New information about the secretome pattern is reported in this paper. Human amniotic fluid stem cells (hAFSCs) possess immunomodulatory properties involving hepatocyte growth factor production. hAFSCs could be used in immunotherapies and might be able to avoid allogenic rejection.
Keywords: Hepatocyte growth factor, Immune reaction, Human amniotic fluid stem cell, Apoptosis
Abstract
Human amniotic fluid stem cells (hAFSCs) may be useful for regenerative medicine because of their potential to differentiate into all three germ layers and to modulate immune response with different types of secretion molecules. This last issue has not been completely elucidated. The aim of this study was to investigate the secretome profile of the hAFSC, focusing on the role of hepatocyte growth factor (HGF) in immunoregulation through short and long cocultures with human peripheral blood mononuclear cells. We found that HGF produced by hAFSCs exerts a cytoprotective role, inducing an increase in caspase-dependent apoptosis in human immune cells. This study provides evidence supporting the hypothesis that amniotic fluid is an ideal source of stem cells for expansion and banking properties for therapeutic use. hAFSCs not only are less immunogenic but also can secrete immunoregulatory factors that may be useful in autoimmune diseases or allogenic implants.
Significance
New information about the secretome pattern is reported in this paper. Human amniotic fluid stem cells (hAFSCs) possess immunomodulatory properties involving hepatocyte growth factor production. hAFSCs could be used in immunotherapies and might be able to avoid allogenic rejection.
Introduction
Mesenchymal stem cells (MSCs) show immunomodulatory activity and secrete a wide spectrum of cytokines and chemokines that suppress inflammatory responses, that block mixed lymphocyte reactions and other immune reactions, and that have proven therapeutic effects against conditions such as graft-versus-host disease [1]. Immunoregulation by bone marrow MSCs (BM-MSCs) is thought to result from both direct interactions between the stromal and immune cells [2–4] and the actions of anti-inflammatory soluble factors released by the stromal cells [5, 6]. However, the relatively limited proliferation of BM-MSCs under standard conditions, suitable for manufacture of a clinical product, presents a potential drawback for their medical application [7]. Tissue engineering and cell therapy will be enhanced by improved cell sources.
Human amniotic fluid stem cells (hAFSCs) are broadly multipotent, can be expanded extensively in culture, are not tumorigenic, and can be readily cryopreserved for cell banking. hAFSCs resemble MSCs in many respects including surface marker expression and differentiation potential. The population of multipotent stem cells from amniotic fluid is obtained by immunoselection for c-Kit (CD117) [8], the cell surface receptor for stem cell factor, and is characterized by a high capacity for self-renewal and the ability to differentiate toward lineages representative of all three germ layers including glial cells, neurons, hepatocytes, osteocytes, chondrocytes, and adipocytes [8–11].
Both hAFSCs and BM-MSCs express surface markers CD29, CD44, CD73, CD90, and CD105, but hAFSCs also express the more primitive stem cell markers SSEA4 [8], Oct4, Nanog, and Sox2 [12]. The two cell types also have a similar immune antigen surface profiles with positive major histocompatibility complex (MHC) class I expression but little to no MHC class II expression [13]. Moreover, amniotic fluid cells may have an immunopriviledged status because fetal cells must possess mechanisms to avoid destruction by the maternal immune system during development [14].
In vivo experiments with hAFSCs have not had successful results. hAFSCs xenotransplanted in a rat model of myocardial infarction, with or without cyclosporine treatment, or in intact heart from immunocompetent or immunodeficient animals were acutely rejected [15]. These authors have continued to investigate the immune regulatory potential of hAFSCs [16] because the prior experiment was cross-species. It may be possible to use amniotic fluid cells in an allogenic fashion, in addition to a potential autologous use for congenital defects. The authors found that exposure to an inflammatory milieu leads to activation of the tryptophan-catabolizing enzyme indoleamine 2,3-dioxygenase (IDO), which becomes the central immunosuppressive enzyme affecting T-cell proliferation in several stem cells types, including hAFSCs [17], even if some other molecules may be involved [16, 18]. In fact, c-Kit-positive cells from amniotic fluid were described as exerting an immunomodulatory effect through cytokines separate from IDO secretion [18]. Moorefield et al. demonstrated that AFSCs secrete multiple factors in common with MSCs that are known to be involved in immune regulation, including growth-related oncogene and monocyte chemotactic protein (MCP) family members and interleukin-6 (IL-6). AFSCs, activated by exposure to peripheral blood mononuclear cells (PBMCs), released several additional cytokines compared with BM-MSCs [1].
Among secreted cytoprotective factors, hepatocyte growth factor (HGF) and matrix metalloproteinases 2 and 9 were identified [1, 19, 20]. HGF is a cytokine with angiogenic potential and is likely released in the blood, suggesting its endocrine role in targeting the injury site. Endogenous HGF is required for self-repair of injured liver, kidneys, lungs, and other organs. In addition, HGF exerts protective effects on epithelial and nonepithelial organs (including heart and brain) through antiapoptotic and anti-inflammatory signals. In line with other soluble factors associated with regenerative processes, HGF-1 possesses immune-modulatory activity [21]. Treatment of dendritic cells with HGF-1 reduces the ability to induce generation of inflammatory Th1 cells [22]. Furthermore, studies have shown that in vivo administration of HGF-1 protects against autoimmune diseases, such as experimental autoimmune encephalomyelitis and collagen-induced arthritis, through stimulation of regulatory T cells producing immune-suppressive cytokines [23, 24]. MSC production of HGF-1 has been shown to be critical in several in vivo therapeutic activities of MSCs in models of autoimmunity [21].
We investigated the role of HGF, a soluble factor with both regenerative and immune modulatory activities that is secreted by hAFSCs, in the regulation of immune response. This study demonstrated that hAFSCs may counteract allogenic rejection by promoting cell survival, also through HGF secretion.
Materials and Methods
Adult Human Tissue Isolation and Cell Culture
Amniocentesis samples taken during the second trimester of pregnancy (6 back-up flasks obtained from different donors) were provided by the Laboratorio di Genetica, Ospedale Santa Maria Nuova (Reggio Emilia, Italy). All samples were collected with the informed consent of the donors (aged ≥35 years) according to Italian law and ethics committee guidelines.
The hAFSCs were isolated, as described previously [8]. Human amniocentesis cultures were harvested by trypsinization and subjected to c-Kit immunoselection by MACS technology (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). The hAFSCs were subcultured routinely at 1:3 dilution and were not allowed to expand beyond 70% of confluence. The hAFSCs were grown in culture medium (Minimum Essential Medium, α modification, supplemented with 20% fetal bovine serum [FBS], 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin; EuroClone, Milan, Italy, http://www.euroclonegroup.it) [25]. Osteogenic differentiation was obtained after 2 weeks of culture in a classic osteogenic medium, as described by Maraldi et al. [25, 26].
