and the Korean population, likewise, Americans have diverse ancestries, mainly Caucasian, Hispanic, and African. The vast majority of publications (75%) investigated the genetic susceptibility in ALL only, and 25% in AML + ALL; no one has investigated AML solely.
Table 1. Genetic susceptibility publications in childhood leukaemia involving genes related to xenobiotic system.
| Continent | Country | Leukaemia subtype | Number of cases/controls | Ages of cases (years) | Investigated genes | Significant results | First author, year | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Genetic variation | OR (95% CI) | P-value | ||||||||
| Europe | United Kingdom | ALL, AML | 36/100 | <15 | NQO1 |
NQO1 609-T in AL MLL+ NQO1 609-T in AL MLL/AF4 |
2.54 (1.08–5.96) 8.63 (2.45–33.22) |
0.015 <0.001 |
Wiemels, 1999 | [47] |
| Portugal | ALL | 47/102 | ≤18 | GSTM1, GSTT1 | GSTM1-null | 2.20 (1.10–4.50) | 0.035 | Alves, 2002 | [50] | |
| Poland | ALL | 113/175 | ≤18 | MDR1 | MDR1 3435-TT | 1.80 (1.10–3.10) | 0.037 | Jamroziak, 2004 | [62] | |
| Germany/Austria/Czech Republic | ALL | 209/190 | ≤19 | NQO1 | No significant associations were found. | – | – | Kracht, 2004 | [41] | |
| Italy | ALL | 156/147 | <15 | NQO1 | NQO1 609-CT+TT in AL MLL- ≤12 months | 4.22 (1.43–12.49) | 0.006 | Lanciotti, 2005 | [42] | |
| Italy | ALL | 323/384 | <18 | GSTM1, GSTT1, GSTP1 | No significant associations were found. | – | – | Pigullo, 2007 | [54] | |
| Hungary | ALL | 396/192 | 1–15 | MDR1, BCRP |
MDR1 2677G-3435T haplotype MDR1 2677T-3435C haplotype |
2.50 (1.40–4.40) 0.40 (0.20–0.80) |
0.002 0.006 |
Semsei, 2008 | [63] | |
| Hungary | ALL | 543/529 | 1–15 | AhR, NQO1, NQO2 | No significant associations were found. | – | – | Lautner-Csorba, 2012 | [43] | |
| Denmark/Norway | ALL | 616/203 | 1–15 | CYP3A5 | CYP3A5*3 (6986-A allele) | 1.64 (1.01–2.66) | 0.049 | Borst, 2011 | [36] | |
| France | ALL, AML | 493/549 | <15 | CYP1A1, CYP2E1, NQO1, NAT2, EPHX1 | NAT2*5 in ALL | 1.80 (1.30–2.50) | NA | Bonaventure, 2012 | [22] | |
| Asia | Turkey | ALL, AML | 177/185 | ≤17 | GSTM1, GSTT1, GSTP1, CYP1A1 | No significant associations were found. | – | – | Balta, 2003 | [20] |
| Turkey | ALL, AML | 273/286 | 1–16 | NQO1 | No significant associations were found. | – | – | Sirma, 2004 | [45] | |
| Turkey | ALL, AML | 163/140 | 2–18 | GSTM1, GSTT1, CYP1A1, CYP2D6, CYP2E1 |
CYP2E1*5B (–/+) in ALL CYP2E1*5B (–/+) in AML |
3.40 (1.30–9.10) 4.90 (1.60–15.20) |
0.010 0.006 |
Aydin-Sayitoglu, 2006 | [19] | |
| Turkey | ALL | 168/207 | 1.5–15.5 | CYP2E1 | Co presence of at least 2 variant CYP2E1 alleles (*5B and *6; *6 and *7B; *5B, *6 and *7B) | 3.90 (1.40–11.00) | <0.050 | Ulusoy, 2007 | [34] | |
| Turkey | ALL | 167/190 | 1.5–15.5 | EPHX1 |
EPHX1 113-His (28T>C) EPHX1 113-His (28T>C) + XRCC1 399-Gln |
1.40 (1.00–2.00) 2.10 (NA) |
0.030 0.030 |
Tumer, 2012 | [49] | |
| India (South) | ALL | 118/118 | ≤14 | GSTM1, GSTT1, CYP1A1, CYP2D6 |
CYP1A1*2A (+/+) CYP1A1*2A (–/+) CYP1A1*2C (+/+) CYP1A1*2C (–/+) GSTM1-null |
6.22 (1.30–29.71) 2.58 (1.41–4.72) 4.28 (1.14–16.11) 2.18 (1.16–4.10) 2.10 (1.21–3.67) |
0.022 0.002 0.032 0.015 0.009 |
Joseph, 2004 | [26] | |
| Japan | ALL, AML | 103/197 | <1.5 | NQO1 |
NQO1 465-CT/TT in ALL MLL+ NQO1 465-CT/TT in ALL MLL-AF4 |
3.55 (1.13–11.10) 6.36 (1.84–21.90) |
0.020 | Eguchi-Ishimae, 2005 | [40] | |
| Japan | ALL | 157/96 | 1–15 | MDR1 |
MDR1 -2352A in children ≥ 6 years of age MDR1 3435T |
0.34 (0.20–0.77) 1.61 (1.09–2.39) |
0.012 0.020 |
Hattori, 2007 | [61] | |
| Thailand | ALL | 107/320 | ≤14 | GSTM1, GSTT1, CYP1A1, CYP3A4, CYP3A5 |
GSTM1-null GSTM1-null + GSTT1-null |
1.70 (1.00–2.70) 1.70 (1.10–2.90) |
0.040 0.020 |
Pakakasama, 2005 | [29] | |
| Thailand | ALL | 100/100 | ≤14 | GSTP1 | No significant associations were found. | – | – | Gatedee, 2007 | [56] | |
| Thailand | ALL | 99/100 | 1–14 | GSTO1, GSTO2 |
GSTO1*140A/D GSTO2*142N/D in high risk ALL |
2.24 (1.16–4.35) 5.52 (1.72–17.71) |
0.009 0.004 |
Pongstaporn, 2009 | [65] | |
| Russia | ALL, AML | 403/490 | ≤17 | GSTM1, GSTT1, CYP1A1, CYP2D6, CYP2C9, CYP2C19, NQO1, NAT2 |
GSTT1-null+GSTM1-null NAT2 341C+C481T+G590A |
3.09 (2.05–4.65) 0.55 (0.33–0.93) |
<0.001 0.026 |
Gra, 2008 | [25] | |
| Philippines | ALL | 60/60 | <18 | GSTM1, GSTT1, NQO1 |
GSTM1-null NQO1 609-CC GSTM1-null + NQO1 609-CC |
2.37 (1.11–5.04) 4.82 (2.18–10.60) 11.9 (3.45–41.09) |
0.020 <0.001 NA |
Rimando, 2008 | [44] | |
| Taiwan | ALL, AML | 114/220 | <20 | AKR1C3 | rs10508293 A > G in the child rs10508293 A > G in both child and mother |
2.46 (1.69–3.58) 1.63 (1.30–2.04) |
<0.001 <0.001 |
Liu, 2008 | [66] | |
| Korea | ALL, AML | 176/298 | ≤18 | CYP1A1 | Absence of haplotype CYP1A1 CGACC (-T1761C, -G9893A,Ex7+A131G, C1188T, C11599G) in children with father’s smoking or at least one smoker at home, respectively (risk for ALL) |
2.80 (1.50–5.30) 2.30 (1.20–4.40) |
0.030 0.020 |
Lee, 2009 | [28] | |
| China | ALL | 67/146 | 0.8–18 | GSTM1, GSTT1 |
GSTM1-null GSTM1-T1-null |
2.86 (1.49–5.46) 3.15 (1.71–5.79) |
<0.001 <0.001 |
Wang, 2004 | [55] | |
| China/Malaysia | ALL | 756/756 | med. 4.8 | GSTM1, GSTT1, NQO1, MDR1 | NQO1 609-CT in Malay boys | 0.38 (0.22–0.66) | 0.001 | Yeoh, 2010 | [48] | |
| Indonesia (Javanese children) | ALL | 185/177 | ≤14 | GSTM1, GSTT1, GSTP1, NQO1 |
GSTM1-null in boys GSTT1-null in girls GSTP1*B in girls |
1.89 (1.04–3.44) 2.20 (1.10–4.37) 0.43 (0.21–0.89) |
0.050 0.027 0.031 |
Chan, 2011 | [38] | |
| Iran | ALL | 85/94 | <16 | CYP1A1 | No significant associations were found. | – | – | Razmkhah, 2011 | [30] | |
| North America | Canada | ALL | 177/304 | 1–21 | GSTM1, GSTT1, CYP1A1, CYP2D6 |
GSTM1-null CYP1A1*2A (+/+, +/–) GSTM1-null + CYP1A1*2A (+/+, +/–) |
1.80 (1.20–2.60) 1.80 (1.10–3.10) 3.30 (1.60–6.90) |
0.004 0.030 0.002 |
Krajinovic,1999 | [27] |
| Canada | ALL | 176/306 | med. 6.0 | NAT1, NAT2 |
NAT2*4 allele NAT2*5C allele NAT2*7B allele NAT1*4/*4 + NAT2-slow |
0.60 (0.50–0.90) 3.10 (1.10–8.50) 2.90 (1.10–7.40) 1.90 (1.10–3.40) |
0.010 0.020 0.030 0.030 |
Krajinovic,2000 | [58] | |
| Canada | ALL | 174/337 | med. 5.2 | NQO1, CYP2E1, MPO |
CYP2E1*5B (–/+) NQO1*2 (C609T) or *3 (C465T) CYP2E1*5B (–/+) + NQO1*2/*3 + MPO*2 (–/–) |
2.80 (1.20–6.70) 1.70 (1.20–2.40) 5.40 (1.20–23.40) |
0.020 0.008 0.003 |
Krajinovic, 2002 | [33] | |
| Canada | ALL | 278/302 | med. 4.9 | GSTP1 |
GSTP1*A/B; B/B GSTP1*A/B; B/B in girls GSTP1*A/B; B/B + GSTM1 null |
1.50 (1.10–2.00) 1.90 (1.20–3.10) 2.20 (1.30–3.50) |
0.020 0.010 0.002 |
Krajinovic, 2002 | [57] | |
| United States | ALL | 197/416 | ≤18 | GSTM1, GSTT1 | GSTM1-null + GSTT1-null in Blacks | 7.36 (2.61a) | <0.001 | Chen, 1997 | [52] | |
| United States | ALL, AML | 39/56 | ≤18 | NQO1 |
NQO1 609-CT/TT in AL MLL+ NQO1 609-CT/TT in ALLMLL+ |
2.47 (1.08–5.68) 3.35 (1.13–9.82) |
0.033 0.028 |
Smith, 2002 | [46] | |
