The aim of this open-label phase I clinical trial is to evaluate the safety of two repeated intrathecal injections of autologous bone marrow (BM)-derived mesenchymal stromal cells (MSCs) in amyotrophic lateral sclerosis patients. With the exception of 1 patient who died before the bone marrow extraction, no serious adverse events were observed during the follow-up period. Two repeated intrathecal injections of autologous MSCs were safe and feasible throughout the duration of the 12-month follow-up period.
Keywords: Amyotrophic lateral sclerosis, Clinical trials, Mesenchymal stromal cells, Intrathecal
Abstract
Stem cell therapy is an emerging alternative therapeutic or disease-modifying strategy for amyotrophic lateral sclerosis (ALS). The aim of this open-label phase I clinical trial was to evaluate the safety of two repeated intrathecal injections of autologous bone marrow (BM)-derived mesenchymal stromal cells (MSCs) in ALS patients. Eight patients with definite or probable ALS were enrolled. After a 3-month lead-in period, autologous MSCs were isolated two times from the BM at an interval of 26 days and were then expanded in vitro for 28 days and suspended in autologous cerebrospinal fluid. Of the 8 patients, 7 received 2 intrathecal injections of autologous MSCs (1 × 106 cells per kg) 26 days apart. Clinical or laboratory measurements were recorded to evaluate the safety 12 months after the first MSC injection. The ALS Functional Rating Scale-Revised (ALSFRS-R), the Appel ALS score, and forced vital capacity were used to evaluate the patients’ disease status. One patient died before treatment and was withdrawn from the study. With the exception of that patient, no serious adverse events were observed during the 12-month follow-up period. Most of the adverse events were self-limited or subsided after supportive treatment within 4 days. Decline in the ALSFRS-R score was not accelerated during the 6-month follow-up period. Two repeated intrathecal injections of autologous MSCs were safe and feasible throughout the duration of the 12-month follow-up period.
Significance
Stem cell therapy is an emerging alternative therapeutic or disease-modifying strategy for amyotrophic lateral sclerosis (ALS). To the authors' best knowledge, there are no clinical trials to evaluate the safety of repeated intrathecal injections of autologous bone marrow mesenchymal stromal cells in ALS. After the clinical trial (phase I/II) was conducted, the stem cell (HYNR-CS, NEURONATA-R) was included in the revision of the regulations on orphan drug designation (number 160; December 31, 2013) and approved as a New Drug Application (Department of Cell and Gene Therapy 233; July 30, 2014) by the Korean Food and Drug Administration. The phase II trial is expected to be reported later.
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder involving the motor neurons in the cerebral cortex, the brainstem, and the spinal cord. The disease is characterized by weakness, which culminates in death within 3–5 years [1, 2]. There is no effective therapeutic regimen for ALS. Recent clinical trials using various type of stem cells, including mesenchymal stromal cells (MSCs) [3], neural stem cells [4], and peripheral blood mononuclear cells [5, 6], represent promising strategies for stem cell-based treatment in ALS.
Several mechanisms have been suggested to explain the positive effects of MSCs, including their potent anti-inflammatory capacity, direct release of antiapoptotic and neurotrophic factors, and ability to induce the proliferation of local neural progenitor cells [7, 8]. Additionally, it has been reported that MSCs have the potential benefit of modulating the functions of immune cells involved in both adaptive and innate immunity [9–12]. Altogether, this wide range of effects of MSCs might be beneficial for the treatment of ALS.
In our previous study using superoxide dismutase 1 (SOD1) mutant mice, we evaluated the dose-dependent effects of human bone marrow (BM)-derived MSCs. Administration of MSCs (1 × 106 cells) into the cisterna magna significantly prolonged the life span and slowed the disease progression [13]. These results suggest that intrathecal injection of an optimized number of MSCs might demonstrate a therapeutic potential for ALS. Recently, animal studies have shown that repeated intrathecal administrations of MSC were better than single administration [14, 15].
Previous clinical trials in ALS have showed that single intrathecal administration of MSCs is safe and feasible [16, 17]. The pilot clinical study (Hanyang University Hospital [HYUH] Institutional Review Board [IRB] 2005-452 and 2006-339) was performed; we investigated whether two intrathecal injections of an optimized number of MSCs into ALS patients would be safe and feasible during a 6-month follow-up period. The results of the pilot study suggested that repeated intrathecal injections of MSCs might be useful for altering the disease progression in ALS patients [18, 19]. In the present study, we performed a phase I clinical trial to assess the safety of two repeated intrathecal injections of autologous BM-derived MSCs in patients with ALS during a 12-month follow-up period.
Materials and Methods
Study Design and Overview
This study was an open-label, single-arm, phase I trial to evaluate the safety of two repeated intrathecal injections of MSCs, conducted in Hanyang University Hospital in Seoul, Republic of Korea. The study was approved by the IRB of Hanyang University Hospital (HYUH IRB 2010-C-70) and by the Korean Food and Drug Administration (KFDA-2413). This study was registered at http://www.clinicaltrials.gov (NCT01363401).