Neurogenic differentiation occurred after 3 weeks in medium containing retinoic acid [9].
Assessment of Cytokines/Chemokines Levels by Antibody Microarrays
The levels of cytokines IL-1α, IL-1β, IL-4, IL-6, IL-10, IL-13, interferon-β (IFN-β), tumor necrosis factor-α (TNF-α) and chemokines IL-8 and MCP-1 were assessed in conditioned media obtained after 5 days of culture of hAFSCs (preactivated or not) with the Quantibody Human Inflammation Array 1 (Ray Biotech, Norcross, GA, http://www.raybiotech.com). In detail, the kit provides microarray slides, each spotted with 16 copies of a cytokine/chemokine antibody array. The slide comes with a 16-well removable gasket that allows for the process of 16 samples in 1 slide. Every array consists of 6 × 8 matrices in quadruplicate for each antibody and 2 positive controls.
Prior to processing, the samples were diluted 1:10 in the appropriate diluent buffer provided in the kit, and the assays were conducted according to a sandwich enzyme-linked immunosorbent assay (ELISA) protocol, according to the manufacturer’s instructions.
The fluorescent signal was read with a ScanArray GX scanner (PerkinElmer, Waltham, MA, http://www.perkinelmer.com) and quantified with the ScanArray Express software (PerkinElmer).
The quantification of each cytokine or chemokine was achieved by interpolating the fluorescent signals to the standard curve, generated by processing some arrays with specific standards containing predetermined cytokines concentrations (picograms per milliliter) [27].
Immunofluorescence and Confocal Microscopy
For immunofluorescence analysis, samples were processed as described previously [28]. Confocal imaging was performed by a Nikon A1 confocal laser scanning microscope (Nikon, Tokyo, Japan, http://www.nikon.com), as described previously [29]. Primary antibody against HGF (rabbit anti-HGF H-170, catalog number sc-13087) was purchased from Santa Cruz Biotechnology (Dallas TX, http://www.scbt.com). The primary antibody was diluted 1:50 and incubated for 1 hour at room temperature.
The confocal serial sections were processed with ImageJ software (NIH, Bethesda, MD, http://imagej.nih.gov/ij/) to obtain three-dimensional projections, as described previously [30]. The image rendering was performed with Adobe Photoshop software (Adobe Systems, San Jose, CA, http://www.adobe.com).
Mononuclear Cell Separation
Human PBMCs were separated from peripheral blood of healthy donors by gradient centrifugation (Ficoll-Hypaque, Lymphoprep; Axis-Shield, Dundee, U.K., http://www.axis-shield.com) at room temperature [31].
The concentration of isolated PBMCs was adjusted to 2 × 106 cells per milliliter in RPMI 1640 (EuroClone) including 10% FBS. Twenty hours later, PBMCs were washed and used for the experiments described below.
Coculture Experiments
The hAFSCs were seeded for all experiments in multiwell plates (6-well culture plates) when confluence was reached (approximately 300,000 cells per well) and were pre-exposed or not to PBMCs for 24 hours prior to coculture with fresh isolated PBMCs.
Cocultures in contact were obtained by adding 2 ml of PBMC suspension (2 × 106cells per milliliter) to hAFSC culture plates. For Transwell culture, PBMCs were seeded onto polycarbonate membrane cell culture inserts (Corning, Corning, NY, http://www.corning.com). For conditioned medium (CM) experiments, PBMCs were suspended in RPMI obtained from hAFSC culture (i.e., CM) for up to 5 days. In some experiments, 1 μg/ml of anti-HGF neutralizing antibody (Sigma-Aldrich, St. Louis, MO, https://www.sigmaaldrich.com) was added to the coculture. In general, cocultured cells and controls were maintained for 5 days.
Western Blotting
Whole-cell lysates from hAFSCs and PBMCs were processed as described previously [32]. Some sample were pretreated for 30 minutes with inhibitors of the Akt pathway [33]: a reversible inhibitor of PI3K LY294002 (25 μM, Calbiochem no. 440202; EMD Millipore, Billerica, MA, http://www.emdmillipore.com), an allosteric Akt inhibitor MK-2206 (0.1 μM, no. 51078; Selleck Chemicals, Houston, TX, http://www.selleckchem.com), and a dual ATP-competitive PI3K and mammalian target of rapamycin inhibitor BEZ235 (0.1 μM, no. 10565; Cayman Chemical, Ann Arbor, MI, https://www.caymanchem.com).
Primary antibodies were used against phospho-c-MET, phospho-Akt, phospho-ERK (Cell Signaling Technology, Beverly, MA, http://www.cellsignal.com); PARP, HGF, β-actin (Santa Cruz Biotechnology); and cleaved caspase 3 and 9 (Sigma-Aldrich).
HGF Immunoassay
Concentration of HGF in the conditioned media of cultured hAFSCs was measured by using a sandwich ELISA, according to the manufacturer’s instructions (Boster Biological Technology, Pleasanton, CA, http://www.bosterbio.com) [34]. Conditioned media were centrifuged before testing. Samples were run in duplicate. A standard curve was constructed using known concentrations of recombinant human HGF (0–8,000 pg/ml).
Evaluation of Apoptosis in Lymphocytes by Flow Cytometry
Apoptosis was quantitatively measured using propidium iodide. The staining process was performed according to the manufacturer’s instructions. Data were obtained using flow cytometric analysis with fluorescence-activated cell sorting within 1 hour.
Apoptosis Assay
PBMCs (106 cells) were removed from coculture and washed with phosphate-buffered saline, and 50 μl of this suspension (0.5 × 106cells) was allowed to adhere to the slide for 10 minutes at room temperature. After 3 washes with 50 μl of binding buffer (10 mM HEPES, pH 7.5, containing 140 mM NaCl and 2.5 mM CaCl2), the specimen was incubated for 15 minutes with 50 μl of double staining solution (binding buffer containing 0.25 μl of annexin V-Cy3 and 0.25 μl of CFDA; Sigma-Aldrich). Finally, the specimen was washed 5 times with 50 μl of binding buffer, mounted with 15 μl of binding buffer, and visualized under fluorescence microscopy.
Statistical Analysis
In vitro experiments were performed in triplicate. For quantitative comparisons, values were reported as mean ± SD based on triplicate analysis for each sample. To test the significance of observed differences among the study groups, analysis of variance with post hoc Bonferroni correction or Student’s t test were applied. A p value <.05 was considered statistically significant.
