Abstract
Background
The mosquito net existed long before it was known that mosquitoes transmitted malaria. Therefore it was not intended for malaria control.
Objectives
To scrutinise the patterns of prevalence and identify any hitherto unknown factors that could explain the findings.
Methods
Retrieval of records on malaria prevalence.
Findings
Households sprayed in the previous 12 months or owning at least one ITN: 77.8% and IRS: 31.6% in mid-northern districts. Paradoxically, this was the highest malaria prevalence at 80.1%, hence the phenomenon of diminishing-returns. The urban children (28.6%), those of post-secondary education mothers (14.3%) and in the highest wealth quintile (33.3%) had a lower malaria prevalence than those without education (55.8%) and the less wealthy (67.6%), (p < 0.001). In all, the connection was that the urban (77.4%) and the wealthy (63.8%) sought health care first from hospitals, for proper treatment. Hence the low prevalence is most likely to be due to anti-malarial medicines and not to bed-nets and IRS, since the other findings of the survey show that there are no significant differences in bed nets ownership and usage and IRS in both groups.
Recommendation
Antimalarial medicines should therefore be used to control malaria instead of the nets and IRS.
Keywords: Malaria, Control, Bed-nets, Phenomenon, Diminishing-returns
Introduction
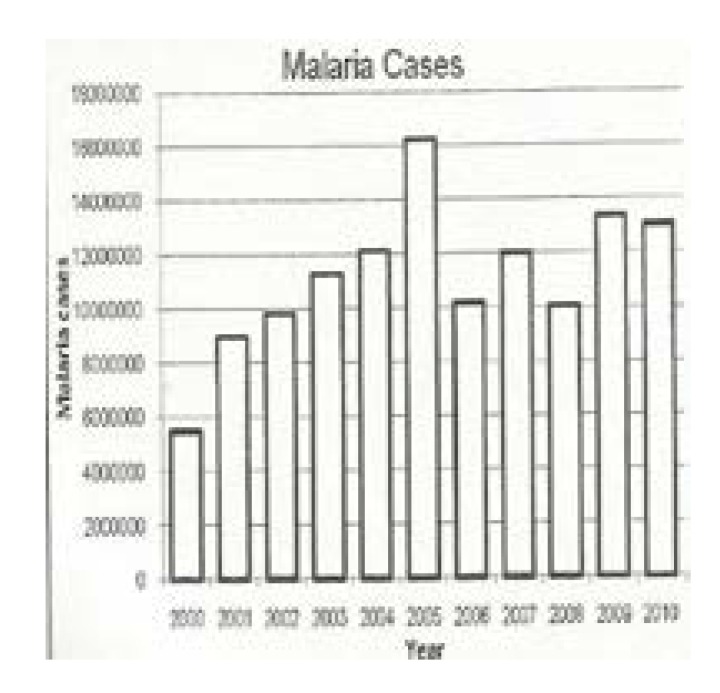
The author having been associated with the subject of malaria for over half a century wishes to combine his long experience with the knowledge from Uganda Malaria Programme Review Report of 2001–2010 published by the Uganda Ministry of Health in May 2011 (Figure 1) and the Uganda Malaria Indicator Survey of 2009 also published by the same Ministry to hold the view that there is no evidence at the present time to suggest that the current control measures of malaria based on the bed net and Indoor Residual Spraying (IRS) have made any impact on the control of malaria or will ever reduce malaria transmission to low levels, hence the need for newer evidence-based ideas on the control of the disease. In support of this view, recent publications have emerged. These include the work of Okiro EA et al1 titled “increasing malaria hospital admissions between 1999 and 2009”; Talisuna A et al2 in the article titled “Malaria in Uganda: challenges to control on the long road to elimination. II.
Figure 1.
Malaria Cases
Source: MoH, HMIS Research Centre
The path forward” states that“the malaria burden is not declining and has likely increased in the last decade”. Prosanna Jaganathan et al3 in the article titled “Increasing incidence of malaria despite insecticide treated bed nets and prompt malaria therapy in Tororo Uganda, concluded the article by saying that despite reports of decreasing malaria morbidity and mortality across many parts of Africa, the incidence of malaria continues to be very high in Tororo, Uganda even in the setting of long lasting insecticide nets (LLINs) and artemesinin combination therapy (ACTs) and this incidence appears to be rising. Thus additional malaria control interventions among young children living in high transmission settings are needed”. However such additional malaria control interventions are not known. Paul Edward Okello et al4 in their paper titled variation in malaria transmission intensity in seven sites throughout Uganda” concludes that based on the observed behavior of the vectors insecticide treated bed nets will be highly effective in controlling malaria.
