Abstract
The ATP-binding cassette transporter MRP4 (encoded by ABCC4) regulates membrane cyclic nucleotides concentrations in arterial cells including smooth muscle cells. MRP4/ABCC4 deficient mice display a reduction in smooth muscle cells proliferation and a prevention of pulmonary hypertension in response to hypoxia. We aimed to study gene transfer of a MRP4/ABCC4 silencing RNA via intratracheal delivery of aerosolized adeno-associated virus 1 (AAV1.shMRP4 or AAV1.control) in a monocrotaline-induced model of pulmonary hypertension in rats. Gene transfer was performed at the time of monocrotaline administration and the effect on the development of pulmonary vascular remodeling was assessed 35 days later. AAV1.shMRP4 dose-dependently reduced right ventricular systolic pressure and hypertrophy with a significant reduction with the higher doses (i.e., >1011 DRP/animal) as compared to AAV1.control. The higher dose of AAV1.shMRP4 was also associated with a significant reduction in distal pulmonary arteries remodeling. AAV1.shMRP4 was finally associated with a reduction in the expression of ANF, a marker of cardiac hypertrophy. Collectively, these results support a therapeutic potential for downregulation of MRP4 for the treatment of pulmonary artery hypertension.
Introduction
Pulmonary artery hypertension (PAH) is characterized by a chronic increase in mean pulmonary arterial pressure and resistance due to pulmonary vascular remodeling. PAH results in progressive right heart failure and premature death.1,2 PAH is classified as idiopathic, heritable, induced by drugs and toxins, or associated to other conditions such as connective tissue disorders, HIV infection, and portal hypertension. Heart diseases can also cause pulmonary hypertension.2
PAH is characterized by abnormal smooth muscle and endothelial cells proliferation in small pulmonary arteries.3 Different studies have shown that PAH is associated with abnormalities in the homeostasis of cyclic nucleotides (namely cAMP and cGMP) that are critical regulators of vascular tone and pulmonary artery smooth muscle cells (PASMCs) proliferation. Due to a reduction in endothelial nitric oxide production and to an increase in phosphodiesterase 5 expression and activity in PASMC, PASMC from PAH patients are characterized by decreased intracellular levels of cGMP.4,5 This, in turn, promotes PASMC vasoconstriction and proliferation. The levels of cAMP are also reduced in PASMC from PAH patient, in line with decreased blood level of vasoactive intestinal peptide and decreased production of prostacyclin (both coupled to adenylyl cyclase), and an increased expression of other cAMP phosphodiesterases type 1 and 3,6,7 promoting also PASMC proliferation. Prostacyclin and its analogues (that increase cAMP levels) as well as phosphodiesterase type 5 (PDE5) inhibitors or stimulators of soluble guanylate cyclase (to increase cGMP level) have thus been approved for the treatment of PAH.8
