This study addressed whether freshly thawed mesenchymal stromal cells (MSCs) are as effective in in vivo settings as those that have been continuously cultured. It also provided further data demonstrating that xenogeneic use of MSCs in immunocompetent mice is as effective as murine MSCs. This information provides further support and direction for potential clinical use of MSCs in patients with severe asthma.
Keywords: Mesenchymal stromal cells, Cryopreservation, Asthma, Mouse
Abstract
Recent data suggest that freshly thawed previously frozen mesenchymal stromal cells (MSCs) may not have the same effectiveness or breadth of anti-inflammatory activities as do continuously cultured MSCs. This has significant implications for clinical use, in which many infusion schemes use frozen cells thawed at the bedside for administration. The available data, however, predominantly evaluate in vitro MSC properties, and so far there has been limited in vivo analysis. To further assess this issue, we compared freshly thawed (thawed) versus continuously cultured (fresh) human bone marrow-derived MSC (hMSC) administration in a mouse model of mixed Th2/Th17 allergic airway inflammation induced by Aspergillus hyphal extract (AHE) exposures in immunocompetent C57Bl/6 mice. Control cell populations included fresh versus thawed murine bone marrow-derived MSCs (mMSCs) and human lung fibroblasts (HLFs). Systemic administration of both thawed and fresh hMSCs and mMSCs, but not HLFs, at the onset of antigen challenge in previously sensitized mice significantly ameliorated the AHE-provoked increases in airway hyper-reactivity, lung inflammation, and antigen-specific CD4 T-cell Th2 and Th17 phenotype. Notably, there was no difference in effects of fresh versus thawed hMSCs or mMSCs on any outcome measured except for some variability in the effects on the bronchoalveolar lavage fluid composition. These results demonstrated potent xenogeneic effects of human MSCs in an immunocompetent mouse model of allergic airways inflammation and that thawed MSCs are as effective as fresh MSCs. The question of fresh versus thawed MSC effectiveness needs to be investigated carefully and may differ in different in vivo disease-specific models.
Significance
This study addressed whether freshly thawed mesenchymal stromal cells (MSCs) are as effective in in vivo settings as those that have been continuously cultured. It also provided further data demonstrating that xenogeneic use of MSCs in immunocompetent mice is as effective as murine MSCs. This information provides further support and direction for potential clinical use of MSCs in patients with severe asthma.
Introduction
Mesenchymal stromal cells (MSCs) isolated from bone marrow, adipose tissue, umbilical cord blood, and other sources secrete a range of anti-inflammatory mediators and have other anti-inflammatory actions in response to different inflammatory stimuli [1–3]. As such, when isolated from the different sources and readministered either systemically or directly in an organ-specific manner, they can have significant anti-inflammatory and disease-ameliorating actions in a wide variety of preclinical inflammatory and autoimmune disease models [4–6]. This has led to an increasing number of clinical investigations of both autologous and allogeneic MSC administration and some suggestions of successful desired anti-inflammatory actions in clinical use, most notably in refractory pediatric graft-versus-host disease [4–7]. However, despite abundant literature describing the actions of MSCs in both in vitro assays and preclinical disease models, fundamental questions remain about the mechanisms of MSC actions in clinical applications. Issues include a relative lack of information correlating in vitro MSC potency on any given inflammatory pathway with the desired disease-specific actions for a given clinical indication. In parallel, optimal approaches for isolating, expanding, preparing, and administering MSCs for disease-specific clinical indications have not yet been determined [8–10].
A simple yet fundamental approach for MSC use has recently come under renewed scrutiny. For practical reasons, systemic administration of MSCs, particularly non-human leukocyte antigen-matched allogeneic MSCs, has involved freezing of cell preparations after isolation and expansion. The frozen MSCs, suspended at the desired concentration in a cryopreservation medium suitable for infusion, are then thawed just prior to administration. However, a significant number of the frozen MSCs may undergo apoptosis during the freeze-thaw process, although much subsequent study has attempted to minimize this occurrence with improved freeze-thaw approaches and cryopreservatives [11–14]. A recent report demonstrated that freshly thawed MSCs were not as effective in in vitro potency assays as continuously cultured MSCs of the same passage number [15]. Data from this study further demonstrated that it took up to 24 hours for the thawed MSCs to regain potency in the in vitro assays. A second recent study found that freeze-thawed MSCs demonstrated reduced initial responsiveness to proinflammatory stimuli, impaired production of anti-inflammatory mediators, and strong activation of the complement cascade compared with continuously cultured cells [16]. This report included a retrospective analysis of systemic MSC administration in patients with complications of hematopoietic stem cell transplantation at the Karolinska Institute between 2002 and 2007 and demonstrated that the use of continuously cultured MSCs at low passage had a response rate twice that observed in a comparable group of patients treated with freshly thawed cells at higher passage (100% vs. 50%) [16]. These results suggest potential disadvantages to the use of freshly thawed MSCs and may significantly affect clinical investigations and uses of MSCs for which it is not uncommon to use freshly thawed cells. However, there have been no other detailed investigations of potential effects of thawing on the immediate anti-inflammatory actions of MSCs either in vitro or in vivo or of whether this might differ depending on the nature of the disease-specific inflammatory environment.
To address this issue, we investigated the question of freshly thawed (thawed) versus continuously cultured (fresh) MSC potency in an in vivo mouse model of allergic airway inflammation. We and others have demonstrated that systemic administration of either syngeneic or allogeneic MSCs during either sensitization or challenge ameliorates airways hyper-reactivity and lung inflammation provoked by a variety of different antigens [17–27]. We have most recently demonstrated the efficacy of MSC administration in a mucosal immunization model involving intratracheal administration of Aspergillus hyphal extract (AHE) [28]. This provokes a mixed Th2/Th17 model of eosinophilic and neutrophilic allergic airway inflammation and is used as a mouse model of severe refractory neutrophilic asthma [29, 30]. Furthermore, because an increasing number of studies have demonstrated efficacy and thus potential usefulness as preclinical models of human MSC (hMSC) administration in immunocompetent mouse models of lung and other diseases [31–35], both fresh and thawed human and syngeneic mouse MSCs were assessed in AHE sensitized and challenged immunocompetent C57Bl/6 mice.