Results
The hAFSCs were isolated from a heterogeneous cell population of second-trimester amniotic fluid. After the selection of c-Kit-positive cells, the culture maintained markers typical not only of the mesechymal profile. In Table 1 we present a summary of the characterization of hAFSCs obtained in our laboratory. These data were previously published in part [9, 35] and were obtained with different techniques, such as Western blot and immunofluorescence. As already reported by other authors, we confirmed that hAFSCs exhibit positivity for stromal mesenchymal markers, such as CD73, CD90, and CD105 [8, 18, 35, 36] and Stro-1 and CD271 [9].
Table 1.
Summary of the characterization of hAFSCs isolated in our laboratory
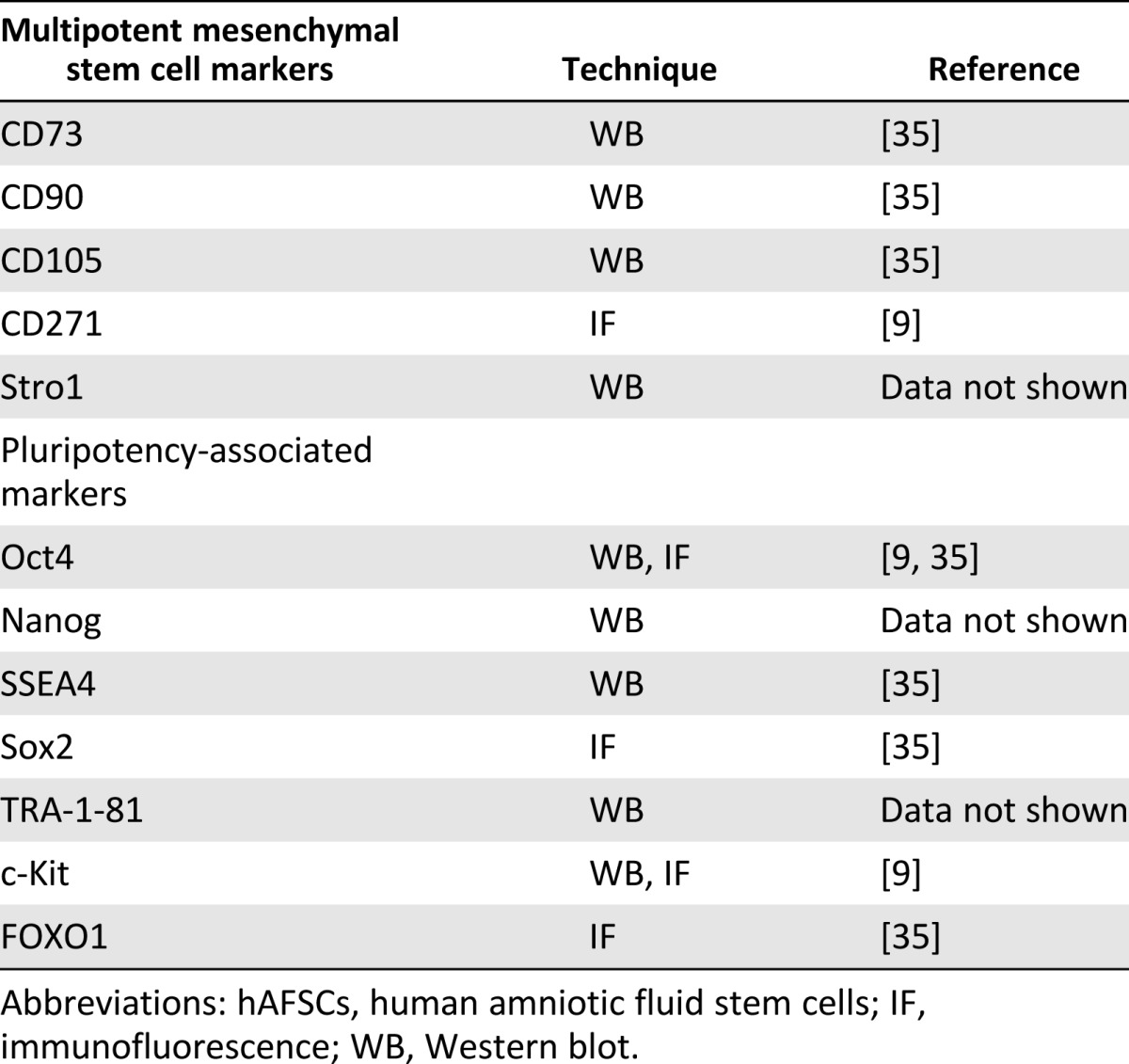
Moreover, hAFSCs selected for c-Kit and maintained in culture for several passages expressed proteins typical of more primitive stem cells features, such as SSEA4, Oct4, TRA-1-81, FOXO1, Sox2 [35], and Nanog [18]. Consequently, the differentiation potential observed for hAFSCs was consistent with the expression of some pluripotency-associated markers. We previously reported successful differentiation in osteoblasts [25, 26]; in adipocytes, myocytes, and pancreatic cells [34]; and in glial and neuronal cells [9].
Regarding the immunoregulatory potential of second-trimester hAFSCs, it has been noted that human leukocyte antigen A (HLA-A), HLA-B, and HLA-C are expressed, unlike HLA-DR [1, 36]. Moreover, the production in the secretum of some immune-modulating molecules has been proved for hAFSCs [1, 18, 36].