However in high transmission areas, additional measures will be needed to reduce the malaria burden to acceptable levels. Both these additional measures and acceptable malaria levels are unknown. Proietti C et al5 states that malaria control remains a tremendous challenge in areas that have not benefited from large scale interventions, illustrated here by the district of Apac. Kigozi R et al6 concluded their paper on residual indoor spraying that IRS was associated with a reduction in the malaria morbidity but had observed that “overall, the impact of IRS was less pronounced among patients 5 years or older” and this being the case, it cannot be a valuable method of control since it works for only a particular group of people and not for all. For these reasons this paper is written to describe the hitherto unknown phenomenon of diminishing-returns in the control of malaria by the bed net and IRS and to show the part so far played by the anti malarial medicines in the control of malaria which may provide an answer to Jaganathan P et al3 on the additional malaria interventions they are interested in. However the findings of all these authors concur with the views of the author that malaria burden is continuing as a problem and is probably on the increase.
Background
Malaria is the single curable disease in Uganda causing very significant morbidity and mortality as well as economic loss. Children under five years of age (Table 2) and pregnant women are particularly affected. In 1998, the then Director General of the World Health Organization, Dr. Gro Harlem Brundtland in her inaugural speech on May 13th 1998 described malaria as “the single largest disease in Africa and a primary cause of poverty”7. Even today, this statement still holds. On the 14th July 1898 two members of the Italian Parliament Giustino Fortunato and Leopoldo Franchetti in their circular letter to sponsor activity of Societa Italiana per gli Studi della Malaria described the disease as follows; “malaria disease leaves uncultivated two million hectares of land in Italy; it strikes more or less 63 provinces and 2823 municipalities. It poisons every year about 2 million inhabitants and kills 15,000 of them. It is impossible to estimate the economic loss to our country due to this infection”7. The same now applies to Uganda.
Table 2.
Parasites and spleen rates in healthy children aged 2–9 years.
| Site | Altitude in m. |
Number | Parasite rate (%) |
Spleen rate (%) |
Endemicity Level |
| Bundibugyo | 700–910 | 1453 | 89.0 | 72.1 | Holo |
| Kamwenge | 1210–1290 | 1501 | 84.6 | 86.4 | Holo |
| Ruteete | 1420–1550 | 1201 | 27.8 | 19.5 | Meso |
| Kichwamba | 1580–1680 | 1490 | 6.7 | 5.3 | Hypo |
Source: Albert Kilian (Personal communication)
The control of malaria in Uganda revolves largely on the use of the bed nets and IRS which actually contravenes the three time honoured principles of disease control, namely vaccination, cure and quarantine and yet cure for malaria actually exists but is not used for the control. Therefore, our current methods for the control of malaria do not depend on the well known time honoured principles for the control of diseases which may perhaps explain the lack of success in the current control of malaria. However, the question whether the bed net and IRS will ever terminate the spread of this disease remains unanswered and the author expresses great scepticism in their value to eliminate malaria from the globe.
The use of mosquito net dates back to ancient times. It is documented in the Holy Bible in the book of Judith 10:21 “Holofernes was resting in bed under a mosquito net woven of purple and gold threads decorated with emeralds and other precious stones”. Again Judith 13:9 states “She rolled his body off the bed and took down the mosquito net from the bed posts”. During those days the mosquito nets were not used for the control of malaria since it was not known at that time that malaria was caused by a parasite nor was it known that it was transmitted by a mosquito as its vector but they were used to stop or reduce the nuisance and painful effects of the insect. The association of the mosquito and malaria was first established by Grassi and Ross in the year 1898, each working independently. It then became apparent that the removal of either the mosquito or the parasite or both from the man-parasite-mosquito cycle would terminate the transmission of the disease, hence the beginning of the scientific control of malaria. Thereafter, the mosquito was selected for destruction in order to control malaria.