Materials and Methods
Mice
C57Bl/6 mice (male, 8–12 weeks, n = 72; Jackson Laboratories, Bar Harbor, ME, http://www.jax.org) were housed in microisolator cages and used in accordance with the University of Vermont (UVM) institutional animal care and use committee under all applicable Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
Cells and Cell Culture
Murine bone marrow-derived mesenchymal stromal cells (mMSCs) from C57Bl/6 mice were obtained from the Texas A&M stem cell core facility [36]. Human mesenchymal stem cells derived from bone marrow of normal human volunteers were obtained from the National Heart, Lung, and Blood Institute’s Production Assistance for Cellular Therapies program (D.H.M.). These cells have been extensively characterized previously for cell surface marker expression and differentiation capacity [36–38]. mMSCs were expanded in culture using Iscove’s Modified Dulbecco’s Medium (Hyclone; GE Healthcare Bio-Sciences, Pittsburgh, PA, http://www.gelifesciences.com), 10% fetal bovine serum (FBS; Hyclone), 10% horse serum (Hyclone), 1% penicillin/streptomycin (Invitrogen, Life Technologies; Thermo Fisher Scientific, Waltham, MA, http://www.thermofisher.com), and 2 mM l-glutamine (Invitrogen) and used at passages 4–6. hMSCs were cultured in Minimal Essential Medium with Earle’s Balanced Salts (Hyclone; GE Healthcare Bio-Sciences, Pittsburgh, PA, http://www.gelifesciences.com), 20% FBS, 1% penicillin/streptomycin, and 2 mM l-glutamine and used at passage 6 or lower. Human and mouse MSCs were passaged every 3 days for these studies. We routinely used mouse and human bone marrow-derived MSCs in passages 2–6 and in previous studies [23, 28]; these cells have been considered low passage, and anything beyond is considered high passage and is not used for in vivo studies. We have never observed any significant difference in behavior of the MSCs in the in vivo studies within this range of passages. Moreover, we were careful to not let individual culture plates go beyond 70% passage to minimize any potential paracrine signaling, so the cells are still actively growing at the time of harvest. Normal adult HLFs were expanded in culture with Dulbecco’s Modified Eagle’s Medium: Nutrient Mixture F-12 (Sigma-Aldrich. St. Louis, MO, https://www.sigmaaldrich.com), 10% FBS, 1% penicillin/streptomycin, and 2 mM l-glutamine and used at passage 6 or lower.
For use in experiments, the cells were harvested for injection using 2.5% Trypsin/EDTA (Invitrogen). Cell density and viability was determined after washing using trypan blue staining and counted using a hemocytometer. Cell pellets were then resuspended in sterile 1× phosphate-buffered saline (PBS) to a final concentration of 1 × 106 cells per 200 μl immediately prior to injection. Cells suspended in sterile PBS were kept on ice until administration within 15 minutes of thawing and washing. Cells (hMSCs, mMSCs, HLFs) in parallel plates from the same batches and passage number as those harvested from continuous culture were similarly harvested and then cryopreserved at −80°C for 48 hours at 1.5 × 106 cells in 1 ml of freezing solution (50% medium, 40% FBS, and 10% dimethyl sulfoxide), followed by 7 days of storage in liquid nitrogen. Cells were thawed immediately prior to injection and washed three times with PBS. Cell viability, density, and final concentration (1 × 106 viable cells per 200 μl PBS) were determined after washing by trypan blue exclusion and counting using a hemocytometer, as described for cultured MSC preparations [23, 28]. Cells were suspended in sterile PBS and kept on ice until administration within 15 minutes of thawing and washing.
Induction of Allergic Airway Inflammation
The study design is shown in schematic form in Figure 1. AHE aliquots at a concentration of 1.466 mg/ml in 1× PBS, generously provided by the Whittaker laboratory at UVM and previously used by us, were thawed and vortexed immediately prior to use, diluted to a final concentration of 5 μg AHE in 40 μl sterile 1× PBS [28–30]. Mice were anesthetized by isoflurane inhalation and received an oropharyngeal administration of PBS (naïve) or AHE solution on days 0 and 7 to initiate the immune response (sensitization), then challenged for 3 days on days 14–16 with oropharyngeal inoculations using the same AHE preparation (Fig. 1A) [28].
Figure 1.
Study design. (A): Schematic of the acute induction of AHR. Mice were sensitized by OP administration of PBS (naïve) or AHE solution on days 0 and 7 and then challenged on days 14–16, with OP AHE inoculations. Cells were administered by systemic (tail vein) injection on the first day of challenge (day 14). Mice were euthanized on day 19 for endpoint assessments. (B): Experimental groups. Animals exposed to AHE were randomly divided to receive treatment with the vehicle PBS, human lung fibroblasts, human mesenchymal stromal cells, and mouse mesenchymal stromal cells. The cells were freshly harvested from continuous culture or immediately thawed before administration. Abbreviations: A, AHE solution; AHE, Aspergillus hyphae extract; F, fresh; HLF, human lung fibroblasts; hMSC, human mesenchymal stromal cells; mMSC, murine mesenchymal stromal cells; MSCs, mesenchymal stromal cells; OP, oropharyngeal; P, vehicle; PBS, phosphate-buffered saline; T, thawed.
Cell Administration
On day 14, immediately after the AHE inoculation, mice were systemically administered by a tail vein injection of fresh or recently thawed mMSCs, hMSCs, or HLFs (1 × 106 viable cells in 200 μl 1× PBS) or of 1× PBS control (Fig. 1B). Mice were euthanized on day 19, and lung function, lung inflammation, and antigen-specific T-cell activity were measured, as described below. Because viability was ∼70% in the thawed cells, 30% more cells were used in mice receiving thawed cells to have ∼1 million viable cells injected. In real numbers, this equates to ∼1.3 million thawed versus 1 million fresh cells.
