Abstract
Background
Naphthoquinone is a class of phenolic compounds derived from naphthalene. 7-Methyljuglone (7-MJ) is a naphthoquinone also known as ramentaceone or 6-Methyl-8-hydroxy-1,4-naphthoquinone or 5-Hydroxy-7-methyl-1,4-naphthoquinone or 7-Methyl-5-hydroxy-1,4-naphthoquinone or 5-Hydroxy-7-methyl-,1,4-naphtoquinone or 7-Methyl-5-hydroxynaphthalene-1,4-dione. This compound is a biologically active naphtoquinone, with a molecular weight of 188 g/mol mostly isolated in the genus Diospyros and Euclea.
Objectives
This review was aimed at providing available chemically and pharmacological data on 7-MJ.
Methods
The chemical and pharmacological data were retrieved from the well-known scientific websites such as Pubmed, Google Scholar, Reaxys, Scirus, Scopus, Sciencedirect, Web-of-knowledge and Scifinder.
Results
7-MJ was reported to have a variety of pharmacological activities such as antibacterial, antifungal, anticancer, antitubercular, anti-inflammatory and antiviral activities. The hemi-synthesis of the compound have been described.
Conclusions
The present review pooled out together the knowledge on 7-MJ, and can serve as the start point for future research and valorization accomplishments.
Keywords: 7-methyljugulone, biosynthesis, in vitro synthesis, pharmacology
Introduction
Naphthoquinone is a class of phenolic compounds derived from naphthalene occurring to some extent in fungi and are extremely common in higher plants and contain the naphthalene nucleus with two carbonyl groups on one nucleus usually at the ortho or para position (bicyclic). Naphthoquinones are widely distributed in plants, fungi and some animals and many were found to exhibit interesting range of pharmacological properties such as antibacterial1,2, antiviral3, trypanocidal4, anticancer5, antimalarial6,7 and antifungal8 activities. Other quinone-related scaffolds such as 2-H-pyran-3(6H)-one derivatives8 and 2,3-dideoxyhexenopyranosides9 are also known for their biological activities against Staphylococcus species and Mycobacterium tuberculosis. Interestingly, 7-MJ, a naphthoquinone exhibits most of these activities. Therefore, we undertook the present review to bring out pharmacological knowledge on 7-MJ. We also discussed herein the biosynthesis and the in vitro synthesis of this compound.
Methods
The chemical and pharmacological data were collected from the well-known scientific websites such as Pubmed, Google Scholar, Scirus, Scopus, Sciencedirect, Reaxys, Web-of-knowledge and Scifinder. The chemical names, 7-methyjulglone, ramentaceone or 6-Methyl-8-hydroxy-1,4-naphthoquinone or 5-Hydroxy-7-methyl-1,4-naphthoquinone or 7-Methyl-5-hydroxy-1,4-naphthoquinone or 5-Hydroxy-7-methyll-,1,4-naphtoquinoneor 7-Methyl-5-hydroxynaphthalene-1,4-dione were used as key word in the above websites and all relevant research papers were retrieved. The collected documents served as necessary tools and were analyzed for data collection.
