This study developed a human plasma-based epidermal substitute (hPBES) as an alternative to traditional cultured epithelial autografts for epidermal coverage in cases of massive burn, and critical quality controls were put in place for preclinical and clinical studies. This work highlights the importance of integrating relevant multiparameter quality controls into the bioengineering of new skin substitutes before they reach clinical development.
Keywords: Skin, Artificial, Burns, Keratinocytes, Fibrin, Tissue Engineering, Quality Control
Abstract
Cultured epithelial autografts (CEAs) produced from a small, healthy skin biopsy represent a lifesaving surgical technique in cases of full-thickness skin burn covering >50% of total body surface area. CEAs also present numerous drawbacks, among them the use of animal proteins and cells, the high fragility of keratinocyte sheets, and the immaturity of the dermal-epidermal junction, leading to heavy cosmetic and functional sequelae. To overcome these weaknesses, we developed a human plasma-based epidermal substitute (hPBES) for epidermal coverage in cases of massive burn, as an alternative to traditional CEA, and set up critical quality controls for preclinical and clinical studies. In this study, phenotypical analyses in conjunction with functional assays (clonal analysis, long-term culture, or in vivo graft) showed that our new substitute fulfills the biological requirements for epidermal regeneration. hPBES keratinocytes showed high potential for cell proliferation and subsequent differentiation similar to healthy skin compared with a well-known reference material, as ascertained by a combination of quality controls. This work highlights the importance of integrating relevant multiparameter quality controls into the bioengineering of new skin substitutes before they reach clinical development.
Significance
This work involves the development of a new bioengineered epidermal substitute with pertinent functional quality controls. The novelty of this work is based on this quality approach.
Introduction
Burns, including superficial and severe cases, constitute a major public health concern with high incidence (11 million people required medical attention for burns in 2004 [1]) and related costs of care. Despite significant improvements in terms of lethality, severe burns (with an annual incidence of 0.2 to 2.9 per 10,000 inhabitants of Europe) are usually the cause of substantial functional, cosmetic, and psychological sequelae [2]. In cases of full-thickness burns of >1 cm in diameter [3], burned tissues are excised as soon as possible to remove heat-induced toxic lipid-protein complexes [4], creating a loss of tissue that needs to be replaced [5]. Classical therapeutic approaches rely on split-thickness skin autografts, considered the gold standard treatment, which allow efficient wound coverage and functional results. In cases of massive burns, when unburned donor sites are too restricted to allow autograft harvesting, surgeons must consider alternative techniques for replacement of burned areas.
Other skin-coverage strategies should be considered, including combining skin autografts or allografts for dermal regeneration as a first step and subsequent permanent epidermal grafts as a second step, consisting of autologous treatment by split-thickness autografts or tissue-engineered epidermal substitutes. Cultured epithelial autografts (CEAs) have been used since the 1980s and are considered the only alternative when split-thickness autograft strategies are not feasible (usually for burn surfaces >50% of the total body surface area). CEAs consist of a cultured immature epidermis generated with autologous keratinocytes on a murine fibroblast feeder layer that forms sheets harvested enzymatically before grafting, as initially described by Rheinwald and Green [6]. This original model is still widely used by burn centers with successful results in full-thickness burns in terms of survival and permanent coverage [7–10]. Even if this methodology has proven its success, it has yet to reach the functional recovery obtained with autografts. The main weaknesses reported are poor dermal-epidermal junction maturation, fragility, use of animal proteins and/or cells in the culture process, and variable grafting efficiency [11–15]. In addition, this technique does not restore all of the functionality of healthy skin, and the reformed skin usually lacks lubrication, temperature regulation, sensitivity, and pigmentation [16]. Consequently, researchers are now focusing on whole-skin substitutes containing not only epidermis but also functional dermis. Ultimately, skin grafts may contain all the different components of native skin including nerve cells or appendages [17].
Retrospective clinical studies showed variable grafting efficiency, with graft-take surfaces varying from 0% to 100% [8, 13, 18, 19]. In addition to clinical aspects, it is of great importance to improve and standardize the biological characteristics of bioengineered grafts. There is a lack of comparative studies analyzing the processes and results obtained in different cell therapy centers. Furthermore, there is no universal standard concerning quality controls suitable for the qualification of a CEA before grafting. The functionality of grafted keratinocytes is critical, particularly their ability to adhere and anchor in the dermis, creating a new dermal-epidermal junction; to proliferate and differentiate in a well-organized pluristratified epidermis; and to migrate to re-epithelialize burned areas. Moreover, maintenance of functional stem and progenitor cell compartments within grafts is critical to ensure long-term epidermal homeostasis. Preservation of all of these characteristics may limit the variability of graft take. Functional assays such as clonogenic studies, long-term cultivation, in vitro stratification, or in vivo regeneration are relevant ways to further evaluate the presence of stem cells in epidermal substitutes [20, 21].
Another aspect of the skin-bioengineering domain concerns the matrix, or support, used to generate epidermal grafts. According to the original method of Rheinwald and Green, epidermal sheets are cultivated on a plastic surface. Matrices of fibrin obtained from purified fibrinogen have been introduced into the process in an attempt to improve the quality and grafting potential of the bioengineered epidermis [22, 23]. The proof of concept of fibrin for clinical interest was shown in different reports suggesting a gain in improvement of the dermal-epidermal junction with favorable graft-take efficiency [24, 25]. Despite increasing interest, the impact of fibrin on keratinocyte biology remains to be elucidated. More recently, clotted human plasma has been used instead of purified fibrinogen and has great potential as a skin matrix in vivo in animals [26, 27] and in human patients [28, 29]; however, to our knowledge, neither in vitro nor in vivo evaluation of plasma-based fibrin epidermal substitute is described in the literature.
In this report, we describe a process for culturing an immature epidermis that is able to reach final stratification once placed in physiological conditions and leads to a functional epidermal graft. The specificity of this work is in the integration of different levels of quality controls for the potency and identity of epidermal substitutes. We demonstrated the proof of concept that our new plasma-based epidermal substitute allows for good regeneration in a mouse model, and then we demonstrated that the clonogenicity and the regenerative potential of our epidermal substitute are in a promising range, using functional assays and phenotypical analyses promoting the standardization of the bioengineering process.
Material and Methods
Study Design
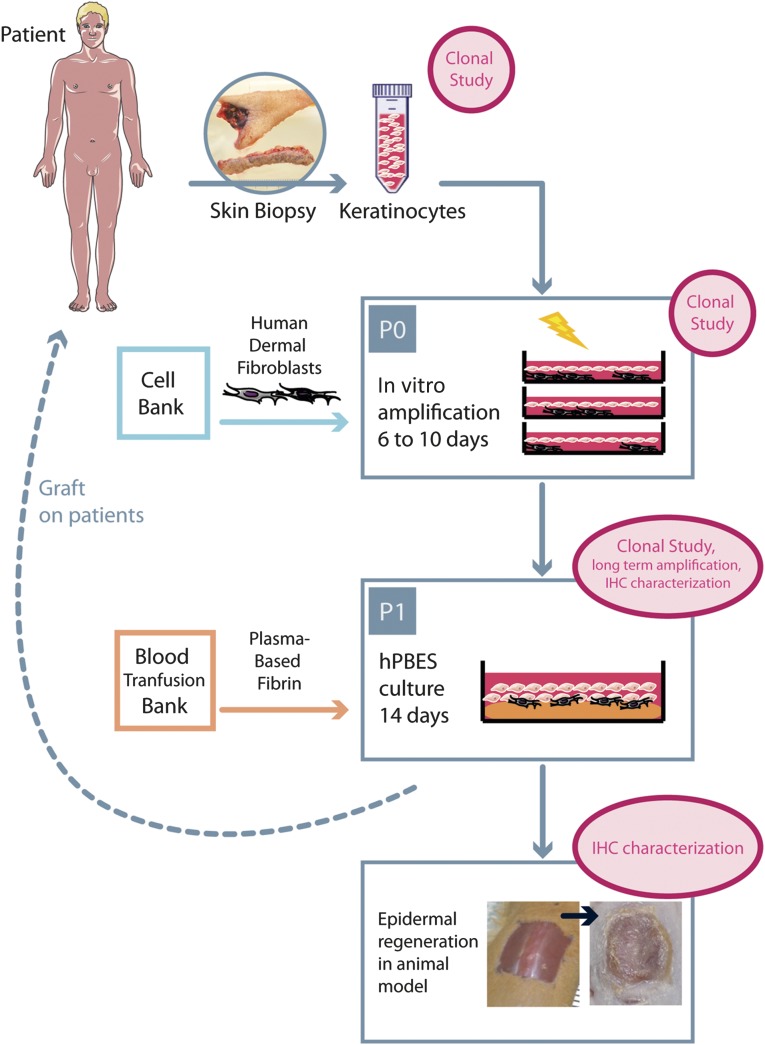
We developed a human plasma-based epidermal substitute (hPBES) relevant for clinical third-degree burn treatment. hPBESs obtained from two different donors were grafted on acute wounds in NOD-SCID mice to assess grafting capacity (Fig. 1). The in vitro culture process for hPBES was controlled by four keratinocyte donors using long-term cell cultivation, clonal analysis, histology, immunohistochemistry (IHC), and in vitro stratification. As an in vitro control, we used Epicel (Genzyme, Boston, MA, http://www.genzyme.com), the CEA medical gold standard, from four patients.