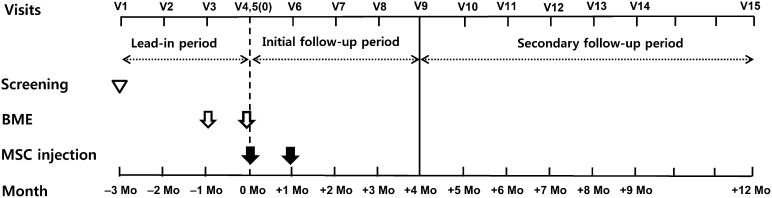
This study was divided into a 3-month lead-in period, a 4-month initial follow-up period and an 8-month secondary follow-up period (Fig. 1; supplemental online Table 1). During the 3-month lead-in period (visits 1–5 [V1–V5], −3 to 0 months), the patients visited the clinic every month to evaluate the natural progression of the disease. After the first MSC injection (V5, 0 months), all patients were assessed at monthly intervals during the 4-month initial follow-up period (V5–V9, 0 to +4 months). After that period, we extended the study (HYUH IRB 2013-06-019 and 2013-08-022) to further evaluate the safety for up to 12 months after the first MSC injection.
Figure 1.
Study design of the trial. This study consists of a 3-month lead-in period, a 4-month initial follow-up period, and an 8-month secondary follow-up period. The first MSC injection was performed at V5 (+0 months). The BM extraction at V3 (−1 months) was for the first injection at V5. The BM extraction at V4 (2 days prior to V5) was for cells for the first injection at V6. Abbreviations: BME, bone marrow extraction; MSC, mesenchymal stromal cell; Mo, months; V, visit.
The essential procedures of this clinical trial were composed of two bone marrow extractions (BME) and two intrathecal injections. To allow sufficient time for MSC expansion ex vivo, each BME was performed 28 days prior to each MSC injection. The BM was extracted at V3 and V4. The MSCs were injected 28 days after each BME, at V5 and V6.
The overall trial-related activities and documents were monitored by an independent auditing board (Dream Clinical Investigation Services, Inc., Seoul, Republic of Korea, http://www.dreamcis.com). An external trial monitor was enlisted to protect the rights and wellbeing of the participants, to verify the accuracy of the trial data, and to guarantee that the conduct of the trial was in compliance with the approved protocol according to good clinical practice guidelines.
Selection Criteria
Patients between 25 and 75 years of age who were diagnosed with clinically probable or definite ALS, according to the revised El Escorial criteria, were eligible for this study [20]. The other inclusion criteria included an Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ALSFRS-R) score between 31 and 46, riluzole treatment at the stable dose (50 mg, twice daily) at least 3 months prior to screening, and a disease duration no longer than 5 years prior to the first diagnosis. Patients who had participated in other clinical trials were excluded. The other exclusion criteria were a forced vital capacity (FVC) of less than 40% of the predicted value, the presence of any concomitant disease that might interfere with the outcome (neurological disease other than ALS, psychiatric disorders, cancer, systemic disease, cardiovascular disease, hepatic or renal disorder, or any other disease), tracheostomal ventilation or noninvasive ventilation (NIV) for more than 12 hours per day, a hemorrhagic tendency at the time of screening, and the administration of any drug that could affect the BM. All patients provided their written informed consent prior to the screening.
Bone Marrow Extraction
Each patient was admitted prior to the BME procedure. Under local anesthesia, BME was performed at the posterior superior iliac crest while the patient was lying in a left or right lateral decubitus position. Approximately 50 ml of BM inocula was collected from each patient. On the day following BM extraction, the puncture site was examined, and the patient was then discharged if there were no adverse events (AEs).
MSC Preparation and Culture
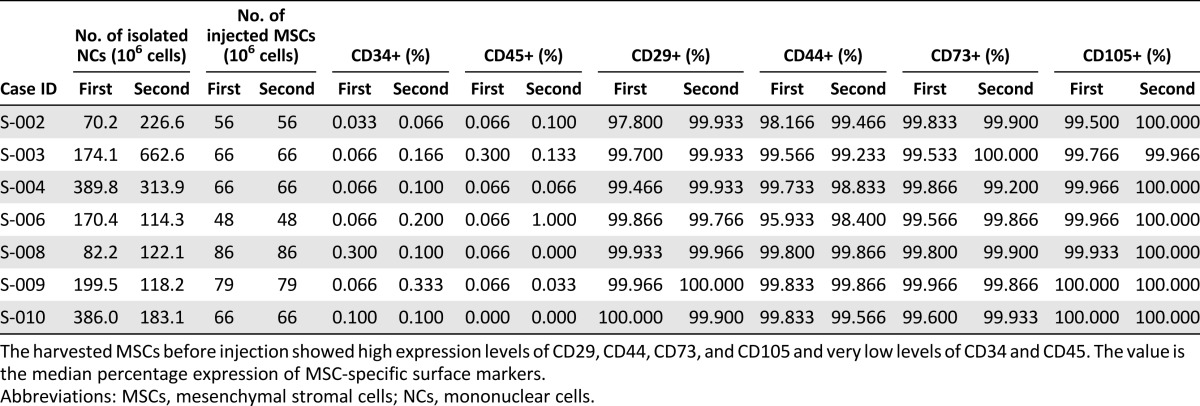
The MSCs were isolated, expanded, and analyzed under good manufacturing practice conditions at Corestem, Inc. (Seoul, Republic of Korea, http://corestem.com). BM mononuclear cells were isolated using Ficoll (Ficoll-Paque Premium; GE Healthcare Bio-Sciences AB, Uppsala, Sweden, http://www.gehealthcare.com) density gradient centrifugation. The mononuclear cells (2 × 105 cells) were placed in a 175-cm2 flask (Thermo Scientific Nunc, Roskilde, Denmark, http://www.nuncbrand.com) and cultured in CSBM-A06 medium (Corestem, Inc.) containing 10% fetal bovine serum (Life Technologies, Grand Island, NY, http://www.lifetech.com), 2.5 mM l-alanyl-l -glutamine (Biochrom AG, Berlin, Germany, http://www.biochrom.de), and 1% penicillin-streptomycin (Biochrom AG) in a