It has recently been shown that beyond hydrolysis by PDEs, cAMP and cGMP levels are also determined by a process involving an active trans-membrane efflux from the cytosol.9 MRP4 is a member of a large family of transmembrane proteins (ATP-binding cassette transporter family class C energy-dependent transporters) that was recently shown as an endogenous regulator of intracellular cyclic nucleotides levels in smooth muscle cells.10,11 In previous experiments, we have shown that MRP4 silencing inhibits smooth muscle cells proliferation in vitro and in vivo.12,13 Furthermore, we have reported increased MRP4 expression in pulmonary arteries from patients with idiopathic PAH as well as in mice developing pulmonary hypertension in response to hypoxia.14 Consistent with a pathogenic role for MRP4 in PAH, MRP4-deficient mice were protected from hypoxia-induced PH.14
To expand upon these first observations, we conducted the present study to determine whether gene transfer of a MRP4/ABCC4 silencing RNA via intratracheal delivery of aerosolized adeno-associated virus 1 (AAV1.shMRP4) would impact the development of monocrotaline (MCT)-induced pulmonary hypertension in rats. Adeno-associated virus serotype 1 was selected for its good tropism for pulmonary vessels as previously reported.15
Results
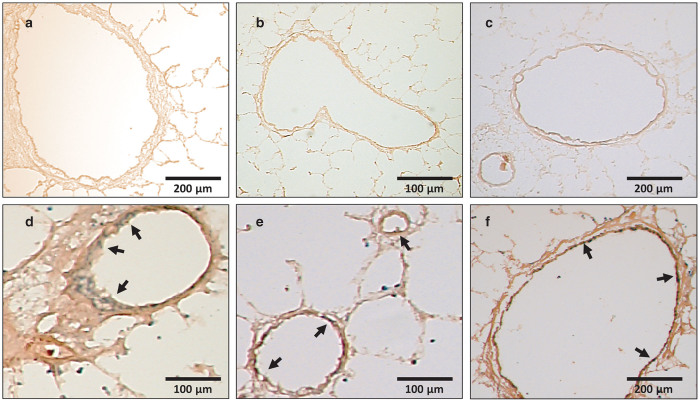
We firstly tested the efficiency of AAV1-mediated gene transfer to transduce the lung vasculature after intratracheal delivery in a monocrotaline-induced pulmonary hypertension model in rats. Rats were injected intraperitoneally with monocrotaline and were at the same time administered with inhalable AAV1 carrying LacZ encoding the β-galactosidase protein (AAV1.βGal, 1 × 1011 DRP/animal) or saline using a MicroSprayer Aerosolizer. Animals were sacrificed 35 days later and X-gal staining was performed on lung sections. No X-gal staining was observed in lungs of MCT-treated animals who received inhalable saline (Figure 1a–c). In contrast, X-gal staining was clearly observed in bronchial smooth muscle cells (Figure 1d) and in the intima and media of large and small vessels (Figure 1e,f) from MCT-treated animals who received inhalable AAV1.βGal.
Figure 1.
Efficacy of intratracheal targeted delivery of AAV1 vectors to the lung vasculature. Representative X-Gal stained sections of lung tissue 35 days after intratracheal delivery of saline (a–c) or of AAV1.βGal (d–f) in MCT-treated animals. Arrows indicate the localization of βGal protein in bronchial smooth muscle cells (d) and in large or small pulmonary vessels smooth muscle cells (e,f).
We then aimed to evaluate the effect of targeted vascular gene transfer of a silencing RNA against ABCC4 mRNA on pulmonary hemodynamics and vascular remodeling. We selected an ABCC4 shRNA sequence that was previously validated16 and generated a double-stranded AAV1 encoding the ABCC4 shRNA (AAV1.shMRP4). The efficiency of the produced vector to silence MRP4 was firstly assessed in vitro in isolated rat smooth muscle cells (Supplementary Figure S1).
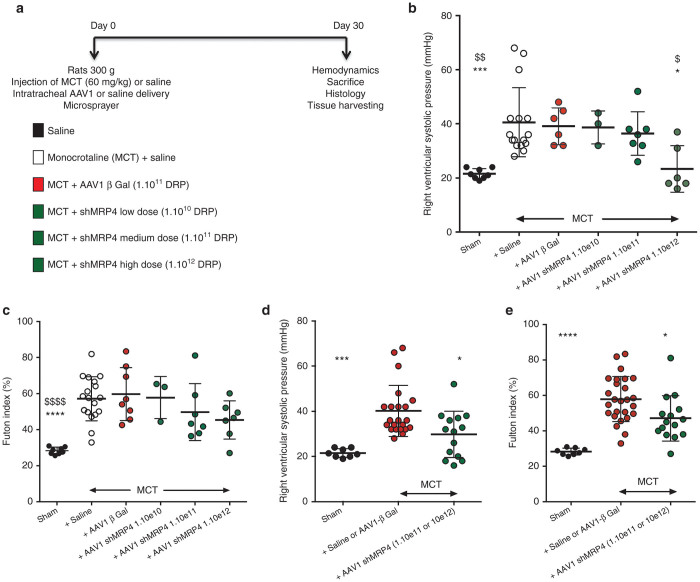
As previously described, rats were injected intraperitoneally with monocrotaline and were administered with inhalable AAV1.βGal or AAV1.shMRP4 or saline at the same time (Figure 2a). In a first approach, three different doses of AAV1-shMRP4 were tested (low dose 1.1010, mid-dose 1.1011, and high dose 1.1012 DRP/animal). As anticipated, MCT-PAH rats treated with aerosolized saline demonstrated increased right ventricular systolic pressure consistent with the development of PAH (Figure 2b). MCT-treated rats infected with aerosolized AAV1.