Figure 1.
Human plasma-based epidermal substitute culture process and evaluation strategy at critical points. Abbreviations: hPBES, human plasma-based epidermal substitute; IHC, immunohistochemistry; P, passage.
Isolation and Culture of Skin Cells
Skin biopsies were obtained from female patients undergoing breast-reduction surgeries after informed consent. Skin pieces were incubated overnight at 4°C in an enzymatic solution of 1.8 IU/ml dispase II (Roche, Basel, Switzerland, http://www.roche.com) and 0.0625% trypsin (Biochrom, Cambridge, U.K., http://www.biochrom.co.uk). Epidermis pieces were separated from dermis using forceps and further digested in 0.05% trypsin/EDTA (Gibco; Thermo Fisher Scientific, Waltham, MA, http://www.lifetechnologies.com/us/en/home/brands/gibco.html). The dermal tissue was digested in 2.4 IU/ml dispase II and 2.4 mg/ml collagenase II (Gibco; Thermo Fisher Scientific).
Fibroblasts were amplified in Dulbecco’s modified Eagle’s medium (Gibco; Thermo Fisher Scientific) supplemented with 5% platelet lysate [30], 10 μg/ml ciprofloxacin (Bayer HealthCare Pharmaceuticals, Berlin, Germany, http://pharma.bayer.com), and 10 IU/ml heparin Choay (Sanofi, Paris, France, http://en.sanofi.com). Cultures were incubated at 37°C in a fully humidified atmosphere containing 5% CO2. At 80% of confluence, fibroblasts were either replated using 0.05% trypsin/EDTA, frozen in liquid nitrogen, or irradiated with a 60-Gy dose of gamma rays to be used as a growth-arrested feeder layer for keratinocyte culture.
Keratinocytes were cultured in a medium described previously [21]. Primary keratinocytes were plated at a density of 2,400 cells per cm2 on growth-arrested fibroblasts (plated 4–12 hours earlier at 20,000 cells per cm2). Before reaching 70% keratinocyte confluence, the remaining fibroblasts were removed from the flasks by flushing, and keratinocytes were detached from plastic with 0.017% and 0.05% trypsin/EDTA, respectively.
Epidermal Substitutes
hPBES
Fresh frozen human plasma was collected at the Centre de Transfusion Sanguine des Armées (Clamart, France) from 10 donors that were biologically qualified in accordance with French legislation and pooled. A solution of pooled plasma mixed with 4.68 mg/ml sodium chloride (Fresenius, Bad Homburg, Germany, http://www.fresenius.com), 0.8 mg/ml calcium chloride (Laboratoire Renaudin, Itxassou, France), and 0.39 mg/ml Exacyl (Sanofi) was left to polymerize at 37°C for a minimum of 3 hours.
After plasma-based fibrin polymerization, growth-arrested fibroblasts and then keratinocytes were plated at the same density as for the primary culture. After 14 days of culture, epidermal substitutes were analyzed directly through colony-forming efficiency (CFE) assay, clonal microculture assay, or histology and IHC analyses; grafted in vivo after <10 hours of transport; or directly subjected to a stratification assay (placed at an air-liquid interface using Transwells; EMD Millipore, Billerica, MA, http://www.emdmillipore.com).
Control Epidermal Substitute
Redundant Epicel (Genzyme) keratinocyte sheets were provided by the Percy Hospital burn unit, with informed consent from family of burned patients receiving this exceptional treatment (we collected superfluous Epicel substitutes from four donors over 2 years). Epicel substitutes had traveled from the U.S. and were studied within producer-specified time limits.
In Vivo Animal Model
All procedures were carried out under a protocol approved by the ethics committee of “Paris Sud” University no 26. NOD/SCID mice were anesthetized via intraperitoneal injection of xylazine (6 mg/kg; Bayer HealthCare Pharmaceuticals) and ketamine (80 mg/kg; Virbac, Carros, France, http://www.virbac.com). hPBESs were cut to 1.21 cm2 to obtain a final size of 1 cm2 after retraction, grafted on full-thickness wounds of 1 cm2 on the back of each mouse, and protected by a silicon device (Interchim, Montluçon, France, http://www.interchim.com) [31]. Silicon protection was left in place until day 21 after grafting, when all animals were sacrificed with an overdose of anesthetics after sedation, according to the French institutional animal guidelines. hPBESs from two different donors were evaluated in vivo. Ten NOD-SCID mice were used per donor. All experiments and housing were performed under aseptic conditions. Wounds were excised and fixed in formalin for histology preparation.
Growth Factor Quantitation and CFE Assay
Matrices of plasma-based fibrin or purified fibrinogen (final solution of 1.5 IU/ml thrombin and 9 mg/ml fibrinogen; Tissucol; Baxter, Deerfield, IL, http://www.baxter.com) were immersed for 1 day in culture medium before medium collection. Growth factor concentration in media with or without matrix was evaluated by protein multiplex quantitation by Quantibody array (Tebu-Bio, Le-Perray-en-Yvelines, France, http://www.tebu-bio.com). For CFE evaluation, keratinocytes from 3 donors were plated at low densities (1,000 after extraction from epidermal substitute or 200 in primary culture) in 60-cm2 petri dishes coated with collagen I (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) on a growth-arrested murine 3T3-NIH feeder layer (60,000 cells per cm2). Keratinocytes were grown over 12 days in culture medium alone or complemented with growth factors: granulocyte colony-stimulating growth factor (G-CSF) at 3.1 pg/ml (R&D Systems, Minneapolis, MN, http://www.rndsystems.com), platelet-derived growth factor-BB (PDGF-BB) at 23.6 pg/ml (R&D Systems), hepatocyte growth factor (HGF) at 4.5 pg/ml (PeproTech, Rocky Hill, NJ https://www.peprotech.com), interleukin 1α (IL-1α) at 2.2 pg/ml (PeproTech), or IL-6 at 6.1 pg/ml (R&D Systems). Colony surface area was measured using ImageJ freeware (NIH, Bethesda, MD, http://imagej.nih.gov/ij/).