| United States | ALL | 171/NA | ≤18 | GSTM1, GSTM3, GSTT1, GSTP1 |
GSTM1*A GSTM1*B GSTT1*1 |
5.66b (2.58–12.41) 4.28 b (1.79–10.19) 2.59 b (1.07–6.29) |
<0.001 0.001 0.035 |
Barnette, 2004 | [51] | |
| United States | ALL | 76/76 | ≤6 | GSTM1, GSTT1 | No significant associations were found. | – | – | Klotz, 2006 | [53] | |
| United States | ALL | 294/369 | <15 | MDR1 |
MDR1 1236-TT MDR1 2677-TA/TT/AA MDR1 3435-TT in non-Hispanic White hyperdiploid ALL MDR1 haplotype CGC(C1236T, G2677T/A, C3435T) x indoor insecticide exposure |
40.35 (3.00–542.60) 6.01 (1.12–32.23) 8.86 (1.35–58.03) 0.37 (0.15–0.88) |
NA NA NA 0.025 |
Urayama, 2007 | [64] | |
| United States | ALL | 163/251 | <21 | CYP1A1, NQO1 | No significant associations were found. | – | – | Beuten, 2011 | [21] | |
| United States | B-ALL | 258/646 | med. 6.3 | CYP1A1 |
CYP1A1*2C (+/+) CYP1A1*2B (+/+) rs4886605 (–/+; +/+) in Caucasians CYP1A1*2A (+/+) in Hispanics CYP1A1*2B (+/+) in Hispanics CYP1A1*2C (+/+) in Hispanics |
2.51 (1.18–5.33) 3.24 (1.43–7.34) 1.58 (1.01–2.46) 2.70 (1.27–5.74) 3.28 (1.40–7.69) 2.47 (1.13–5.38) |
0.016 0.005 0.043 0.010 0.006 0.023 |
Swinney, 2011 | [31] | |
| United States | ALL | 377/448 | <14 | 250 SNPs in42 genesc + GSTM1, GSTT1 |
MDR1 haplotype ARNT haplotype CYP2C8 haplotype CYP1A2 haplotype in non-Hispanics CYP1B1 haplotype in non-Hispanics GSTM1-null in non-Hispanics IDH1 haplotype in Hispanics GSTM1-null in Hispanics CYP2C8 haplotype in Hispanics associated with paint use MDR1 haplotype in Hispanics associated with indoor insecticides |
0.44 (0.23–0.85) 4.93 (1.94–12.53) 3.18 (1.45–6.95) 2.19 (1.28–3.77) 0.11 (0.02–0.56) 0.62 (0.43–0.89) 6.12 (1.75–21.36) 1.85 (1.19–2.88) 1.67 (1.21–2.30) 3.03 (1.59–5.78) |
0.015 0.001 0.004 0.005 0.007 0.010 0.005 0.007 0.001 0.005 |
Chokkalingam, 2012 | [24] | |
| South America | Brazil | ALL | 113/221 | ≤18 | GSTM1, GSTT1, GSTP1, CYP1A1, CYP2E1 | GSTP1*B + GSTM1-null + CYP1A1*2 + CYP2E1*5B | 10.30 (1.00–111.80) | 0.050 | Canalle, 2004 | [23] |
| Brazil | ALL | 99/99 | med. 4.0 | CYP1A1, NQO1 |
NQO1 609-CT+TT NQO1 609-CT+TT CYP1A1 *2A/*2B/*2C |
2.64 (1.46–4.80) 10.71 (1.20–95.46) |
0.001 0.030 |
Yamaguti, 2010 | [32] | |
| Brazil | ALL | 206/364 | <18 | CYP2D6,EPHX1,MPO,NQO1 |
EPHX1*2 (–/+; +/+) CYP2D6*1 + EPHX1*2 + MPO*2 + NQO1*1 CYP2D6*1 + EPHX1*2 + MPO*1 + NQO1*2 CYP2D6*1 + EPHX1*2 + MPO*2 + NQO1*2 CYP2D6*1 + EPHX1*2 and *3 + MPO*2 + NQO1*2 |
0.26 (0.16–0.42) 0.20 (0.05–0.70) 0.46 (0.04–0.60) 0.06 (0.01–0.40) 0.20 (0.04–0.90) |
0.001 0.003 0.001 <0.001 0.010 |
Silveira, 2010 | [35] | |
| Brazil | ALL | 132/131 | ≤1.75 | NAT2 |
NAT2*5 NAT2*6 NAT2*5/*6 (slow) NAT2*5/*7 (slow) NAT2*5/*14 (slow) NAT2 slow in both child andmother |
2.41 (1.23–4.78) 2.32 (1.13–4.80) 11.70 (2.00–118.40) 6.95 (1.08–74.20) 13.50 (1.37–174.20) 30.00 (5.870–279.70) |
NA NA NA NA NA NA |
Zanrosso, 2010 | [60] | |
| Brazil | ALL, AML | 232/303 | ≤10 | NAT2 |
NAT2 341-C in ALL NAT2 341-C in AML NAT2 590-A in AML NAT2 slow phenotypes NAT2 rapid phenotypes |
2.30 (1.51–3.51) 2.48 (1.38–4.51) 1.57 (1.07–2.30) 2.42 (1.71–3.44) 0.41 (0.29–0.59) |
0.000 0.000 0.030 NA NA |
Zanrosso, 2012 | [59] | |
| Brazil | ALL, AML | 626/401 | ≤12 | NQO1, PON1 |
PON1-55M in non-Whites PON1-55M in ALL >1–10 years PON1-192R in ALL >1–10 years NQO1-609T in AML ≤ 1 year NQO1-609T in ALL MLL- ≤12 months NQO1-609T in ALL MLL- >12–24 months |
2.52 (1.49–4.26) 1.99 (1.17–3.39) 0.57 (0.33–0.97) 0.26 (0.10–0.68) 0.36 (0.16–0.81) 2.36 (1.02–5.72) |
NA NA NA NA NA NA |
De Aguiar Gonçalves, 2012 | [39] | |
| Brazil | ALL | 204/364 | mean3.9 | CYP3A5, NAT2 |
CYP3A5*3 (+/+) in White children CYP3A5*3 (–/+; +/+) in White children CYP3A5*6 (–/+; +/+) in White children CYP3A5*6 (–/+; +/+) in non-White children |
0.38 (0.16–0.90) 0.43 (0.18–1.00) 3.80 (1.10–13.59) 0.32 (0.11–0.90) |
0.030 0.050 0.020 0.050 |
Silveira, 2012 | [37] | |
med.: median of age.
MLL+: positive for MLL gene rearrangement.
MLL–: negative for MLL gene rearrangement.
(–/+): heterozygote for the variant allele.
(+/+): homozygote for the variant allele.
AL: acute leukaemia.
ALL: acute lymphoblastic leukaemia.
AML: acute myeloid leukaemia.
OR: odds ratio. 95%CI: 95% confidence interval.
NA: not available.
only lower bound of confidence interval was presented by the authors.
relative risk (RR).
ABCB1 (MDR1), ABCC1 (MRP1), ABCC2 (MRP2), AhR, ARNT, COMT, CYP1A1, CYP1A2, CYP1B1, CYP2A6, CYP2B6, CYP2C19, CYP2C8, CYP2D6, CYP2E1, CYP3A4, CYP3A5, EPHX1, EPHX2, FMO3, GCLC, GGT1, GPX6, GSR, GSS, GSTA1, GSTO2, GSTP1, IDH1, MPO, NAT1, NAT2, NQO1, PON1, PTGS1, PTGS2, SULT1A1, TPMT, UGT1A1, UGT1A7, UGT1A9, UGT2B7.
Regarding phase I metabolism, fourteen publications [19–32] explored polymorphisms of CYP1A1, mainly the variant alleles *2A, *2B, *2C, and *4; only six (42.9%) found significant associations between these alleles and childhood ALL. CYP1A1*2A allele was associated with increased risk for ALL among Canadians [27], Indians [26], and Hispanics in North-America [31]. CYP1A1*2B was associated with increased risk for B-cell precursor ALL (Bcp-ALL) among north-Americans [31]. CYP1A1*2C allele was also associated with increased risk for ALL among Indians [26] and north-Americans [31]. Two Brazilian papers found significant associations of CYP1A1 variant alleles and ALL only with combined genotypes: CYP1A1*2 + CYP2E1*5B + GSTP1*B + GSTM1-null [23], and CYP1A1*2A/*2B/*2C + NQO1 609-CT/ CT + TT [32].
Six publications [19, 22–24, 33, 34] explored variant alleles of CYP2E1, mainly *5B, *6 and *7B; in four out of six (66.7%), significant associations were found: CYP2E1*5B was related to increased risk for ALL/AML in Canadians [33] and Turkish [19]. The presence of at least two variant alleles (*5B and *6; *6 and *7B; or *5B, *6, and *7B) was related to increased risk for ALL in Turkish [34]. Besides, the combined genotype CYP2E1*5B + CYP1A1*2 + GSTP1*B + GSTM1-null was associated with increased risk for ALL in Brazilians [23].
Six publications [19, 24–27, 35] explored CYP2D6*3 and *4 alleles. The wild-type allele (CYP2D6*1) was negatively associated with ALL in Brazilians [35]. Polymorphisms of CYP3A genes (CYP3A4*1B, CYP3A5*3, and CYP3A5*6) were investigated in four papers [24, 29, 36, 37], and no significant associations were found for CYP3A4*1B allele [24, 29]. The wild-type allele CYP3A5*1 was associated with ALL increased risk in Denmark and Norway [36], whereas alleles CYP3A5*3 and CYP3A5*6 were associated with ALL protection in Brazilians [37]. The same Brazilian study found increased risk association for the CYP3A5*6 allele only in Whites, leading to speculation that CYP3A5 may also be involved in detoxification as well as activation mechanisms of carcinogens. Alleles that do not produce a functional protein, such as CYP3A5*6, would contribute to accumulation of potentially harmful substances [36].