Results
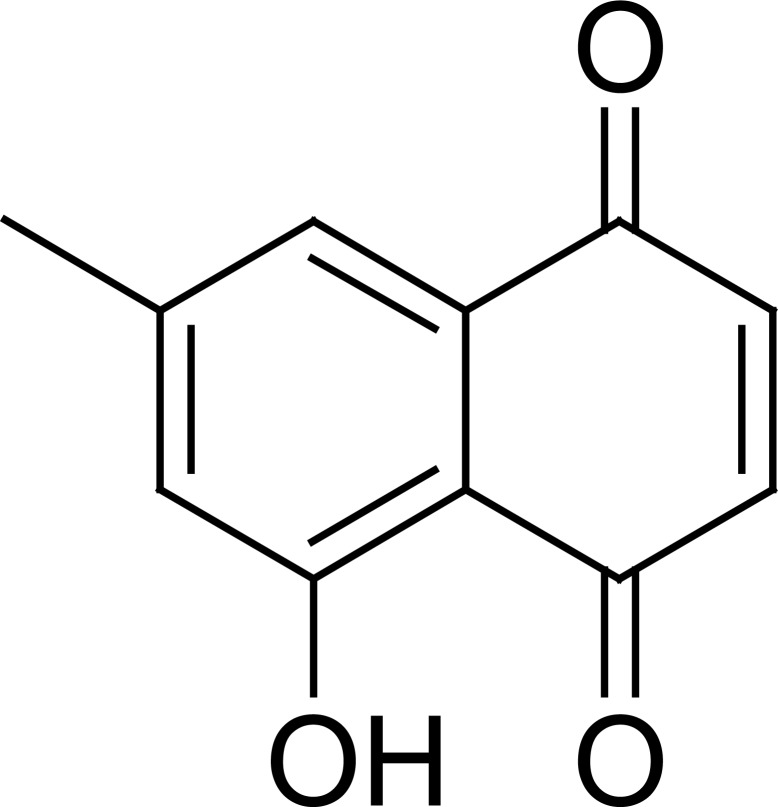
The naphthoguinone 7-MG has been isolated from different parts of the plants of the family Ebenaceae; This includes the bark of Diospyros maritima10, the twigs of Diospyros lycioides Desf.11, the roots of Diospyros lotus L.12, Euclea natalensisA.DC.13, Euclea undulata14 and Diospyros virginiana.15 7-MG (Fig. 1) appeared as an orange needles [m.p. 150–152°C,11 or 155°C16 or 156°C, or yellow needles; mp. 126°C; density 1.371g/cm3; boiling point: 427.2°C at 760 mmHg ; refractive index: 1.642]. IR(KBr, cm−1): 1670, 1645 (C=O); δH (CDCl3, 200 MHz); 11.84 (1H, s, 5-OH), 7.42 (1H, s, H-8), 7.06 (1H, s, H-6), 6.89 (2H, s, H-2, H-3), 2.41 (3H, s, CH3); found: (EI) 188.0475, C11H8O3 requires 188.0473.17
Fig. 1.
Chemical structure of 7-methyljuglone (7-MJ)
Biosynthesis and in vitro synthesis
It has been shown that higher plants have developed at least three separate pathways for the synthesis of the naphthoquinone carbon skeleton, including the shikimate, the homogentisate and the polyacetate-malonate pathways. The two main ways of naphthoquinones biosynthesis in higher plants include direct incorporation of shikimate17 or the homogentisic acid pathway involving the condensation of mevalonic acid and most probably toluhydroquinone17. It was demonstrated that 2-Methyl-juglone (plumbagin) by its striking structural similarity with juglone and menadion was expected to be formed via the shikimate pathway, by C-methylation of Juglone in the 2 position18. According to Durand and Zenk19 plumbagin and 7-MJ are the first napthoquinones in higher plants shown to be formed according to the acetate pathway which has long been known for the formation of naphthoquinones in fungi20. An hexaacetyl chain leads to the formation of these naphthalenes19.
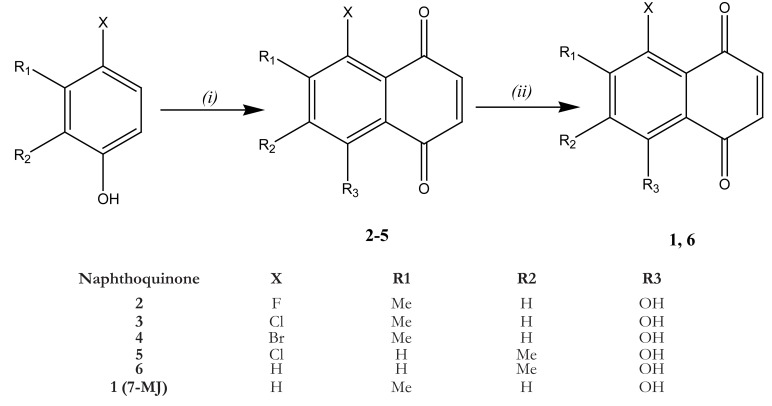
The synthesis of 7-MJ was realized by Mahapatra21 as follows: A mixture of anhydrous AlCl3 (40 g, 300 mmol) and NaCl (8 g, 137 mmol) were heated to 180°C. A mixture of appropriate 4-halo-3-methyl phenol (10.7 mmol) or 4-halo-2-methyl phenol and maleicanhydride (4 g, 40.8 mmol) was added to the above melt with vigorous stirring for 2 min, and then poured into a mixture of ice and 12 M HCl. The mixture was kept for 30 min, and the precipitate was filtered and dried at room temperature overnight. The residue obtained was powdered and extracted with n-hexane with vigorous stirring at 50°C. The extract was concentrated under reduced pressure and crystallized from chloroform to afford the halogenated products 2–5. A solution of 3 (200 mg, 0.90 mmol) in THF (20 mL) was added drop-wise to a solution of SnCl2 (1.0 g, 51 mmol) in 4 M HCl (70 mL) and THF (20 mL) at 60 °C and stirred for 3 h. It was then cooled and filtered into a solution of FeCl3. The resulting precipitate was filtered and dried to afford 7-MJ (Fig. 2).