Clonal Microculture Assay
Microcultures were performed in 96-well plates coated with collagen I (Biocoat; Becton-Dickinson, Franklin Lakes, NJ, http://www.bd.com), as previously described [21]. The day before keratinocyte plating, growth-arrested fibroblasts were plated at 6,000 cells per cm2 in microculture wells. Single keratinocytes (primary keratinocytes, expanded keratinocytes, or keratinocytes extracted from epidermal substitutes) were deposited automatically in individual microwells using a flow cytometer coupled with a Cyclon automatic cell-deposition unit (MoFlo, Cytomation; Beckman Coulter, Pasadena, CA, https://www.beckmancoulter.com), with a sort droplet envelope between 1/2 and 5. In order to control the cell-deposition procedure in every independent experiment, additional single-cell plating was performed in series of microwells filled with phosphate-buffered saline containing 10 µg/ml Hoechst 33342 (Sigma-Aldrich). Staining of cell nuclei with Hoechst enabled counting of keratinocytes effectively deposited in multiple control wells under ultraviolet light with an inverted fluorescence microscope. Plating efficiency routinely exceeded 70% of wells filled with single keratinocytes. Two-cell wells were rarely observed (<1%). For size quantification of individual clones, clones were trypsinized individually, and keratinocytes were counted manually using a Malassez chamber after 2 weeks of culture.
Long-Term Growth
Keratinocytes extracted from hPBESs and Epicel were serially passaged, as described previously [21]. Briefly, cells were plated at 1,000 cells per cm2 in 25-cm2 culture flasks coated with collagen I (Biocoat; Becton-Dickinson). Before reaching 70% of confluence, keratinocytes were trypsinized, counted, and replated at a density of 1,000 viable cells per cm2 until growth capacity was exhausted. The cumulative number of population doublings (PDs) was calculated as follows: PD = (log N/N0)/log2. N0 represents the number of plated cells, and N represents the number of cells obtained after each culture step.
Histology and IHC
Samples were rinsed, fixed with buffered 4% formalin (Labonord, Templemars, France, http://www.labonord.com) for 1 day and then dehydrated with a graded series of ethanol treatments before paraffin embedding. Paraffin sections of 5-μm thickness were dried, deparaffinized, and stained with hematoxylin, phloxine, and safranin.
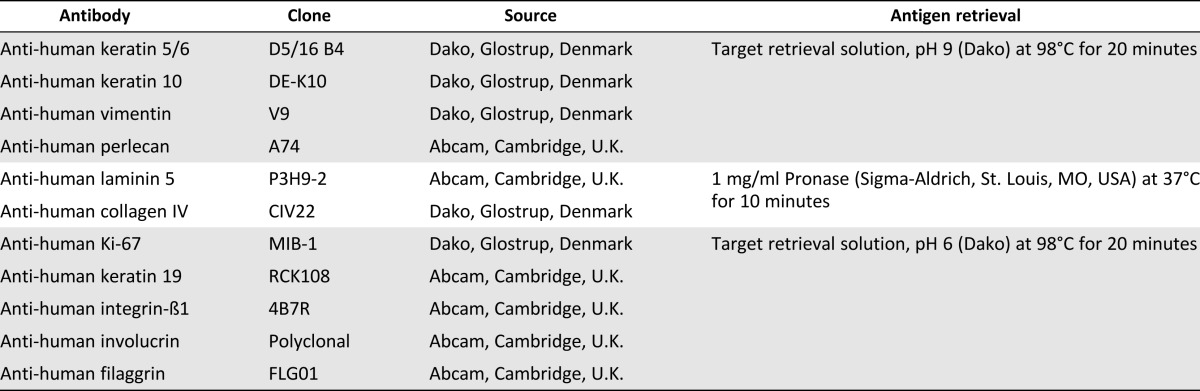
For immunohistochemistry, paraffin sections of 5-μm thickness were dried and deparaffinized. Antigen retrieval was achieved differently depending on the antibody used (Table 1). endogenous peroxidases were blocked with 3% H2O2 (Dako, Glostrup, Denmark, http://www.dako.com). Sections were then incubated at room temperature for 30 minutes with primary antibodies (Table 1). The following steps were performed using the LSAB2 kit (Dako) with a Dako autostainer instrument.
Table 1.
Antibodies and antigen retrieval
The percentages of human and mouse epidermal coverage were obtained by measuring the new epidermis (stained or not with integrin-β1 antibody specifically against human epitope) formed on acute wounds:
Ki-67 proliferation index and keratin 19% were obtained by counting stained cells in basal keratinocytes on 8 randomly chosen fields of a histology section for each donor of hPBES and Epicel (magnification ×20).
Vimentin and integrin-β1-stained areas were measured using Fiji freeware (Fiji Project, http://fiji.sc/Fiji). Stainings with 3,3′-diaminobenzidine (DAB) and hematoxylin were separated using color deconvolution on 8 randomly chosen fields (magnification ×20). Areas of both DAB and hematoxylin in keratinocyte layers were measured.
Mean percentage of markers for each donor was graphically represented with the median.
Statistical Analysis
Statistical analysis was achieved using GraphPad Prism software (GraphPad, San Diego, CA, http://www.graphpad.com). Data on CFE assays were analyzed using two-way analysis of variance or t tests for comparison with the effect of medium alone. Mann-Whitney tests were used to compare four donors of hPBES with four donors of Epicel for IHC quantifications.
Results
hPBES Is Able to Regenerate a Fully Differentiated Epidermis While Retaining its Stemness and Proliferation Potential After 3 Weeks In Vivo
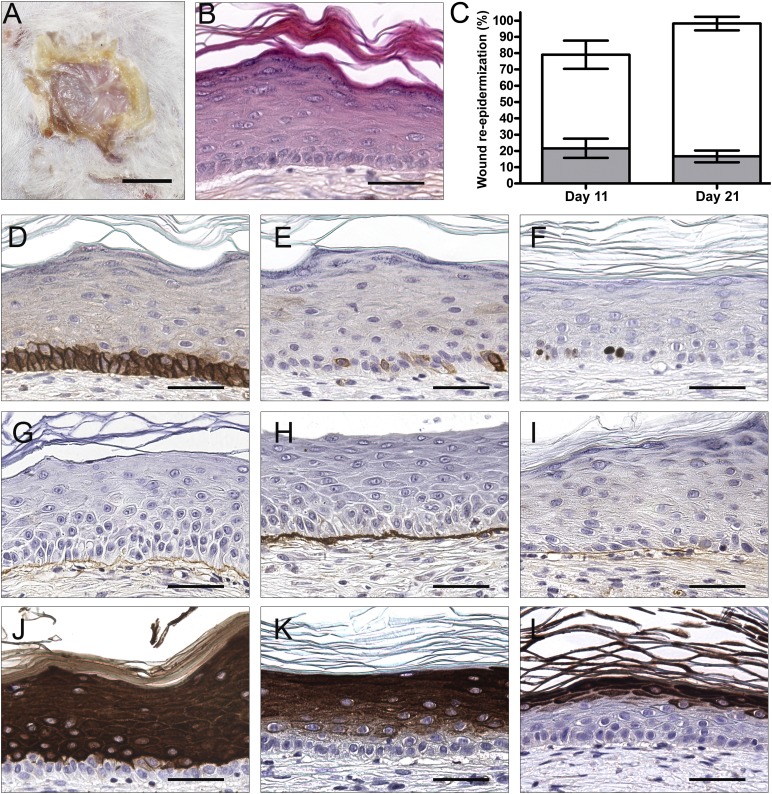
We developed a new epidermal substitute for clinical burn treatment with human keratinocytes and fibroblasts on a matrix based on human plasma. The culture process, starting from a patient skin biopsy, requires 6–10 days of patient keratinocyte amplification on growth-arrested human dermal fibroblasts from our cell bank and then 14 days of epidermal substitute formation on a plasma-based matrix (Fig. 1). We decided to investigate the regenerative potential of our hPBES in an in vivo model of acute wounds in NOD-SCID mice. At 11 (data not shown) and 21 days (Fig. 2A) after grafting, newly formed epidermis of pearly pink aspect with fine wrinkles could be clearly distinguished macroscopically in hPBES-grafted zones compared with the white surrounding mice epidermis. We observed hPBES graft take on the totality of the 20 mice grafted with hPBES (2 donors tested). Histologically, we observed the complete stratification of human keratinocytes, forming several layers up to the stratum corneum and showing signs of desquamation. Nonetheless, a well-organized basal layer with cuboidal keratinocytes was observed, as expected in a healthy epidermis (Fig. 2B).