humidified incubator at 37°C under 5% CO2 conditions. The nonadherent cells were removed by replacing the medium. After removing the nonadherent cells, the culture medium was changed twice a week. When the MSC primary cultures reached 80% confluence, the cells were harvested using 0.125% trypsin-EDTA (Life Technologies) and subcultured. Every harvest of MSCs resulted in a homogenous population of cells that displayed high expression levels of CD29, CD44, CD73, and CD105 and low expression levels of CD34 and CD45 (Table 1). To confirm sterility, the samples were cultured for bacteria, fungi, viruses, and mycoplasma, and real-time polymerase chain reaction was also performed to detect contaminating mycoplasma. No evidence of bacterial, fungal, viral, or mycoplasmal contamination was found (supplemental online Table 2). The MSCs were supplied as a suspension (concentration, 1 × 107 cells per ml) in autologous cerebrospinal fluid (CSF) and delivered to the hospital for administration to the patient in a container that was maintained at 2–8°C for 30 minutes or less.
Table 1.
MSC-specific cell count and immunophenotypes of autologous bone marrow derived MSCs before injection
Treatment Procedure
The patients in the study received two successive intrathecal injections of autologous MSCs at 26-day intervals, at V5 (0 months) and V6 (+1 months). The MSCs were injected 28 days after each BME. Each injection consisted of approximately 1 × 106 cells per kg in autologous CSF (Table 1). Using a standard lumbar puncture at the level of L2–4, the MSCs were slowly injected over approximately 2 minutes. After the injection of the MSCs, 1 ml of CSF was injected to flush the syringe and spread the MSCs. This treatment procedure had been safely performed in our previous pilot study [18, 19]. Vital sign and physical examinations were performed at 6-hour intervals for 48 hours after the administration of the MSCs. The patient was discharged if there were no AEs. According to the following procedures, we monitored the AEs. All patients received standard treatment with riluzole (50 mg, twice daily) throughout the duration of the clinical trial.
Assessment
The safety was assessed on the basis of the occurrences of serious adverse events (SAEs), AEs, and laboratory abnormalities as defined by the CONSORT group [21]. The patients were evaluated every month for 15 months from V1 (−3 months) to V15 (+12 months) (supplemental online Table 1). To evaluate the safety of intrathecal injections of MSCs, we monitored AEs and SAEs on a monthly basis, beginning at V5 (0 months). The Common Terminology Criteria for Adverse Events (CTCAE, version 3.0) was used to grade the AEs (grades I–V) [22]. Before trial onset, the investigators were instructed to determine whether the therapy should be continued in the setting of an SAE. Physical and neurological examinations were performed at every visit for 15 months. Blood counts, blood chemistry, renal function, liver function, and urine were examined at V1 (−3 months), V5 (0 month), V7 (+2 months), and V9 (+4 months). For patients who were unable to visit the ALS clinic, their clinical status was acquired over the telephone or by a home visit by the doctor.
Additionally, to evaluate the disease progression and functional changes, the ALSFRS-R scores (48 [normal] to 0 [maximally impaired]), the Appel ALS (AALS, 30 [normal] to 164 [maximally impaired]) scores, and the FVC were measured. The ALSFRS-R, the most widely used assessment in clinical trials of ALS, measures the clinical impact of disease severity [23].
The ALSFRS-R score was assessed at monthly intervals from V1 (−3 months) to V11 (+6 months). The FVC and the AALS score were assessed at V1, V5, and V9 (−3, 0, and +4 months, respectively). All outcome measures were determined by well-trained neurologists and graders. The dates of percutaneous endoscopic gastrostomy (PEG), NIV, or tracheostomy were also recorded.
CSF Cytokine Assay
To obtain biochemical evidence in ALS patients with MSC injection, we performed a comparative study of CSF cytokines (baseline vs. 1 month after treatment) in 2 patients (S-008 and S-010) with remnant CSF. On the basis of our recent studies [20], CSF cytokine levels including transforming growth factor (TGF)-β1, TGF-β2, TGF-β3, interleukin (IL)-6, IL-10, and monocyte chemoattractant protein (MCP)-1 were measured in 2 patients using the Bio-Rad Bioplex assay (Bio-Rad, Hercules, CA, http://www.bio-rad.com).