Respiratory Mechanics
Pulmonary function was analyzed using the forced oscillation technique (flexiVent; SCIREQ Scientific Respiratory Equipment, Montreal, Canada, http://www.scireq.com) as previously described [23, 28, 39, 40]. The peak responses for airway resistance, overall tissue resistance, and elastance within the lung were determined in response to sequential inhalation of nebulized saline, followed by 3.125 mg/ml, 12.5 mg/ml, and 25 mg/ml of methacholine (MCh) diluted in saline.
Assessment of Airway Inflammation
Following evaluation of lung mechanics, mice were euthanized by lethal intraperitoneal injection of sodium pentobarbital. Bronchoalveolar lavage fluid (BALF) was collected by administering 1 ml of sterile 1× PBS to the airways through a tracheal cannula and rinsing the lungs three times prior to recovery. BALF was centrifuged at 2,000g, for 5 minutes at 4°C, and the supernatant was collected in separate tubes and stored at −80°C. A Bio-Plex cytokine assay system (Bio-Rad, Hercules, CA, http://www.bio-rad.com) was used to examine undiluted BALF samples for soluble inflammatory cytokines using a mouse 23-plex panel. Concentrations were determined using the Bio-Plex Manager software. The cell pellet was resuspended, and an aliquot was used to determine total cell count with an ADVIA hematology analyzer (Siemens, Munich, Germany, http://usa.healthcare.siemens.com). Cytospins were made using 3 × 104 cells centrifuged onto precleaned, pretreated glass slides (Corning, Corning, NY, http://www.corning.com) at 300g for 8 minutes, dried overnight, and stained using Hema 3 Manual Staining System (Fisher Scientific, Pittsburgh, PA, http://www.thermofisher.com). Different cell populations were determined by blinded manual count of 200 cells performed by three separate persons. Following BALF collection, the trachea and heart/lung block were removed and the right lobes of the lung were removed and snap frozen on liquid nitrogen. The left lobe was then gravity fixed (20 cm H2O) for 1 hour with 4% paraformaldehyde and 5-μm paraffin sections subsequently stained with hematoxylin and eosin. In a blinded fashion, 3 separate individuals evaluated airways inflammation, 10 airways per animal, based on the presence and intensity of peribronchial cell infiltrates compared with positive and negative controls using an established semiquantitative scoring system, using a 0–3 range as previously described. No individual knew which group was analyzed [23, 28].
Mediastinal Lymph Node Mixed Lymphocyte Assessments
Mediastinal lymph nodes (MLNs) were isolated by dissection from each mouse and placed in T-cell medium (RPMI, 5% FBS, 1× penicillin/streptomycin, 2 mM l-glutamine, 2,500 mg/ml glucose, 1 mg/ml folate in 2 g/l sodium bicarbonate, 1 mM sodium pyruvate, and 50 μM β-mercaptoethanol). To ensure enough cells for assay, MLN cells from mice of the same experimental group were pooled and pressed through a 40-μm mesh filter into a single-cell suspension. Cells were then washed twice in 1× PBS and resuspended for counting. One million cells per time point (24, 48, and 72 hours) were plated in duplicate for each group in a 24-well dish in 500 μl of T-cell medium. In half of the wells, cells were stimulated with 1 μg of AHE in the medium for 24 or 48 hours; the other wells were left unstimulated for the same time points. Total contents of each well were collected at the indicated time points and were centrifuged for 5 minutes at 5,000 rpm to pellet cells and debris. Supernatants were moved to a new tube and frozen at −20°C. Content of representative Th1, Th2, and Th17 soluble mediators (interleukin 4 [IL-4], IL-5, IL-17 and interferon-γ) were assessed by commercially available enzyme-linked immunosorbent assay (ELISA) kits (BioLegend, San Diego, CA, http://www.biolegend.com). Sensitivity of ELISA kits was IL-4, 1 pg/ml; IL-5, 4 pg/ml; IL-17, 8 pg/ml; and IFNγ, 4 pg/ml.
Statistical Analyses
All data were graphed and analyzed using the GraphPad Prism v6.0 statistical software package (GraphPad Software, La Jolla, CA, http://www.graphpad.com). The normality of the data (Kolmogorov-Smirnov test with Lilliefors correction) and the homogeneity of variances (Levene median test) were tested. Parametric data are expressed as mean ± SD. Differences between the groups were evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s test. Nonparametric data were analyzed using ANOVA on ranks followed by Dunn’s post hoc test. Statistical significance was established at p ≤ .05.
Results
Systemic Administration of Fresh or Thawed hMSCs and mMSCs Comparably Ameliorate AHE-Induced Airway Hyper-Responsiveness
AHE sensitization and challenge resulted in a significant increase compared with naïve mice in each measure of methacholine-stimulated airway hyper-reactivity (AHR): large airway resistance, lung elastance, and tissue resistance (Fig. 2). Administration of either mMSCs or hMSCs significantly decreased each measure of AHR, whereas administration of the HLF control cell population had no effect. Notably, hMSCs were as effective as mMSCs, and no differences were observed in effects of fresh versus thawed for mMSCs, hMSCs, or HLFs.
Figure 2.
Systemic administration of either fresh or thawed hMSCs or mMSCs significantly ameliorated airway hyper-responsiveness provoked by AHE sensitization and challenge. Airway resistance, overall tissue resistance, and lung elastance following methacholine challenge of naïve and AHE-exposed mice treated with the vehicle phosphate-buffered saline or with fresh or thawed cells. (A): HLF: 17 naïve, 15 A-P, 6 A-HLF-F, 6 A-HLF-T. (B): hMSC: 17 naïve, 15 A-P, 10 A-hMSC-F, 6 A-hMSC-T. (C): mMSC: 17 naïve, 15 A-P, 6 A-mMSC-F, 6 A-mMSC-T. Data are presented as peak response normalized to the baseline and then expressed as percentage increase over the baseline ±SD. p ≤ .05. ∗Significance compared with naïve. #Significance compared with A-P. Abbreviations: A, AHE-exposed; A-P, AHE-exposed mice treated with PBS; AHE, Aspergillus hyphae extract; F, fresh; G, overall tissue resistance; H, lung elastance; HLF, human lung fibroblasts; hMSC, human mesenchymal stromal cells; mMSC, murine mesenchymal stromal cells; N, naïve; P, vehicle; PBS, phosphate-buffered saline; Rn, airway resistance; T, thawed.