Figure 2.
Human plasma-based epidermal substitutes (hPBESs) are able to form and maintain a differentiated epidermis when implanted in vivo for 3 weeks. Representative macroscopic view (A) and hematoxylin, phloxine, and safranin staining (B) of resulting epidermis 3 weeks after hPBES (2 donors) grafting in NOD-SCID mice (10 mice per donor). (C): Percentage of wound re-epithelialization for human epidermis (white) and mouse epidermis (gray) in mean ± SEM. Immunohistochemistry staining was used to characterize the resulting epidermis (positive staining is brown): integrin-β1 (D), keratin 19 (E), Ki-67 (F), collagen IV (G), laminin 5 (H), perlecan (I), keratin 10 (J), involucrin (K), filaggrin (L). Scale bars = 500 μm (A), 100 μm (B–L).
We further characterized this new epidermis by immunohistochemistry. We demonstrated the human origins of the keratinocytes composing this new epidermis, with an antibody specific for human integrin-β1 (supplemental online Fig. 1). Human and mouse re-epithelialization was quantified based on integrin-β1 expression at 11 and 21 days after grafting. The percentage of new mouse epidermis remained stable between days 11 and 21, whereas the percentage of human epidermis increased, showing that complete re-epithelialization was achieved mainly by human keratinocytes from hPBES at day 21 (Fig. 2C). We characterized the newly formed human epidermis resulting from hPBES graft by immunohistochemistry. At 21 days after grafting, basal keratinocytes expressed integrin-β1 in a pattern similar to healthy mature skin (Fig. 2D). Stem cells expressing keratin 19 were noticed in the basal layer 3 weeks after grafting (Fig. 2E). Similarly, proliferating cells (Ki-67) could be noticed in basal keratinocytes (Fig. 2F). Dermal-epidermal junction proteins, such as collagen IV (Fig. 2G), laminin 5 (Fig. 2H), and perlecan (Fig. 2I), were deposited along the keratinocyte basement membrane on the mouse granulation tissue. The differentiation pattern was close to normal skin, with keratin 10 expressed in all suprabasal layers (Fig. 2J), involucrin became more and more restricted to the external layers (Fig. 2K), and filaggrin was restricted to the superficial layers (Fig. 2L).
These results show that hPBES has the regenerative potential to stratify and maintain a fully differentiated epidermis with characteristics close to healthy skin at 3 weeks after grafting.
Plasma-Based Matrix Releases Growth Factors Involved in Keratinocyte Clonogenicity Enhancement
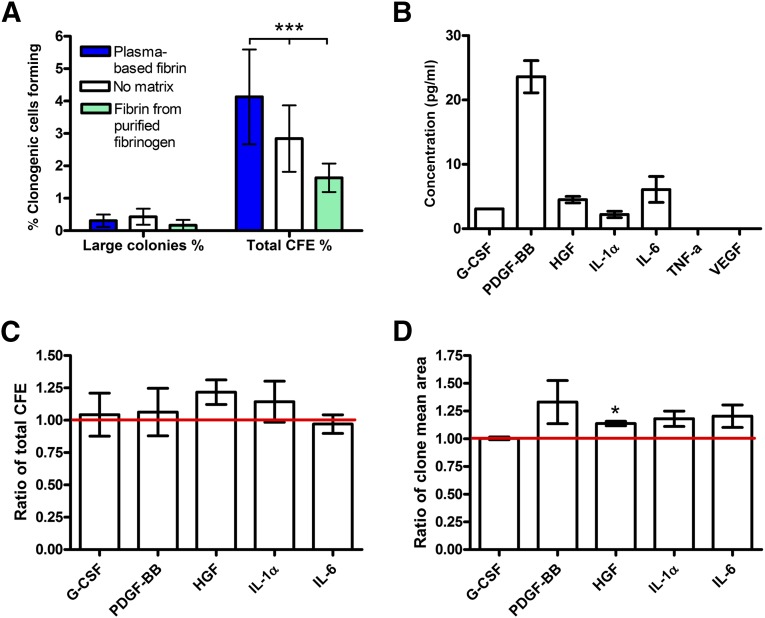
After validating the ability of hPBES to regenerate an epidermis and to ensure an engraftment process in vivo, we evaluated the advantages brought by the plasma-based fibrin in terms of clonogenicity in vitro. We extracted the keratinocytes from epidermal substitutes cultivated on plasma-based fibrin (hPBES), on fibrin from purified fibrinogen, or without matrix support and evaluated their CFE (Fig. 3A). We observed no variation in the number of large colonies, whereas a higher total CFE percentage (CFE%) for keratinocytes extracted from hPBES (4.1%) was observed compared with total CFE% from condition without matrix (2.8%) or from fibrin of purified fibrinogen (1.6%). We then investigated whether the presence of plasma factors contained in plasma-based fibrin could explain these differences. We showed that G-CSF, PDGF-BB, HGF, IL-1α, and IL-6 were released in the culture medium after 1 day of submersion of plasma-based matrix, whereas none of these factors were released by fibrin from purified fibrinogen (except for IL-6 used in further experiments as a control) (Fig. 3B). We further investigated whether these concentrations of growth factors had an effect on keratinocyte CFE and could explain the difference observed in Figure 3A. We demonstrated that HGF increased keratinocyte total CFE by 1.22 fold (p = .235, n = 3). The release of this factor could partially explain the relatively greater CFE% of keratinocytes from hPBES than from epidermal substitutes with no matrix. Moreover PDGF-BB (p = .231, n = 3), HGF (p = .021, n = 3), IL-1α (p = .122, n = 3), and IL-6 (p = .181, n = 3) slightly increased the mean area for each clone. These results show that using our human plasma-based matrix instead of fibrin from purified fibrinogen or no matrix is favorable for keratinocyte clonogenicity.
Figure 3.