βGal demonstrated the same increase in right ventricular pressure than the MCT-treated animals receiving saline aerosols further confirming that AAV1 infection does not modify the development of MCT-induced PAH. We then observed a dose–response reduction in right ventricular pressure in MCT-PAH rats treated with AAV1.shMRP4 with significant reduction in rats receiving high-dose AAV1.shMRP4. A similar trend was observed when analyzing the right ventricle remodeling through the Fulton index (Figure 2c) although not reaching statistical significance. In a secondary approach, we performed additional analyses by pooling the MCT+saline and MCT+AAV1.βGal groups (reference group) and the MCT+AAV1.shMRP4 mid- and high-doses (experimental group). This approach was supported by the lack of statistical differences between the MCT+saline and MCT+AAV1.βGal groups and by the lack of apparent effect in the MCT-PH rats treated with low dose of AAV1.shMRP4. We consistently found a significant reduction in the right ventricular (RV) pressure and remodeling in MCT-PAH rats treated with AAV1.shMRP4 mid- and high-doses (Figure 2d,e).
Figure 2.
Effect of AAV1.shMRP4 on cardiac parameters. Experimental design of the study (a), right ventricular (RV) systolic pressure (mmHg) (b,d) and RV hypertrophy reflected by the RV weight over LV plus interventricular septum (S) weight ratio (defined as RV/(LV+S) = Fulton index) (c,e) measured 5 weeks postinjections (comparisons versus MCT + Saline *P < 0.05; ***P < 0.001, ****P < 0.0001; versus MCT + AAV1.βGal: $P < 0.05, $$P < 0.01, $$$$P < 0.0001).
We then measured mRNA levels for ANF and SERCA2a, two heart failure markers, in right ventricles isolated from rat hearts. The values observed in MCT+saline and MCT+AAV1.βGal groups were pooled and used as a reference group (Figure 3a). As expected, MCT treatment induced an increase in ANF mRNA level in the control group, whereas the level of SERCA2a mRNA was decreased (Figure 3b). AAV1.shMRP4 partially prevented the increase in ANF mRNA but did not significantly influence SERCA2a mRNA expression level (Figure 3b).
Figure 3.
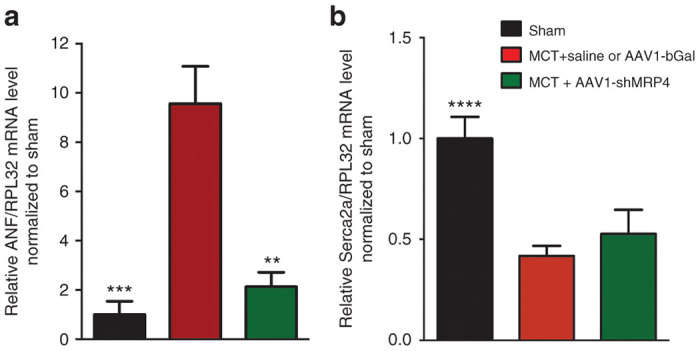
Effect of AAV1.shMRP4 on markers of cardiac hypertrophy. Atrial natriuretic factor (ANF) and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a) mRNA levels relative to RPL32 mRNA detected by reverse transcriptase polymerase chain reaction. ANF mRNA expression (a); SERCA2a mRNA expression (b) were normalized to the level of each mRNA in sham (comparisons versus MCT + saline or AAV1.βGal: **P < 0.01, ***P < 0.001, ****P < 0.0001).
The remodeling of distal pulmonary arteries was finally assessed in the control, MCT+saline, MCT+AAV1.βGal, and MCT+AAV1.shMRP4 high-dose groups (n = 3–4 animals/group). Vessels were stained with an endothelial marker (Von Willebrand factor, in green) and a smooth muscle cell marker (calponin, in red) (Figure 4a). Morphometric analysis of pulmonary arteries was performed blindly of the allocated group and demonstrated a significant increase in the medial thickness in MCT+saline as well as in MCT+AAV1.βGal treated rats compared to sham, regardless of vessel diameters. By contrast, the vessel remodeling was significantly reduced in the MCT+AAV1.shMRP4 treated animals (Figure 4b). There was no significant difference in the number of muscularized vessels between the sham and the AAV1.shMRP4 groups.