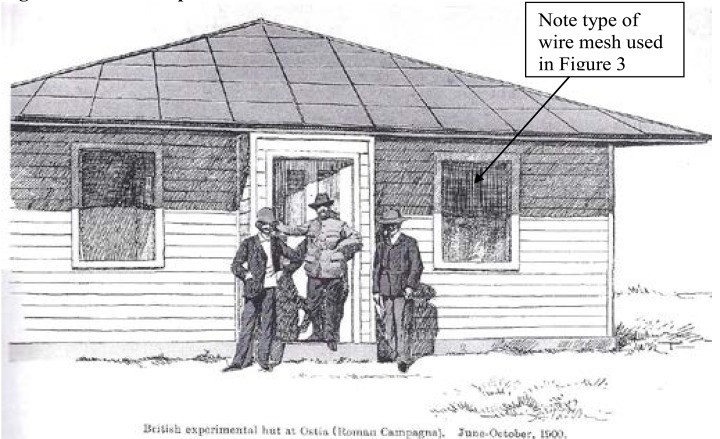
Thus, the selection of the mosquito at that time for persecution and destruction, which has persisted to the present day unquestioned about its efficiency was, however dictated by circumstances of the day as the only option since the parasite could not be attacked because microscopes for its detection and anti-malarial medicines, apart from the then rare cinchona bark for its cure were not readily available to the common man, hence the concentration of action on the mosquito. It cannot be said that before selecting the mosquito for persecution or elimination any comprehensive experiments were ever undertaken since such work does not seem to exist. However after implicating the mosquito as the vector for malaria in 1898 a British Team including C. Low, LW Sambon and AJI Terzi in the summer of 1900 was based for three months at Castel Fusano, a highly malaria endemic locality near Ostia to verify the mosquito malaria hypothesis.(8) (Figure 2) shows the British Experimental wire screened hut at Ostia which was used to protect the Experimental Team for three months from the bite of mosquitoes. Such small numbers cannot prove that the net is instrumental in the control of malaria. Even today, we cannot clearly state whether the bed nets and IRS will end the malaria saga.
Figure 2.
British Experimental wire screened hut at Ostia
British experimental hut at Ostia (Roman Campagna). June–October, 1900
Source: Dobson MJ, The Malariology Centenary
The bed net operates by providing a mere physical barrier which makes the mosquito-man-contact difficult while IRS reduces the mosquito population, thereby reducing the number of mosquito bites, infective and non-infective. Neither the net nor IRS have any lethal or other effect at all on parasites that may be existing within the body and which should be dealt with anti malarial medicines and yet we are bent on the destruction of the mosquito which is a mere vector but not the cause of malaria. Malaria is caused by parasites and if these are removed from the population with appropriate antimalarial medicines, the mosquito will have no source of infection and will therefore cease to be a danger as is the case in parts of the world where malaria existed but no longer exists but mosquitoes still exist there.
Methods and subjects
Search for records particularly of our own work on the incidence or prevalence of malaria in Uganda at different times was undertaken by the author and were looked at carefully compared and contrasted and any differences noted. Causal factors for any differences in prevalences such as altitude would be noted so that wild conclusions were not made. Findings that seemed to lead to no conclusions were also overlooked.
The Malaria Indicator Survey, 2009 published by the Ministry of Health of Uganda, the first of its kind in Uganda was accessed. This used a nationally representative sample of 4760 household. All women aged 15–49 Years in these households where eligible to be individually interviewed about various aspects of malaria. A diversity of investigations where carried out and these included examination of blood from children aged 0–59 months. These were examined for malaria parasites using RDT and microscopy. The 2009 UMIS was designed to monitor all key malaria indicators in Uganda. The Uganda Bureau of Statistics Kampala managed the figures.
Various aspects of malaria as laid out in the UMIS 2009 report were looked at and any irregularity in pattern scrutinized with the intention of finding causes for the irregularities. We selected what we considered particularly relevant in the control of malaria which included the incidences or prevalence of malaria in the under fives, the ownership and use of bed nets, the spraying of the houses, the care seeking behaviour for the first place to seek care and the intermittent preventive treatment during pregnancy. The place of residence, whether rural or urban, the region of residence, mothers' education and wealth quintile of the households were also scrutinized and any deviation noted.
Results
The findings are summarized in tables 1–6 Nevill C., Ochen K., Munafu C., Bek'Obita D and Sezi C.L (9) during 1988 examined blood from 3,999 primary school children from 6 centres scattered throughout Uganda for malaria parasites, 3 and 20% had parasites.
Table 1.