Fig. 2.
Synthesis of 7-methyljugulone. Reagents and conditions: (i) maleic anhydride, AlCl3, NaCl, 180°C, 2 min; (ii) SnCl2, 4 M HCl/THF, 60°C, 2–4 h17.
The antifungal activities of 7-MJ were reported against Cryptococcus neoformans [50% inhibition of growth concentration (GI50) of 0.3 µg/mL and a Minimal Inhibitory Concentration (MIC) of 1 µg/mL], Candida albicans (GI50: 0.3 µg/mL; MIC: 20 µg/mL), Saccharomyces cerevisiae (GI50: 0.3 µg/mL; MIC: 1 µg/mL) and Aspergillus niger (GI50: 5 µg/mL; MIC: 300 µg/mL).10 7-MJ also displayed antifungal activities against Phomopsis obscurans [inhibition percentage (IP) of 97%] and Phomopsis viticola (IP of 53.4 – 54.3%) at 30 µM.15
7-MJ also demonstrated inhibitory activities against the Gram-positive oral streptococci, Streptococcus mutans (MIC of 156 µg/mL) and S. sanguis (MIC: 78 µg/mL) as well as against the Gram-negative anaerobic rods Prevotella gingivalis (MIC: 39 µg/mL) and P. intermedia (MIC: 78 µg/mL) frequently associated with human peritonitis known as gum disease.11 The antibacterial activity of this compound was also reported against M. luteus (GI50: 20 µg/mL; MIC: 1000 µg/mL).10
7-MJ showed an exceptional antitubercular inhibitory effects against Mycobacterium tuberculosis H37Rv with a MIC value of 0.5 µg/mL combined to a very good selectivity index of 30.22 on normal vero cells.21 This compound was found to react as potent subversive substrate for the NADPH-dependent enzyme mycothiol disulfide reductase of M. tuberculosis, which is one of several potential biological targets for it anti-mycobacterial activity17. In fact, M. tuberculosis lacks glutathione, instead it maintains millimolar concentrations of the structurally distinct low molecular weight thiol mycothiol (MSH).17 analogous to glutathione, MSH plays an important role in oxidative stress management and is oxidized to the symmetrical disulfide (MSSM) in the process. The NADPH-dependent enzyme mycothiol disulfide reductase (Mtr)22 helps to maintain an intracellular reducing environment by reducing MSSM back to MSH. MSH is essential for the growth of M. tuberculosis23 and MSH-deficient mycobacteria exhibit increased sensitivity to oxidative stress making this redox pathway a potential biological target for novel antitubercular chemotherapies.21 7-MJ also showed a very good antimycobacterial activity on other mycobacteria, with MIC values as low as 1.55 µg/mL against M. bovis, 1.57 µg/mL against M. smegmatis and 1.55 µg/mL on M. fortuitum.14 a 50% inhibition of growth concentration was also reported to be 5 µg/mL against M. avium.10
7-MJ displayed antiviral activities through the inhibition of recombinant reverse transcriptase of HIV-1 with 80 to 100% inhibition at the concentration ranges of 12.5 to 100 µg/mL.24 This phytochemical also inhibited the human rhinovirus 3C protease with an IC50 value of 6.4 µM.25