Figure 4.
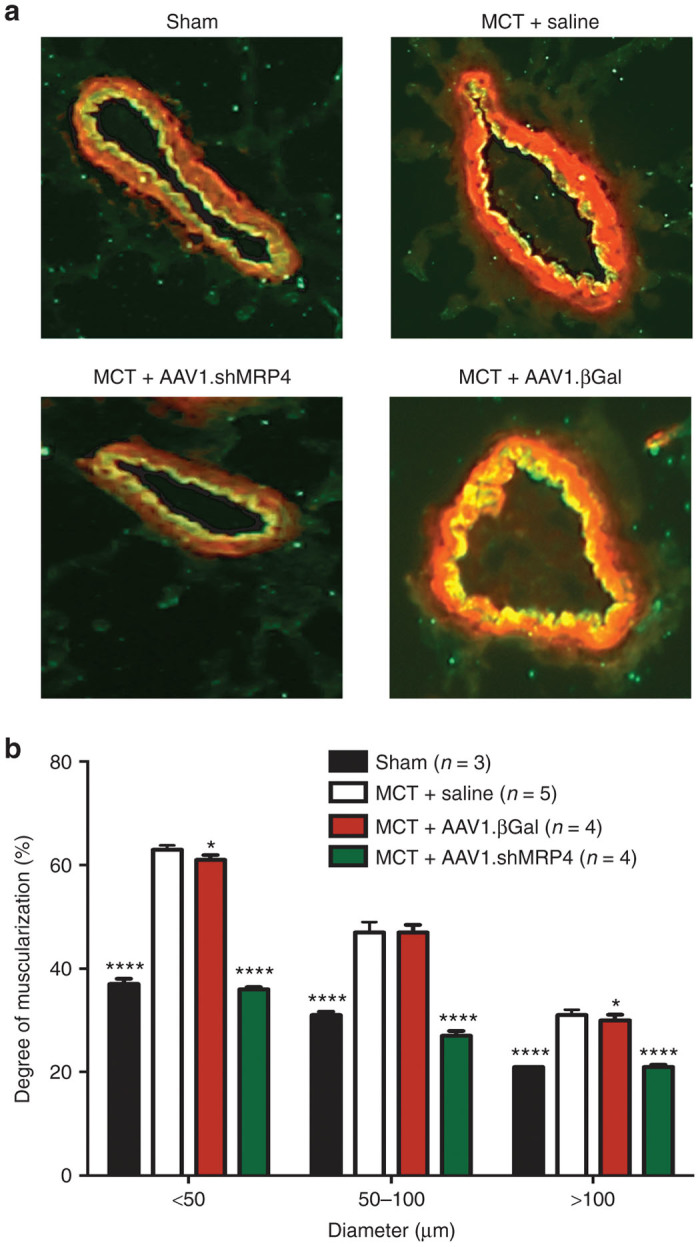
Prevention of monocrotaline-induced pulmonary vessels remodeling with MRP4 inhibition by shMRP4. (a) Representative immunofluorescence staining in sections of pulmonary vessels from the different groups. (b) Percentage of medial thickness of arteries in relation to cross-sectional diameter (comparisons versus MCT + Saline: *P < 0.05, ****P < 0.0001).