Systemic Administration of Fresh or Thawed hMSCs and mMSCs Comparably Ameliorate AHE-Induced Lung Inflammation
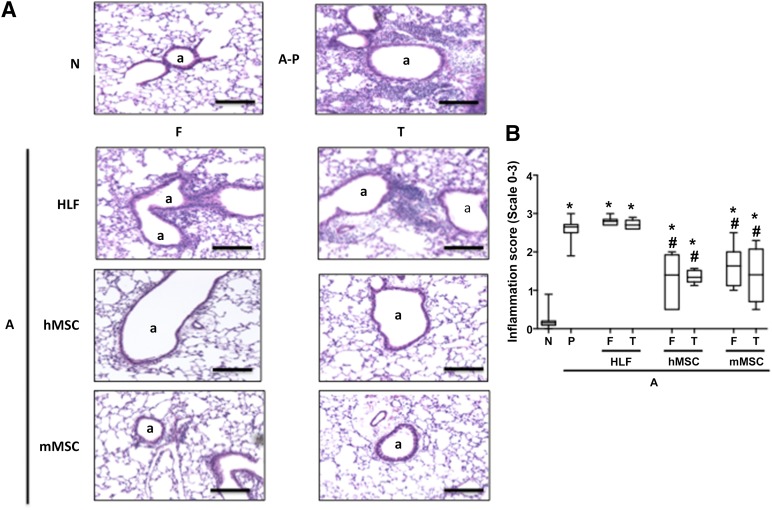
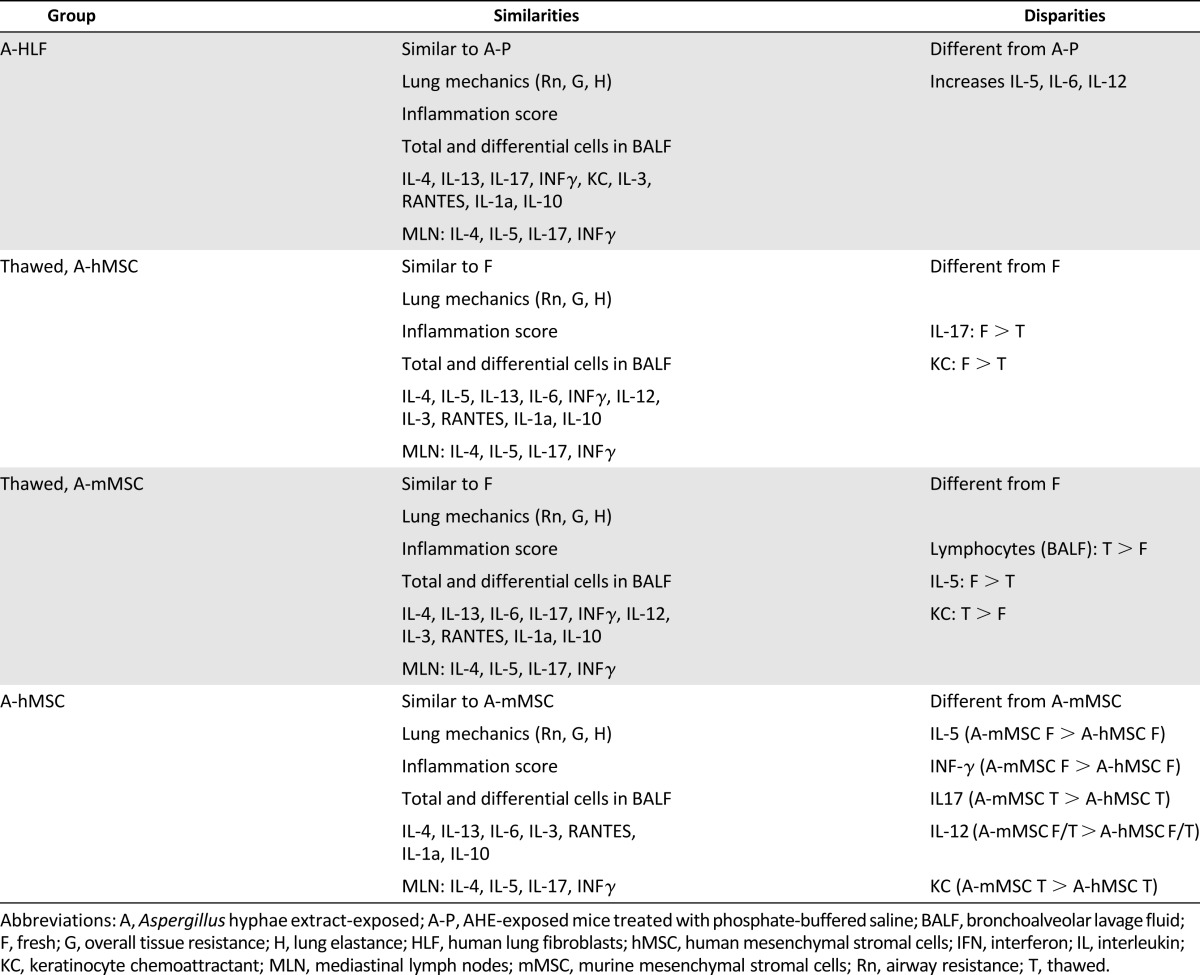
AHE sensitization and challenge resulted in a significant increase in histologic and BALF inflammation compared with naïve mice (Fig. 3–5). Administration of either mMSCs or hMSCs comparably and significantly decreased both histologic inflammation (Fig. 3) and total and differential BALF cell counts (Fig. 4). No difference was observed between fresh and thawed cells, except for a more pronounced reduction of lymphocytes using fresh mMSCs compared with thawed cells (Fig. 4B). HLF administration had no effects on the AHE-provoked histologic or BALF inflammation.