The cytotoxicity of 7-MJ was reported on several cancer cell lines including human oral epidermoid carcinoma [KB (IC50 value of 4.1 µM)], the human lung cancer cells [Lu1 (IC50: 13.2 µM)] and hormone-dependent human prostate cancer cells [LNCaP] (IC50: 3.7 µM).10 Though this compound was less toxic against the normal monkey kidney vero cells21, its toxicity toward the Umbilical Vein Endothelial Cells (HUVEC) was rather found to be higher (IC50: 5.7 µM) clearly indicating that possible chemotherapy involving pregnant women should be taken with caution. At 10 µM, 7-MJ induced apoptosis against leukemia HL60 cells with the percentage of Sub-G1 phase ranged from 10.3 – 27.5% whilst the IC50 value of 8.75 µM was reported on this cell line.26
Discussion
7-MJ was isolated mostly in the family Moraceae. especially in the genus Diospyros and Euclea. The occurrence of naphthoquinones in the two genera has been documented10–15. Its partial synthesis has also been described21, suggesting that the compounds can easily be available for any in vivo and possible clinical studies. Phytochemicals are routinely classified as antimicrobials on the basis of susceptibility tests that produce MIC in the range of 100 to 1000 mg/mL27. Activity is considered to be significant if MIC values are below 10 µg/mL for pure compound, moderate when 10<MIC<100 µg/mL and low when the MIC values is above 100 µg/mL28,29. On this basis, 7-MJ can be considered as significantly effective antimicrobial compound against Saccharomyces cerevisiae (MIC: 1 µg/mL)10, M. bovis (1.55 µg/mL), M. smegmatis (1.57 µg/mL) and M. fortuitum (1.55 µg/mL) 14. This clearly shows that this compounds can be explored more as potential drug against some pathogenic fungi and bacteria. In the US NCI screening program, a compound is generally considered to have in vitro cytotoxic activity, if the IC50 value following incubation between 48 and 72 h, is less than 4 µg/ml or 10 µM30.
On this basis, 7-MJ can also be considered as a good cytotoxic compound against several cancer cell lines such as KB, Lu1 and LNCaP10. Its selectivity toward cancer cells as compared with the normal vero monkey kidney cells 17 suggests that this compound is relatively safe for a potential anticancer treatment. Nevertheless, its toxicity toward the HUVEC indicated that caution should be taken when a potential treatment involve a pregnant women.
Conclusion
The present review obviously showed that 7-MJ could move forward into in vivo studies, in regards to its enormous pharmacological activities. Also, the study of the possible drug-drug interaction of 7-MJ is to be investigated. However, 7-MJ displayed high toxicity in HUVEC cells, clearly indicating that caution should be taken for any treatment involving pregnant women. The easy way of the in vitro chemical synthesis of 7-MJ ensure the availability of the compound for necessary future studies. Finally, the present work can served as a the future starting point for the developments of pharmaceutical from this potentially potent biological molecule.
Acknowledgment
The authors are thankful to Mr. Seydina Diene (University of Marseille, France); Mr. Arnaud T. D. Tchatchou of the University of the Witwatersrand, Johannesburg and Louis P. Sandjo of the University of Mainz-Germany for their fruitful contribution in the literature search.