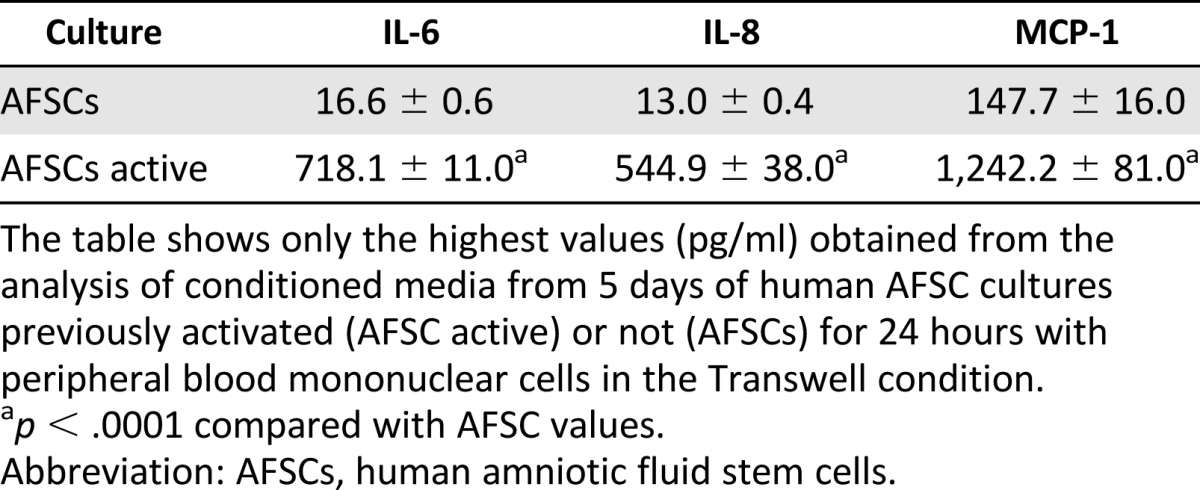
In order to shed a light on this issue, we used a microarray designed to test the presence in the secretum of several cytokines and chemokines: IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-13, MCP-1, IFN-γ, and TNF-α. Table 2 shows only the highest values obtained from the analysis of hAFSC culture in standard growth conditions or pre-exposed to PBMCs (AFSC active). This analysis confirmed the presence of IL-6 in hAFSC secretum [1], mostly in activated hAFSC medium. In this last condition, we also observed a detectable value of IL-8. The presence of MCP-1 is still evident in unactivated hAFSCs, but pre-exposure to PBMCs causes a large increase (10 times).
Table 2.
Inflammation microarray of conditioned media
Immune modulation can be exerted by other soluble factors, IDO [18] and HGF [37]. HGF has been shown to exert regenerative activity outside the liver, including stimulation of angiogenesis. In line with other soluble factors associated with regenerative processes, HGF possesses immune modulatory activity. Consequently, we focused our attention on the role of HGF in the immunosuppressive effects of hAFSCs.
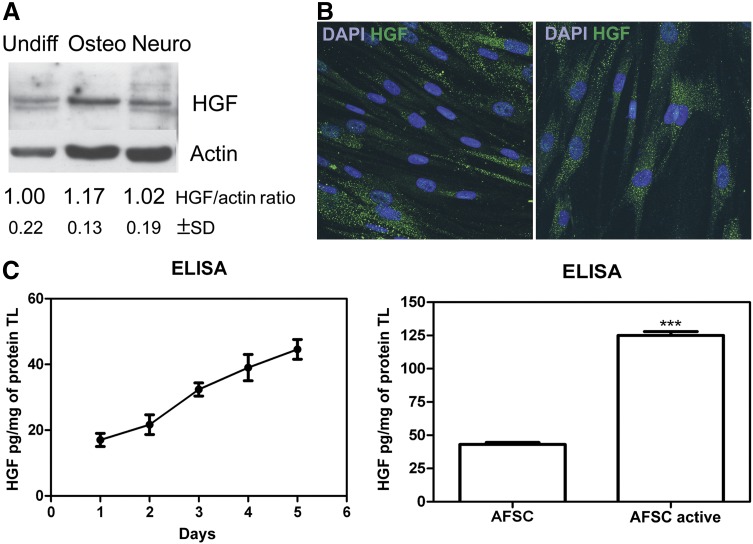
HGF expression in hAFSCs was evaluated in different culture conditions. Figure 1A shows the Western blot analysis of total lysates of hAFSCs, even after 2 weeks of culture in osteogenic differentiation medium or after 3 weeks in neurogenic medium. In all of these conditions, HGF was detectable at comparable intensities, indicating that this property was maintained during differentiation.
Figure 1.
HGF expression by human AFSCs (hAFSCs). (A): Western blot analysis with anti-HGF revealed total lysates of undifferentiated hAFSCs, after 2 weeks in culture with osteogenic medium, and after 3 weeks in culture with neurogenic medium. Actin detection was performed to show the amount of protein loaded in each lane. Presented data are representative of three independent experiments, and the gray density values were normalized to that of actin. (B): Representative immunofluorescence images obtained at different magnifications of undifferentiated hAFSCs labeled with DAPI (blue) and HGF (green). Scale bar = 10 μm. (C): ELISA quantification (normalized to protein content) of HGF in media obtained from hAFSCs after 1–5 days in culture (left) and compared with hAFSCs preactivated for 24 hours and after 5 days in culture (right). Student’s t test showed a significant difference between samples. ∗∗∗, p ≤ .0001. Presented data are the average of three independent experiments. Abbreviations: AFSC, amniotic fluid stem cells; DAPI, 4′,6-diamidino-2-phenylindole; ELISA, enzyme-linked immunosorbent assay; HGF, hepatocyte growth factor; Neuro, neurogenic; Osteo, osteogenic; TL, total lysate; Undiff, undifferentiated.
Results obtained by Western blot were confirmed by immunofluorescence. Undifferentiated hAFSCs strongly expressed HGF within the cytoplasm in a spot-like distribution (Fig. 1B). HGF production by hAFSCs seeded at confluence was measured in culture media obtained after 1–5 days in culture. The graph on the left of Figure 1C shows time-dependent accumulation of HGF within the medium. Comparing media of hAFSCs grown in normal conditions versus preactivated for 24 hours with exposure to PBMCs, the ELISA assay showed an increase (3 times) of HGF level that was also observed with microarray for other soluble factors (Table 2).
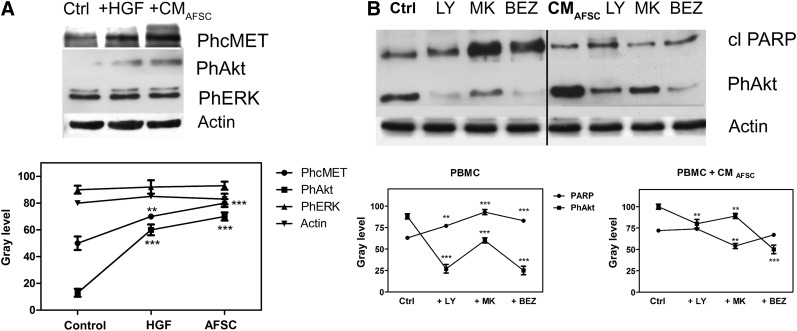
We compared the effect of exogenous HGF and hAFSC CM on the PBMC signaling pathway downstream of HGF binding to its receptor. As expected, HGF caused an increase in the phosphorylation of its receptor c-MET, exposed on PBMC membranes (Fig. 2A). In the same way, hAFSC CM exposure induced activation of the HGF receptor. Akt and ERK1/2 phosphorylation were then analyzed. Compared with PBMC control, samples incubated with pure HGF or hAFSC CM for 24 hours showed activation of the Akt pathway, unlike ERK1/2. Consequently, we tested the efficacy of different Akt pathway inhibitors, such as LY294002, MK-2206, and BEZ235.