Data Analysis
The continuous variables were expressed as the means and standard deviations. The categorical variables were expressed as absolute values and relative frequency. All adverse events were categorized according to the affected organ system and the specific event. The paired t test was used to compare the changes of functional parameters (ALSFRS-R, AALS, and FVC) from baseline at the follow-up visits and the safety parameters (laboratory data and vital signs) between visits (V1 vs. V9 and V5 vs. V7). p values <.05 were considered to be statistically significant. AEs were coded using MedDRA version 12.0. SPSS statistical software version 21.0 (SPSS Inc., Chicago, IL, http://www-01.ibm.com/software/analytics/spss) was used.
Results
Study Flow and Baseline Characteristics
Between February and August 2011, 10 patients were screened, and 8 patients were enrolled. Two patients were excluded because of NIV and severe proteinuria (supplemental online Fig. 1). One patient (S-001) was enrolled but died before the first BM extraction.
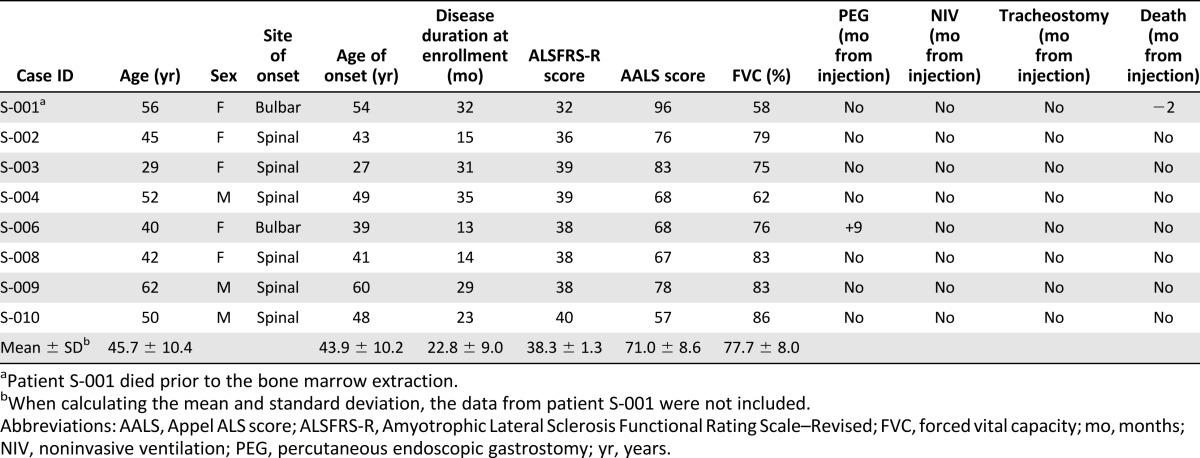
The baseline characteristics and functional changes of the initial 8 patients are described in Table 2. The mean age of the 7 patients (3 male and 4 female) who completed the study was 45.7 ± 10.4 years. The mean age of clinical onset was 43.9 ± 10.2 years (1 patient with bulbar onset ALS and 6 with spinal-onset ALS). None of the patients reported a familial history of ALS. For the 7 included ALS patients, the period from the onset of symptoms to study enrollment was 22.8 ± 9.0 months. At V1 (−3 months), the mean ALSFRS-R score, AALS score, and FVC were 38.3 ± 1.3, 71.0 ± 8.6, and 77.7% ± 8.0%, respectively. Laboratory tests and physical examinations detected no disorders other than ALS (supplemental online Tables 3 and 4).
Table 2.
Clinical characteristics of the patients at the time of enrollment
Safety and AEs
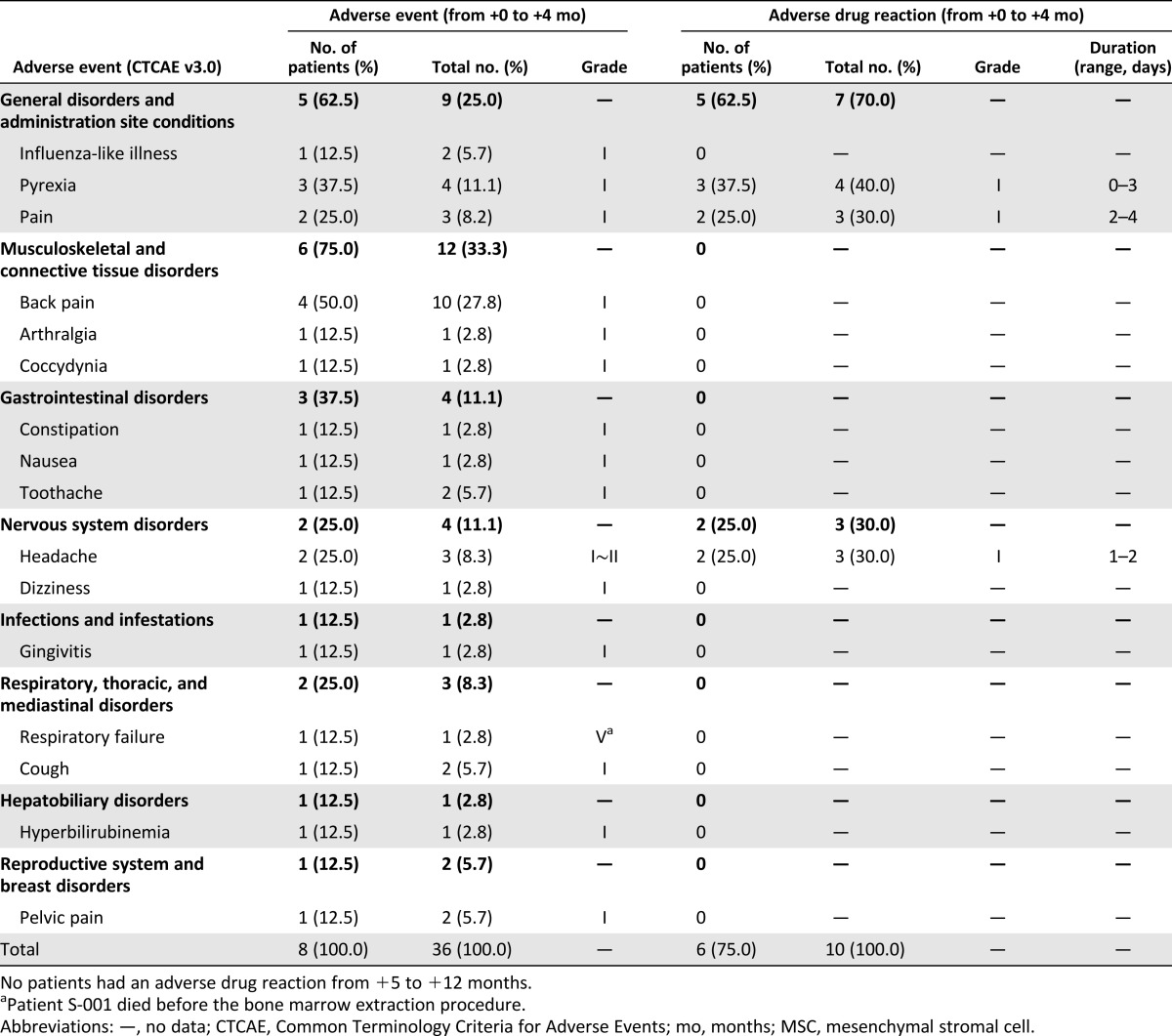