References
- 1.Roushdi IM, Ibrahim ESA, Habib NS. Synthesis of 1.4-naphthoquinones-4-aryl(aroyl)hydrazones of potential antimicrobial activity. Pharmazie. 1976;31:856–859. [PubMed] [Google Scholar]
- 2.Osman SAA, Abdalla AA, Alaib MOJ. Synthesis of sulfanilamido-naphthoquinones as potential antituberculous agents. Pharm Sci. 1983;72:68–71. doi: 10.1002/jps.2600720116. [DOI] [PubMed] [Google Scholar]
- 3.Brinkworth RI, Fairlie DP. Hydroxyquinones are competitive non-peptide inhibitors of HIV-1 proteinase. Biochim Biophys Acta. 1995;1253:5–8. doi: 10.1016/0167-4838(95)00183-u. [DOI] [PubMed] [Google Scholar]
- 4.Salmon-Chemin L, Buisine E, Yardley V, Kohler S, Debreu MA, Landry V, Sergheraert C, Croft SL, Krauth-Siegel RL, Davioud-Charvet E. 2- and 3-substituted 1,4-naphthoquinone derivatives as subversive substrates of trypanothione reductase and lipoamide dehydrogenase from Trypanosoma cruzi: synthesis and correlation between redox cycling activities and in vitro cytotoxicity. J Med Chem. 2001;44:548–565. doi: 10.1021/jm001079l. [DOI] [PubMed] [Google Scholar]
- 5.Hazra B, Sur P, Roy DK, Sur B, Banerjee A. Biological activity of diospyrin towards Ehrlich ascites carcinoma in Swiss A mice. Planta Med. 1984;51:295–297. doi: 10.1055/s-2007-969713. [DOI] [PubMed] [Google Scholar]
- 6.Perry NB, Blunt JW, Munro MH. A cytotoxic and antifungal 1,4-naphthoquinone and related compounds from a New Zealand brown algae, Landsburgia quercifolia. J Nat Prod. 1991;54:978–985. doi: 10.1021/np50076a009. [DOI] [PubMed] [Google Scholar]
- 7.Yardley V, Snowdon D, Croft S, Hazra B. In vitro activity of diospyrin and Derivatives against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei brucei. Phytother Res. 1996;10:559–562. [Google Scholar]
- 8.Georgiadis MP, Couladouros EA, Delitheos AKJ. Synthesis and antimicrobial properties of 2H-pyran-3(6H)-one derivatives and related compounds. Pharm Sci. 1992;81:1126–1131. doi: 10.1002/jps.2600811117. [DOI] [PubMed] [Google Scholar]
- 9.Saquib M, Gupta MK, Sagar R, Prabhakar YS, Shaw AK, Kumar R, Maulik PR, Gaikwad AN, Sinha S, Srivastava AK, Chaturvedi V, Srivastava R, Srivastava BS. C-3 alkyl/arylalkyl-2,3-dideoxy hex-2-enopyranosides as antitubercular agents: synthesis, biological evaluation, and QSAR study. J Med Chem. 2007;50:2942–2950. doi: 10.1021/jm070110h. [DOI] [PubMed] [Google Scholar]
- 10.Gu JQ, Graf TN, Lee D, Chai HB, Mi Q, Kardono LB, Setyowati FM, Ismail R, Riswan S, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kroll DJ, Falkinham JO, 3rd, Wall ME, Wani MC, Kinghorn AD, Oberlies NH. Cytotoxic and antimicrobial constituents of the bark of Diospyros maritima collected in two geographical locations in Indonesia. J Nat Prod. 2004;67:1156–1161. doi: 10.1021/np040027m. [DOI] [PubMed] [Google Scholar]
- 11.Cai L, Wei GX, van der Bijl P, Wu CD. Namibian chewing stick, Diospyros lycioides, contains antibacterial compounds against oral pathogens. J Agric Food Chem. 2000;48:909–914. doi: 10.1021/jf9909914. [DOI] [PubMed] [Google Scholar]
- 12.Yoshihira K, Tezuka M, Natori S. Naphthoquinone derrivatives from the Ebenaceae. II. Isodiospyrin, bisisodiospyrin, and mamegakinone from Diospyros lotus L. and D. morrisiana Hance. Chem Pharm Bull. 1971;19:2308–2313. [Google Scholar]
- 13.Lall N, Meyer JJM, Wang Y, Bapela NB, van Rensburg CEJ, Fourie B, Franzblau SG. Characterization of intracellular activity of antitubercular constituents the roots of Euclea natalensis. Pharm Biol. 2005;43:353–357. doi: 10.1080/13880200590951829. [DOI] [PubMed] [Google Scholar]