Evaluation of plasma-based matrix growth factor release and its role in enhancing keratinocyte clonogenicity. (A): Percentage of clonogenic cells forming large colonies (>4 mm in diameter) and total CFE from epidermal substitutes cultured on plasma-based fibrin, with no matrix, or with fibrin from purified fibrinogen. Data shown as mean ± SEM for three donors. ∗∗∗, p < .0001, two-way analysis of variance. (B): Released growth factor concentration (picograms per milliliter) in culture medium after 1 day of plasma-based matrix immersion. Data shown as mean ± SEM for four independent experiments. Percentage of total CFE with G-CSF, PDGF-BB, HGF, IL-1α, or IL-6 on percentage of total CFE in culture medium alone (C) or ratio of mean area of colonies in the presence of factors on mean area of colony in culture medium alone (D). Data shown as mean ± SEM for three donors. ∗, p < .05, t test with culture medium alone. Abbreviations: CFE, colony-forming efficiency; G-CSF, granulocyte colony-stimulating growth factor; HGF, hepatocyte growth factor; IL-1α, interleukin 1α; IL-6, interleukin 6; PDGF-BB, platelet-derived growth factor-BB; TNF-a, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Follow-up of Keratinocyte Clonogenic Potential Throughout the hPBES Bioengineering Process
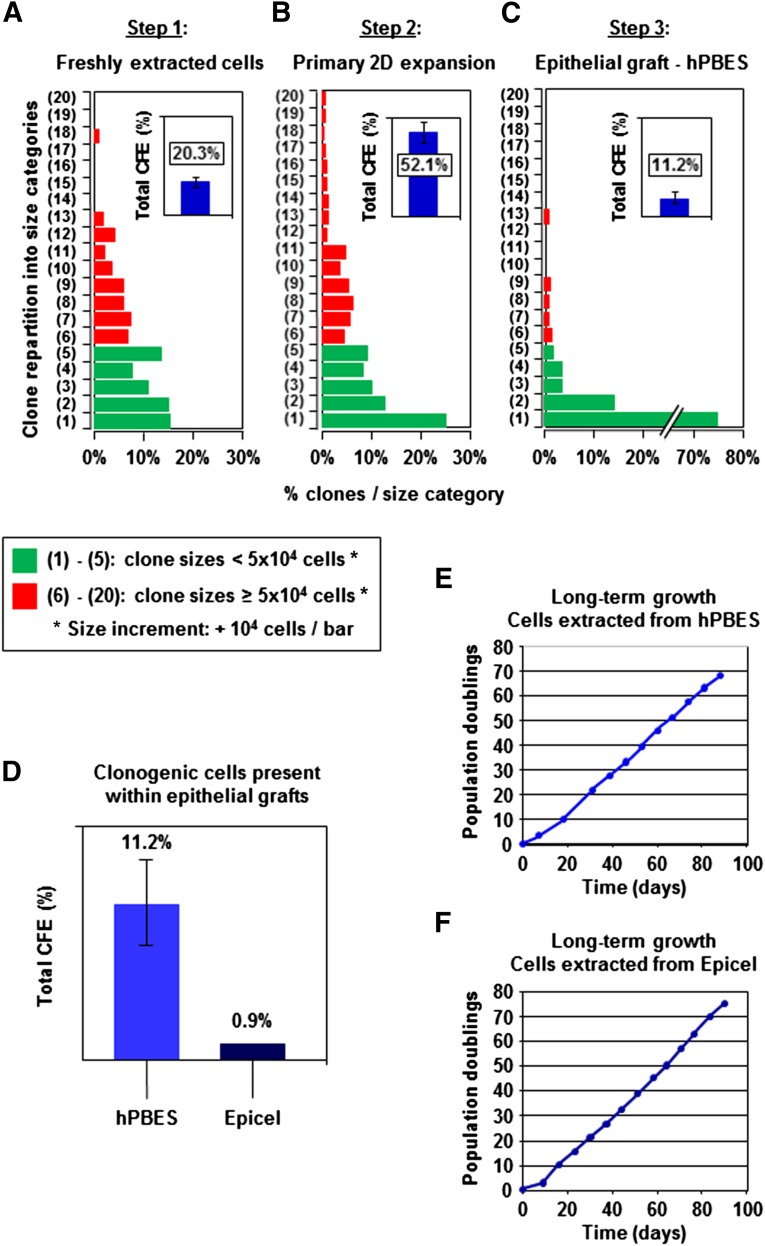
Our investigations of clonogenicity of hPBES were deepened using a clonal microculture assay compared with an epidermal clinical reference: Epicel. Keratinocyte CFE was characterized at three critical steps of the hPBES bioengineering process leading to production of that epidermal substitute. Analysis performed on keratinocytes freshly extracted from native skin samples provided a quality control for this initial cellular material (Fig. 4A). Next, estimation of CFE was performed after the step of two-dimensional (2D) culture (Fig. 4B) and provided estimation of the growth capacity of the expanded keratinocytes used to generate the three-dimensional (3D) epidermis substitute. Finally, CFE analysis was performed on keratinocytes extracted from bioengineered 3D hPBES (Fig. 4C) to determine the presence of immature clonogenic keratinocytes. Freshly extracted keratinocytes exhibited the classical clonal growth profile, ranging from low proliferative colonies to large colonies containing >105 cells. As expected, clonogenic keratinocytes were enriched during 2D expansion, and total CFE increased from 20.3% ± 3.1% at culture initiation to 52.1% ± 6.0% by the end of this culture step. Importantly, the short expansion step used in our bioengineering process preserved the keratinocyte fraction with the highest clonogenic capacity, as large colonies containing >105 cells were easily detected. In contrast, total CFE was markedly reduced in bioengineered 3D hPBES, decreasing to 11.2% ± 3.7% (Fig. 3D). A critical finding is that this CFE diminution in hPBES was associated with a relative rarefaction of large colonies containing >5 × 105 cells. Although the marked CFE reduction detected in hPBES (probably due to the confluence and stratification occurring during 3D culture) appeared to be of great importance, it remained higher than CFE detected in typical samples of the CEA model Epicel, which has been used successfully for burn treatment for decades (Fig. 4D). Importantly, we could show that the observed CFE reduction did not reach a critical level because keratinocytes extracted from hPBES and Epicel were both capable of sustaining long-term proliferation exceeding 60 population doublings in 2D culture (Fig. 4E, 4F).
Figure 4.
Follow-up of keratinocyte clonogenic potential throughout the hPBES bioengineering process. Total CFE and clone-size distribution characterized in keratinocytes freshly extracted from skin samples (A), after 2D expansion (B), and in bioengineered 3D hPBES (C). Clone size distributions: pooled data from three independent experiments. Total CFE estimations: mean ± SEM for four independent experiments. (D): Estimation of total CFE in hPBES and Epicel (four samples for hPBES, two samples for Epicel). Long-term growth potential of keratinocytes extracted from hPBES (E) and Epicel (F) (typical experiments shown). Abbreviations: 2D, two dimensional; CFE, colony-forming efficiency; hPBES, human plasma-based epidermal substitute.
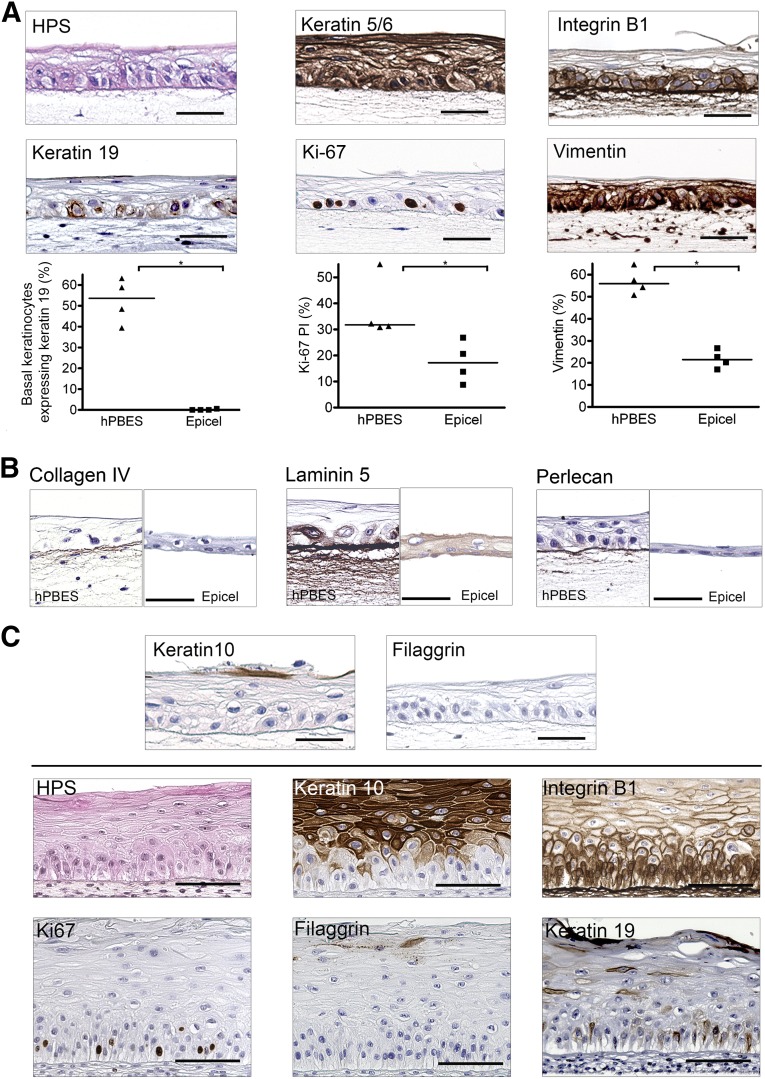
hPBES Exhibits In Vitro Characteristics of Skin Basal Layer Enriched With Highly Proliferative Keratinocytes
We aimed to characterize the phenotype of our hPBES by histology and IHC after culture in immersion before grafting (Fig. 5A–5C, upper part) or after an in vitro stratification assay at the air-liquid interface as a quality control (Fig. 5C, lower part). We observed a well-organized basal layer of cuboidal or columnar keratinocytes similar to that of healthy skin (Fig. 5A). To further characterize the basal layer of hPBES, we used IHC to analyze the expression of markers (in brown) linked with adhesion, stemness, or activation. hPBES keratinocytes expressed keratin 5/6 (marker of basal and activated cells) through all the layers and integrin-β1 (marker of basal cells) restricted to the basal layer (Fig. 5A). A strong deposition of integrin-β1 was seen at the basal lamina (Fig. 5A). The mean number of keratin 19-expressing keratinocytes in the basal layer was high in hPBES (52.46% ± 5.33%), whereas keratin 19 stem cells were scarcely observed in Epicel (0.15% ± 0.15) (Fig. 4A). The measurement of hPBES Ki-67 proliferation index showed an important number of proliferative basal keratinocytes (37.34% ± 5.89%), more than twice the Epicel Ki-67 proliferation index (Fig. 4A). Next, vimentin expression was analyzed to determine whether keratinocytes in epidermal substitutes were in epithelial-mesenchymal transition (EMT) such as during wound healing. Image analysis showed that a large percentage of hPBES cells were expressing vimentin (56.83% ± 2.95%), almost 3 times more than in Epicel (Fig. 5A).