Figure 3.
Systemic administration of either fresh or thawed hMSCs or mMSCs significantly reduced histologic lung inflammation provoked by AHE sensitization and challenge. (A): Representative photomicrographs of hematoxylin and eosin-stained lung section, Original magnification ×10. Scale bar indicates 100 μm. (B): Inflammation score of airways in N and A mice treated with fresh and thawed HLF, hMSC, and mMSC: 17 N, 15 A-P, 6 A-HLF-F, 6 A-HLF-T, 10 A-hMSC-F, 6 A-hMSC-T, 6 A-mMSC-F, 6 A-mMSC-T. Data are presented as mean ±SD. p ≤ .05. Significance compared with ∗N and #A-P. Abbreviations: a, airway; A, AHE-exposed; A-P, AHE-exposed mice treated with PBS; AHE, Aspergillus hyphae extract; F, fresh; HLF, human lung fibroblasts; hMSC, human mesenchymal stromal cells; mMSC, murine mesenchymal stromal cells; N, naïve; P, vehicle (PBS); PBS, phosphate-buffered saline; T, thawed.
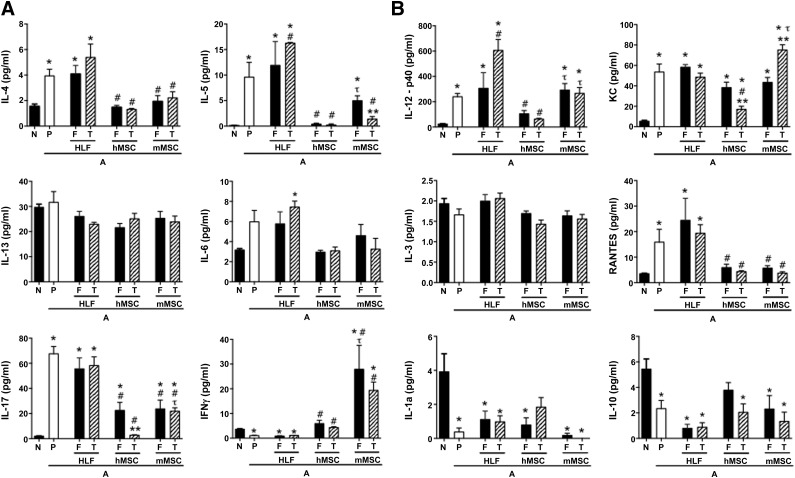
Figure 5.
Systemic administration of either fresh or thawed hMSCs or mMSCs significantly reduced the increased bronchoalveolar lavage fluid content of proinflammatory soluble cytokines and chemokines provoked by AHE sensitization and challenge. (A): Cytokines associated with Th2 (IL-4, IL-5, IL-13), Th17 (IL-6, IL-17a), and Th1 inflammation (IFNγ). (B): Further Th17 inflammation associated cytokines (IL-12, KC), alternative inflammatory cytokines (IL-3, RANTES), and cytokines previously identified as secreted by MSCs in immunomodulation (IL-1A, IL-10). n = 17 N, 15 A-P, 6 A-HLF-F, 6 A-HLF-T, 10 A-hMSC-F, 6 A-hMSC-T, 6 A-mMSC-F, 6 A-mMSC-T. Data are presented as mean ±SD. p ≤ .05. Significance compared with ∗N and #A-P. τA-mMSC versus A-hMSC. ∗∗F versus T. Abbreviations: A, AHE-exposed; A-P, AHE-exposed mice treated with PBS; AHE, Aspergillus hyphae extract; F, fresh; HLF, human lung fibroblasts; hMSC, human mesenchymal stromal cells; IL, interleukin; IFN, interferon; mMSC, murine mesenchymal stromal cells; N, naïve; P, vehicle (PBS); PBS, phosphate-buffered saline; T, thawed.
Figure 4.
Systemic administration of fresh or thawed hMSCs or mMSCs significantly reduces increases in BALF inflammatory cells provoked by AHE sensitization and challenge. (A): Total BALF cell numbers in N and A mice treated with fresh and thawed HLF, hMSC, and mMSC. (B): Differential BALF cell populations normalized to total cell numbers: 17 N, 15 A-P, 6 A-HLF-F, 6 A-HLF-T, 10 A-hMSC-F, 6 A-hMSC-T, 6 A-mMSC-F, 6 A-mMSC-T. Data are presented as mean ±SD. p ≤ .05. Significance compared with ∗N and #A-P. ∗∗F versus T. Abbreviations: A, AHE-exposed; A-P, AHE-exposed mice treated with PBS; AHE, Aspergillus hyphae extract; BALF, bronchoalveolar lavage fluid; F, fresh; HLF, human lung fibroblasts; hMSC, human mesenchymal stromal cells; mMSC, murine mesenchymal stromal cells; N, naïve; P, vehicle (PBS); PBS, phosphate-buffered saline; T, thawed.
Administration of hMSCs, mMSCs, and HLFs had more mixed effects on levels of BALF cytokines (Fig. 5). HLFs generally had no effect on levels of any cytokine except for increases in IL-5, IL-6, and IL-12 produced by thawed but not fresh cells over those produced by AHE exposure alone. Both fresh and thawed hMSCs and mMSCs had similar effects in decreasing AHE-provoked increase in BALF IL-4 and RANTES. Fresh mMSCs were less effective than either thawed mMSCs or either fresh or thawed hMSCs in reducing IL-5, whereas both fresh and thawed hMSCs were both comparably more potent in reducing IL-12p40. In contrast, mMSCs, both fresh and thawed, were more potent in increasing IFNγ levels. Thawed mMSCs were more potent in reducing IL-5 than fresh mMSCs, and thawed hMSCs were more potent in reducing both IL-17 and keratinocyte chemoattractant compared with fresh hMSCs. No effect of any cell types was observed on levels of IL-3 or IL-13. The results of the different cell effects on BALF cytokine measures are summarized in Table 1. The number of experimental samples in which measurable levels of cytokines were detected is included in supplemental online Table 1.