Eighteen publications [21, 22, 24, 25, 32, 33, 35, 38–48] addressed polymorphisms of NQO1 gene (C609T and C465T). In ten studies (55.5%), divergent associations were found. NQO1 609T allele was associated with increased risk for ALL among Canadians [33] and Brazilians [32]. In Filipinos, the genotype NQO1 609CC was associated with increased risk for ALL [44], whereas the variant allele NQO1 609T was negatively associated with ALL in Malaysian boys [48] and Brazilians [35]. The 609T allele has been associated with infant leukaemia and MLL gene rearrangements in Caucasians [39, 42, 46, 47]. Otherwise, very few publications demonstrated increased risk for ALL in Canadians [33], and Japanese [40] with NQO1 465T allele.
Few publications [22, 24, 35, 49] have explored polymorphisms of EPHX1*2 (T28C–Tyr113His) and EPHX1*3 (A52G–His139Arg); two out of four showed divergent results: variant alleles *2 and *3 were associated with protection for ALL in Brazilians [35], whereas the *2 allele was associated with increased risk for ALL in Turkish [49]. Given the duality of functions that enzyme EPHX1 performs, its interconnection with CYP450 family, the diversity of xenobiotics presented in the environment, and the differences in allele frequencies among populations, gene polymorphisms of EPHX1 may contribute in an unpredictable way in activation or detoxification of xenobiotics.
Finally, three publications [24, 33, 35] demonstrated that the variant alleles of MPO, mainly MPO*2 (G-463A) have a protective effect when combined to other gene polymorphisms [33, 35]. No study has ever demonstrated an independent effect of MPO*2 in childhood leukaemia susceptibility.
Regarding phase II metabolism, the GST gene family was the most investigated (20 case-control publications), regarding association with childhood leukaemia risk all over the world. In twelve out of seventeen [19, 20, 23–27, 29, 38, 44, 48, 50–55] the homozygous deletion of GSTM1 and GSTT1 alleles (null genotype) was related to childhood leukaemia risk: GSTM1-null genotype was associated with increased risk for ALL in ten papers [23, 26, 27, 29, 38, 44, 50, 52, 55]; GSTT1-null genotype was associated with increased risk for ALL, mostly when combined with GSTM1-null genotype [29, 38, 52, 55]. However, two studies performed in US children disclosed conflicting results: GSTM1-null was found to be associated with protection for ALL among non-Hispanic American children [24], whereas non-null alleles GSTM1*A, GSTM1*B and GSTT1*1 were associated with increased risk for ALL [51]. These discrepancies may be because of differences in allele frequencies in mixed populations and the different patterns of environmental exposures.
From eight publications [20, 23, 24, 38, 51, 54, 56, 57] that explored GSTP1 polymorphisms (A1578G–GSTP1*B; C2293T–GSTP1*C), only three (37.5%) showed significant associations, but with opposite results: GSTP1*B was related to increased risk for ALL among Canadians [57] and Brazilians [23], while a protective effect was observed for Indonesian girls [38]. Seven publications [22, 24, 25, 37, 58–60] analysed the complexity of NAT2 polymorphisms. Overall, haplotypes that result in low activity phenotype were associated with increased risk for childhood leukaemia [59]. The only exception was a protective effect of NAT2 341C-481T-590A, that results in slow activity phenotype, among Russians [25]. Besides, the combination of slow phenotype in both child and mother intensified the risk for early age ALL [60]. The rapid allele *4 was associated with protection for ALL among Canadians [58], as well as for AML in Brazilian children [59].
Besides the detoxifying function of some metabolising enzymes, membrane transporter proteins also act protectively against carcinogens. Six publications [24, 48, 61–64] explored polymorphisms of MDR1 (ABCB1 family) gene, mainly C1236T, G2677T/A, C3435T, and T-129C, that encodes an efflux membrane transporter (P-glycoprotein) with childhood ALL. MDR1 3435T allele was consensually associated with increased risk for ALL in four publications [61–64], while the haplotype GAGT (rs2520464, rs12334183, rs1202179, rs17327442) was associated with protection for ALL in north-Americans [24].
Finally, few publications have explored other genes, such as ARNT, CYP1A2, CYP1B1, CYP2C8 and IDH1 [24], PON1 [39], NAT1 [58], GSTO1, and GSTO2 [65], and AKR1C3 [66], in childhood ALL. Their results, however, need to be replicated in further studies.
DNA repair
The data from genotyping studies in genes related to DNA repair system are summarised in Table 2. The main investigated gene polymorphisms comprised the genes ERCC2, MLH1, MSH3, NBN, and XRCC1. Six publications (37.5%) were performed in Europeans, five (31.25%) in Asians, four (25%) in north-Americans, and one (6.25%) in Brazilians. The majority of them focused only in ALL.
Table 2. Genetic susceptibility publications in childhood leukaemia involving genes related to DNA repair.
| Continent | Country | Leukaemia subtype | Numberof cases/controls | Ages of cases(years) | Investigated genes | Significant results | First author, year | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Genetic variation | OR (95% CI) | P-value | ||||||||
| Europe | Poland | ALL | 270/6984 | mean6.65 | NBN | NBN 657del5 carriers | 1.85 (1.42–2.65) | 0.035 | Chrzanowska, 2006 | [76] |
| Poland | ALL | 398/731 | med. 4.9 | NBN | NBN 657-wt/del5 NBN 657-del5/del5 | 3.01 (2.42–3.85) 1325.21 (859.84–2167.90) |
0.004 0.003 |
Pastorczak, 2011 | [77] | |
| Poland | ALL | 97/131 | mean5.4 | OGG1, MUTYH, XRCC1 |
OGG1 326-Ser/Ser OGG1 326-Cys/Cys OGG1 326-Cys XRCC1 399-Arg/Arg + OGG1 326-Ser/Ser XRCC1 399-Arg/Gln + OGG1 326-Cys/Cys OGG1 326-Ser/Ser + MUTYH 165-Tyr/Tyr OGG1 326-Cys/Cys + MUTYH 165-Tyr/Tyr |
0.45 (0.26–0.76) 5.36 (1.90–15.09) 2.33 (1.53–3.55) 0.40 (0.19–0.83) 3.83 (1.00–14.86) 0.43 (0.25–0.73) 6.75 (2.19–20.77) |
0.003 0.001 <0.001 0.013 0.050 0.001 <0.001 |
Stanczyk, 2011 | [74] | |
| Turkey | Pre-B ALL | 52/60 | mean5.9 | XRCC1, ERCC2 | No significant associations were found. | – | – | Celkan, 2008 | [69] | |
| Turkey | ALL | 70/75 | ≤15 | XRCC1, ERCC2 | XRCC1 codon 194-Arg/Trp + Trp/Trp in girls | 5.47 (1.49–20.10) | 0.008 | Batar, 2009 | [67] | |
| Turkey | ALL | 167/190 | 1.5–15.5 | XRCC1(+ CYP2E1) |
XRCC1 codon 399-Arg/Gln +Gln/Gln XRCC1 codon 399-Arg/Gln +Gln/Gln in girls XRCC1 codon 399-Gln + CYP2E1*5B-*6 XRCC1 codon 399-Gln +CYP2E1*5B-*6 in girls |
1.60 (1.00–2.40) 2.10 (1.10–3.90) 3.70 (NA) 17.40 (1.90–153.70) |
0.040 0.020 0.049 0.001 |
Tumer, 2010 | [75] | |
| Asia | India | ALL | 117/117 | ≤14 | XRCC1 |
XRCC1 codon 399-Gln/Gln XRCC1 codon 399-Arg/Gln XRCC1 codons 194-Trp + 399Gln |
2.42 (1.00–5.89) 1.90 (1.08–3.35) 4.41 (1.83–10.61) |
0.050 0.030 0.009 |
Joseph, 2005 | [71] |
| China | ALL | 183/190 | ≤18 | ERCC1 |
ERCC1 8092-CC ERCC1 8092-CC in boys ERCC1 19007-GG in boys ERCC1 8092-CC in children <8 years of age |
1.61 (1.03–2.50) 1.94 (1.09–3.41) 2.36 (1.05–5.27) 1.87 (1.04–3.37) |
0.030 0.020 0.040 0.040 |
Wang, 2006 | [80] | |
| China | ALL | 415/511 | 1–18 | OGG1 | OGG1 326-Ser/Ser +Ser/Cys | 0.66 (0.49–0.88) | 0.005 | Li, 2011 | [79] | |
| Thailand | ALL | 108/317 | ≤14 | XRCC1,ERCC2 |
XRCC1 codon 194-Trp/Trp XRCC1 codon 399-Arg/Gln +Gln/Gln XRCC1 haplotype B(194Trp-280Arg-399Arg) XRCC1 haplotype C(194Arg-280Arg-399Gln) |
0.22 (0.05–0.96) 2.18 (1.39–3.42) 0.62 (0.42–0.90) 1.59 (1.14–2.23) |
0.030 0.001 0.010 0.008 |
Pakakasama, 2007 | [73] | |
| Taiwan | ALL, AML | 266/266 | <18 | XRCC4 |
XRCC4 rs6869366rs28360071: TT/DD XRCC4 rs6869366rs28360071: GT/II XRCC4 rs6869366rs28360071: GT/ID XRCC4 rs6869366rs28360071: GT/DD |
2.82 (1.03–7.70) 2.16 (1.29–3.61) 2.26 (1.22–4.17) 4.94 (1.01–24.27) |
0.048 0.003 0.009 0.040 |
Wu, 2010 | [81] | |
| North America | Canada | ALL | 287/320 | med. 5 | MLH1, MSH3(+ GSTM1, CYP1A1, CYP2E1, NQO1, NAT2) |