Figure 2.
Human AFSC (hAFSC) CM modulation of immune cell signaling pathways. (A): Western blot analysis of lysates of PBMCs exposed to 1 ng/ml HGF or hASCF CM for 24 hours revealed phospho-c-MET, phospho-Akt, and phospho-ERK. (B): Western blot analysis of cleaved PARP and phospho-Akt in samples of PBMCs exposed or not to hAFSC CM for 24 hours and pretreated for 30 minutes with LY294002, MK-2206, and BEZ235. Actin detection was performed to show the amount of protein loaded in each lane. Presented data are representative of three independent experiments, and quantification analysis is shown in the graphs. ∗∗∗, p < .0001, ∗∗, p < .01 compared with the control or AFSC values. Abbreviations: AFSC, amniotic fluid stem cells; cl, cleaved; BEZ, BEZ235; CM, conditioned medium; Ctrl, control; HGF, hepatocyte growth factor; LY, LY294002; MK, MK-2206; PBMC, peripheral blood mononuclear cells; Ph, phospho.
Figure 2B shows (on the left) the effect of these inhibitors on PBMCs at the concentration usually reported in literature and in the Materials and Methods section. The phosphorylation of Akt decreased, and, in parallel, the cleaved PARP (marker of apoptosis) increased, confirming the prosurvival role of Akt in PBMCs. In contrast, samples of PBMCs exposed to hAFSC CM showed a different reaction to inhibitor incubation; in fact, although a reduction in phosphorylation of Akt still occurred, an increase in cleaved PARP levels was not evident. This observation could suggest that hAFSC CM exposure induced activation of c-MET, leading to phosphorylation of Akt despite a negative effect on PBMC viability. The major arm of c-MET signaling is the PI3K/Akt signaling axis. When c-MET is phosphorylated, it binds and activates PI3K, which probably promotes cell viability and motility [38], also linking c-MET signaling to the MAPK cascade [39]. Functional effects of HGF depend mostly on cell context, and thus both apoptotic and antiapoptotic effects of HGF have been observed [40].
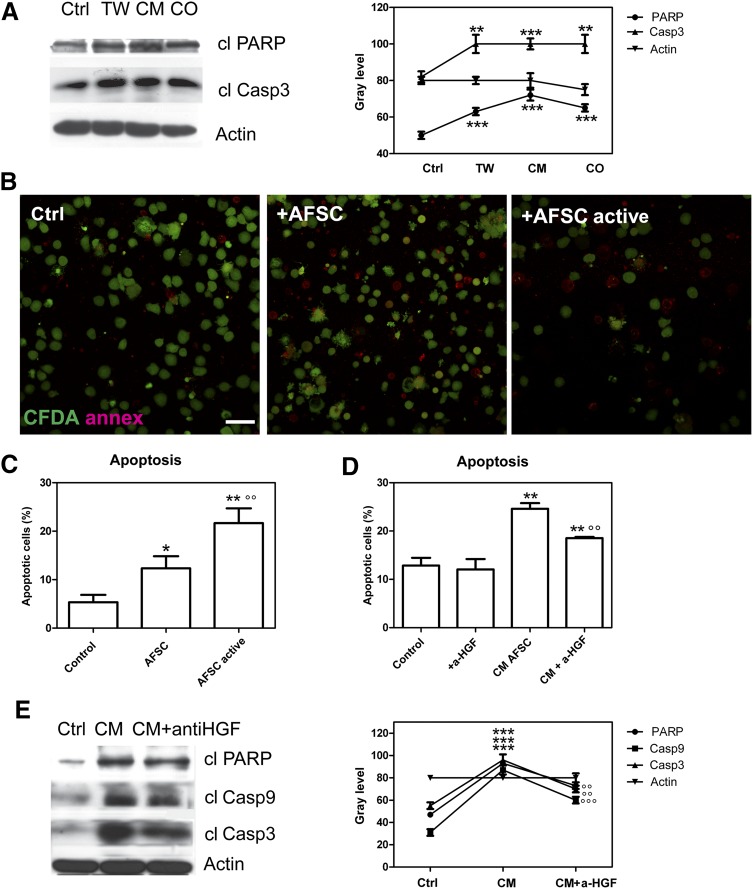
In order to better investigate the effect of interaction between hAFSCs and PBMCs, coculture experiments were performed for longer times. Samples of immune cells exposed to different kinds of coculture: (a) in CM, culture medium was derived after 5 days of hAFSCs in RPMI serum free; (b) in the Transwell condition, hAFSCs were in coculture with PBMCs but physically separated by a membrane; (c) in coculture of hAFSCs and PBMCs, PBMCs and hAFSCs were physically in contact.
PBMCs were then collected and analyzed by fluorescence-activated cell sorting (FACS), Western blot, and confocal microscopy.
Figure 3A shows representative images of PBMCs alone or exposed to CM, Transwell, and hAFSC-PBMC coculture with hAFSCs analyzed by Western blot for apoptotic markers. The cleaved form of PARP and caspase 3 significantly increased in the presence of hAFSC secretum in all the three coculture conditions.
Figure 3.
Apoptosis of immune cells exposed to human AFSC (hAFSC) coculture. (A): Western blot analysis of lysates of peripheral blood mononuclear cells (PBMCs) exposed for 3 days to hAFSCs by conditioned medium, Transwell condition, or direct contact. Then immunoblot directed to cleaved PARP and caspase 3 was performed. Presented data are representative of three independent experiments, and the gray density values are shown in the graph. ∗∗∗∗, p < .0001; ∗∗, p < .01. (B): Representative immunofluorescence images of annexin V (red) and CFDA (green) labeling of PBMCs exposed for 3 days to media (CM) obtained from 5 days of hAFSCs preactivated (AFSC active) or not (AFSC). (C): Representative fluorescence-activated cell sorting (FACS) analysis of propidium iodide (PI) fluorescence of lymphocytes exposed for 3 days to media (CM) obtained from 5 days of hAFSCs preactivated (AFSC active) or not (AFSC) for 24 hours. ∗, p < .05; ∗∗, p < .01 vs. control; °°, p < .01 vs. AFSC. (D): Representative FACS analysis of PI fluorescence of lymphocytes cultured alone, after exposure to CM of activated hAFSCs, and in presence of anti-HGF neutralizing antibodies. Histograms represent the means of three different experiments ± SD. ∗∗, p < .01 vs control; °°, p < .01 vs. AFSC CM. (E): Western blot analysis of lysates of PBMCs exposed to CM of activated hAFSCs and in the presence of anti-HGF neutralizing antibodies revealed cleaved PARP and caspase 3 and 9. Actin detection was performed to show the amount of protein loaded in each line. Presented data are representative of three independent experiments, and the gray density values are shown in the graph. ∗∗∗, p < .0001 vs. control; °°°, p < .0001; °°, p < .01 vs. CM. Abbreviations: AFSC, amniotic fluid stem cells; a-HGF, anit-hepatocyte growth factor; Casp3, caspase 3; cl, cleaved; CM, conditioned medium; CO, direct contact; Ctrl, control; TW, Transwell.