Age Distribution of malaria parasites in 539 people at Buwenge in 1996
| Age in years Cohort |
Numbers in | No. with parasites |
Percentage with parasites |
||
| 0–10 | 212 | 132 | 62.3 | ||
| 11–20 | 127 | 40 | 31.4 | ||
| 21–30 | 86 | 13 | 15.1 | ||
| 31–40 | 43 | 6 | 14 | ||
| 41–50 | 30 | 4 | 13.3 | ||
| 51–60 | 27 | 3 | 11.1 | ||
| 61–70 | 14 | 3 | 21.4 | ||
| All ages | Total | 539 | Total | 201 | -- |
Source: Sezi C.L.
Dr. Albert Kilian (Personal communication) in the then Kabarole District before its division undertook a malaria incidence study. The results are shown in Table 2.
Arua had 52% as the highest incidence During 1996 the author screened 539 asymptomatic people of all ages in a sleeping sickness prone area of Buwenge for haemoparasites. The results are shown in Table 1
The results of care seeking behavior (Table 3a) were scrutinised with hardly any useful information but when the government and private hospital attendances were added together (Table 3) useful information came to light.
Table 3(a).
 |
Table 3.
Extracted from Table No. 4.9, page 40 UMIS 2009 on care seeking behaviour, first place to go to. (Only for residence and wealth quintiles).
| Background Characteristics |
Percentage attending Government Hospitals |
Percentage attending Private Hospitals |
Total Hospital Attendances (Percentage) |
No. of children under age 5 with fever in the 2 weeks preceding survey. |
| Residence | ||||
| Urban | 18.5 | 58.9 | 77.4 | 160 |
| Rural | 8.2 | 23.1 | 31.3 | 1209 |
| Kampala | 22.6 | 39.8 | 62.4 | 28 |
| Wealth quintile | ||||
| Lowest | 6.0 | 19.1 | 25.1 | 384 |
| Highest | 19.3 | 44.5 | 63.8 | 177 |
Source: UMIS 2009 (as modified from Table N.o 4.9 page 40).
In the mid-northern districts, namely Gulu, Amuru, Kitgum, Pader, Apac, Oyam, Lira, Amolatar and Dokolo, 31.6% of the households were exposed to IRS. It is in these same districts where the use of bed nets was highest at 77.8%. Furthermore, the prevalence of malaria in these districts was also the highest in the whole country at 62.5% by microscopy and 81.8% by RDT which is paradoxical. This brings out clearly what we refer to as the phenomenon of diminishing returns inspite of such high bed nets supply and usage and IRS which are considered the standard methods of malaria control and yet prevalence is the highest in the whole country.
The results of the prevalence of malaria among children aged 6–59 months by residence, region, mothers' education and wealth quintile are shown in Table 4.
Table 4.
Prevalence of malaria in children
| Percentage of children age 0–59 months classified as having malaria, by background characteristics, Uganda MIS 2009. | ||||
| Malaria prevalence | ||||
| Background characteristic |
RDT positive | Number of children tested |
Microscopy positive |
Number of children tested |
| Age: | ||||
| <12 | 32.5 | 673 | 24.4 | 678 |
| 0–5 | 18.8 | 312 | 16.3 | 316 |
| 6–11 | 44.3 | 361 | 31.5 | 362 |
| 12–17 | 53.1 | 399 | 38.5 | 401 |
| 18–23 | 47.1 | 404 | 35.8 | 406 |
| 24–35 | 57.5 | 767 | 45.2 | 768 |
| 36–47 | 58.6 | 778 | 49.5 | 782 |
| 48–59 | 58.3 | 812 | 53.2 | 814 |
| Sex | ||||
| Male | 50.0 | 1,887 | 41.1 | 1,892 |
| Female | 53.9 | 1,946 | 43.7 | 1,955 |
| Residence | ||||
| Urban | 28.6 | 563 | 15.3 | 564 |
| Rural | 56.0 | 3,270 | 47.1 | 3,283 |
| Region | ||||
| Central 1 | 44.6 | 293 | 39.1 | 294 |
| Central 2 | 62.1 | 344 | 50.7 | 344 |
| Kampala | 7.4 | 116 | 4.9 | 118 |
| East Central | 65.2 | 539 | 56.2 | 539 |
| Mid Eastern | 40.1 | 471 | 37.5 | 472 |
| North East | 54.5 | 350 | 40.0 | 351 |
| Mid Northern | 80.1 | 550 | 62.5 | 554 |
| West Nile | 60.2 | 343 | 45.7 | 343 |
| Mid Western | 48.4 | 373 | 42.7 | 374 |
| South Western | 17.7 | 455 | 11.6 | 460 |
| Mother's education | ||||
| No education | 55.8 | 718 | 46.5 | 719 |
| Primary | 53.8 | 2,075 | 45.0 | 2,080 |
| Secondary | 39.3 | 448 | 24.0 | 449 |
| More than secondary | 14.3 | 75 | 5.1 | 75 |
| Missing | 55.7 | 517 | 47.4 | 523 |
| Wealth quintile | ||||
| Lowest | 67.6 | 877 | 56.1 | 880 |
| Second | 55.7 | 824 | 47.8 | 826 |
| Middle | 52.3 | 791 | 43.6 | 791 |
| Fourth | 44.1 | 723 | 38.0 | 727 |
| Highest | 33.5 | 618 | 19.5 | 623 |
| Total 0–59 | 52.0 | 3,833 | 42.4 | 3,847 |
| Total 6–59 | 54.9 | 3,521 | 44.7 | 3,532 |
Source: UMIS 2009 (as Table 6.3, page 60).