MLH1 219-Ile/Ile + CYP2E1*5 (–/+, +/+) MLH1 219-Ile/Ile + GSTM1-null + CYP1A1*2A (–/+, +/+) |
15.80 (2.00–122.60) 6.00 (1.90–18.90) |
<0.001 0.002 |
Mathonnet, 2003 | [78] |
| Mexico(Hispanics) | ALL | 120/120 | ≤14 | XRCC1 |
XRCC1 haplotype B(194Trp-280Arg-399Arg) XRCC1 haplotype B(194Trp-280Arg-399Arg) in boys |
1.95 (1.13–3.37) 2.65 (1.25–5.63) |
0.016 0.010 |
Meza-Espinoza, 2009 | [72] | |
| United States (Caucasians) | ALL | 163/251 | <21 | MLH1, MSH2, MSH3 | No significant associations were found. | – | – | Beuten, 2011 | [21] | |
| United States (Hispanics/non-Hispanics) | ALL | 335/490 | mean 5.5 mean 5.6 | Haplotypes of 32 genes(21 genes related to DNA repair systemsa) |
ERCC2 (rs3916874,rs238416, rs171140) GAA APEX1 (rs11160711, rs3120073) AA in non-Hispanics BRCA2 (rs4942448,rs9943876) GA in non-Hispanics RAD51 (rs2304579,rs7177265, rs2304580) AAA in Hispanics RAD51 (rs2304579,rs7177265, rs2304580) AGA in Hispanics NBN (rs12680687, rs6470522, rs7840099, rs1805812,rs709816) rare haplotypes in ALL with t (12; 21) XRCC4 (rs7711825, rs1193695, rs301276, rs301287, rs3777018)CGAGA in ALL with t (12; 21) XRCC4 (rs7711825, rs1193695, rs301276, rs301287, rs3777018) CGGGA in ALL with t (12; 21) XRCC4 (rs1193695, rs301276, rs301287) GAG in ALL with any structural change XRCC4 (rs1193695, rs301276, rs301287) GGGin ALL with any structural change |
0.59 (0.38–0.91) 1.90 (1.25–2.89) 1.77 (1.10–2.85) 1.55 (1.01–2.42) 1.51 (1.01–2.26) 0.38 (0.16–0.88) 0.56 (0.31–1.00) 0.39 (0.16–0.95) 0.60 (0.42–0.86) 0.55 (0.35–0.88) |
0.018 0.003 0.020 0.050 0.040 0.025 0.050 0.039 0.006 0.012 |
Chokkalingam, 2011 | [70] | |
| South America | Brazil (Whites/non-Whites) | ALL | 206/364 | 0.3–18 | XRCC1, ERCC2 (+ TYMS) |
TYMS 2R/3R; 3R/3R + XRCC1 194-Arg/Arg + XRCC1 399-Arg/Gln; Gln/Gln TYMS 2R/3R; 3R/3R + XRCC1 194-Arg/Arg + XRCC1 399-Arg/Gln; Gln/Gln + ERCC2 751-Lys/Gln; Gln/Gln |
0.25 (0.08–0.76) 0.25 (0.08–0.76) |
0.005 0.005 |
Canalle, 2011 | [68] |
med.: median of age. ALL: acute lymphoblastic leukaemia. AML: acute myeloid leukaemia. OR: odds ratio. 95%CI: 95% confidence interval.
APEX1, MUTYH, UNG2, XRCC1, ERCC2, LIG4, PRKDC, XRCC4, XRCC5, XRCC6, BRCA1, BRCA2, MRE11, NBN, RAD50, RAD51, RAD54B, RAD54L, XRCC2, XRCC3 and MGMT.
Nine case-control publications [67–75] addressed polymorphisms of XRCC1 (Arg194Trp, Arg280His, and Arg399Gln), which encodes a protein involved in BER pathway. Seven of them (77.8%) have shown significant associations with childhood ALL. XRCC1 194Trp allele was related to increased risk among Turkish girls [67], Indians [71], and Mexicans [72], while it was related to protection among Thai [73]. No publication showed association of 280His allele with childhood ALL. XRCC1 399Gln allele was related to increased risk for ALL among Indians [71], Thai [73], Turkish [75], and Poles [74]. However, when combined with XRCC1 194Arg wild-type allele and the variant alleles ERCC2 751Gln and TYMS 3R, the XRCC1 399Gln allele was related to protection for ALL among Brazilians [68].
The ERCC2 gene encodes a DNA helicase involved in NER pathway, and its polymorphisms (Asp312Asn and Lys751Gln), were explored in five case-control publications [67–70, 73]. No one was able to show an independent association with leukaemia risk. ERCC2 751Gln allele was related to protection for ALL among Brazilians when combined with XRCC1 399Gln-194Trp and TYMS 3R [68]. The haplotype GAA (rs3916874, rs238416, rs171140) was also associated with protection for ALL among north-Americans [70].
Genetic polymorphisms of NBN, which is involved in DNA repair by HR, were reported by three publications [70, 76, 77]. Two of them showed that 657del5 mutation was related to increased risk for ALL among Poles [76, 77]. Recently, five SNPs of NBN gene (rs12680687, rs6470522, rs7840099, rs1805812, rs709816) were associated with protection for a subset of Bcp-ALL in north-Americans [70], reinforcing that the interaction of multiple polymorphisms can influence paediatric leukaemia risk.
Polymorphisms of two genes involved in MMR, MLH1, and MSH3, were explored in two papers [21, 78], but both failed to demonstrate any independent association with childhood ALL. However, the combination of MLH1 219-Ile/Ile with genetic variants of CYP2E1 or GSTM1 and CYP1A1 increased the risk for ALL [78]. Other genotyping studies of OGG1 [74, 79], MUTYH [74], ERCC1 [80], XRCC4 [70, 81], APEX1, BRCA2, and RAD51 [70] found increased associations with childhood ALL.
Regulatory genes and GWAS
To date, the majority of publications regarding regulatory genes and childhood leukaemia were performed to validate GWAS results. The main investigated gene polymorphisms are summarised in Table 3. The majority of publications focused only in ALL (87.5%) and is from North America, followed by Asians (25%), and Europeans (25%).
Table 3. Genetic susceptibility publications in childhood leukaemia involving genes related to cell cycle regulation, signaling, proliferation and differentiation.
| Continent | Country | Leukaemia subtype | Number of cases/controls | Age of cases(years) | Investigate dgenes | Significant results | First author, year | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Genetic variation | OR (95%CI) | P-value | ||||||||
| Europe | United Kingdom | ALL | 114/414 | ≤14 | TP53, MDM2, and others(DAXX, BAT3, LTA, DDR1, IER3) |
TP53 codon 72-Arg/Pro +Pro/Pro BAT3 rs805303 BAT3 rs2077102 DAXX rs2239839-rs1059231rs2073524 |
1.67 (1.21–2.30) 0.68 (0.49–0.95) 0.62 (0.39–0.99) 2.45 (1.22–4.91) |
0.002 0.020 0.040 0.010 |
Do, 2009 | [83] |
| Germany/United Kingdom | Pre-B ALL | 1384/1877 | mean 6 | IKZF1, ARID5B, CEBPE |
IKZF1 rs4132601-AC IKZF1 rs4132601-CC ARID5B rs7089424-AC ARID5B rs7089424-CC CEBPE rs2239633-GG |
1.80 (1.50–2.00) 2.80 (2.20–3.60) 1.80 (1.50–2.10) 3.20 (2.60–4.00) 1.60 (1.30–1.90) |
<0.001 <0.001 <0.001 <0.001 <0.001 |
Prasad, 2010 | [95] | |
| Poland | ALL | 398/731 | med. 4,9 | IKZF1, ARID5B, CEBPE, CDKN2A |
IKZF1 rs4132601-G ARID5B rs7089424-G |
1.34 (1.11–1.61) 1.33 (1.10–1.61) |
0.002 0.003 |
Pastorczak, 2011 | [77] | |
| Hungary | ALL | 543/529 | 1–15 | 16 genesa |
ARID5B rs10821936 in B-ALL ARID5B rs7089424 in B-ALL ARID5B rs4506592 in B-ALL IKZF1 rs6964969 in B-ALL IKZF1 rs11978267 in B-ALL IKZF1 rs4132601 in B-ALL STAT3 rs3816769 in hyperdiploid ALL STAT3 rs12949918 in hyperdiploid ALL |
1.53 (1.26–1.85) 1.52 (1.25–1.84) 1.51 (1.24–1.83) 1.70 (1.40–2.08) 1.68 (1.38–2.05) 1.69 (1.38–2.06) 0.62 (0.49–0.79) 0.64 (0.50–0.81) |
<0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 |
Lautner-Csorba, 2012 | [43] | |
| Asia | Israel | T-ALL | 39/200 | NA | ATMTP53 |
ATM-T1229C, T1744C, T4388G ATM-C103T, -30del215, 2284delCT |
4.90 (1.20–18.20) 12.90(2.50–42.70) |
0.030 0.004 |
Liberzon, 2004 | [84] |
| China | ALL | 183/190 | mean 9.32 | CCND1 | CCND1 870AA versus. AG+GG | 3.29 (1.99–9.02) | 0.021 | Hou, 2005 | [82] | |
| China | ALL | 570/673 | 1–18 | TERT |
TERT rs2735940-TT TERT rs2853676-AG TERT rs2736100-CC TERT rs10069690-AA TERT rs4246742-TA |
1.38 (1.00–1.90) 1.36 (1.06–1.74) 1.56 (1.11–2.21) 2.00 (1.03–3.88) 0.78 (0.61–1.00) |
0.034 0.010 0.006 0.032 0.029 |
Sheng, 2013 | [85] | |
| Thailand | ALL | 190/182 | mean 6.0 | IKZF1, ARID5B, CEBPE, CDKN2A |
IKZF1 rs4132601-C ARID5B rs10821938-C inpre-B ALL |
1.57 (1.01–2.44) 0.73 (0.55–0.97) |
0.040 0.030 |
Vijayakrishnan, 2010 | [97] | |
| North America | Canada | Pre-B ALL | 240/277 | 0.4–18 | CDKN2A, CDKN2B, CDKN1A, CDKN1B |
CDKN2A -222-A CDKN2A -222-TA CDKN2B -593-T CDKN2B -1270/-593/-287CTG haplotype CDKN2B -1270/-593/-287CAG haplotype CDKN1B -1608-GA |
2.20 (1.20–4.00) 2.60 (1.10–4.30) 0.70 (0.60–1.00) 0.80 (0.60–1.00) 1.70 (1.20–2.40) 1.70 (1.00–2.80) |
0.008 0.010 0.020 0.040 0.004 0.030 |
Healy, 2007 | [16] |
| Canada | Pre-B ALL | 284/270 | med. 4.2 | ARID5B |
ARID5B rs7073837-AA ARID5B rs10994982-AA ARID5B rs10740055-CC ARID5B rs10821936-CC ARID5B rs7089424-CC ARID5B-AACCG haplotype |