Table 3 presents the AEs that occurred from the first MSC injection to V9 (+4 months) in the 8 patients. One SAE occurred in patient S-001. Patient S-001 died of respiratory failure associated with ALS progression prior to the BM extraction. With the exception of patient S-001, there were no procedure- or MSC-related SAEs during the 12 months. All 36 AEs in the 7 patients were classified as grade I or grade II according to the CTCAE. Most of the AEs were musculoskeletal and connective tissue disorders (12 of 36, 33%) or general disorders (9 of 36, 25%). The most common AE were back pain (4 patients) and pyrexia (3 patients). The 26 AEs during the initial follow-up period were not related to the MSCs. The 10 AEs associated with the MSCs were pyrexia, pain, or headache; these AEs were mild and transient and occurred within 4 days of the MSC injection; all were self-limited or subsided within 4 days after treatment with simple analgesics. Pyrexias occurred in patients S-003 (within 24 hours after the second MSC injection), S-004 (within 24 hours after the first and second MSC injection), and S-006 (within 48 hours after the first MSC injection). Additionally, no MSC- or procedure-related SAEs were observed during the 8-month secondary follow-up period (from +5 months to +12 months). During the entire follow-up period, there were no deaths (except for patient S-001), and neither NIV nor tracheostomy was required in any of the patients. PEG placement was required for 1 patient at 9 months after the first MSC injection because of worsening of a bulbar symptom.
Table 3.
Adverse events and MSC-related adverse events for all participants from the time of the first MSC injection to +12 months
Supplemental online Table 3 shows the laboratory findings (blood counts and blood chemistry, renal function, and liver function assessments) of V1, V5, V7, and V9. Supplemental online Table 4 shows the vital signs during the lead-in and initial follow-up periods of the visits. There were significant differences between visits: 2 months after the first MSC injection (hematocrit, red blood cell count, alanine aminotransferase, albumin, total protein, chloride, and calcium); V9 from V1 (albumin, chloride, and systolic blood pressure). However, these differences were not related to adverse events. Altogether, it seems that these findings were not clinically significant differences. Neither the EKG nor the simple chest x-ray displayed any abnormalities.