Discussion
Despite novel treatments, improvement in survival of patients with PAH remains unsatisfactory and PAH remains a progressive, fatal disease2,17 urging for the development of novel therapies. Here, we provide evidence that a single administration of aerosolized AAV1 encoding MRP4 silencing RNA limits the progression of the disease in a rat model of pulmonary hypertension induced by monocrotaline administration. We did not evaluate the ability of AAV1.shMRP4 to reverse established PAH in this study thus limiting the immediate clinical impact of our study. However, the present results confirm and expand upon our previous data on PAH prevention in MRP4 deficient mice.14 In addition to our previous observations that the development of hypoxic pulmonary hypertension was prevented in MRP4 knockout mice,14 we here report that MRP4 knockdown is sufficient to achieve similar effect which further supports MRP4 as a new target in the treatment of this disease. Phosphodiesterase type 5 (PDE5) inhibitors (sildenafil and tadalafil) are currently approved for the treatment of patients with PH5,8,18–20 and inhibitors of MRP4 could be an additional way to increase cyclic nucleotides levels and limits pulmonary arterial hypertension.14,16
The mechanism involved in the protective effect of AAV1.shMRP4 has not been deciphered in the present study. Indeed, we previously demonstrated that MRP4 silencing is associated with an increase in both cAMP and cGMP levels in vascular and in pulmonary artery smooth muscle cells and with a consequent activation of cyclic-nucleotide-mediated signaling pathways.14,16 In vascular smooth muscles, MRP4 thus promotes cellular proliferation and acts by activating the smooth muscle cells proliferative phenotype. The significant reduction in pulmonary vessels muscularization after AAV1.shMRP4 delivery is consistent with the reduction in smooth muscle cell proliferation after MRP4 silencing as previously reported.14,16 The importance of MRPs in lowering global intracellular cyclic nucleotides level is disputed given the efficiency of PDEs compared with the low affinity of MRPs for cAMP and cGMP.9,21,22 However, MRP4 is localized in specific regions of the membrane13,23 and is likely to control cyclic nucleotides pools in regions where expression of PDEs is low. Manipulation of MRP4 expression was consistently associated with significant activation of cyclic-nucleotide signaling pathways also suggesting that MRP4 is a critical player of the cyclic nucleotides signalosome.9 MRPs are however able to transport out of the cell, at the expense of ATP hydrolysis, other organic anionic compounds such as antiviral, cytostatic drugs, antibiotics, as well as a wide variety of endogenous metabolites and signaling molecules such as leukotrienes, steroids, glutathione.11 We cannot thus exclude that inactivation of MRP4 prevents extrusion of other metabolites in addition to cyclic nucleotides, a phenomenon that may also contribute to the prevention of pulmonary vascular remodeling.24,25
The present study also evaluated a new therapeutic strategy to target MRP4 based on intra-tracheal gene transfer of a specific silencing RNA. Gene therapies have been previously proposed for PAH such as overexpression of the gene encoding prostaglandin I2 synthase. Injection of either AAV1 or AAV2 encoding prostaglandin I2 synthase significantly inhibited the hypoxia-induced cardiac and vascular remodeling in mice,26 as well as monocrotaline-induced PAH in rats.27 In these studies the vectors were injected into the tight muscle and no difference was seen between the two AAV serotypes used. AAV2 containing an extracellular fragment of the TIE2 receptor (AAV-sTIE2), injected in the pulmonary artery of rats, was also shown to prevent MCT- and hypoxia-induced PH in rats.28 Intramuscular injection of AAV1-IL10 also prevented MCT-induced right ventricular hypertrophy, increase in pulmonary pressure and pulmonary artery remodeling.29 The originality of our study is to propose gene transfer through aerosolized intratracheal delivery. Gene transfer of SERCA2a via a similar way (i.e., intratracheal delivery of aerosolized AAV1) was recently shown to prevent MCT- induced PAH in rats.15 AAV1 has tropism for endothelial and vascular smooth muscle cells30 and was shown to efficiently target the intima and media of small pulmonary arteries as well as in bronchial smooth muscle cells after a single aerosolized intratracheal administration.15 AAV1 has also tropism for primary airway epithelial cells.31 Noteworthy, we assessed the efficiency of MRP4 silencing to prevent the development of PAH but it remains to be determined if MRP4 silencing would reverse established PAH. The present study will provide critical evidence for the development of future reversal studies.
The use of intratracheal instillation of aerosolized AAV might also restrict gene delivery to the lung and limit possible off-target transduction into unwanted organs. MRP4 expression has been reported in different organs including lung and heart.9,14,32 In the present study, we found a reduction in right ventricular remodeling after MRP4 silencing. Importantly, viral genome copies after administration of aerosolized AAV1.Serca2a were detected in lung but not in right ventricles samples,15 suggesting that this reduction in right ventricular remodeling is directly linked to the reduction in pulmonary vessels remodeling.