The other significant findings show some urban-rural variation in taking antimalarial medicines to prevent malaria during pregnancy. Women in urban areas are more likely to use malaria prophylaxis during pregnancy than women in rural areas.
Also women with more education as well as those in higher wealth quintiles are more likely than women with no or little education and women in lower wealth quintiles to use malaria prophylaxis during pregnancy.
The findings of increased usage of prophylactic anti-malarials by urban women compared to the rural, by women with more education compared to those with no education and by women in higher wealth quintiles compared to those in lower wealth quintiles have children with a lower prevalence of malaria although bed net ownership and usage and IRS coverage do not reveal significant differences in these groups. It still remains unclear how the mothers' malaria prophylaxis leads to a low prevalence of malaria in their children, in case it is the responsible factor.
The results of care seeking behaviour for the first place to report to are summarized in Table 4 which is derived from Table 3a.
Discussion
The children of urban residence (28.6%), of Kampala residence (17.4%), of mothers with the highest education (14.3%) and in the highest wealth quintile (33.3%) were found to have a lower malaria prevalence than the rural (56.0%), than those in the lowest wealth quintile (67.7%) or no education (55.8%), the differences being highly statistically significant, p< 0.001 in all cases. All these differences require a satisfactory explanation since they cannot be attributed to chance and neither to bed nets, treated or untreated or IRS since it was observed in the survey that there are no significant differences in long lasting insecticidal nets ownership and usage by rural and urban residence or by wealth quintile. Therefore the significant differences in the prevalences should be divorced from bed net ownership and use. IRS (Table 5), is also very unlikely to have been responsible for the differences in malaria prevalences since the report summarizes it as “there is very little difference by urban or rural residence or by wealth” [Page 34, (UMIS, 2009)]. However, the findings in the mid-northern districts of Uganda make it absolutely clear that it is in the districts where the percentage of households sprayed in the previous 12 months prior to the survey was highest in the whole country at 31.6% (Table 5) and that is also the same districts where the percentage of households sprayed in the previous 12 months prior to the survey or having at least 1 insecticide treated net was highest in the whole country at 77.8% is paradoxically where the highest prevalence of malaria was found at 62.5% by microscopy and 80.1% by the Rapid Diagnostic test. While the report is silent about this paradoxical finding, it raises pertinent issues why this phenomenon of diminishing returns should occur and whether either indoor spraying of houses or the use of bed nets or both are truly control measures for malaria in a heavily endemic area for malaria where around 80% of the under fives are having parasites. With such findings in an area where large numbers of nets have been used and IRS applied, it is hard to convince anybody that those are genuine measures for the control of malaria.
Table 5.