Annexin V analysis was performed on PBMCs cultured alone (control) and PBMCs cocultured with normal hAFSC culture or activated hAFSCs to confirm apoptotic cell death in immune cells. Figure 3B shows representative images of PBMCs cultured alone or exposed to hAFSCs or activated hAFSCs, labeled by annexin V. The presence of cells stained with red rings increased in both exposure conditions with respect to the PBMCs cultured alone, indicating the onset of an apoptotic process, mostly in cells exposed to activated hAFSC conditioned medium. In order to quantify apoptosis occurring in PBMCs, FACS analysis was performed after staining with propidium iodide (Fig. 3C). The percentage of apoptotic cells in the presence or absence of CM obtained from normal hAFSC culture or activated hAFSCs is shown; in both conditions, the cells in the sub-G1 phase reach ∼10% and 20%, respectively. These values are significantly different from the control and between each other.
The conditioned medium obtained after 5 days was chosen to test the efficacy of anti-HGF neutralizing antibody in reducing the apoptotic process. In fact, the conditioned medium is cell free; therefore, it is possible to perform preincubation for 1 hour with the antibody in a shaking condition. Apoptosis was quantitatively assessed by cytofluorimetric analysis (Fig. 3D). The percentage of apoptotic PBMCs obtained from CM of hAFSCs in the presence of anti-HGF significantly decreased, although it did not reach the percentage of leukocytes cultured alone. Furthermore, no significant differences in the sub-G1 level were observed in PBMCs with the addition of anti-HGF neutralizing antibody in comparison with PBMCs cultured alone.
This effect has been confirmed by Western blot analysis. The expression of PARP and active caspase 9 and 3 was assessed in whole leukocyte lysates cultured alone and cocultured with hAFSCs active in the Transwell condition (Fig. 3E). The increase of these markers confirms the presence of apoptotic death.
Discussion
MSCs can give rise to differentiated cells of the mesodermal lineage including bone, fat, cartilage, tendon, and muscle [41–43]. In addition, their ability to evade immunosurveillance after cell transplantation and to suppress immune response has made BM-MSCs a particularly attractive candidate for clinical use [44, 45]. In particular, it was observed that BM-MSCs could suppress lymphocyte proliferation and activation in response to the allogenic activation or chemical stimulation in vitro or in vivo [43, 46, 47].
Among stem cell sources, the amniotic fluid contains multiple cell types derived mainly from exfoliating surfaces of the developing fetus [48]. These include cells from the fetal skin, respiratory system, and urinary and gastrointestinal tracts, along with populations of MSCs [49]. Immunoselection for c-Kit (CD117), the cell surface receptor for stem cell factor, designated AFSCs with no signs of malignant transformation, chromosomal abnormalities, or loss of differentiation potential [8].
In this study, we confirmed that hAFSCs cultured in our experimental conditions were positive for the mesenchymal stem cell markers CD73, CD90, CD105, CD271, and Stro-1 but also for typical markers of embryonic stem cells, such as SSEA4, Oct4, Nanog, FOXO1, Sox2, and TRA-1-81.
CD271, also known as low affinity nerve growth factor receptor (LNGFR) or p75NTR, belongs to the low-affinity neurotrophin receptor and tumor necrosis factor receptor superfamily [50]. This cell surface marker potentially defines an MSC precursor subpopulation with immunosuppressive and lymphohematopoietic engraftment-promoting properties [51]. The expression of this marker in most amniotic fluid stem cell populations should suggest immune-regulation potential.
Differentiation plasticity can be justified by the expression of several pluripotent stem cell markers. SSEA4, an early embryonic glycolipid antigen, is commonly used as a marker for undifferentiated pluripotent human embryonic stem cells and cleavage to blastocyst-stage embryos but also identifies the adult mesenchymal stem cell population.
The transcription factor Oct4 has essential functions for the maintenance of pluripotent embryonic and germ cells of mammals [52]. Sox2, a member of the SoxB1 transcription factor family, is an important transcriptional regulator in pluripotent stem cells. Together with Oct4 and Nanog, they cooperatively control gene expression in pluripotent stem cells and maintain their pluripotency. The FOXO transcription factors have an essential role in maintaining stem cell identity [53]. Finally, all human pluripotent stem cells express TRA-1-81 antigen.
AFSCs are able to suppress inflammatory responses in vitro, and soluble factors are an essential for communication between lymphocytes and AFSCs. Considering their extensive self-renewal capacity, possibility for banking, and absence of tumorigenicity, AFSCs can be evaluated as a superior source of stable, well-characterized, “off the shelf” immunomodulatory cells for a variety of immunotherapies and have the ability to avoid allogenic rejection [1].
It has been proven that AFSCs modulate lymphocyte proliferation in a different manner according to gestational age. Second-trimester AFSCs showed low expression of HLA class I molecules and absence of HLA II, and their features were associated with lower sensitivity to natural killer (NK) cell-mediated lysis. Second-trimester AFSCs did not efficiently inhibit T and NK cell proliferation but could suppress B-cell proliferation, which was not affected by the first- and third-trimester AFSCs [36]. In contrast, the mechanism of hAFSC immunoregulation by soluble factors has been elucidated only in part. Under growth conditions, amniotic fluid stem cells and BM-MSCs release relatively low levels of very few cytokines, but on activation by PBMCs, high levels of cytokines are released [1]. In fact, the microarray shown in Table 2 demonstrated the activating effect of pre-exposure to PBMCs on the secretion of immunomodulatory factors.
Based on these observations, we decided to evaluate the immunosuppressive actions of hAFSCs directed toward the whole peripheral blood leukocyte population, focusing on the secreted factor HGF. HGF is a potent immunomodulatory factor that inhibits dendritic cell function along with differentiation of IL-10-producing regulatory T cells, decrease in IL-17-producing T cells, and downregulation of surface markers of T-cell activation [23]. Apoptotic effects of HGF and c-Met are not yet fully understood [39] but have been observed in several cell lines such as ovarian carcinoma cells, breast carcinoma cells, mouse sarcoma cells, and mouse hepatocarcinoma cells [54–57]. Yang et al. [58] found that hepatic stellate cells send an apoptotic message to T cells and, at the same time, inhibit antigen-specific T-cell activity. Bottai et al. [37] demonstrated the secretion of HGF by hAFSCs isolated from third trimester amniotic fluid and focused on its endocrine role in targeting the injury site in an animal model without evaluating its possible immunoregulating capacity.