In conclusion, we demonstrate that localized viral gene silencing of MRP4 in pulmonary vessels help to limit pulmonary vessels remodeling in a model of monocrotaline-induced PAH in rats.
Materials and Methods
AAV1 vector
The sequence of the rat short hairpin RNA against MRP4/ABCC4 was described previously.13 It was first inserted in pSIREN-DNR dsRed express. Then the MLu1-Apa1 fragment containing the U6 promoter + shMRP4 was cloned in the double stranded pds AAV2-EGFP vector to generate pds-AAV2-ShMRP4. AAV1.βgal was described previously.15 HEK 293 T cells were transfected with pds AAV2-shMRP4 (50 µg) plus the helper vector PXYZ (150 µg) coding for AAV1 capsid proteins. Three days later, virus were purified and quantified as described by Kohlbrenner et al.33
Animal model of PH
Adult male Wistar rats (300 g body weight were obtained from Janvier Laboratories, Le Genest-Saint-Isle, France). Animals were housed in an environmentally-controlled animal facility for the duration of the experiment. Rats were injected (intraperitoneal) with either monocrotaline (MCT 60 mg/kg, Sigma Aldrich, Saint-Louis, MO) or saline (1 ml). Immediately after, an intratracheal injection of saline or of AAV1-βGal (2 × 1011 DRP) or three doses of AAV1-shMRP4 (1 × 1010, 1 × 1011, 1 × 1012 DRP) under a volume of 300 µl was performed using a micro-sprayer (Penn-Century MicroSprayer Aerosolizer - Model IA-1B, Glenside, PA). The animals were sacrificed 5 ± 0.5 weeks later. Care of the animals and surgical procedures were performed according to the Directive 2010/63/EU of the European Parliament and had been approved by the Ministry of Agriculture, France, (authorization for surgery C-75-665-R). The project was submitted to the Institutional review board and obtained the authorization Ce5/2012/050.
Hemodynamic measurements
Hemodynamic measurements and assessment of right heart hypertrophy were performed 5 ± 0.5 weeks postinjections. Rats were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg). For hemodynamic measurements, a 24-gauge catheter was advanced to the RV through the right jugular vein for measurement of RV pressure with fluid-filled force transducers. After hemodynamic measurements, animals were euthanized using penthobarbital (150 mg/kg), and a thoracotomy was performed. Hearts were collected the right ventricle was separated from the left ventricle + septum, and each was frozen in liquid nitrogen. After exsanguination, lungs were also collected, the left lung was filled with 50% optimum cutting temperature (OCT) embedding matrix in phosphate-buffered saline and fixed in 10% neutral buffered formalin. The right lung was snap-frozen in liquid nitrogen.
Cardiac and vascular remodeling
The heart was dissected and weighed. Cardiac right ventricular hypertrophy was evaluated by the right ventricle to left ventricle plus septum weight ratio (RV/(LV + S)) – Fulton index.
Sections were performed in three different portions of the lung: proximal, medial, and distal (compared to the trachea). To assess AAV1 infection level in lungs, X-Gal staining was made on MCT-AAV1.βGal-treated rat lungs. Sections were fixed in 0.2% glutaraldehyde in phosphate-buffered saline containing 20 mmol/l MgCl2. After washes, sections were incubated in X-Gal (5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside) solution containing: 10 mg X-Gal, 15 mmol/l MgCl2, 30 mmol/l K4Fe(CN)6 (potassium ferrocyanide), 30 mmol/l K3Fe(CN)6 (potassium ferricyanide), 100 mmol/l Na-P, and 10 mmol/l KCl (pH 7,5), during 3 hours (or overnight), at 37 °C, in the dark. After new washes, sections were immersed in 100% ethanol for 2 hours. An eosin staining was performed during 40 seconds. Then, several successive baths of ethanol were made for 5 minutes and sections were immersed in Xylene 2 minutes.