2.37 (1.45–3.85) 2.29 (1.42–3.69) 2.76 (1.68–4.53) 3.11 (1.90–5.10) 3.11 (1.89–5.12) 1.93 (1.47–2.53) |
<0.001 <0.001 <0.001 <0.001 <0.001 <0.001 |
Healy, 2010 | [94] | |
| United States (Caucasians) | AML | 432/496 | <21 | MDM2 | MDM2 309-GG | 1.50 (1.03–2.20) | NA | Phillips, 2010 | [86] | |
| United States (Caucasians) | ALL | 163/251 | <21 | 23 genesb |
LMO1 rs442264 LMO1 rs442264 in B-ALL |
1.90 (1.41–2.56) 1.98 (1.44–2.73) |
<0.001 <0.001 |
Beuten, 2011 | [21] | |
| United States (Hispanics/non-Hispanics) | B-ALL | 203/414 | mean6.8/6.0 | MLL, CREBBP, TOP2A,EP300 |
MLL rs525549-AA versus AT/TT in Hispanics MLL rs6589664-GG versusAG/AA in Hispanics MLL rs6589664-AA versus AG/GG in Whites EP300 rs5758222-GG/AG versus AA in Hispanics EP300 rs7286979-AA/AG versus GG in Hispanics EP300 rs20551-AA/AG versus GG in Hispanics MLL-EP300 AG-GAA haplotype |
2.56 (1.02–6.41) 2.49 (1.23–5.04) 0.39 (0.17–0.91) 2.79 (1.46–5.32) 2.67 (1.40–5.07) 2.79 (1.26–6.18) 5.68(2.82–11.44) |
0.044 0.011 0.029 0.002 0.003 0.011 <0.001 |
Piwkham, 2011 | [87] | |
| United States (Hispanics/non-Hispanics) | ALL | 335/490 | mean 5.5/5.6 | Haplotypes of 32 genes(8 genes related to cell cycle and to poisomerase c) |
CDKN2A (rs3731257rs2518719) GG in hyperdiploid AL CDKN2A (rs3731257rs2518719) AA and GG in AL with any numerical ploidy change |
0.30 (0.14–0.64) 0.67 (0.50–0.90) 0.44 (0.26–0.73) |
0.0020.0080.001 | Chokkalingam, 2011 | [70] | |
| United States (Hispanics/non-Hispanics) | Pre-B ALL | 1308/1587 | <21 | ARID5B |
ARID5B rs10821936 in non-Hispanics ARID5B rs10821936 in Hispanics ARID5B rs7915732 in Hispanics |
2.13 (1.77–2.58) 1.92 (1.50–2.45) 2.58 (1.27–3.52) |
<0.001 <0.001 0.009 |
Xu, 2012 | [98] | |
| United States (non-Hispanics) | ALL, AML | 171/384 | <1 | IKZF1, ARID5B, CEBPE |
IKZF1 rs11978267-GG ARID5B rs10821936-C allele in ALL MLL- ARID5B rs10994982-A allele in AML MLL+ |
2.30 (1.30–4.20) 2.80 (1.60–5.00) 0.50 (0.30–0.90) |
<0.050 <0.050 <0.050 |
Ross, 2013 | [96] | |
med.: median of age.
MLL+: positive for MLL gene rearrangement.
MLL–: negative for MLL gene rearrangement.
AL: acute leukaemia.
ALL: acute lymphoblastic leukaemia.
AML: acute myeloid leukaemia.
OR: odds ratio. 95% CI: 95% confidence interval.
NA: not available.
ARID5B, BAX, BCL2A, BCL2B, CCR5, CEBPA, CEBPE, IKZF1, JAK1, JAK3, NOTCH1, STAT1, STAT3, STAT5A, STAT5B, STAT6.
BCR, ABL1, ETV6, FBXW7, LMO1, LYL1, EP300, CREBBP, MLL, JAK2, RUNX1, TCF3, CHEK2, ATM, CCND1, TOP2A, CDKN1B, IKZF1, NR3C1, TP53, BLNK, CD6, SAMSN1.
TP53, TP53BP1, CCND1, CDKN2A (p16), CDKN2B (p15), TOP1, TOP2A, TOP2B.
Prior to GWAS publications, a few genetic polymorphisms of regulatory genes were investigated by candidate gene approach. CCND1 870AA genotype (homozygous wild-type) was related to increased risk for ALL among Chinese [82]. Polymorphisms in promoter regions of genes CDKN2A (T-222A), CDKN2B (C-1270T, A-593T, C-287G) and CDKN1B (G-1608A) were also associated with childhood pre-B ALL risk among Canadians [16]. Furthermore, the Arg72Pro polymorphism in TP53 gene was associated with increased risk for ALL among children from the United Kingdom [83]. Polymorphisms of other genes were also related to childhood acute leukaemia (CAL) risk, like BAT3 and DAXX [83], ATM [84], TERT [85], MDM2 [86], LMO1 [21], MLL and EP300 [87], but the associations need to be replicated in further studies.
The first two GWAS regarding genetic susceptibility to childhood leukaemia were published in 2009, which observed that SNPs in regions 7p12.2 (IKZF1 rs4132601, rs11978267), 10q21.2 (ARID5B rs7089424, rs10821936, rs10994982), and 14q11.2 (CEBPE rs2239633) were associated with childhood ALL risk, specifically with B-cell acute lymphoblastic leukaemia (B-ALL), and hyperdiploid subsets, with ORs ranging from 1.34 to 1.91, and P <10–7 [88, 89]. In subsequent analysis, the association of CDKN2A rs3731217 (T allele) with protection for ALL (OR 0.71, P = 3.01 x 10–11) was validated [90], and ARID5B rs10821936 was associated with ALL among Blacks (OR 2.08, P = 0.0015), mainly hyperdiploid B-ALL (OR 6.62, P <0.001) in the US [91]. Also, it was demonstrated that 24% of the total variation in B-cell precursor acute lymphoblastic leukaemia (Bcp-ALL) risk is accounted for common genetic variation, which supports for a polygenic basis for susceptibility to Bcp-ALL [92]. French studies have shown similar associations regarding IKZF1 and ARID5B, and also found associations between CDKN2A rs3731217 (OR 0.8) and CEBPE rs2239633 (OR 0.9) with ALL risk [93].
Since then, other research groups have aimed to replicate the risk associations previously identified by the GWAS. As shown in Table 3, seven papers confirmed ARID5B rs10821936 association with increased ALL risk, mainly B-ALL [43, 77, 94–98]. From six publications that explored IKZF1 SNPs [21, 43, 77, 95–97], five of them confirmed the risk associations for ALL. CDKN2A and CEBPE SNPs associations were replicated by one study respectively [70, 95].
SNPs in other genes were also identified as risk variants for ALL by other GWAS, but replication of these results are still needed: HAO1 (rs6140264, OR 8.84), EPB41L2 (rs9388856, rs9388857, rs1360756, OR 8.97), C2orf3 (rs12105972, OR 0.13), and MAN2A1 (rs3776932, OR 0.11) were associated with ALL risk among Koreans (P = 0.0001) [99]; TP63 (rs17505102, OR 0.63, P = 4.87 x 10–7), PTPRJ (rs3942852, OR 0.77, P = 2.54 x 10–4), and EPOR (rs4804164, OR 0.58, P = 0.008; rs317913, OR 0.60, P = 0.019) were associated with ETV6/RUNX1 ALL risk among Europeans [100, 101].
Gene-environment interaction
Few publications addressed the interaction between gene polymorphisms, environmental exposures, and childhood leukaemia. Studies performed in children exposed directly and/or indirectly throughout maternal exposures are scarce. To date, environmental exposures that have been explored were smoking (tobacco exposure), pesticides, insecticides, trihalomethanes (chlorination by-products of drinking water) alcohol consumption, paint use and x-rays. The absence of CYP1A1 CGACC haplotype, consisting of five SNPs (-T1761C, -G9893A, Ex7+A131G, C1188T, C11599G), was associated with increased risk for ALL among children with father’s smoking or at least one smoker at home [28]. Also, haplotypes of CYP2C8 and MDR1 (ABCB1) were related to increased risk for ALL when associated with paint use and indoor insecticides, respectively among Hispanics living in the US [24]. Another finding was that children exposed to indoor insecticides carrying MDR1 haplotype CGC (C1236T, G2677T/A, C3435T) presented a lower risk for ALL [64], indicating that pesticides’ toxic effects may be influenced by efflux through P-glycoprotein complex.
Six publications [102–107] estimated risk associations by calculating IOR in a case-only cohort. It was observed that CYP1A1*2A/*2B increased the risk for ALL by five-fold among children exposed to pesticides during maternal pregnancy and childhood, while CYP1A1*2B was related to a protective effect among children with mothers who had smoked 1–20 cigarettes during the first trimester of pregnancy (IOR = 0.1; IC95% 0.01–0.9) and with fathers who had smoked >20 cigarettes between birth and diagnosis (IOR 0.2; IC95% 0.04–0.9) [102, 103]. Also, it was observed that among children exposed to higher levels of trihalomethanes in drinking water, the risk for ALL was increased in the presence of the polymorphic variant CYP2E1*5 (IOR 9.75; IC95% 1.10–86.01), and GSTT1 deletion (IOR 9.13; IC95% 1.44–57.82), in pre and post-natal periods, respectively [104]. GSTM1-null genotype and CYP2E1*5 variant were related to increased risk for ALL among children with mothers who had consumed alcoholic beverages during the third trimester of pregnancy (IOR 2.4; IC95% 1.1–5.4) and nursing period (IOR 4.9; IC95% 1.4–16.6), respectively [105]. Thus, it is notable that variations in xenobiotic metabolism resulted from genetic polymorphisms can modulate childhood leukaemia risk.