Table 1.
Summary of similarities and disparities between groups
Systemic Administration of Fresh or Thawed hMSCs and mMSCs Comparably Ameliorate Antigen-Specific CD4 T-Cell Release of Th2 and Th17 Mediators
AHE sensitization and challenge resulted in a significant increase in release of IL-4, IL-5, and IL-17 by mixed MLN cultures following ex vivo antigen stimulation (Fig. 6). This was most notable at 48 hours, particularly for the increase in IL-17. Administration of either mMSCs or hMSCs, but not HLFs, comparably decreased levels of all three cytokines, and no difference was observed in effects of fresh versus thawed cells. In contrast, both fresh and thawed mMSCs were more potent that hMSCs in increasing IFNγ release.
Figure 6.
Systemic administration of either fresh or thawed hMSCs or mMSCs significantly altered IL-4, IL-5, IL-17, and INFγ production in ex vivo restimulation of mediastinal lymphocytes. Assessment of IL-4, IL-5, IL-17, and INFγ levels in supernatants from mixed mediastinal lymph node cell populations ex vivo restimulated for 48 hours with AHE antigen. n = 17 N, 15 A-P, 6 A-HLF-F, 6 A-HLF-T, 10 A-hMSC-F, 6 A-hMSC-T, 6 A-mMSC-F, 6 A-mMSC-T. All units are picograms per milliliter. Data are represented by the average ±SD. p ≤ .05. Significance compared with ∗N and #A-P. τA-mMSC versus A-hMSC. ∗∗F versus T. Abbreviations: A, AHE-exposed; A-P, AHE-exposed mice treated with PBS; AHE, Aspergillus hyphae extract; F, fresh; HLF, human lung fibroblasts; hMSC, human mesenchymal stromal cells; IL, interleukin; IFN, interferon; mMSC, murine mesenchymal stromal cells; N, naïve; P, vehicle (PBS); PBS, phosphate-buffered saline; T, thawed.
Discussion
The first notable finding of these studies is that hMSCs were as effective, if not more so, than syngeneic mMSCs in ameliorating experimentally induced mixed Th2/Th17 AHR and lung inflammation in an immunocompetent mouse model. The second notable finding is that freshly thawed MSCs were as effective overall as freshly harvested continuously cultured MSCs in decreasing the AHE-induced AHR, lung inflammation, and antigen-specific CD4 Th2/Th17 phenotype.
A growing number of preclinical studies demonstrate that xenogeneic administration of human MSCs is feasible and can be effective in mitigating disease-specific endpoints in different preclinical disease models [41, 42]. Whether these reflect specific anti-inflammatory actions of the administered hMSCs or the newly appreciated instant blood-mediated inflammatory reaction or other reactions to the hMSCs is not yet clear [16, 43, 44]. Whatever the underlying mechanisms, use of xenogeneic hMSC administration in immunocompetent mice provides a novel model with which to investigate these pathways. These approaches may also provide more direct approaches for determining in vivo potency measures of hMSCs for any disease indication. Recent data suggest that in vitro expression of indoleamine 2,3-dioxygenase may correlate with in vivo MSC potency for certain disease applications [45]. This may aid in the selection of appropriate MSC preparations that can be tailored to specific diseases and perhaps even individual patients [8].
A growing number of studies demonstrates efficacy of human MSCs in lung injury models in both immune-deficient and immune-competent mice [31–35]. However, a recent comparison demonstrated that human MSCs were more effective in ameliorating bleomycin-induced lung injury in immune-deficient mice than in immune-competent mice [46]. The current study demonstrates that hMSCs are potent in ameliorating mixed Th2/Th17 AHR, lung inflammation, and antigen-specific Th2/Th17 phenotype. At present, the specific mechanistic actions of the hMSCs in this model have not yet been elucidated. One interesting finding is that administration of syngeneic mMSCs were more potent than hMSCs in inducing the Th1 phenotype (i.e., increased BALF and mixed lymphocyte production of IFNγ). We had previously found that amelioration of Th2 eosinophilic allergic airway inflammation by both syngeneic and allogeneic MSCs involved an IFNγ-dependent upregulation of the Th1 phenotype [23]. Upregulation of antigen-specific IFNγ-producing Th1 CD4 cells is recognized as counterbalancing Th2-mediated allergic airway inflammation [47, 48]. Whether this also a significant mechanism by which mMSCs and hMSCs ameliorate allergic airway inflammation in the mixed Th2/Th17 AHE model remains to be determined.
The findings that freshly thawed MSCs were as potent, if not even more so, than freshly harvested continuously cultured MSCs from the same passage number and plating in ameliorating AHR, lung inflammation, and antigen-specific Th phenotype is in contrast to recent in vitro data and a retrospective analysis of clinical MSC investigations [5, 6]. A number of factors could potentially explain these differing observations that will need further investigation. In the current study, cell viability was ∼70% after thawing and harvest compared with ∼100% viability of the freshly harvested, continuously cultured cells. These are comparable to experiences in other studies, as are the freezing medium and the freeze-thaw technique used [11–14]. As such, there is no obvious discrepancy in the technical approaches. We used equal numbers of viable cells because it seemed an apt comparison. As such, 30% more cells were used in mice receiving thawed cells so as to have ∼1 million viable cells injected. In real numbers, this equates to ∼1.3 million thawed versus 1 million fresh cells. Acknowledging the differences, the total cell numbers are still well within the range of 1 million to 2 million cells per mouse administered in previous studies [23, 28], and so we do not think the total cell numbers accounted for any significant effects in the outcome measures. Although the increased number of cells and perhaps the presence of apoptotic and likely necrotic cells may have stimulated inflammatory host reactions, they did not appear to influence effects on the AHE-stimulated AHR, lung inflammation, and antigen-specific Th phenotype. The length of freezing prior to thaw in the current studies was relatively short (7 days) and may not have had the same effect as longer freezing. Longer freeze time prior to thawing and use has been demonstrated to have detrimental effects on initial cell viability after thawing and on proliferative and differentiation capacities [11–14]. However, length of freeze time on anti-inflammatory actions is not yet well understood.