To assess remodeling of muscular pulmonary arteries, immuno-fluorescence was performed on cryosections using anti-Von-Willebrand factor (Ab11713, 1/2,000, Abcam, Cambridge, UK) to visualize the endothelium and a-calponin (Ab 466794, 1/500, Abcam) to label the media. Secondary antibodies were from Invitrogen (1/400). Microscopic images were analyzed using a computerized morphometric system (Leica, Solms, Germany) and muscularization was assessed as external diameter (Calponin-labeled) − internal diameter (Von-Willebrand factor labeled)/external diameter × 100. Arteries were categorized according to their external diameters: <50 µm, 50–100 µm, >100 µm. Analysis was done in a blinded fashion. At least three sections (on the proximal, median, and distal lungs) were performed per animal. About 100 small arteries per animal were detected, measured, and analyzed according to their diameter (small <50, medium 50–100, or high >100). All measures were performed blindly of the allocated group.
Quantitative real-time PCR
Total RNA was extracted from the left ventricle using the trizol protocol. Reverse transcription was performed on 500 ng total RNA and PCR was performed on 1/20 of the first strand. The following primers were used: (i) rat RPL32 f) CCA GAG GCA TCG ACA ACA, r) GCA CTT CCA GCT CCT TGA CAT; (ii) rat ANF f) ATG GGC TCC TTC TCC ATC ACC, r) TCC GCT CTG GGC TCC AAT CCT GT; rat SERCA2a f) 5′-ATGGACGAGACGCTCAAGTT-3′, r) 5′-TTTCTCTTTCCCCAAGCTCA-3′. The following protocol was used: 95 °C—15 minutes then 40 cycles at 95 °C—30 seconds, 60 °C—1 minute, 72 °C—40 seconds and terminated by 1 cycle of 1 minute at 95 °C to obtain the dissociation curve.
Statistical analysis
Quantitative data are reported as means ± standard error of the mean. Statistical analysis was performed with the Prism software. Kruskal-Wallis one-way analysis of variance test was performed for multiple comparisons of values. Data were compared to MCT + saline or MCT + AAV1.bGal groups as indicated. Nonparametric post hoc Dunn test was used for pairwise multiple comparisons to identify which group differences accounted for overall analysis of variance results. All values with P < 0.05 were considered significant.
Acknowledgments
This study was funded through a grant-in-aid from Bayer Healthcare Pharmaceuticals (Berlin, Germany). J.-S.H. and A.-M.L. have a patent on the use of MRP4 inhibitors for the treatment of cardiovascular disorders (EP07290433.7, INSERM). J.-S.H, A.-M.L, and D.B. designed the study and planned the experiments. J.-S.H. and A.-M.L. supervised the experiments, analyzed the data, and wrote the manuscript. C.C. performed the histological, biochemical and molecular experiments with the help of J.B. N.M. performed the gene transfer and animal experiments. M.C. and F.A. produced the AAV1 vectors. We would like to thank Yannis Hara for the help in preliminary experiments.
References
- Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43 12 Suppl S:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2008;118:2372–2379. doi: 10.1172/JCI33452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Mason NA, Morrell NW, Kojonazarov B, Sadykov A, Maripov A. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001;104:424–428. doi: 10.1161/hc2901.093117. [DOI] [PubMed] [Google Scholar]
- Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, Yuan JX. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol. 2007;292:L294–L303. doi: 10.1152/ajplung.00190.2006. [DOI] [PubMed] [Google Scholar]
- Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation. 2007;115:2331–2339. doi: 10.1161/CIRCULATIONAHA.106.676809. [DOI] [PubMed] [Google Scholar]
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- Cheepala S, Hulot JS, Morgan JA, Sassi Y, Zhang W, Naren AP. Cyclic nucleotide compartmentalization: contributions of phosphodiesterases and ATP-binding cassette transporters. Annu Rev Pharmacol Toxicol. 2013;53:231–253. doi: 10.1146/annurev-pharmtox-010611-134609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Sassi Y, Hara Y, Lompré AM, Hulot JS. Multi-drug resistance protein 4 (MRP4/ABCC4) and cyclic nucleotides signaling pathways. Cell Cycle. 2009;8:962–963. [PubMed] [Google Scholar]