Concerning DNA repair genes, it was observed that variants of APEX1 (Asp148Glu) and MLH1 (Ile219Val) were associated with a protective effect for ALL among girls exposed to x-rays (one or more exposures) during postnatal period (MLH1 IOR 0.2; IC95% 0.1–0.8; and APEX1 IOR 0.1; IC95% 0.0–0.7) [106, 107]. Also, the protective effect for ALL of XRCC4 GGG haplotype, consisting of SNPs rs1193695, rs301276, and rs301287, was modulated by number of postnatal x-rays (P = 0.027) [70]. Again, cancer susceptibility resulted from the interaction of environmental exposure and genetic polymorphism, which highlights the multifactorial aetiology of paediatric leukaemia.
Conclusions
Great scientific advances in the understanding of paediatric leukaemia have been made. Unlike the adult, who usually develops cancer because of the cumulative effect of environmental exposures during his life, the child, which manifests leukaemia with a short latency period, does not have enough exposure time to allow the initiation of a long carcinogenic process. Thus, genetic susceptibility may play an important role in modulating environmental exposures’ effects.
This systematic review gathered publications up to 2013 and was an attempt to overview the risk associations between several gene polymorphisms and paediatric AL. It was possible to collect from the selected studies significant amount of data, which is considered to be a fair representation of international scientific literature on this subject. The vast majority of studies so far focused on evaluating the magnitude of risk of genetic polymorphisms in ALL, mainly because Bcp-ALL is the most frequent type of leukaemia in children. In this context, we also realised that there is still a great need for further investigations on the risk factors for paediatric AML.
Regarding the xenobiotic system, gene polymorphisms of CYP2E1, GSTM1, NQO1, NAT2, and ABCB1 (MDR1) were more frequently associated with childhood leukaemia risk, which also showed interaction effect with environmental exposures such as paints, household pesticides, insecticides, smoking, alcohol, and trihalomethanes. Gene polymorphisms related to DNA repair have been little investigated in paediatric leukaemia, maybe because of its association with genetic diseases. However, it was noticed that XRCC1 polymorphisms play an important role in the development of ALL, and postnatal exposure to x-rays can modulate leukaemia risk in the presence of APEX1, MLH1, and XRCC4 gene variants. While interpreting these results, one has to consider that fetuses and infants are naturally more affected than adults by a variety of environmental toxicants, mainly because of differential exposure and physiologic immaturity, which makes them more susceptible to suffer from DNA damage and less capable of detoxifying carcinogenic compounds [108]. And so, genetic polymorphisms involving xenobiotic and DNA repair systems have a major role in modulating the effects of environmental agents in children.
Some limitations were observed in the studies that might make the consolidation of scientific evidence difficult, such as: 1) relatively small number of cases, making it difficult to obtain statistically significant results; 2) ethnic and racial differences between populations, which are reflected in distinct polymorphic allele frequencies and patterns of exposure to environmental agents; 3) little information regarding the effect of gene polymorphisms on the encoded protein; 4) and few considerations about gene–environment interactions. Also, we could observe that the majority of associations provided low risk estimates (OR <2.0), which showed that gene polymorphisms are of low penetrance, and conceptually, are minor parts of multifactorial pathways to childhood leukaemia [6].
More recently, GWAS have identified new gene polymorphisms potentially related to paediatric ALL, particularly involving IKZF1 and ARID5B, which were subsequently replicated in independent studies. As a result, we see genetic susceptibility clearly contributes to childhood leukaemia risk, mainly through gene–gene and gene–environment interactions. Further studies are still needed to confirm the observed associations in different populations and to characterise environmental agents as risk factors for childhood leukaemia.
Conflicts of interest
The authors declare no conflict of interest.
Authors’ contributions
The study was designed by GDB, LRA, and MSPO. The literature search and data analysis were carried out by GDB, with the supervision of LRA and MSPO. The manuscript was prepared by GDB, LRA, and MSPO.
References
- 1.Greaves M. Childhood leukaemia. BMJ. 2002;324(7332):283–7. doi: 10.1136/bmj.324.7332.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullighan CG, Flotho C, Downing JR. Genomic assessment of pediatric acute leukemia. Cancer J. 2005;11(4):268–82. doi: 10.1097/00130404-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Wiemels J. Perspectives on the causes of childhood leukemia. Chem Biol Interact. 2012;196(3):59–67. doi: 10.1016/j.cbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greaves M. Molecular genetics, natural history and the demise of childhood leukaemia. Eur J Cancer. 1999;35(14):1941–53. doi: 10.1016/S0959-8049(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 5.Greaves M. In utero origins of childhood leukaemia. Early Hum Dev. 2005;81(1):123–9. doi: 10.1016/j.earlhumdev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Chokkalingam AP, Buffler PA. Genetic susceptibility to childhood leukaemia. Radiation protection dosimetry. 2008;132(2):119–29. doi: 10.1093/rpd/ncn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. [14 August 2014]. Polymorphism [ http://ghr.nlm.nih.gov/glossary=polymorphism]
- 8.What are single nucleotide polymorphisms (SNPs)? [14 August 2014]. [ http://ghr.nlm.nih.gov/handbook/genomicresearch/snp]
- 9.Belson M, Kingsley B, Holmes A. Risk factors for acute leukemia in children: a review. Environ Health Perspect. 2007;115(1):138–45. doi: 10.1289/ehp.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinnett D, Krajinovic M, Labuda D. Genetic susceptibility to childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2000;38(5–6):447–62. doi: 10.3109/10428190009059264. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, et al. X-ray repair cross-complementing group 1 (XRCC1) genetic polymorphisms and risk of childhood acute lymphoblastic leukemia: a meta-analysis. PloS one. 2012;7(4):e34897. doi: 10.1371/journal.pone.0034897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronen A, Glickman BW. Human DNA repair genes. Environ Mol Mutagen. 2001;37(3):241–83. doi: 10.1002/em.1033. [DOI] [PubMed] [Google Scholar]
- 13.Morita R, et al. Molecular mechanisms of the whole DNA repair system: a comparison of bacterial and eukaryotic systems. J Nucleic Acids. 2010;2010:179594. doi: 10.4061/2010/179594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seif AE. Pediatric leukemia predisposition syndromes: clues to understanding leukemogenesis. Cancer Genet. 2011;204(5):227–44. doi: 10.1016/j.cancergen.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1(3):222–31. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 16.Healy J, et al. Promoter SNPs in G1/S checkpoint regulators and their impact on the susceptibility to childhood leukemia. Blood. 2007;109(2):683–92. doi: 10.1182/blood-2006-02-003236. [DOI] [PubMed] [Google Scholar]
- 17.Khoury MJ, et al. Transforming epidemiology for 21st century medicine and public health. Cancer Epidemiol Biomarkers Prev. 2013;22(4):508–16. doi: 10.1158/1055-9965.EPI-13-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aydin-Sayitoglu M, et al. Role of CYP2D6, CYP1A1, CYP2E1, GSTT1, and GSTM1 genes in the susceptibility to acute leukemias. Am J Hematol. 2006;81(3):162–70. doi: 10.1002/ajh.20434. [DOI] [PubMed] [Google Scholar]
- 20.Balta G, et al. Characterization of MTHFR, GSTM1, GSTT1, GSTP1, and CYP1A1 genotypes in childhood acute leukemia. Am J Hematol. 2003;73(3):154–60. doi: 10.1002/ajh.10339. [DOI] [PubMed] [Google Scholar]
- 21.Beuten J, et al. Candidate gene association analysis of acute lymphoblastic leukemia identifies new susceptibility locus at 11p15 (LMO1) Carcinogenesis. 2011;32(9):1349–53. doi: 10.1093/carcin/bgr091. [DOI] [PubMed] [Google Scholar]
- 22.Bonaventure A, et al. Maternal smoking during pregnancy, genetic polymorphisms of metabolic enzymes, and childhood acute leukemia: the ESCALE study (SFCE) Cancer Causes Control. 2012;23(2):329–45. doi: 10.1007/s10552-011-9882-9. [DOI] [PubMed] [Google Scholar]
- 23.Canalle R, et al. Genetic polymorphisms and susceptibility to childhood acute lymphoblastic leukemia. Environ Mol Mutagen. 2004;43(2):100–9. doi: 10.1002/em.20003. [DOI] [PubMed] [Google Scholar]
- 24.Chokkalingam AP, et al. Variation in xenobiotic transport and metabolism genes, household chemical exposures, and risk of childhood acute lymphoblastic leukemia. Cancer Causes Control. 2012;23(8):1367–75. doi: 10.1007/s10552-012-9947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gra OA, et al. [Genetic polymorphism in GST, NAT2, and MTRR and susceptibility to childhood acute leukemia] Molekuliarnaia biologiia. 2008;42(2):214–25. [PubMed] [Google Scholar]
- 26.Joseph T, et al. Genetic polymorphism of CYP1A1, CYP2D6, GSTM1 and GSTT1 and susceptibility to acute lymphoblastic leukaemia in Indian children. Pediatr Blood Cancer. 2004;43(5):560–7. doi: 10.1002/pbc.20074. [DOI] [PubMed] [Google Scholar]
- 27.Krajinovic M, et al. Susceptibility to childhood acute lymphoblastic leukemia: influence of CYP1A1, CYP2D6, GSTM1, and GSTT1 genetic polymorphisms. Blood. 1999;93(5):1496–501. [PubMed] [Google Scholar]