Initially we demonstrated that HGF is released by hAFSCs in the medium and after different types and durations of differentiation protocols, suggesting that the potential immunomodulatory property could be maintained in vivo during differentiation and after engraftment. HGF production is time dependent and enhanced by hAFSC preactivation with PBMCs, a condition reflecting the realistic situation occurring after the in vivo implant.
We next examined the effect of short time exposure to hAFSC secretum on PBMCs compared with pure HGF. Figure 2 shows that 24 hours with hAFSC CM activate the typical HGF pathway of c-MET/Akt in PBMCs and induce exogenous HGF; however, this phenomenon is not linked to the obvious survival stimulus, as demonstrated by the unexpected effect of Akt inhibitors.
PBMC apoptotic portion was increased by the presence of hAFSCs in all coculture conditions via a caspase-dependent process. The apoptotic effect was augmented by increased HGF production after hAFSC activation. By neutralizing the HGF activity, the immunosuppressive action of the hAFSCs was partially abolished. These results suggest that hAFSCs possess immunomodulatory properties involving HGF production.
Conclusion
Our findings have provided new information about the immune modulatory properties of hAFSCs. It is clear that immunomodulation is not a peculiar feature of MSC-like cells but actually general property of stem cells that may be induced or enhanced by inflammatory stimuli. Understanding these mechanisms may help identify novel therapeutic strategies and recognize the most effective stem cell class for interfering with damage-mediated inflammation and inducing tissue regeneration and organ repair [16]. Consequently, further in vivo studies will clarify whether these considerations for MSCs may be applied to c-Kit-positive stem cell populations such as AFSCs.
We conclude that AFSCs can be considered as a new therapeutic tool for many pathologies, taking into account the differentiation potential in vitro toward cell lineages belonging to the three germ layers and the different ways to regulate immune responses.
Acknowledgments
This work was supported by grants from Ministero dell'Istruzione, dell'Università e della Ricerca Fondo per gli Investimenti della Ricerca di Base Accordi di Programma 2010 (MIUR FIRB) Prot: RBAP10Z7FS. E. We thank Dr. Ardizzoni at the Department of Diagnostics, Clinical and Public Health Medicine, University of Modena and Reggio Emilia, Modena, Italy, for providing expertise in microarray experiments.
Author Contributions
T.M.: conception/design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; F.B., M.G., and M.Z.: collection of assembly of data; A.D.P.: manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Moorefield EC, McKee EE, Solchaga L, et al. Cloned, CD117 selected human amniotic fluid stem cells are capable of modulating the immune response. PLoS One. 2011;6:e26535. doi: 10.1371/journal.pone.0026535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 3.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 4.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 5.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 6.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 7.Sensebé L, Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87(suppl):S49–S53. doi: 10.1097/TP.0b013e3181a28635. [DOI] [PubMed] [Google Scholar]
- 8.De Coppi P, Bartsch G, Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 9.Maraldi T, Bertoni L, Riccio M, et al. Human amniotic fluid stem cells: Neural differentiation in vitro and in vivo. Cell Tissue Res. 2014;357:1–13. doi: 10.1007/s00441-014-1840-x. [DOI] [PubMed] [Google Scholar]
- 10.Riccio M, Maraldi T, Pisciotta A, et al. Fibroin scaffold repairs critical-size bone defects in vivo supported by human amniotic fluid and dental pulp stem cells. Tissue Eng Part A. 2012;18:1006–1013. doi: 10.1089/ten.TEA.2011.0542. [DOI] [PubMed] [Google Scholar]
- 11.Kolambkar YM, Peister A, Soker S, et al. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol. 2007;38:405–413. doi: 10.1007/s10735-007-9118-1. [DOI] [PubMed] [Google Scholar]
- 12.Rossi B, Merlo B, Colleoni S, et al. Isolation and in vitro characterization of bovine amniotic fluid derived stem cells at different trimesters of pregnancy. Stem Cell Rev. 2014;10:712–724. doi: 10.1007/s12015-014-9525-0. [DOI] [PubMed] [Google Scholar]
- 13.Ryan JM, Barry FP, Murphy JM, et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betz AG. Immunology. Have you seen your mother, baby. Science. 2010;330:1635–1636. doi: 10.1126/science.1200406. [DOI] [PubMed] [Google Scholar]
- 15.Chiavegato A, Bollini S, Pozzobon M, et al. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J Mol Cell Cardiol. 2007;42:746–759. doi: 10.1016/j.yjmcc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Di Trapani M, Bassi G, Ricciardi M, et al. Comparative study of immune regulatory properties of stem cells derived from different tissues. Stem Cells Dev. 2013;22:2990–3002. doi: 10.1089/scd.2013.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sessarego N, Parodi A, Podestà M, et al. Multipotent mesenchymal stromal cells from amniotic fluid: Solid perspectives for clinical application. Haematologica. 2008;93:339–346. doi: 10.3324/haematol.11869. [DOI] [PubMed] [Google Scholar]
- 18.Luo C, Jia W, Wang K, et al. Human amniotic fluid stem cells suppress PBMC proliferation through IDO and IL-10-dependent pathways. Curr Stem Cell Res Ther. 2014;9:36–45. doi: 10.2174/1574888x113086660067. [DOI] [PubMed] [Google Scholar]
- 19.Yeung TY, Seeberger KL, Kin T, et al. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One. 2012;7:e38189. doi: 10.1371/journal.pone.0038189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang JW, Tsai HL, Chen CW, et al. Conditioned mesenchymal stem cells attenuate progression of chronic kidney disease through inhibition of epithelial-to-mesenchymal transition and immune modulation. J Cell Mol Med. 2012;16:2935–2949. doi: 10.1111/j.1582-4934.2012.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260. doi: 10.1186/s12967-014-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: Cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 23.Benkhoucha M, Santiago-Raber ML, Schneiter G, et al. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2010;107:6424–6429. doi: 10.1073/pnas.0912437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okunishi K, Dohi M, Fujio K, et al. Hepatocyte growth factor significantly suppresses collagen-induced arthritis in mice. J Immunol. 2007;179:5504–5513. doi: 10.4049/jimmunol.179.8.5504. [DOI] [PubMed] [Google Scholar]