Figure 5.
hPBESs exhibit basal keratinocyte phenotype with high proliferative and migratory potential while keeping differentiation ability. Each view represents independent experiments performed with four donors of keratinocytes for hPBES and four Epicel substitutes. Hematoxylin, phloxine, and safranin and immunohistochemistry staining (positive staining is brown) and quantification showed basal properties (individual donors and mean represented four donors, ∗, p = .03, U = 0.00, Mann-Whitney tests) (A), the presence of dermal-epidermal junction proteins (B), and low expression of differentiation markers (C) (upper bar) of hPBES keratinocytes. When placed at the air-liquid interface, hPBESs are able to stratify and differentiate (C) (lower section). Scale bars = 50 μm. Abbreviations: hPBES, human plasma-based epidermal substitute; PI, proliferation index.
We further investigated, by immunohistochemistry, the influence of our culture process on dermal-epidermal junction maturation. Collagen IV, laminin 5, and perlecan were deposited at the junction of basal keratinocytes and fibrin matrix of our hPBES (Fig. 5B). Laminin 5 was greatly expressed by basal keratinocytes and diffused through the fibrin scaffold. These results could explain the good adhesion of our hPBES to the dermal bed grafted in vivo (via the conservation of dermal-epidermal junction proteins). The use of a fibrin matrix allowed the conservation of these basal proteins, which are usually removed from CEA during enzymatic detachment such as that observed in Epicel (Fig. 5B).
These results suggest that hPBES exhibits morphological characteristics and proliferative and EMT phenotypes more prone to regenerate a fully mature epidermis once grafted in vivo compared with the medical reference Epicel.
hPBES Keratinocytes Express a Low Differentiated Phenotype While Maintaining Stratification Potential
The basal phenotype of hPBES keratinocytes in immersion was also confirmed by the absence of the terminal differentiation marker filaggrin and the very scarce expression of the suprabasal marker keratin 10 (Fig. 5C, upper bar). We evaluated the potential of stratification of hPBESs in vitro by placing them at the air-liquid interface (Fig. 5C, lower section). When subjected to these tests, hPBES keratinocytes can form several layers of healthy skin, such as the stratum basale, stratum spinosum, and stratum granulosum, while conserving columnar basal cells. Basal cells were maintained in a proliferative state, as assessed by the presence of Ki-67-expressing basal keratinocytes. Moreover, hPBES differentiation can be attested by the suprabasal expression of markers such as keratin 10. The late differentiation marker filaggrin was scarcely expressed at this stage of the stratification process. Despite showing differentiation characteristics, keratinocytes of the basal layer were still expressing integrin-β1 like healthy skin epidermal basal cells. Furthermore, keratinocyte stem cells expressing keratin 19 persisted in the basal layer of stratified hPBES. These results suggest that, in vitro at the air-liquid interface, our hPBES is able to differentiate and stratify like mature skin.
Discussion
Autologous epidermal substitutes are intended to completely and quickly regenerate the epidermal barrier that is missing on burn wounds, sustaining maintenance over the long term. To induce rapid and complete wound healing with high grafting efficiency, epidermal substitutes should be characterized by a high potential for cell proliferation and subsequent differentiation close to healthy skin. Our in vivo results, achieved with a combination of quality controls, showed that the hPBES culture process produced epidermal substitutes fulfilling these criteria. In addition, we observed good grafting quality with the regeneration of a mature epidermis in vivo. Consequently, this new substitute could provide an interesting alternative treatment for epidermal regeneration in massively burned patients. In our study design, we focused on setting efficient potency and identity quality controls to obtain thorough characterization of hPBES. To our knowledge, this study is the first to evaluate the grafting potential of an epidermal substitute by using clonal assays and phenotypical analyses before grafting. Moreover, these results were compared with a medical reference, Epicel.
When engineering an epidermal substitute for treatment of massive burns, the first challenge faced is the very high culture amplification ratio needed to obtain large surfaces of engineered epidermis (total body surface area is 1.8 m2) from the small skin biopsy harvested initially (2–6 cm2). The risk of this high expansion rate could be exhaustion or alteration of clonal cell diversity within epidermal substitutes. In our study, we analyzed the clonogenic potential [32] of cultured keratinocytes in two dimensions during the expansion step and in three dimensions after epidermal substitute formation (Fig. 4A–4C). Despite a clonogenic reduction in hPBES keratinocytes inherent to multilayered epidermal substitute culture (Fig. 4C), greater performance for clonogenic cell maintenance was obtained with our process compared with Epicel, which was analyzed for reference (Fig. 4). In addition, we showed that plasma growth factors such as PDGF-BB, HGF, and IL-1α, released from the plasma-based matrix in culture medium, could be involved in the better clonogenicity of keratinocytes from hPBES (Fig. 3) and in keratinocyte proliferation and/or migration in general [33, 34]. In our study, we also combined these functional assays with a phenotypical evaluation of the stemness marker keratin 19 [35, 36] and observed enrichment in basal stem keratinocytes expressing keratin 19 in our hPBES (Fig. 4A) compared with Epicel. The link between long-term engraftment and clonogenic content of cellular therapy products has been emphasized in different medical applications. Notably, the presence of highly proliferative clones (holoclones) within cultured epithelial grafts has been associated with good regeneration prognosis in the treatment of massive full-thickness skin burns [24]. Moreover, during wound healing, interfollicular epidermis stem cells stably contribute to epidermal repair, whereas progenitor contribution is transient [37]. A parallel could be done with hematopoietic stem cell (HSC) transplantation, in which both short-term and long-term repopulating cells are needed [38, 39], and research is ongoing for ex vivo expansion of HSCs to avoid alteration of the stem cell compartment [40–42]. Another possible risk associated with rarefaction of the clonogenic compartment is forced proliferation centered on a few remaining progenitors, creating strong stress and potential unfortunate events such as cell senescence or genomic abnormalities; however, the classical process for epidermal substitutes does not generate artifacts, leading to the selection of transformed cells [43]. These criteria favor long-term engraftment and homeostasis of hPBES resulting from the conservation of clonal diversity and keratin 19-positive stem cell enrichment during our culture process.