- 28.Lee KM, et al. Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res. 2009;33(2):250–8. doi: 10.1016/j.leukres.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pakakasama S, et al. Polymorphisms of drug-metabolizing enzymes and risk of childhood acute lymphoblastic leukemia. Am J Hematol. 2005;79(3):202–5. doi: 10.1002/ajh.20404. [DOI] [PubMed] [Google Scholar]
- 30.Razmkhah F, et al. Frequency of CYP1A1*2C Polymorphism in Patients with Leukemia in the Iranian Population. Lab Med. 2011;42:220–3. doi: 10.1309/LM337JWOSVNEHPUI. [DOI] [Google Scholar]
- 31.Swinney RM, et al. Polymorphisms in CYP1A1 and ethnic-specific susceptibility to acute lymphoblastic leukemia in children. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1537–42. doi: 10.1158/1055-9965.EPI-10-1265. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguti GG, et al. Increased risk for acute lymphoblastic leukemia in children with cytochrome P450A1 (CYP1A1)- and NAD(P)H:quinone oxidoreductase 1 (NQO1)-inherited gene variants. Acta Haematol. 2010;124(3):182–4. doi: 10.1159/000320275. [DOI] [PubMed] [Google Scholar]
- 33.Krajinovic M, et al. Role of NQO1, MPO and CYP2E1 genetic polymorphisms in the susceptibility to childhood acute lymphoblastic leukemia. Int J Cancer. 2002;97(2):230–6. doi: 10.1002/ijc.1589. [DOI] [PubMed] [Google Scholar]
- 34.Ulusoy G, et al. Significance of genetic polymorphisms at multiple loci of CYP2E1 in the risk of development of childhood acute lymphoblastic leukemia. Oncology. 2007;72(1–2):125–31. doi: 10.1159/000111131. [DOI] [PubMed] [Google Scholar]
- 35.Silveira Vda S, et al. Role of the CYP2D6, EPHX1, MPO, and NQO1 genes in the susceptibility to acute lymphoblastic leukemia in Brazilian children. Environ Mol Mutagen. 2010;51:48–56. doi: 10.1002/em.20510. [DOI] [PubMed] [Google Scholar]
- 36.Borst L, et al. The impact of CYP3A5*3 on risk and prognosis in childhood acute lymphoblastic leukemia. Eur J Haematol. 2011;86(6):477–83. doi: 10.1111/j.1600-0609.2011.01608.x. [DOI] [PubMed] [Google Scholar]
- 37.Silveira VS, et al. CYP3A5 and NAT2 gene polymorphisms: role in childhood acute lymphoblastic leukemia risk and treatment outcome. Mol Cell Biochem. 2012;364(1–2):217–23. doi: 10.1007/s11010-011-1220-8. [DOI] [PubMed] [Google Scholar]
- 38.Chan JY, et al. Xenobiotic and folate pathway gene polymorphisms and risk of childhood acute lymphoblastic leukaemia in Javanese children. Hematol Oncol. 2011;29(3):116–23. doi: 10.1002/hon.965. [DOI] [PubMed] [Google Scholar]
- 39.De Aguiar Goncalves BA, et al. NQO1 rs1800566 (C609T), PON1 rs662 (Q192R), and PON1 rs854560 (L55M) polymorphisms segregate the risk of childhood acute leukemias according to age range distribution. Cancer Causes Control. 2012;23(11):1811–9. doi: 10.1007/s10552-012-0060-5. [DOI] [PubMed] [Google Scholar]
- 40.Eguchi-Ishimae M, et al. The association of a distinctive allele of NAD(P)H:quinone oxidoreductase with pediatric acute lymphoblastic leukemias with MLL fusion genes in Japan. Haematologica. 2005;90(11):1511–5. [PubMed] [Google Scholar]
- 41.Kracht T, et al. NQO1 C609T polymorphism in distinct entities of pediatric hematologic neoplasms. Haematologica. 2004;89(12):1492–7. [PubMed] [Google Scholar]
- 42.Lanciotti M, et al. Genetic polymorphism of NAD(P)H:quinone oxidoreductase is associated with an increased risk of infant acute lymphoblastic leukemia without MLL gene rearrangements. Leukemia. 2005;19(2):214–6. doi: 10.1038/sj.leu.2403613. [DOI] [PubMed] [Google Scholar]
- 43.Lautner-Csorba O, et al. Candidate gene association study in pediatric acute lymphoblastic leukemia evaluated by Bayesian network based Bayesian multilevel analysis of relevance. BMC Med Genomics. 2012;5:42. doi: 10.1186/1755-8794-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rimando MG, et al. Prevalence of GSTT1, GSTM1 and NQO1 (609C > T) in Filipino children with ALL (acute lymphoblastic leukaemia) Biosci Rep. 2008;28:117–24. doi: 10.1042/BSR20070010. [DOI] [PubMed] [Google Scholar]
- 45.Sirma S, Agaoglu L, Yildiz I, Cayli D, Horgusluoglu E, Anak S, et al. NAD(P)H:quinone oxidoreductase 1 null genotype is not associated with pediatric de novo acute leukemia. Pediatr Blood Cancer. 2004;43(5):568–70. doi: 10.1002/pbc.20098. [DOI] [PubMed] [Google Scholar]
- 46.Smith MT, et al. Low NAD(P)H:quinone oxidoreductase activity is associated with increased risk of leukemia with MLL translocations in infants and children. Blood. 2002;100(13):4590–3. doi: 10.1182/blood-2001-12-0264. [DOI] [PubMed] [Google Scholar]
- 47.Wiemels JL, et al. A lack of a functional NAD(P)H:quinone oxidoreductase allele is selectively associated with pediatric leukemias that have MLL fusions. Cancer Res. 1999;59(16):4095–9. [PubMed] [Google Scholar]
- 48.Yeoh AE, et al. Genetic susceptibility to childhood acute lymphoblastic leukemia shows protection in Malay boys: results from the Malaysia-Singapore ALL Study Group. Leuk Res. 2010;34(3):276–83. doi: 10.1016/j.leukres.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Tumer TB, Sahin G, Arinc E. Association between polymorphisms of EPHX1 and XRCC1 genes and the risk of childhood acute lymphoblastic leukemia. Arch Toxicol. 2012;86(3):431–9. doi: 10.1007/s00204-011-0760-8. [DOI] [PubMed] [Google Scholar]
- 50.Alves S, et al. The GSTM1 and GSTT1 genetic polymorphisms and susceptibility to acute lymphoblastic leukemia in children from north Portugal. Leukemia. 2002;16(8):1565–7. doi: 10.1038/sj.leu.2402543. [DOI] [PubMed] [Google Scholar]
- 51.Barnette P, et al. High-throughput detection of glutathione s-transferase polymorphic alleles in a pediatric cancer population. Cancer Epidemiol Biomarkers Prev. 2004;13(2):304–13. doi: 10.1158/1055-9965.EPI-03-0178. [DOI] [PubMed] [Google Scholar]
- 52.Chen CL, et al. Higher frequency of glutathione S-transferase deletions in black children with acute lymphoblastic leukemia. Blood. 1997;89(5):1701–7. [PubMed] [Google Scholar]
- 53.Klotz J, et al. Population-based retrieval of newborn dried blood spots for researching paediatric cancer susceptibility genes. Paediatr Perinat Epidemiol. 2006;20(5):449–52. doi: 10.1111/j.1365-3016.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- 54.Pigullo S, et al. Are genotypes of glutathione S-transferase superfamily a risk factor for childhood acute lymphoblastic leukemia? Results of an Italian case-control study. Leukemia. 2007;21(5):1122–4. doi: 10.1038/sj.leu.2404617. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, et al. Genetic polymorphisms analysis of glutathione S-transferase M1 and T1 in children with acute lymphoblastic leukemia. Journal of Huazhong Univ Sci Technolog Med Sci. 2004;24(3):243–4. doi: 10.1007/BF02832001. [DOI] [PubMed] [Google Scholar]
- 56.Gatedee J, et al. Glutathione S-transferase P1 genotypes, genetic susceptibility and outcome of therapy in thai childhood acute lymphoblastic leukemia. Asian Pac J Cancer Prev. 2007;8(2):294–6. [PubMed] [Google Scholar]
- 57.Krajinovic M, Labuda D, Sinnett D. Glutathione S-transferase P1 genetic polymorphisms and susceptibility to childhood acute lymphoblastic leukaemia. Pharmacogenetics. 2002;12(8):655–8. doi: 10.1097/00008571-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Krajinovic M, et al. Genetic polymorphisms of N-acetyltransferases 1 and 2 and gene-gene interaction in the susceptibility to childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev. 2000;9(6):557–62. [PubMed] [Google Scholar]
- 59.Zanrosso CW, et al. Genetic variability in N-acetyltransferase 2 gene determines susceptibility to childhood lymphoid or myeloid leukemia in Brazil. Leuk Lymphoma. 2012;53(2):323–7. doi: 10.3109/10428194.2011.619605. [DOI] [PubMed] [Google Scholar]
- 60.Zanrosso CW, et al. N-acetyltransferase 2 polymorphisms and susceptibility to infant leukemia with maternal exposure to dipyrone during pregnancy. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3037–43. doi: 10.1158/1055-9965.EPI-10-0508. [DOI] [PubMed] [Google Scholar]
- 61.Hattori H, et al. Regulatory polymorphisms of multidrug resistance 1 (MDR1) gene are associated with the development of childhood acute lymphoblastic leukemia. Leukemia Res. 2007;31(12):1633–40. doi: 10.1016/j.leukres.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 62.Jamroziak K, et al. Functional C3435T polymorphism of MDR1 gene: an impact on genetic susceptibility and clinical outcome of childhood acute lymphoblastic leukemia. Eur J Haematol. 2004;72(5):314–21. doi: 10.1111/j.1600-0609.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 63.Semsei AF, et al. Association of some rare haplotypes and genotype combinations in the MDR1 gene with childhood acute lymphoblastic leukaemia. Leuk Res. 2008;32(8):1214–20. doi: 10.1016/j.leukres.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 64.Urayama KY, et al. MDR1 gene variants, indoor insecticide exposure, and the risk of childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1172–7. doi: 10.1158/1055-9965.EPI-07-0007. [DOI] [PubMed] [Google Scholar]
- 65.Pongstaporn W, et al. Polymorphism of glutathione S-transferase Omega gene: association with risk of childhood acute lymphoblastic leukemia. J Cancer Res Clin Oncol. 2009;135(5):673–8. doi: 10.1007/s00432-008-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu CY, et al. Maternal and offspring genetic variants of AKR1C3 and the risk of childhood leukemia. Carcinogenesis. 2008;29(5):984–90. doi: 10.1093/carcin/bgn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Batar B, et al. DNA repair gene XPD and XRCC1 polymorphisms and the risk of childhood acute lymphoblastic leukemia. Leuk Res. 2009;33(6):759–63. doi: 10.1016/j.leukres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Canalle R, et al. Impact of thymidylate synthase promoter and DNA repair gene polymorphisms on susceptibility to childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2011;52(6):1118–26. doi: 10.3109/10428194.2011.559672. [DOI] [PubMed] [Google Scholar]