- 25.Maraldi T, Riccio M, Resca E, et al. Human amniotic fluid stem cells seeded in fibroin scaffold produce in vivo mineralized matrix. Tissue Eng Part A. 2011;17:2833–2843. doi: 10.1089/ten.tea.2011.0062. [DOI] [PubMed] [Google Scholar]
- 26.Maraldi T, Riccio M, Pisciotta A, et al. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res Ther. 2013;4:53. doi: 10.1186/scrt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Sala GB, Ardizzoni A, Capodanno F, et al. Protein microarrays on midtrimester amniotic fluids: A novel approach for the diagnosis of early intrauterine inflammation related to preterm delivery. Int J Immunopathol Pharmacol. 2012;25:1029–1040. doi: 10.1177/039463201202500420. [DOI] [PubMed] [Google Scholar]
- 28.Pisciotta A, Riccio M, Carnevale G, et al. Human serum promotes osteogenic differentiation of human dental pulp stem cells in vitro and in vivo. PLoS One. 2012;7:e50542. doi: 10.1371/journal.pone.0050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guida M, Maraldi T, Beretti F, et al. Nuclear Nox4-derived reactive oxygen species in myelodysplastic syndromes. Biomed Res Int. 2014;014:456937. doi: 10.1155/2014/456937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zavatti M, Resca E, Bertoni L, et al. Ferutinin promotes proliferation and osteoblastic differentiation in human amniotic fluid and dental pulp stem cells. Life Sci. 2013;92:993–1003. doi: 10.1016/j.lfs.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Portolani M, Beretti F, Bartoletti AM, et al. Propagation of a transmissible cytotoxic activity on cultures of human peripheral blood lymphocytes. New Microbiol. 2008;31:417–422. [PubMed] [Google Scholar]
- 32.Guida M, Maraldi T, Resca E, et al. Inhibition of nuclear Nox4 activity by plumbagin: Effect on proliferative capacity in human amniotic stem cells. Oxid Med Cell Longev. 2013;2013:680816. doi: 10.1155/2013/680816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maraldi T, Prata C, Vieceli Dalla Sega F, et al. NAD(P)H oxidase isoform Nox2 plays a prosurvival role in human leukaemia cells. Free Radic Res. 2009;43:1111–1121. doi: 10.1080/10715760903186132. [DOI] [PubMed] [Google Scholar]
- 34.Carnevale G, Riccio M, Pisciotta A, et al. In vitro differentiation into insulin-producing β-cells of stem cells isolated from human amniotic fluid and dental pulp. Dig Liver Dis. 2013;45:669–676. doi: 10.1016/j.dld.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Maraldi T, Guida M, Zavatti M, et al. Nuclear Nox4 role in stemness power of human amniotic fluid stem cells. Oxid Med Cell Longev. 2015 doi: 10.1155/2015/101304. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Trapani M, Bassi G, Fontana E, et al. Immune regulatory properties of CD117(pos) amniotic fluid stem cells vary according to gestational age. Stem Cells Dev. 2015;24:132–143. doi: 10.1089/scd.2014.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bottai D, Scesa G, Cigognini D, et al. Third trimester NG2-positive amniotic fluid cells are effective in improving repair in spinal cord injury. Exp Neurol. 2014;254:121–133. doi: 10.1016/j.expneurol.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 38.Maulik G, Madhiwala P, Brooks S, et al. Activated c-Met signals through PI3K with dramatic effects on cytoskeletal functions in small cell lung cancer. J Cell Mol Med. 2002;6:539–553. doi: 10.1111/j.1582-4934.2002.tb00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: Cytokines and factors. Am J Reprod Immunol. 2012;67:1–8. doi: 10.1111/j.1600-0897.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- 40.Tulasne D, Foveau B. The shadow of death on the MET tyrosine kinase receptor. Cell Death Differ. 2008;15:427–434. doi: 10.1038/sj.cdd.4402229. [DOI] [PubMed] [Google Scholar]
- 41.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 42.Haynesworth SE, Goshima J, Goldberg VM, et al. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 43.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 44.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 45.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 46.Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 47.Maitra B, Szekely E, Gjini K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 48.Gosden CM. Amniotic fluid cell types and culture. Br Med Bull. 1983;39:348–354. doi: 10.1093/oxfordjournals.bmb.a071847. [DOI] [PubMed] [Google Scholar]
- 49.Fauza D. Amniotic fluid and placental stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18:877–891. doi: 10.1016/j.bpobgyn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Thomson TM, Rettig WJ, Chesa PG, et al. Expression of human nerve growth factor receptor on cells derived from all three germ layers. Exp Cell Res. 1988;174:533–539. doi: 10.1016/0014-4827(88)90323-0. [DOI] [PubMed] [Google Scholar]
- 51.Kuçi S, Kuçi Z, Kreyenberg H, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95:651–659. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tolkunova E, Malashicheva A, Parfenov VN, et al. PIAS proteins as repressors of Oct4 function. J Mol Biol. 2007;374:1200–1212. doi: 10.1016/j.jmb.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 53.Chaudhari P, Ye Z, Jang YY. Roles of reactive oxygen species in the fate of stem cells. Antioxid Redox Signal. 2014;20:1881–1890. doi: 10.1089/ars.2012.4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rasola A, Anguissola S, Ferrero N, et al. Hepatocyte growth factor sensitizes human ovarian carcinoma cell lines to paclitaxel and cisplatin. Cancer Res. 2004;64:1744–1750. doi: 10.1158/0008-5472.can-03-2383. [DOI] [PubMed] [Google Scholar]
- 55.Arakaki N, Kazi JA, Kazihara T, et al. Hepatocyte growth factor/scatter factor activates the apoptosis signaling pathway by increasing caspase-3 activity in sarcoma 180 cells. Biochem Biophys Res Commun. 1998;245:211–215. doi: 10.1006/bbrc.1998.8397. [DOI] [PubMed] [Google Scholar]
- 56.Matteucci E, Modora S, Simone M, et al. Hepatocyte growth factor induces apoptosis through the extrinsic pathway in hepatoma cells: Favouring role of hypoxia-inducible factor-1 deficiency. Oncogene. 2003;22:4062–4073. doi: 10.1038/sj.onc.1206519. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y, Difrancesca D, Wang X, et al. Promotion of Fas-mediated apoptosis in type II cells by high doses of hepatocyte growth factor bypasses the mitochondrial requirement. J Cell Physiol. 2007;213:556–563. doi: 10.1002/jcp.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang HR, Chou HS, Gu X, et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-gamma signaling. Hepatology. 2009;50:1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]