It is noteworthy that similar effects of fresh versus thawed cells were seen not just with syngeneic mMSC but also with xenogeneic hMSC administration. This suggests that whatever actions that MSCs are exerting to ameliorate inflammation in this specific disease model are not affected by species difference or by any potential detrimental effects of the freeze-thaw on relevant anti-inflammatory actions of the MSCs. As such, these results complement increasing data demonstrating that effects such as those of xenogeneic versus syngeneic or allogeneic cells and the use of fresh versus thawed MSCs will be disease specific. This reflects the increasing recognition of the complexity of potential MSC actions and will need to be carefully investigated in appropriate disease-specific contexts.
Conclusion
Even accounting for some difference in the actual numbers of cells administered, freshly thawed bone marrow-derived MSCs were as potent as, if not more potent than, continuously cultured MSCs in ameliorating airways hyper-responsiveness, lung inflammation, and activity of antigen-specific CD4 T lymphocytes in an in vivo model of mixed Th2/Th17 allergic airway inflammation. These data suggest that administration of freshly thawed MSCs may be effective in appropriate clinical scenarios. However, this needs to be investigated in each disease system for which the MSCs might conceivably be used. In parallel, human bone marrow-derived MSCs were as, if not more, potent than mouse MSCs in this model. This observation adds to a growing number of reports demonstrating efficacy of xenogeneic human MSC administration in immunocompetent mouse models of disease. However, the mechanisms by which the human MSCs act have not yet been elucidated and may be different from those by which mouse MSCs act.
Supplementary Material
Acknowledgments
We thank Nirav Daphtary and Minara Aliyeva of the Vermont Lung Center Core facility for assistance with Flexivent technical support and Joseph Platz and Melissa Lathrop for technical advice. This research was supported by NIH American Recovery and Reinvestment Act (ARRA) RC4HL106625 (D.J.W.), National Heart, Lung, and Blood Institute (NHLBI) R21HL108689 (D.J.W.), the Vermont Lung Center of Biomedical Research Excellence (CoBRE) grant (P20RR15557), the Brazilian Council for Scientific and Technological Development (CNPq)—Science Without Borders, and the NIH Production Assistance for Cellular Therapies (PACT) program (contract HHSN268201000008C). Mouse mesenchymal stromal cells used in this work were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through NIH/National Center for Research Resources (NCRR) Grant P40RR017447.
Author Contributions
F.F.C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; Z.D.B., M.G., D.S., and D.W.: collection and/or assembly of data, final approval of manuscript; D.H.M.: provision of study materials, manuscript writing, final approval of manuscript; P.R.M.R.: conception and design, final approval of manuscript; D.J.W.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
D.H.M. is an uncompensated consultant with Erytech Pharma and Data and Safety Monitoring Board and has compensated research funding from PACT NIH/NHLBI, contract; Novartis contract. The other authors indicated no potential conflicts of interest.
References
- 1.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 2.Keating A. Mesenchymal stromal cells: New directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Bahr L, Sundberg B, Lönnies L, et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant. 2012;18:557–564. doi: 10.1016/j.bbmt.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Cyranoski D. Canada approves stem cell product. Nat Biotechnol. 2012;30:571. [Google Scholar]
- 8.Krampera M, Galipeau J, Shi Y, et al. Immunological characterization of multipotent mesenchymal stromal cells--the International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054–1061. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Menard C, Pacelli L, Bassi G, et al. Clinical-grade mesenchymal stromal cells produced under various good manufacturing practice processes differ in their immunomodulatory properties: Standardization of immune quality controls. Stem Cells Dev. 2013;22:1789–1801. doi: 10.1089/scd.2012.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viswanathan S, Keating A, Deans R, et al. Soliciting strategies for developing cell-based reference materials to advance mesenchymal stromal cell research and clinical translation. Stem Cells Dev. 2014;23:1157–1167. doi: 10.1089/scd.2013.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong CY, Subramanian A, Biswas A, et al. Derivation efficiency, cell proliferation, freeze-thaw survival, stem-cell properties and differentiation of human Wharton’s jelly stem cells. Reprod Biomed Online. 2010;21:391–401. doi: 10.1016/j.rbmo.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Eini F, Foroutan T, Bidadkosh A. The effects of freeze/thawing process on cryopreserved equine umbilical cord blood-derived mesenchymal stem cells. Comp Clin Pathol. 2012;21:1713–1718. [Google Scholar]
- 13.Naaldijk Y, Staude M, Fedorova V, et al. Effect of different freezing rates during cryopreservation of rat mesenchymal stem cells using combinations of hydroxyethyl starch and dimethylsulfoxide. BMC Biotechnol. 2012;12:49. doi: 10.1186/1472-6750-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginis I, Grinblat B, Shirvan MH. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng Part C Methods. 2012;18:453–463. doi: 10.1089/ten.TEC.2011.0395. [DOI] [PubMed] [Google Scholar]
- 15.François M, Copland IB, Yuan S, et al. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy. 2012;14:147–152. doi: 10.3109/14653249.2011.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moll G, Alm JJ, Davies LC, et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32:2430–2442. doi: 10.1002/stem.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho KS, Park HK, Park HY, et al. IFATS collection: Immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells. 2009;27:259–265. doi: 10.1634/stemcells.2008-0283. [DOI] [PubMed] [Google Scholar]