- 69.Celkan T, et al. The difference between pre-B cell acute lymphoblastic leukemia and Burkitt lymphoma in relation to DNA damage repair gene polymorphisms in childhood. Leuk Lymphoma. 2008;49(8):1638–40. doi: 10.1080/10428190802140063. [DOI] [PubMed] [Google Scholar]
- 70.Chokkalingam AP, et al. Haplotypes of DNA repair and cell cycle control genes, X-ray exposure, and risk of childhood acute lymphoblastic leukemia. Cancer Causes Control. 2011;22(12):1721–30. doi: 10.1007/s10552-011-9848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joseph T, et al. DNA repair gene XRCC1 polymorphisms in childhood acute lymphoblastic leukemia. Cancer Lett. 2005;217(1):17–24. doi: 10.1016/j.canlet.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 72.Meza-Espinoza JP, et al. XRCC1 polymorphisms and haplotypes in Mexican patients with acute lymphoblastic leukemia. Genet Mol Res. 2009;8(4):1451–8. doi: 10.4238/vol8-4gmr687. [DOI] [PubMed] [Google Scholar]
- 73.Pakakasama S, et al. Genetic polymorphisms and haplotypes of DNA repair genes in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;48(1):16–20. doi: 10.1002/pbc.20742. [DOI] [PubMed] [Google Scholar]
- 74.Stanczyk M, et al. The association of polymorphisms in DNA base excision repair genes XRCC1, OGG1 and MUTYH with the risk of childhood acute lymphoblastic leukemia. Mol Biol Rep. 2011;38(1):445–51. doi: 10.1007/s11033-010-0127-x. [DOI] [PubMed] [Google Scholar]
- 75.Tumer TB, et al. DNA repair XRCC1 Arg399Gln polymorphism alone, and in combination with CYP2E1 polymorphisms significantly contribute to the risk of development of childhood acute lymphoblastic leukemia. Leuk Res. 2010;34(10):1275–81. doi: 10.1016/j.leukres.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 76.Chrzanowska KH, et al. Carrier frequency of mutation 657del5 in the NBS1 gene in a population of Polish pediatric patients with sporadic lymphoid malignancies. Int J Cancer. 2006;118(5):1269–74. doi: 10.1002/ijc.21439. [DOI] [PubMed] [Google Scholar]
- 77.Pastorczak A, et al. Role of 657del5 NBN mutation and 7p12.2 (IKZF1), 9p21 (CDKN2A), 10q21.2 (ARID5B) and 14q11.2 (CEBPE) variation and risk of childhood ALL in the Polish population. Leuk Res. 2011;35(11):1534–6. doi: 10.1016/j.leukres.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 78.Mathonnet G, et al. Role of DNA mismatch repair genetic polymorphisms in the risk of childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;123(1):45–8. doi: 10.1046/j.1365-2141.2003.04551.x. [DOI] [PubMed] [Google Scholar]
- 79.Li Q, et al. hOGG1 Ser326Cys polymorphism and risk of childhood acute lymphoblastic leukemia in a Chinese population. Cancer Sci. 2011;102(6):1123–7. doi: 10.1111/j.1349-7006.2011.01928.x. [DOI] [PubMed] [Google Scholar]
- 80.Wang SL, et al. Polymorphisms in ERCC1 and susceptibility to childhood acute lymphoblastic leukemia in a Chinese population. Leuk Res. 2006;30(11):1341–5. doi: 10.1016/j.leukres.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 81.Wu KH, et al. Significant association of XRCC4 single nucleotide polymorphisms with childhood leukemia in Taiwan. Anticancer Res. 2010;30(2):529–33. [PubMed] [Google Scholar]
- 82.Hou X, et al. Cyclin D1 gene polymorphism and susceptibility to childhood acute lymphoblastic leukemia in a Chinese population. Int J Hematol. 2005;82(3):206–9. doi: 10.1532/IJH97.A10418. [DOI] [PubMed] [Google Scholar]
- 83.Do TN, et al. TP53 R72P and MDM2 SNP309 polymorphisms in modification of childhood acute lymphoblastic leukemia susceptibility. Cancer Genet Cytogenet. 2009;195(1):31–6. doi: 10.1016/j.cancergencyto.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 84.Liberzon E, et al. Germ-line ATM gene alterations are associated with susceptibility to sporadic T-cell acute lymphoblastic leukemia in children. Genes Chromosomes Cancer. 2004;39(2):161–6. doi: 10.1002/gcc.10306. [DOI] [PubMed] [Google Scholar]
- 85.Sheng X, et al. TERT polymorphisms modify the risk of acute lymphoblastic leukemia in Chinese children. Carcinogenesis. 2013;34(1):228–35. doi: 10.1093/carcin/bgs325. [DOI] [PubMed] [Google Scholar]
- 86.Phillips CL, et al. MDM2 polymorphism increases susceptibility to childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2010;55(2):248–53. doi: 10.1002/pbc.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piwkham D, et al. Multilocus association of genetic variants in MLL, CREBBP, EP300, and TOP2A with childhood acute lymphoblastic leukemia in Hispanics from Texas. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1204–12. doi: 10.1158/1055-9965.EPI-11-0059. [DOI] [PubMed] [Google Scholar]
- 88.Papaemmanuil E, et al. Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet. 2009;41(9):1006–10. doi: 10.1038/ng.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trevino LR, et al. Germline genomic variants associated with childhood acute lymphoblastic leukemia. Nat Genet. 2009;41(9):1001–5. doi: 10.1038/ng.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sherborne AL, et al. Variation in CDKN2A at 9p21.3 influences childhood acute lymphoblastic leukemia risk. Nat Genet. 2010;42(6):492–4. doi: 10.1038/ng.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang W, et al. ARID5B SNP rs10821936 is associated with risk of childhood acute lymphoblastic leukemia in blacks and contributes to racial differences in leukemia incidence. Leukemia. 2010;24(4):894–6. doi: 10.1038/leu.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Enciso-Mora V, et al. Common genetic variation contributes significantly to the risk of childhood B-cell precursor acute lymphoblastic leukemia. Leukemia. 2012;26(10):2212–5. doi: 10.1038/leu.2012.89. [DOI] [PubMed] [Google Scholar]
- 93.Orsi L, et al. Genetic polymorphisms and childhood acute lymphoblastic leukemia: GWAS of the ESCALE study (SFCE) Leukemia. 2012;26(12):2561–4. doi: 10.1038/leu.2012.148. [DOI] [PubMed] [Google Scholar]
- 94.Healy J, et al. Replication analysis confirms the association of ARID5B with childhood B-cell acute lymphoblastic leukemia. Haematologica. 2010;95(9):1608–11. doi: 10.3324/haematol.2010.022459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prasad RB, et al. Verification of the susceptibility loci on 7p12.2, 10q21.2, and 14q11.2 in precursor B-cell acute lymphoblastic leukemia of childhood. Blood. 2010;115(9):1765–7. doi: 10.1182/blood-2009-09-241513. [DOI] [PubMed] [Google Scholar]
- 96.Ross JA, et al. Genetic variants modify susceptibility to leukemia in infants: a Children’s Oncology Group report. Pediatr Blood Cancer. 2013;60(1):31–4. doi: 10.1002/pbc.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vijayakrishnan J, et al. Variation at 7p12.2 and 10q21.2 influences childhood acute lymphoblastic leukemia risk in the Thai population and may contribute to racial differences in leukemia incidence. Leuk Lymphoma. 2010;51(10):1870–4. doi: 10.3109/10428194.2010.511356. [DOI] [PubMed] [Google Scholar]
- 98.Xu H, et al. ARID5B genetic polymorphisms contribute to racial disparities in the incidence and treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2012;30(7):751–7. doi: 10.1200/JCO.2011.38.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han S, et al. Genome-wide association study of childhood acute lymphoblastic leukemia in Korea. Leuk Res. 2010;34(10):1271–4. doi: 10.1016/j.leukres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 100.Ellinghaus E, et al. Identification of germline susceptibility loci in ETV6-RUNX1-rearranged childhood acute lymphoblastic leukemia. Leukemia. 2012;26(5):902–9. doi: 10.1038/leu.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hosking FJ, et al. Genome-wide homozygosity signatures and childhood acute lymphoblastic leukemia risk. Blood. 2010;115:4472–7. doi: 10.1182/blood-2009-09-244483. [DOI] [PubMed] [Google Scholar]
- 102.Infante-Rivard C, et al. Parental smoking, CYP1A1 genetic polymorphisms and childhood leukemia (Quebec, Canada) Cancer Causes Control. 2000;11(6):547–53. doi: 10.1023/A:1008976116512. [DOI] [PubMed] [Google Scholar]
- 103.Infante-Rivard C, et al. Risk of childhood leukemia associated with exposure to pesticides and with gene polymorphisms. Epidemiology. 1999;10(5):481–7. doi: 10.1097/00001648-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 104.Infante-Rivard C, Amre D, Sinnett D. GSTT1 and CYP2E1 polymorphisms and trihalomethanes in drinking water: effect on childhood leukemia. Environmental health perspectives. 2002;110(6):591–3. doi: 10.1289/ehp.02110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Infante-Rivard C, et al. Childhood acute lymphoblastic leukemia associated with parental alcohol consumption and polymorphisms of carcinogen-metabolizing genes. Epidemiology. 2002;13(3):277–81. doi: 10.1097/00001648-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 106.Infante-Rivard C. Diagnostic x rays, DNA repair genes and childhood acute lymphoblastic leukemia. Health Phys. 2003;85(1):60–4. doi: 10.1097/00004032-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 107.Infante-Rivard C, Mathonnet G, Sinnett D. Risk of childhood leukemia associated with diagnostic irradiation and polymorphisms in DNA repair genes. Environ Health Perspect. 2000;108(6):495–8. doi: 10.1289/ehp.00108495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perera FP, et al. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ Health Perspect. 1999;107(Suppl3):451–60. doi: 10.1289/ehp.99107s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]