Changes in Functional Outcome
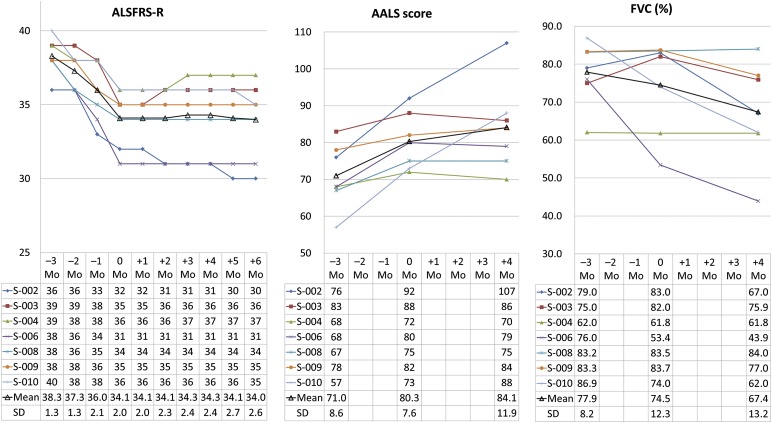
The changes in ALSFRS-R scores are presented in Figure 2. The ALSFRS-R scores of 5 patients did not decrease during the 6 months after the first administration of the MSCs. The ALSFRS-R scores of 2 patients were increased at V7 (+2 months, patient S-003) and V8 (+3 months, patient S-004) but remained stable thereafter. The mean decrease in the ALSFRS-R score after the 3-month lead-in period was 4.1 ± 1.3 points. The mean decrease in the ALSFRS-R score after the 4-month initial follow-up period was −0.1 ± 0.7 points (and 0.1 ± 1.1 at 6 months). The rate of ALSFRS-R score declined over 4 months of the initial follow-up period (−0.04 ± 0.17 point per month, p < .001), and 6 months after the first MSC injection (0.02 ± 0.18 point per month, p < .001) the rate was lower than the rate of decline in the lead-in period (1.38 ± 0.45). The changes in AALS scores and the FVC during the lead-in and follow-up periods are presented in Figure 2. The mean changes in the AALS score and the FVC after the 3-month lead-in period were 9.3 ± 5.4 points and −3.4% ± 10.5%, respectively. The mean changes in the AALS scores and the FVC after the 4-month initial follow-up period were 3.9 ± 7.7 points and −7.1% ± 6.0%, respectively. The rate of deterioration of the AALS score in the initial follow-up period was lower than the rate in the lead-in period (0.96 ± 1.93 vs. 3.10 ± 7.73 points per month, p = .002). The rate of decline in FVC was not different between the 4-month follow-up and the lead-in periods (−1.78 ± 1.51 vs. 1.14% ± 3.50% per month, p = .64).
Figure 2.
Progression of the ALSFRS-R score, the AALS score, and the FVC. Patient S-001 died prior to the bone marrow extraction. When calculating the mean and standard deviation, the data from patient S-001 were not included. Abbreviations: AALS, Appel ALS score; ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised; FVC, forced vital capacity; Mo, months.
CSF Cytokine Assay
After intrathecal MSC injections, the levels of IL-10, TGF-β1, TGF-β2, TGF-β3, and IL-6 were increased compared with the baseline (supplemental online Table 5). In contrast to these cytokines, the level of MCP-1, which is chemokine-related and exacerbates the motor neuron injury in ALS [21], was reduced (supplemental online Table 5).
Discussion
This phase I study show that two intrathecal injections of autologous BM-derived autologous MSCs were safe and well tolerated by 7 patients with ALS. Up to 12 months after the first MSC injection, none of the 7 patients reported any procedure- or MSC-related SAEs, and all of the AEs were mild and transient. The most frequent treatment-related AEs after MSC injection were pyrexia, pain, and headache. Of the 4 cases of pyrexia (1 in patient S-003, 2 in patient S-004, and 1 in patient S-006), 3 occurred within 1 day after MSC injection; this is similar to the previous report demonstrating that fever is among the most common AEs [16]. During the entire follow-up period, there were no deaths, and neither NIV nor tracheostomy was required. Additionally, there was no acceleration in the decline of the ALSFRS-R score, the AALS score, or the FVC. Despite a lack of adequate power to detect a meaningful efficacy, the decrease in the ALSFRS-R score during the 6-month follow-up period was more gradual than the decrease during the lead-in period, and the ALSFRS-R scores remained stable for 6 months after the initial injection of MSCs.
In this study, intrathecal MSC administration elevated TGF-β and IL-10 levels, whereas MCP-1, which is chemokine-related and exacerbates the motor neuron injury in ALS, was reduced. This finding suggests that intrathecal injection of MSCs may be associated with the positive effect on immune response in ALS patients. Previous studies have demonstrated that the IL-10 was increased in the spinal cords of ALS mice (G93A SOD1 mutant mice) during the stable disease phase and was suppressed at the end disease stage [24, 25]. In addition, reduced TGF-β expression levels were associated with rapidly progressing ALS patients and inversely correlated with the progression rate [26]. We also reported that MSCs elevated IL-10 and TGF-β levels in the peripheral blood mononuclear cells of ALS patients [27]. Unfortunately, we could not perform CSF analysis in all patients of the phase I trial because remnants of CSF were not enough to analyze. However, in the phase II trials, we were able to perform in most patients to investigate the immune modulating mechanism of intrathecal administration of MSC.