- 14.McGaw LJ, Lall N, Hlokwe TM, Michel AL, Meyer JJ, Eloff JN. Purified compounds and extracts from Euclea species with antimycobacterial activity against Mycobacterium bovis and fast-growing mycobacteria. Biol Pharm Bull. 2008;31:1429–1433. doi: 10.1248/bpb.31.1429. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Habib E, León F, Radwan MM, Tabanca N, Gao J, Wedge DE, Cutler SJ. Antifungal metabolites from the roots of Diospyros virginiana by overpressure layer chromatography. Chem Biodivers. 2011;8:2331–2340. doi: 10.1002/cbdv.201000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedin PA, Collum DH, Langhans VE, Graves CH. Distribution of juglone and related compounds in pecan and their effect on Fusicladium effusum. J Agric Food Chem. 1980;28:340–344. [Google Scholar]
- 17.Zenk MH, Leistner E. Biosynthesis of quinones. Lloydia. 1968;31:275–292. [Google Scholar]
- 18.Teuscher E. “Pharmakognosie” part II. Berlin: Akademie Verlag; 1970. p. 245. [Google Scholar]
- 19.Durand R, Zenk MH. Biosynthesis of plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) via the acetate pathway in higher plants. Tetrahedron Lett. 1971;32:3009–3012. [Google Scholar]
- 20.Thomson RH. ‘Naturally Occurring Quinones’. London and New York: Academic Press; 1971. pp. 13–15. [Google Scholar]
- 21.Mahapatra A, Mativandlela SP, Binneman B, Fourie PB, Hamilton CJ, Meyer JJ, van der Kooy F, Houghton P, Lall N. Activity of 7-methyljuglone derivatives against Mycobacterium tuberculosis and as subversive substrates for mycothiol disulfide reductase. Bioorg Med Chem. 2007;15:7638–7646. doi: 10.1016/j.bmc.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 22.Patel MP, Blanchard JS. Expression, purification, and characterization of Mycobacterium tuberculosis mycothione reductase. Biochemistry. 1999;38:11827–11833. doi: 10.1021/bi991025h. [DOI] [PubMed] [Google Scholar]
- 23.Sareen D, Newton GL, Fahey RC, Buchmeier NA. Mycothiol is essential for growth of Mycobacterium tuberculosis Erdman. J Bacteriol. 2003;185:6736–6740. doi: 10.1128/JB.185.22.6736-6740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahapatra A, Tshikalange TE, Meyer JJM, Lall N. Synthesis and HIV-1 reverse transcriptase inhibition activity of 1,4-naphthoquinone derivatives. Chem Nat Comp. 2012;47:883–887. [Google Scholar]
- 25.Singh SB, Graham PL, Reamer RA, Cordingley MG. Discovery, total synthesis, HRV 3C-protease inhibitory activity, and structure-activity relationships of 2-methoxystypandrone and its analogues. Bioorg Med Chem Lett. 2001;11:3143–3146. doi: 10.1016/s0960-894x(01)00648-5. [DOI] [PubMed] [Google Scholar]
- 26.Kawiak A, Zawacka-Pankau J, Wasilewska A, Stasilojc G, Bigda J, Lojkowska E. Induction of apoptosis in HL-60 cells through the ROS-mediated mitochondrial pathway by ramentaceone from Drosera aliciae. J Nat Prod. 2012;75:9–14. doi: 10.1021/np200247g. [DOI] [PubMed] [Google Scholar]
- 27.Simões M, Bennett RN, Rosa EA. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat Prod Rep. 2009;26:746–757. doi: 10.1039/b821648g. [DOI] [PubMed] [Google Scholar]
- 28.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 2010;76:1479–1491. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 29.Kuete V, Efferth T. Cameroonian medicinal plants: pharmacology and derived natural products. Front Pharmacol. 2010;1:123. doi: 10.3389/fphar.2010.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuete V, Ngameni B, Wiench B, Krusche B, Horwedel C, Ngadjui BT, Efferth T. Cytotoxicity and mode of action of four naturally occuring flavonoids from the genus Dorstenia: gancaonin Q, 4-hydroxylonchocarpin, 6-prenylapigenin, and 6,8-diprenyleriodictyol. Planta Med. 2011;77:1984–1989. doi: 10.1055/s-0031-1280023. [DOI] [PubMed] [Google Scholar]