- 18.Cho KS, Roh HJ. Immunomodulatory effects of adipose-derived stem cells in airway allergic diseases. Curr Stem Cell Res Ther. 2010;5:111–115. doi: 10.2174/157488810791268681. [DOI] [PubMed] [Google Scholar]
- 19.Bonfield TL, Koloze M, Lennon DP, et al. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol. 2010;299:L760–L770. doi: 10.1152/ajplung.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HK, Cho KS, Park HY, et al. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev. 2010;19:1811–1818. doi: 10.1089/scd.2009.0513. [DOI] [PubMed] [Google Scholar]
- 21.Nemeth K, Keane-Myers A, Brown JM, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Firinci F, Karaman M, Baran Y, et al. Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. Int Immunopharmacol. 2011;11:1120–1126. doi: 10.1016/j.intimp.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin M, Sueblinvong V, Eisenhauer P, et al. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29:1137–1148. doi: 10.1002/stem.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011;66:523–531. doi: 10.1111/j.1398-9995.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Jang AS, Kwon JH, et al. Mesenchymal stem cell transfer suppresses airway remodeling in a toluene diisocyanate-induced murine asthma model. Allergy Asthma Immunol Res. 2011;3:205–211. doi: 10.4168/aair.2011.3.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ou-Yang HF, Huang Y, Hu XB, et al. Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med (Maywood) 2011;236:1461–1467. doi: 10.1258/ebm.2011.011221. [DOI] [PubMed] [Google Scholar]
- 27.Ionescu LI, Alphonse RS, Arizmendi N, et al. Airway delivery of soluble factors from plastic-adherent bone marrow cells prevents murine asthma. Am J Respir Cell Mol Biol. 2012;46:207–216. doi: 10.1165/rcmb.2010-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lathrop MJ, Brooks EM, Bonenfant NR, et al. Mesenchymal stromal cells mediate Aspergillus hyphal extract-induced allergic airway inflammation by inhibition of the Th17 signaling pathway. Stem Cells Translational Medicine. 2014;3:194–205. doi: 10.5966/sctm.2013-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allard JB, Poynter ME, Marr KA, et al. Aspergillus fumigatus generates an enhanced Th2-biased immune response in mice with defective cystic fibrosis transmembrane conductance regulator. J Immunol. 2006;177:5186–5194. doi: 10.4049/jimmunol.177.8.5186. [DOI] [PubMed] [Google Scholar]
- 30.Allard JB, Rinaldi L, Wargo MJ, et al. Th2 allergic immune response to inhaled fungal antigens is modulated by TLR-4-independent bacterial products. Eur J Immunol. 2009;39:776–788. doi: 10.1002/eji.200838932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim ES, Chang YS, Choi SJ, et al. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir Res. 2011;12:108. doi: 10.1186/1465-9921-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, Han ZB, Liao W, et al. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti- and pro-inflammatory factors. Cell Physiol Biochem. 2011;27:587–596. doi: 10.1159/000329980. [DOI] [PubMed] [Google Scholar]
- 33.Chang YS, Choi SJ, Sung DK, et al. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011;20:1843–1854. doi: 10.3727/096368911X565038. [DOI] [PubMed] [Google Scholar]
- 34.Pierro M, Ionescu L, Montemurro T, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013;68:475–484. doi: 10.1136/thoraxjnl-2012-202323. [DOI] [PubMed] [Google Scholar]
- 35.Pati S, Gerber M, Menge TD, et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6:e25171. doi: 10.1371/journal.pone.0025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 37.About PACT. Available at https://secure.emmes.com/pactweb/Facilities. Accessed January 14, 2015.
- 38.Reed W, Noga SJ, Gee AP, et al. Production Assistance for Cellular Therapies (PACT): Four-year experience from the United States National Heart, Lung, and Blood Institute (NHLBI) contract research program in cell and tissue therapies. Transfusion. 2009;49:786–796. doi: 10.1111/j.1537-2995.2008.02027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuessler TF, Bates JH. A computer-controlled research ventilator for small animals: Design and evaluation. IEEE Trans Biomed Eng. 1995;42:860–866. doi: 10.1109/10.412653. [DOI] [PubMed] [Google Scholar]
- 40.Gomes RF, Shardonofsky F, Eidelman DH, et al. Respiratory mechanics and lung development in the rat from early age to adulthood. J Appl Physiol (1985) 2001;90:1631–1638. doi: 10.1152/jappl.2001.90.5.1631. [DOI] [PubMed] [Google Scholar]
- 41.Asmussen S, Ito H, Traber DL, et al. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax. 2014;69:819–825. doi: 10.1136/thoraxjnl-2013-204980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amos PJ, Kapur SK, Stapor PC, et al. Human adipose-derived stromal cells accelerate diabetic wound healing: Impact of cell formulation and delivery. Tissue Eng Part A. 2010;16:1595–1606. doi: 10.1089/ten.tea.2009.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moll G, Rasmusson-Duprez I, von Bahr L, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells. 2012;30:1565–1574. doi: 10.1002/stem.1111. [DOI] [PubMed] [Google Scholar]
- 44.Jitschin R, Mougiakakos D, Von Bahr L, et al. Alterations in the cellular immune compartment of patients treated with third-party mesenchymal stromal cells following allogeneic hematopoietic stem cell transplantation. Stem Cells. 2013;31:1715–1725. doi: 10.1002/stem.1386. [DOI] [PubMed] [Google Scholar]
- 45.François M, Romieu-Mourez R, Li M, et al. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 46.Lim R, Milton P, Murphy SV, et al. Human mesenchymal stem cells reduce lung injury in immunocompromised mice but not in immunocompetent mice. Respiration. 2013;85:332–341. doi: 10.1159/000343078. [DOI] [PubMed] [Google Scholar]
- 47.Gajewski TF, Fitch FW. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–4252. [PubMed] [Google Scholar]
- 48.Parronchi P, De Carli M, Manetti R, et al. IL-4 and IFN (alpha and gamma) exert opposite regulatory effects on the development of cytolytic potential by Th1 or Th2 human T cell clones. J Immunol. 1992;149:2977–2983. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.