High proliferation and migration levels of keratinocytes and low differentiation phenotype are related to rapid and efficient wound healing and thus are key characteristics for epidermal substitutes before grafting. We demonstrated in our study that our process induced high proliferation and expression of vimentin (an epithelial-mesenchymal transition marker linked with high cell motility [44–46]) (Fig. 4A). These levels decreased to normal levels after grafting (data not shown). Use of functional migration assays may be impeded by the use of 3D matrices. Consequently, phenotypical markers associated with epithelialization, such as vimentin or connexins [47], are of interest for 3D tissue-engineered substitute characterization. In our study, hPBES keratinocytes that expressed keratin 10 or that processed profilaggrin into filaggrin [48] before grafting were very rare, showing that the culture process prevents cells from engaging too early in the differentiation pathway (Fig. 4C).We also proved that hPBES keratinocytes were able to rapidly differentiate in vitro when subjected to an air-liquid interface (Fig. 4C) and to form protective overlying terminally differentiated keratinocytes expressing filaggrin in vivo (Fig. 2). This low level of differentiation is required for an epidermal substitute before grafting because it has been shown that undifferentiated keratinocytes are more prone to regenerate a healthy epidermis over the long term [49]. Our in vitro quality controls provide information about the state of keratinocytes in terms of proliferation, migration, and differentiation and indicate that the hPBES phenotype is close to one of the epidermal tongue during wound healing.
In addition to the different cellular aspects, a major challenge with epidermal substitutes after grafting is the reformation and rapid maturation of a functional dermal-epidermal junction. During the culture process, keratinocytes and fibroblasts synthesize dermal-epidermal junction proteins on either a plastic or fibrin matrix. The use of a fibrin matrix presents the advantage of conserving these proteins [22], which are otherwise cleaved by enzymatic treatment during CEA detachment from the culture vessel [50], as confirmed in our study (Fig. 5B). Basement membrane proteins determine sustained basal keratinocyte functionality, growth, and survival and epithelial architectural maturation [51]. Moreover, pre-existing basement membrane proteins are essential to induce rapid and functional assembly of dermal-epidermal junction proteins after grafting [52] and thus may enhance the graft-take rate in vivo [53]. The conservation of basement membrane with the use of fibrin matrix (Fig. 5B) might be the cause of the high graft take observed in vivo with hPBES (Fig. 2) or in other in vivo studies [54]. Finally, plasma-based support combines advantages brought by fibrin support for basement membrane protein conservation with plasma growth factors released by the matrix (Fig. 3) that can improve the deposition of dermal-epidermal junction proteins [55–57]. Consequently, the use of a plasma-based matrix for our epidermal substitute constitutes a promising improvement to solve one of the main drawbacks cited for cultured epidermal autografts in clinic: dermal-epidermal junction immaturity.
Conclusion
In response to medical needs for epidermal coverage for massively burned patients, we developed a new human plasma-based epidermal substitute and evaluated it with a combination of phenotypical and functional quality controls. Our culture process allows the production of epidermal substitutes with efficient graft take, resulting in a well-regenerated epidermis that has a healthy epidermis macroscopic appearance and that fulfills microscopic requirements for normal skin characteristics. These favorable results encourage us to go forward with clinical development in compliance with regulatory requirements. We are confident that our new human epidermal substitute will rapidly reach clinical trial evaluation based on the encouraging proof of concept obtained in in a mouse model and strengthened by the positive results obtained with our comprehensive quality controls.
Supplementary Material
Acknowledgments
We thank Dr. St. Blancard for his advice on anatomopathology and INSERM Unit 1004 for access to the experimental animal facilities. We also thank Drs. Bey and Bargues, heads of the plastic surgery department and the burn treatment unit, respectively, for their active collaboration. M.M.A. is supported by Celogos with funding from the Direction Générale de l’Armement, which belongs to the French Ministry of Defense, and from Association Nationale Recherche Technologie. Finally, we thank Dr. Kyle Lund for his helpful language assistance.
Author Contributions
M.M.A. and M.T.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; M.N. and E.B.: collection and/or assembly of data; T.L. and P.D.: provision of study material or patients, data analysis and interpretation; M.T.M.: conception and design, data analysis and interpretation, final approval of manuscript; C.D.: conception and design, financial support, administrative support, data analysis and interpretation, final approval of manuscript; N.O.F.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; J.-J.L.: conception and design, administrative support, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
M.M.A. had compensated employment at Celogos for her Ph.D. and compensated research funding; Ph.D. was partly funded by Direction Générale de l’Armement. The other authors indicated no potential conflicts of interest.
References
- 1.Peck MD. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns. 2011;37:1087–1100. doi: 10.1016/j.burns.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Brusselaers N, Monstrey S, Vogelaers D, et al. Severe burn injury in Europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit Care. 2010;14:R188. doi: 10.1186/cc9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papini R. Management of burn injuries of various depths. BMJ. 2004;329:158–160. doi: 10.1136/bmj.329.7458.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allgöwer M, Schoenenberger GA, Sparkes BG. Pernicious effectors in burns. Burns. 2008;34(suppl 1):S1–S55. doi: 10.1016/j.burns.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Burke JF, Quinby WC, Jr, Bondoc CC. Primary excision and prompt grafting as routine therapy for the treatment of thermal burns in children. Surg Clin North Am. 1976;56:477–494. doi: 10.1016/s0039-6109(16)40890-x. [DOI] [PubMed] [Google Scholar]
- 6.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 7.Braye F, Dumortier R, Bertin-Maghit M, et al. Cultured epidermis for the treatment of severe burns. A 2-year study (18 patients) Ann Chir Plast Esthet. 2001;46:599–606. doi: 10.1016/s0294-1260(01)00066-8. [DOI] [PubMed] [Google Scholar]
- 8.Cirodde A, Leclerc T, Jault P, et al. Cultured epithelial autografts in massive burns: A single-center retrospective study with 63 patients. Burns. 2011;37:964–972. doi: 10.1016/j.burns.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Odessey R. Addendum: Multicenter experience with cultured epidermal autograft for treatment of burns. J Burn Care Rehabil. 1992;13:174–180. [PubMed] [Google Scholar]
- 10.Sood R, Roggy D, Zieger M, et al. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patients: The Indiana University experience. J Burn Care Res. 2010;31:559–568. doi: 10.1097/BCR.0b013e3181e4ca29. [DOI] [PubMed] [Google Scholar]
- 11.Kirsner RS, Falanga V, Eaglstein WH. The development of bioengineered skin. Trends Biotechnol. 1998;16:246–249. doi: 10.1016/s0167-7799(98)01196-2. [DOI] [PubMed] [Google Scholar]
- 12.Lootens L, Brusselaers N, Beele H, et al. Keratinocytes in the treatment of severe burn injury: An update. Int Wound J. 2013;10:6–12. doi: 10.1111/j.1742-481X.2012.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghunath M, Meuli M. Cultured epithelial autografts: Diving from surgery into matrix biology. Pediatr Surg Int. 1997;12:478–483. doi: 10.1007/BF01258706. [DOI] [PubMed] [Google Scholar]
- 14.Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: A review. Adv Skin Wound Care. 2007;20:493–508; quiz 509–510. doi: 10.1097/01.ASW.0000288217.83128.f3. [DOI] [PubMed] [Google Scholar]