Recently, the number of clinical trials in which MSCs are administered to treat various diseases, such as cardiac disease, orthopedic disease, and neurologic disorders, has increased [28–30]. Additionally, several stem cell delivery methods for ALS patients have been reported, including direct transplantation into the spinal cord [3, 31] or the frontal motor cortex via a stereotaxic surgical procedure [6, 32], intravenous administration [16, 33–35], or intrathecal injection [5, 16–19]. Although possible safety and effectiveness of a single administration of MSCs is demonstrated in ALS, there are few reports of the safety and feasibility of repeated administrations of MSCs [18, 19, 36]. After intrathecal administration, only a few MSCs were observed in brain and spinal cord parenchyma with a time-dependent depletion of cell number [13, 37]. The therapeutic effectiveness of intrathecal administration of MSCs was related with the level of neurotrophic factor and anti-inflammatory cytokines in ALS patients [18]. The potential therapeutic effect of MSCs would not persist long lasting because cells gradually disappear over time in CSF. Thus, repeated injection of MSCs would be needed to sustain therapeutic effects. In the present study, we suggest that the performance of two repeated intrathecal MSC injections into ALS patients is safe and feasible.
The intrathecal approach of MSC administration provides several practical advantages: (a) The intrathecal approach is a less invasive procedure than direct implantation [14]. Moreover, this procedure may avoid the acceleration of ALS progression related to invasive procedure [38]. (b) Because ALS induces widespread degeneration throughout the length of the neural axis, the intrathecal approach may be more likely to influence multiple affected regions in the brain and the spinal cord because of the dynamics of CSF flow [13, 15]. (c) The intrathecal approach may reduce the likelihood of the injected MSC becoming trapped in the lung when compared with intravenous administration (or in the microvasculature, when compared with arterial administration) [39, 40]. For these reasons, we hypothesized that the intrathecal approach would provide a safe and acceptable route to deliver the MSCs. However, the optimal strategy to deliver stem cells to the central nervous system in patients with ALS has not been discovered yet.
This study has some limitations. First, the lack of postmortem material prohibits any definitive conclusion regarding the fate of the MSCs after injections. Second, because our trial was a small-sized, open-label, single-arm, phase I study, it is premature to make conclusions regarding the feasibility of MSC treatment. After an on-going phase II, controlled, randomized trial, which was performed with rigor and integrity, the in-depth safety and biological effect of two repeated intrathecal injections of MSCs to patients with ALS have been determined.
Conclusion
Our results demonstrate that two repeated intrathecal autologous BM-derived MSC injections are safe and feasible for at least 12 months in 7 patients. No significant AEs were monitored. This approach is feasible and well-tolerated, supporting late-stage clinical trial examining therapeutic in-depth safety, biological effect, and efficacy.
Supplementary Material
Acknowledgments
This study was supported by grants from the Korea Healthcare Technology R&D Project of the Ministry for Health & Welfare Affairs, Republic of Korea (HI10C1673). This study would not have been possible without the participation of the patients and their families. We also thank the ALS nurse specialists at the Hanyang ALS Clinic (Ji Won An, Juyeon Oh, Hanna Lim, and Bo Kyeong Hwang) for their helpful support. K.-W.O. and S.H.K. had full access to all the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions
K.-W.O.: administrative support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript, performing procedure; C.M.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; H.Y.K.: conception and design, collection and/or assembly of data, data analysis and interpretation; S.-i.O.: data analysis and interpretation, manuscript writing, performing procedure; J.P.: data analysis and interpretation, final approval of manuscript; J.H.L. and I.Y.C.: administrative support, provision of study material; K.S.K.: conception and design, administrative support, provision of study material; S.H.K.: conception and design, financial support, provision of study patients, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
J.H.L., I.Y.C., and K.S.K. have compensated employment. S.H.K. has uncompensated research funding from the Korea Healthcare Technology R&D Project of the Ministry for Health & Welfare Affairs, Republic of Korea (HI10C1673). The other authors indicated no potential conflicts of interest.
References
- 1.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369:2031–2041. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- 3.Mazzini L, Mareschi K, Ferrero I, et al. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: A long-term safety study. Cytotherapy. 2012;14:56–60. doi: 10.3109/14653249.2011.613929. [DOI] [PubMed] [Google Scholar]
- 4.Glass JD, Boulis NM, Johe K, et al. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: Results of a phase I trial in 12 patients. Stem Cells. 2012;30:1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- 5.Janson CG, Ramesh TM, During MJ, et al. Human intrathecal transplantation of peripheral blood stem cells in amyotrophic lateral sclerosis. J Hematother Stem Cell Res. 2001;10:913–915. doi: 10.1089/152581601317211015. [DOI] [PubMed] [Google Scholar]
- 6.Martínez HR, Molina-Lopez JF, González-Garza MT, et al. Stem cell transplantation in amyotrophic lateral sclerosis patients: Methodological approach, safety, and feasibility. Cell Transplant. 2012;21:1899–1907. doi: 10.3727/096368911X582769. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 8.Koh SH, Noh MY, Cho GW, et al. Erythropoietin increases the motility of human bone marrow-multipotent stromal cells (hBM-MSCs) and enhances the production of neurotrophic factors from hBM-MSCs. Stem Cells Dev. 2009;18:411–421. doi: 10.1089/scd.2008.0040. [DOI] [PubMed] [Google Scholar]