Indoor Residual Spraying
| Percentage of households reporting indoor residual spraying the previous 12 months and percentage of households sprayed in previous 12 months or having at least one ITN, by background characteristics, Uganda MIS 2009. | |||
| Background characteristics |
Percentage of households sprayed in previous 12 months |
Percentage of household sprayed in the previous 12 months or having at least one ITN |
Number of households |
| Residence | |||
| Urban | 3.7 | 48.0 | 710 |
| Rural | 5.8 | 49.4 | 3,711 |
| Region | |||
| Central 1 | 0.2 | 35.3 | 364 |
| Central 2 | 4.6 | 26.2 | 439 |
| Kampala | 5.5 | 52.3 | 273 |
| East Central | 0.4 | 33.8 | 557 |
| Mid Eastern | 0.6 | 59.6 | 530 |
| North East | 4.2 | 77.1 | 335 |
| Mid Northern | 31.6 | 77.8 | 552 |
| West Nile | 0.0 | 52.4 | 288 |
| Western | 0.2 | 34.1 | 377 |
| South Western | 1.8 | 44.7 | 705 |
| Wealth quintile | |||
| Lowest | 8.6 | 51.0 | 871 |
| Second | 8.1 | 47.9 | 931 |
| Middle | 3.4 | 50.5 | 848 |
| Fourth | 2.5 | 46.1 | 852 |
| Highest | 4.6 | 50.3 | 919 |
| Total | 5.5 | 49.2 | 4,421 |
Source: UMIS 2009 (as Table 5.7, page 54)
When one looks at Figure 1 and notices the numbers of cases reported for the years 2009 and 2010, they are well above 12,000,000 per annum for the whole country. The concern then about whether these are effective methods for the control of malaria on the ground becomes greater. Considering the work that has been done especially in the North of Uganda and the resources hitherto used, if the measures were actually effective malaria ought to have been reduced significantly. For these reasons a critical appraisal of the methods for the control of the disease become essential since we should not be forced to believe that bed nets and IRS will ever produce a dent in the malaria picture in Uganda. Malaria in Uganda is endemic which means it is “in us” and not epidemic or “upon us”. Therefore we need to remove the parasites which are already established in us with anti-malarial medicines instead of providing bed nets and IRS which seem to conserve the disease in the population.
The findings in table 3 show that the urban (77.4%) and Kampala City (62.4%) residents and those in the highest wealth quintile (63.8%) seek care in larger numbers than the rural residents (31.3%) and those with the lowest wealth quintile (25.1%) from both government and private hospitals and therefore get proper anti-malarial medicines prescribed by qualified medical doctors hence the lower prevalence of malaria among their children. It is therefore the anti-malarial medicines given to their children that are responsible for the low prevalence of malaria and not the use of bed nets or IRS since use of these shows no differences in these two groups of the high and low prevalence of malaria. From these findings, we can conclude that the control of malaria currently going on in Uganda is most likely due to anti-malarial medicines used for therapy and unlikely to be due to bed nets or IRS since there is no evidence to show that it is due to bed nets or IRS.
The anti-malarial medicines have demonstrated their effects in the urban and wealthy children. We too should take advantage of their action instead of concentrating on bed nets and IRS. Furthermore, the value of the bed-net as a good control measure of malaria is further thinned down when we realize that in scenarios of very high prevalence such as in the Mid-northern districts of Uganda where more than 80% of the under-fives have parasitaemia, a supply of 100 bed-nets will target to protect only less than 20% of the children since 80% of under-fives actually do not need the protection since they are already infected. In such a case, evaluation of the outcome will be based on less than 20% and may therefore not be easily noticed, hence the reality of the phenomenon of diminishing returns in the control of malaria using bed nets. This also explains its causation. Since prevalence in some places are already above 80%, as demonstrated in Table 1 and 4 it is not inappropriate to state that the higher the prevalence the less the numbers to be protected. In such scenarios, supply of nets with intent to protect the population is a mere “much ado about nothing” and there is need for serious urgent review of malaria policy by governments. The results of the malaria status especially in the Mid-northern districts cannot be described as a success story and it is sufficient enough to cause change of policy since their authenticity cannot be doubted and this should be done before they are relegated forever into the archives of history and completely forgotten.
At the present time, the indirect methods of control targeting the vector are cherished and valued as is the custom. However, there is need to re-evaluate the hospital records and see whether there is any realistic control of malaria on the ground, but Figure 1 shows that there is hardly any control of malaria. The whistle is blown and it is for the government through its Ministry of Health and partners to respond.