- Sassi Y, Lipskaia L, Vandecasteele G, Nikolaev VO, Hatem SN, Cohen Aubart F. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118:2747–2757. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Sassi Y, Guibert C, Gambaryan N, Dorfmüller P, Eddahibi S. Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J Clin Invest. 2011;121:2888–2897. doi: 10.1172/JCI45023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadri L, Kratlian RG, Benard L, Maron BA, Dorfmüller P, Ladage D. Therapeutic efficacy of AAV1.SERCA2a in monocrotaline-induced pulmonary arterial hypertension. Circulation. 2013;128:512–523. doi: 10.1161/CIRCULATIONAHA.113.001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi Y, Lipskaia L, Vandecasteele G, Nikolaev VO, Hatem SN, Cohen Aubart F. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118:2747–2757. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LJ, Simonneau G, Badesch D, Galiè N, Humbert M, Keogh A. The study of risk in pulmonary arterial hypertension. Eur Respir Rev. 2012;21:234–238. doi: 10.1183/09059180.00003712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, ESC Committee for Practice Guidelines (CPG) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278:17664–17671. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol. 2003;63:1094–1103. doi: 10.1124/mol.63.5.1094. [DOI] [PubMed] [Google Scholar]
- Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell. 2007;131:940–951. doi: 10.1016/j.cell.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med. 2013;5:200ra117. doi: 10.1126/scitranslmed.3006674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Yoo HY, Earm YE, Kim SJ, Kim JK, Kim SD. Role of arachidonic acid-derived metabolites in the control of pulmonary arterial pressure and hypoxic pulmonary vasoconstriction in rats. Br J Anaesth. 2011;106:31–37. doi: 10.1093/bja/aeq268. [DOI] [PubMed] [Google Scholar]
- Kataoka M, Kawakami T, Tamura Y, Yoshino H, Satoh T, Tanabe T. Gene transfer therapy by either type 1 or type 2 adeno-associated virus expressing human prostaglandin I2 synthase gene is effective for treatment of pulmonary arterial hypertension. J Cardiovasc Pharmacol Ther. 2013;18:54–59. doi: 10.1177/1074248412457046. [DOI] [PubMed] [Google Scholar]
- Ito T, Okada T, Mimuro J, Miyashita H, Uchibori R, Urabe M. Adenoassociated virus-mediated prostacyclin synthase expression prevents pulmonary arterial hypertension in rats. Hypertension. 2007;50:531–536. doi: 10.1161/HYPERTENSIONAHA.107.091348. [DOI] [PubMed] [Google Scholar]
- Kido M, Du L, Sullivan CC, Deutsch R, Jamieson SW, Thistlethwaite PA. Gene transfer of a TIE2 receptor antagonist prevents pulmonary hypertension in rodents. J Thorac Cardiovasc Surg. 2005;129:268–276. doi: 10.1016/j.jtcvs.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Ito T, Okada T, Miyashita H, Nomoto T, Nonaka-Sarukawa M, Uchibori R. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res. 2007;101:734–741. doi: 10.1161/CIRCRESAHA.107.153023. [DOI] [PubMed] [Google Scholar]
- Lompré AM, Hadri L, Merlet E, Keuylian Z, Mougenot N, Karakikes I. Efficient transduction of vascular smooth muscle cells with a translational AAV2.5 vector: a new perspective for in-stent restenosis gene therapy. Gene Ther. 2013;20:901–912. doi: 10.1038/gt.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Lei-Butters DC, Keiser NW, Engelhardt JF. Distinct transduction difference between adeno-associated virus type 1 and type 6 vectors in human polarized airway epithelia. Gene Ther. 2013;20:328–337. doi: 10.1038/gt.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi Y, Abi-Gerges A, Fauconnier J, Mougenot N, Reiken S, Haghighi K. Regulation of cAMP homeostasis by the efflux protein MRP4 in cardiac myocytes. FASEB J. 2012;26:1009–1017. doi: 10.1096/fj.11-194027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlbrenner E, Henckaerts E, Rapti K, Gordon RE, Linden RM, Hajjar RJ. Quantification of AAV particle titers by infrared fluorescence scanning of coomassie-stained sodium dodecyl sulfate-polyacrylamide gels. Hum Gene Ther Methods. 2012;23:198–203. doi: 10.1089/hgtb.2012.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.