- 15.Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn injuries: A critical review of the literature. Burns. 2006;32:395–401. doi: 10.1016/j.burns.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Blais M, Parenteau-Bareil R, Cadau S, et al. Concise review: Tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Translational Medicine. 2013;2:545–551. doi: 10.5966/sctm.2012-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamel RA, Ong JF, Eriksson E, et al. Tissue engineering of skin. J Am Coll Surg. 2013;217:533–555. doi: 10.1016/j.jamcollsurg.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Auxenfans C, Shipkov H, Bach C, et al. Cultured allogenic keratinocytes for extensive burns: A retrospective study over 15 years. Burns. 2014;40:82–88. doi: 10.1016/j.burns.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Atiyeh BS, Costagliola M. Cultured epithelial autograft (CEA) in burn treatment: Three decades later. Burns. 2007;33:405–413. doi: 10.1016/j.burns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Chadli L, Martin MT, Fortunel NO. Investigating human keratinocyte stem cell identity. Eur J Dermatol. 2011;21(suppl 2):4–11. doi: 10.1684/ejd.2011.1269. [DOI] [PubMed] [Google Scholar]
- 21.Fortunel NO, Cadio E, Vaigot P, et al. Exploration of the functional hierarchy of the basal layer of human epidermis at the single-cell level using parallel clonal microcultures of keratinocytes. Exp Dermatol. 2010;19:387–392. doi: 10.1111/j.1600-0625.2009.01046.x. [DOI] [PubMed] [Google Scholar]
- 22.Meana A, Iglesias J, Del Rio M, et al. Large surface of cultured human epithelium obtained on a dermal matrix based on live fibroblast-containing fibrin gels. Burns. 1998;24:621–630. doi: 10.1016/s0305-4179(98)00107-7. [DOI] [PubMed] [Google Scholar]
- 23.Ronfard V, Broly H, Mitchell V, et al. Use of human keratinocytes cultured on fibrin glue in the treatment of burn wounds. Burns. 1991;17:181–184. doi: 10.1016/0305-4179(91)90099-3. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrini G, Ranno R, Stracuzzi G, et al. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation. 1999;68:868–879. doi: 10.1097/00007890-199909270-00021. [DOI] [PubMed] [Google Scholar]
- 25.Ronfard V, Rives JM, Neveux Y, et al. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation. 2000;70:1588–1598. doi: 10.1097/00007890-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 26.Mazlyzam AL, Aminuddin BS, Fuzina NH, et al. Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns. 2007;33:355–363. doi: 10.1016/j.burns.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 27.Monfort A, Soriano-Navarro M, García-Verdugo JM, et al. Production of human tissue-engineered skin trilayer on a plasma-based hypodermis. J Tissue Eng Regen Med. 2013;7:479–490. doi: 10.1002/term.548. [DOI] [PubMed] [Google Scholar]
- 28.Llames S, García E, García V, et al. Clinical results of an autologous engineered skin. Cell Tissue Bank. 2006;7:47–53. doi: 10.1007/s10561-004-7253-4. [DOI] [PubMed] [Google Scholar]
- 29.Gómez C, Galán JM, Torrero V, et al. Use of an autologous bioengineered composite skin in extensive burns: Clinical and functional outcomes. A multicentric study. Burns. 2011;37:580–589. doi: 10.1016/j.burns.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Doucet C, Ernou I, Zhang Y, et al. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Ge J, Tredget EE, et al. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat Protoc. 2013;8:302–309. doi: 10.1038/nprot.2013.002. [DOI] [PubMed] [Google Scholar]
- 32.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peplow PV, Chatterjee MP. A review of the influence of growth factors and cytokines in in vitro human keratinocyte migration. Cytokine. 2013;62:1–21. doi: 10.1016/j.cyto.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 35.Michel M, Török N, Godbout MJ, et al. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: Keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109:1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- 36.Pontiggia L, Biedermann T, Meuli M, et al. Markers to evaluate the quality and self-renewing potential of engineered human skin substitutes in vitro and after transplantation. J Invest Dermatol. 2009;129:480–490. doi: 10.1038/jid.2008.254. [DOI] [PubMed] [Google Scholar]
- 37.Mascré G, Dekoninck S, Drogat B, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 38.Glimm H, Eisterer W, Lee K, et al. Previously undetected human hematopoietic cell populations with short-term repopulating activity selectively engraft NOD/SCID-beta2 microglobulin-null mice. J Clin Invest. 2001;107:199–206. doi: 10.1172/JCI11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domen J, Weissman IL. Self-renewal, differentiation or death: Regulation and manipulation of hematopoietic stem cell fate. Mol Med Today. 1999;5:201–208. doi: 10.1016/S1357-4310(99)01464-1. [DOI] [PubMed] [Google Scholar]
- 40.Flores-Guzmán P, Fernández-Sánchez V, Mayani H. Concise review: Ex vivo expansion of cord blood-derived hematopoietic stem and progenitor cells: Basic principles, experimental approaches, and impact in regenerative medicine. Stem Cells Translational Medicine. 2013;2:830–838. doi: 10.5966/sctm.2013-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norol F, Drouet M, Pflumio F, et al. Ex vivo expansion marginally amplifies repopulating cells from baboon peripheral blood mobilized CD34+ cells. Br J Haematol. 2002;117:924–934. doi: 10.1046/j.1365-2141.2002.03531.x. [DOI] [PubMed] [Google Scholar]
- 42.Horwitz ME, Chao NJ, Rizzieri DA, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014;124:3121–3128. doi: 10.1172/JCI74556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thépot A, Desanlis A, Venet E, et al. Assessment of transformed properties in vitro and of tumorigenicity in vivo in primary keratinocytes cultured for epidermal sheet transplantation. J Skin Cancer. 2011;2011:936546. doi: 10.1155/2011/936546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendez MG, Kojima S-I, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24:1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro-Muñozledo F, Velez-DelValle C, Marsch-Moreno M, et al. Vimentin is necessary for colony growth of human diploid keratinocytes. Histochem Cell Biol. 2015;143:45–57. doi: 10.1007/s00418-014-1262-6. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura M, Tokura Y. Epithelial-mesenchymal transition in the skin. J Dermatol Sci. 2011;61:7–13. doi: 10.1016/j.jdermsci.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Brandner JM, Houdek P, Hüsing B, et al. Connexins 26, 30, and 43: Differences among spontaneous, chronic, and accelerated human wound healing. J Invest Dermatol. 2004;122:1310–1320. doi: 10.1111/j.0022-202X.2004.22529.x. [DOI] [PubMed] [Google Scholar]
- 48.Presland RB, Kuechle MK, Lewis SP, et al. Regulated expression of human filaggrin in keratinocytes results in cytoskeletal disruption, loss of cell-cell adhesion, and cell cycle arrest. Exp Cell Res. 2001;270:199–213. doi: 10.1006/excr.2001.5348. [DOI] [PubMed] [Google Scholar]
- 49.Schlüter H, Paquet-Fifield S, Gangatirkar P, et al. Functional characterization of quiescent keratinocyte stem cells and their progeny reveals a hierarchical organization in human skin epidermis. Stem Cells. 2011;29:1256–1268. doi: 10.1002/stem.675. [DOI] [PubMed] [Google Scholar]
- 50.Matsumura H, Gondo M, Imai R, et al. Chronological histological findings of cultured epidermal autograft over bilayer artificial dermis. Burns. 2013;39:705–713. doi: 10.1016/j.burns.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Segal N, Andriani F, Pfeiffer L, et al. The basement membrane microenvironment directs the normalization and survival of bioengineered human skin equivalents. Matrix Biol. 2008;27:163–170. doi: 10.1016/j.matbio.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andriani F, Margulis A, Lin N, et al. Analysis of microenvironmental factors contributing to basement membrane assembly and normalized epidermal phenotype. J Invest Dermatol. 2003;120:923–931. doi: 10.1046/j.1523-1747.2003.12235.x. [DOI] [PubMed] [Google Scholar]
- 53.Takeda A, Kadoya K, Shioya N, et al. Pretreatment of human keratinocyte sheets with laminin 5 improves their grafting efficiency. J Invest Dermatol. 1999;113:38–42. doi: 10.1046/j.1523-1747.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- 54.Yanaga H, Tai Y, Yamauchi T, et al. Take percentage and conditions of cultured epithelium were improved when basement membrane of the graft was maintained and anchoring mesh was added. Plast Reconstr Surg. 2001;107:105–115. doi: 10.1097/00006534-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 55.Eming SA, Medalie DA, Tompkins RG, et al. Genetically modified human keratinocytes overexpressing PDGF-A enhance the performance of a composite skin graft. Hum Gene Ther. 1998;9:529–539. doi: 10.1089/hum.1998.9.4-529. [DOI] [PubMed] [Google Scholar]
- 56.König A, Bruckner-Tuderman L. Transforming growth factor-beta stimulates collagen VII expression by cutaneous cells in vitro. J Cell Biol. 1992;117:679–685. doi: 10.1083/jcb.117.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amano S, Akutsu N, Ogura Y, et al. Increase of laminin 5 synthesis in human keratinocytes by acute wound fluid, inflammatory cytokines and growth factors, and lysophospholipids. Br J Dermatol. 2004;151:961–970. doi: 10.1111/j.1365-2133.2004.06175.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.