Countries like England had malaria. The disappearance of the disease was not caused by bed nets or IRS. S P James states as follows about the disease in 1929; “The diminution of local malaria in England was due neither to natural causes nor to the intentional application of any particular preventive method reputed to be specific, but to progressive improvements of a social and public health character (10). In Uganda, a similar happening is already taking place as well brought out by the prevalence of malaria among the urban compared to the rural children and the wealthy against the less wealthy, these being the “progressive improvements of a social and public health character referred to by SP James
Recommendations
In the circumstances recommending presumptive treatment for all to remove the source of infection, the malaria parasite from the population, using anti-malarial medicines there by depriving the mosquitoes the source of infection to carry to the people, should not be met with hostility since it is already acceptable for pregnant mothers. However, the chemotherapeutic agent need not be the same and a single dose of an effective medicine against parasites and gametocytes would be ideal, for best compliance. Screening for parasites should be avoided if the programme is to take off and be sustained since the disease prevalence is already above 54% (Table 4). Sezi C.L. (2004) had earlier suggested so (11, 12). This approach on its own if well done should be sufficient to control malaria and should not be done with methods that have failed to control the disease.
Acknowledgement
The author is indeed grateful to the Ministry of Health for producing an excellently done Uganda Malaria Indicator Survey of 2009 which is a treasure of highly informative data whose authenticity is far from the ordinary journal articles.
The same gratitude go to my colleagues with whom we did work on malaria in 1988, namely C. Nevill, K. Ochen, C. Munafu and the late D. Bek'Obita (RIP).
To my colleague Dr. Albert Kilian, I owe gratitude for the discussions we held during meetings.
I wish to thank Dr. Achilles Katamba, statistician Makerere University College of Health Sciences for carrying out the statistical work.
Lastly but not least I wish to thank Dr. Seremba Emmanuel, Consultant Physician Mulago hospital, for his great assistance in the preparation of the electronic version of this manuscript.
References
- 1.Okiro EA, Bitira D, Mbabazi G, Mpimbaza A, Alegana VA, Talisuna A, et al. Increasing malaria hospital admissions in Uganda between 1999 and 2009. BMC medicine. 2011;9:37. doi: 10.1186/1741-7015-9-37. Epub 2011/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talisuna A, Adibaku S, Dorsey G, Kamya MR, Rosenthal PJ. Malaria in Uganda: challenges to control on the long road to elimination. II. The path forward. Acta tropica. 2012;121(3):196–201. doi: 10.1016/j.actatropica.2011.06.013. Epub 2011/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jagannathan P, Muhindo MK, Kakuru A, Arinaitwe E, Greenhouse B, Tappero J, et al. Increasing incidence of malaria in children despite insecticide-treated bed nets and prompt anti-malarial therapy in Tororo, Uganda. Malaria journal. 2012;11:435. doi: 10.1186/1475-2875-11-435. Epub 2013/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. The American journal of tropical medicine and hygiene. 2006;75(2):219–225. Epub 2006/08/10. [PubMed] [Google Scholar]
- 5.Proietti C, Pettinato DD, Kanoi BN, Ntege E, Crisanti A, Riley EM, et al. Continuing intense malaria transmission in northern Uganda. The American journal of tropical medicine and hygiene. 2011;84(5):830–837. doi: 10.4269/ajtmh.2011.10-0498. Epub 2011/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kigozi R, Baxi SM, Gasasira A, Sserwanga A, Kakeeto S, Nasr S, et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PloS one. 2012;7(8):e42857. doi: 10.1371/journal.pone.0042857. Epub 2012/08/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parassitologia. 1999 Sep;41(1–3):10. Editor. [Google Scholar]
- 8.Dobson M. The Malariology Centenary Parassitologia. 41(1–3):21–32. [PubMed] [Google Scholar]
- 9.Nevill C, Ochen K, Munafu C, Bek' Obita D, Sezi CL. Response of P.falciparum to chloroquine and Fansidar in vivo and Chloroquine and amodiaquine in vitro in Uganda. East African Medical Journal. 1995;72(6):349–355. [PubMed] [Google Scholar]
- 10.James S. Parassitologia. 1999 Sep;41(1–3):19. [Google Scholar]
- 11.Sezi C. The Place of Presumptive antimalarial chemotherapy in malaria control in Africa. International Journal of Infectious Diseases; 11th International Congress on Infectious Disease (Abstracts); Official Publication of the International Society for Infectious Diseases; 2004. Mar, ISSN 1201-9712. [Google Scholar]
- 12.Sezi C. The Effect of Age on malaria and Mansonella perstans microfilaria parasitaemia in Uganda. International Journal of Infectious Diseases; 11th International Congress on Infectious Diseases (Abstracts); 2004. Mar, ISSN 1201-9712. [Google Scholar]