- 9.Salem HK, Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.English K, Mahon BP. Allogeneic mesenchymal stem cells: Agents of immune modulation. J Cell Biochem. 2011;112:1963–1968. doi: 10.1002/jcb.23119. [DOI] [PubMed] [Google Scholar]
- 11.Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128–134. doi: 10.1016/j.molmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Keating A. Mesenchymal stromal cells: New directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Kim HY, Choi MR, et al. Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci Lett. 2010;468:190–194. doi: 10.1016/j.neulet.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Zhou C, Teng JJ, et al. Multiple administrations of human marrow stromal cells through cerebrospinal fluid prolong survival in a transgenic mouse model of amyotrophic lateral sclerosis. Cytotherapy. 2009;11:299–306. doi: 10.1080/14653240902806986. [DOI] [PubMed] [Google Scholar]
- 15.Harris VK, Yan QJ, Vyshkina T, et al. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J Neurol Sci. 2012;313:167–177. doi: 10.1016/j.jns.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prabhakar S, Marwaha N, Lal V, et al. Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis: A pilot study. Neurol India. 2012;60:465–469. doi: 10.4103/0028-3886.103185. [DOI] [PubMed] [Google Scholar]
- 18.Kim HY, Kim H, Oh KW, et al. Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: An investigator-initiated trial and in vivo study. Stem Cells. 2014;32:2724–2731. doi: 10.1002/stem.1770. [DOI] [PubMed] [Google Scholar]
- 19.Kim HY, Paek J, Park HK, et al. Efficacy and safety of autologous bone marrow-derived mesenchymal stem cell treatment in patients with amyotrophic lateral sclerosis. J Korean Neurol Assoc. 2009;27:163–169. [Google Scholar]
- 20.Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 21.Ioannidis JPA, Evans SJW, Gøtzsche PC, et al. Better reporting of harms in randomized trials: An extension of the CONSORT statement. Ann Intern Med. 2004;141:781–788. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- 22.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 23.Berry JD, Cudkowicz ME. New considerations in the design of clinical trials for amyotrophic lateral sclerosis. Clin Investig (Lond) 2011;1:1375–1389. doi: 10.4155/cli.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noh MY, Cho KA, Kim H, et al. Erythropoietin modulates the immune-inflammatory response of a SOD1(G93A) transgenic mouse model of amyotrophic lateral sclerosis (ALS) Neurosci Lett. 2014;574:53–58. doi: 10.1016/j.neulet.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Beers DR, Henkel JS, Zhao W, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134:1293–1314. doi: 10.1093/brain/awr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henkel JS, Beers DR, Wen S, et al. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med. 2013;5:64–79. doi: 10.1002/emmm.201201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon MS, Noh MY, Oh KW, et al. The immunomodulatory effects of human mesenchymal stem cells on peripheral blood mononuclear cells in ALS patients. J Neurochem. 2014 doi: 10.1111/jnc.12814. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Trounson A, Thakar RG, Lomax G, et al. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: An update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 30.Le Blanc K, Pittenger M. Mesenchymal stem cells: Progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 31.Mazzini L, Ferrero I, Luparello V, et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A Phase I clinical trial. Exp Neurol. 2010;223:229–237. doi: 10.1016/j.expneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Martinez HR, Gonzalez-Garza MT, Moreno-Cuevas JE, et al. Stem-cell transplantation into the frontal motor cortex in amyotrophic lateral sclerosis patients. Cytotherapy. 2009;11:26–34. doi: 10.1080/14653240802644651. [DOI] [PubMed] [Google Scholar]
- 33.Appel SH, Engelhardt JI, Henkel JS, et al. Hematopoietic stem cell transplantation in patients with sporadic amyotrophic lateral sclerosis. Neurology. 2008;71:1326–1334. doi: 10.1212/01.wnl.0000327668.43541.22. [DOI] [PubMed] [Google Scholar]
- 34.Cashman N, Tan L-Y, Krieger C, et al. Pilot study of granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood stem cells in amyotrophic lateral sclerosis (ALS) Muscle Nerve. 2008;37:620–625. doi: 10.1002/mus.20951. [DOI] [PubMed] [Google Scholar]
- 35.Rice CM, Mallam EA, Whone AL, et al. Safety and feasibility of autologous bone marrow cellular therapy in relapsing-progressive multiple sclerosis. Clin Pharmacol Ther. 2010;87:679–685. doi: 10.1038/clpt.2010.44. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Chen D, Xi H, et al. Olfactory ensheathing cell neurorestorotherapy for amyotrophic lateral sclerosis patients: Benefits from multiple transplantations. Cell Transplant. 2012;21(suppl 1):S65–S77. doi: 10.3727/096368912X633789. [DOI] [PubMed] [Google Scholar]
- 37.Morita E, Watanabe Y, Ishimoto M, et al. A novel cell transplantation protocol and its application to an ALS mouse model. Exp Neurol. 2008;213:431–438. doi: 10.1016/j.expneurol.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Pinto S, Swash M, de Carvalho M. Does surgery accelerate progression of amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry. 2014;85:643–646. doi: 10.1136/jnnp-2013-305770. [DOI] [PubMed] [Google Scholar]
- 39.Toma C, Wagner WR, Bowry S, et al. Fate of culture-expanded mesenchymal stem cells in the microvasculature: In vivo observations of cell kinetics. Circ Res. 2009;104:398–402. doi: 10.1161/CIRCRESAHA.108.187724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.